Plant Metabolites as Potential Agents That Potentiate or Block Resistance Mechanisms Involving β-Lactamases and Efflux Pumps
Abstract
1. Introduction
2. Medicinal Plant Secondary Metabolites as Antimicrobial Agents
3. Bacterial Efflux Pump Mechanisms
4. Mechanism of β-Lactamases
5. Phytochemicals as Efflux Pump and β-Lactamase Inhibitors
6. Plant Metabolite Structure Activity Relationship
7. Synergistic Interactions Between Efflux Pump and β-Lactamase Inhibitor Phytochemicals and Conventional Antibiotics
8. Plant Metabolites Efflux Pump Inhibitor Mechanisms
9. Plant Metabolites β-Lactamase Inhibitor Mechanism
10. Challenges and Future Perspectives
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akova, M. Epidemiology of antimicrobial resistance in bloodstream infections. Virulence 2016, 7, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Goossens, H. Antibiotic consumption and link to resistance. Clin. Microbiol. Infect. 2009, 15, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.L.; Oliver, K.B. Antibiotic resistance threats in the United States: Stepping back from the brink. Am. Fam. Physician 2014, 89, 938–941. [Google Scholar]
- Maragakis, L.L.; Perencevich, E.N.; Cosgrove, S.E. Clinical and economic burden of antimicrobial resistance. Expert Rev. Anti-Infect. Ther. 2008, 6, 751–763. [Google Scholar] [CrossRef]
- Filice, G.A.; Nyman, J.A.; Lexau, C.; Lees, C.H.; Bockstedt, L.A.; Como-Sabetti, K.; Lesher, L.J.; Lynfield, R. Excess costs and utilization associated with methicillin resistance for patients with Staphylococcus aureus infection. Infect. Control. Hosp. Epidemiol. 2010, 31, 365–373. [Google Scholar] [CrossRef]
- Hübner, C.; Hübner, N.O.; Hopert, K.; Maletzki, S.; Flessa, S. Analysis of MRSA-attributed costs of hospitalized patients in Germany. Eur. J. Clin. Microbiol. 2014, 33, 1817–1822. [Google Scholar] [CrossRef]
- Macedo-Vinas, M.; De Angelis, G.; Rohner, P.; Safran, E.; Stewardson, A.; Fankhauser, C.; Schrenzel, J.; Pittet, D.; Harbarth, S. Burden of methicillin-resistant Staphylococcus aureus infections at a Swiss University hospital: Excess length of stay and costs. J. Hosp. Infect. 2013, 84, 132–137. [Google Scholar] [CrossRef]
- Alcalde-Rico, M.; Hernando-Amado, S.; Blanco, P.; Martinez, J.L. Multidrug efflux pumps at the crossroad between antibiotic resistance and bacterial virulence. Front. Microbiol. 2016, 7, 1483. [Google Scholar] [CrossRef]
- Grkovic, S.; Brown, M.H.; Skurray, R.A. Regulation of bacterial drug export systems. Microbiol. Mol. Biol. Rev. 2002, 66, 671–701. [Google Scholar] [CrossRef]
- Avakh, A.; Grant, G.D.; Cheesman, M.J.; Kalkundri, T.; Hall, S. The art of war with Pseudomonas aeruginosa: Targeting mex efflux pumps directly to strategically enhance antipseudomonal drug efficacy. Antibiotics 2023, 12, 1304. [Google Scholar] [CrossRef] [PubMed]
- Du, D.J.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018, 16, 577. [Google Scholar] [CrossRef]
- Hassan, K.A.; Jackson, S.M.; Penesyan, A.; Patching, S.G.; Tetu, S.G.; Eijkelkamp, B.A.; Brown, M.H.; Henderson, P.J.F.; Paulsen, I.T. Transcriptomic and biochemical analyses identify a family of chlorhexidine efflux proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 20254–20259. [Google Scholar] [CrossRef]
- Hassan, K.A.; Liu, Q.; Henderson, P.J.F.; Paulsena, I.T. Homologs of the Acinetobacter baumannii AceI transporter represent a new family of bacterial multidrug efflux systems. mBio 2015, 6, e01982-14. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, R.A. β-Lactamases: A focus on current challenges. Cold Spring Harb. Perspect. Med. 2017, 7, a025239. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Naas, T.; Poirel, L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2011, 17, 1791–1798. [Google Scholar] [CrossRef]
- Naas, T.; Dortet, L.; Iorga, B.I. Structural and functional aspects of Class A carbapenemases. Curr. Drug Targets 2016, 17, 1006–1028. [Google Scholar] [CrossRef]
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef]
- González-Lamothe, R.; Mitchell, G.; Gattuso, M.; Diarra, M.S.; Malouin, F.; Bouarab, K. Plant antimicrobial agents and their effects on plant and human pathogens. Int. J. Mol. Sci. 2009, 10, 3400–3419. [Google Scholar] [CrossRef]
- Keita, K.; Darkoh, C.; Okafor, F. Secondary plant metabolites as potent drug candidates against antimicrobial-resistant pathogens. SN Appl. Sci. 2022, 4, 209. [Google Scholar] [CrossRef]
- Li, J.M.; Feng, S.S.; Liu, X.; Jia, X.; Qiao, F.L.; Guo, J.L.; Deng, S.S. Effects of traditional Chinese medicine and its active ingredients on drug-resistant bacteria. Front. Pharmacol. 2022, 13, 837907. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Khare, T.; Shriram, V.; Bae, H.; Kumar, V. Plant synthetic biology for producing potent phyto-antimicrobials to combat antimicrobial resistance. Biotechnol. Adv. 2021, 48, 107729. [Google Scholar] [CrossRef]
- Radulovic, N.S.; Blagojevic, P.D.; Stojanovic-Radic, Z.Z.; Stojanovic, N.M. Antimicrobial plant metabolites: Structural diversity and mechanism of action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar] [PubMed]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the role of plant natural products in antibiotic drug discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Bin Emran, T.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef]
- Dib, M.E.; Allali, H.; Bendiabdellah, A.; Meliani, N.; Tabti, B. Antimicrobial activity phytochemical screening of Arbutus unedo L. J. Saudi Chem. Soc. 2013, 17, 381–385. [Google Scholar] [CrossRef]
- Tan, K.K.; Khoo, T.J.; Rajagopal, M.; Wiart, C. Antibacterial alkaloids from Artabotrys crassifolius Hook.f. & Thomson. Nat. Prod. Res. 2015, 29, 2346–2349. [Google Scholar]
- Sardessai, Y.; Desai, R.R.; Joshi, A.B.; Bhobe, M.P. Screening for antimicrobial activity of the stem bark of Bauhinia purpurea Linn. Glob. J. Pharmacol. 2013, 7, 288–293. [Google Scholar]
- Azimi, G.; Hakakian, A.; Ghanadian, M.; Joumaa, A.; Alamian, S. Bioassay-directed isolation of quaternary benzylisoquinolines from Berberis integerrima with bactericidal activity against Brucella abortus. Res. Pharm. Sci. 2018, 13, 149–158. [Google Scholar]
- Mandi, F.; Morgan, J.B.; Liu, W.L.; Agarwal, A.K.; Jekabsons, M.B.; Liu, Y.; Zhou, Y.D.; Nagle, D.G. Sampangine (a Copyrine Alkaloid) Exerts Biological Activities through Cellular Redox Cycling of Its Quinone and Semiquinone Intermediates. J. Nat. Prod. 2015, 78, 3018–3023. [Google Scholar]
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; MacNair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the hidden antibiotic potential of cannabis. ACS Infect. Dis. 2020, 6, 338–346. [Google Scholar] [CrossRef]
- Subhadradevi, V.; Asokkumar, K.; Umamaheswari, M.; Sivashanmugam, A.; Ushanandhini, J.; Jagannath, P. Antimicrobial activity of leaves and flowers of Cassia auriculata Linn. Bangladesh J. Sci. Ind. Res. 2011, 46, 513–518. [Google Scholar] [CrossRef]
- Oloyede, G.K.; Onocha, P.A.; Soyinka, J.; Oguntokun, O.; Thonda, E. Phytochemical screening, antimicrobial and antioxidant activities of four Nigerian medicinal plants. Ann. Biol. Res. 2010, 1, 114–120. [Google Scholar]
- Kumar, R.S.; Balasubramanian, P.; Govindaraj, P.; Krishnaveni, T. Preliminary studies on phytochemicals and antimicrobial activity of solvent extracts of Coriandrum sativum L. roots (Coriander). J. Pharmacogn. Phytochem. 2014, 2, 74–78. [Google Scholar]
- Shen, F.G.; Tang, X.D.; Wang, Y.; Yang, Z.Q.; Shi, X.C.; Wang, C.; Zhang, Q.L.; An, Y.A.; Cheng, W.; Jin, K.Q.; et al. Phenotype and expression profile analysis of Staphylococcus aureus biofilms and planktonic cells in response to licochalcone A. Appl. Microbiol. Biotechnol. 2015, 99, 359–373. [Google Scholar] [CrossRef]
- Pájaro-Gonzalez, Y.; Oliveros-Diaz, A.F.; Cabrera-Barraza, J.; Fernández-Daza, E.; Reyes, N.; Montes-Guevara, O.A.; Caro-Fuentes, D.; Franco-Ospina, L.; Quiñones-Fletcher, W.; Quave, C.L.; et al. Mammea B/BA isolated from the seeds of Mammea americana L. (Calophyllaceae) is a potent inhibitor of methicillin-resistant Staphylococcus aureus. Front. Pharmacol. 2022, 13, 826404. [Google Scholar] [CrossRef]
- Mada, S.; Garba, A.; Mohammed, H.a.A.; Muhammad, A.; Olagunju, A.; Muhammad, A. Antimicrobial activity and phytochemical screening of aqueous and ethanol extracts of Momordica charantia L. leaves. J. Med. Plants Res. 2013, 7, 579–586. [Google Scholar]
- Singh, A.R.; Bajaj, V.K.; Sekhawat, P.S.; Singh, K. Phytochemical estimation and antimicrobial activity of aqueous and methanolic extract of Ocimum sanctum L. J. Nat. Prod. Plant Resour. 2013, 3, 51–58. [Google Scholar]
- Dhale, D.; Mogle, U. Phytochemical screening and antibacterial activity of Phyllanthus emblica (L.). Sci. Res. Rep. 2011, 1, 138–142. [Google Scholar]
- Pandey, A. Antibacterial properties of Psidium guajava leaves, fruits and stems against various pathogens. Int. J. Pharm. Res. Dev. 2011, 3, 15–24. [Google Scholar]
- Gosset-Erard, C.; Zhao, M.J.; Lordel-Madeleine, S.; Ennahar, S. Identification of punicalagin as the bioactive compound behind the antimicrobial activity of pomegranate (Punica granatum L.) peels. Food Chem. 2021, 352, 129396. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Liu, H.X.; Wang, L.; Xu, Z.F.; Tan, H.B.; Qiu, S.X. Rhodomyrtosone B, a membrane-targeting anti-MRSA natural acylgphloroglucinol from Rhodomyrtus tomentosa. J. Ethnopharmacol. 2019, 228, 50–57. [Google Scholar] [CrossRef]
- Hamoud, R.; Reichling, J.; Wink, M. Synergistic antibacterial activity of the combination of the alkaloid sanguinarine with EDTA and the antibiotic streptomycin against multidrug resistant bacteria. J. Pharm. Pharmacol. 2015, 67, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, I.T.S.; Neto, A.T.; Pedroso, M.; Mostardeiro, C.P.; Da Cruz, I.B.M.; Silva, U.F.; Ilha, V.; Dalcol, I.I.; Morel, A.F. Antimicrobial activity of Schinus lentiscifolius (Anacardiaceae). J. Ethnopharmacol. 2013, 148, 486–491. [Google Scholar] [CrossRef]
- Webber, M.A.; Piddock, L.J.V. The importance of efflux pumps in bacterial antibiotic resistance. J. Antimicrob. Chemother. 2003, 51, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J.V. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Balzi, E.; Tulkens, P.M. Antibiotic efflux pumps—Commentary. Biochem. Pharmacol. 2000, 60, 457–470. [Google Scholar] [CrossRef]
- Bush, K. Past and present perspectives on beta-lactamases. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Tehrani, K.H.M.E.; Martin, N.I. β-lactam/β-lactamase inhibitor combinations: An update. Medchemcomm 2018, 9, 1439–1456. [Google Scholar] [CrossRef]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.F.; Stephen, J.; Lekshmi, M.; Ojha, M.; Wenzel, N.; Sanford, L.M.; Hernandez, A.J.; Parvathi, A.; Kumar, S.H. Bacterial resistance to antimicrobial agents. Antibiotics 2021, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, S.; Bellucci, M.C.; Mondelli, R. Mode of binding of the cytotoxic alkaloid berberine with the double helix oligonucleotide D(AAGAATTCTT). Bioorg. Med. Chem. 2003, 11, 505–514. [Google Scholar] [CrossRef]
- Imanshahidi, M.; Hosseinzadeh, H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother. Res. 2008, 22, 999–1012. [Google Scholar] [CrossRef]
- Huang, C.G.; Chu, Z.L.; Wei, S.J.; Jiang, H.; Jiao, B.H. Effect of berberine on arachidonic acid metabolism in rabbit platelets and endothelial cells. Thromb. Res. 2002, 106, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Zhan, Y.Y.; Hou, J.; Xu, B.; Chen, B.; Tian, Y.; Wu, D.; Zhao, Y.; Zhang, Y.; Chen, X.; et al. Berberine binds RXRα to suppress β-catenin signaling in colon cancer cells. Oncogene 2017, 36, 6906–6918. [Google Scholar] [CrossRef]
- Ko, W.H.; Yao, X.Q.; Lau, C.W.; Law, W.I.; Chen, Z.Y.; Kwok, W.; Ho, K.; Huang, Y. Vasorelaxant and antiproliferative effects of berberine. Eur. J. Pharmacol. 2000, 399, 187–196. [Google Scholar] [CrossRef]
- Liu, Y.M.; Niu, L.; Wang, L.L.; Bai, L.; Fang, X.Y.; Li, Y.C.; Yi, L.T. Berberine attenuates depressive-like behaviors by suppressing neuro-inflammation in stressed mice. Brain Res. Bull. 2017, 134, 220–227. [Google Scholar] [CrossRef]
- Kim, T.S.; Kang, B.Y.; Cho, D.H.; Kim, S.H. Induction of interleukin-12 production in mouse macrophages by berberine, a benzodioxoloquinolizine alkaloid, deviates CD4+T cells from a Th2 to a Th1 response. Immunology 2003, 109, 407–414. [Google Scholar] [CrossRef]
- Vinod, N.V.; Shijina, R.; Dileep, K.V.; Sadasivan, C. Inhibition of beta-lactamase by 1,4-Naphthalenedione from the Plant Holoptelea integrifolia. Appl. Biochem. Biotechnol. 2010, 160, 1752–1759. [Google Scholar] [CrossRef]
- Elfaky, M.A.; El-Halawany, A.M.; Koshak, A.E.; Alshali, K.Z.; El-Araby, M.E.; Khayat, M.T.; Abdallah, H.M. Bioassay guided isolation and docking studies of a potential β-lactamase inhibitor from Clutia myricoides. Molecules 2020, 25, 2566. [Google Scholar] [CrossRef]
- Zhou, X.; Jia, F.; Liu, X.; Yang, J.; Zhang, Y.; Wang, Y. In vitro synergistic interaction of 5-O-methylglovanon and ampicillin against ampicillin resistant Staphylococcus aureus and Staphylococcus epidermidis isolates. Arch. Pharm. Res. 2011, 34, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.; Oluwatuyi, M.; Kaatz, G.W. A novel inhibitor of multidrug efflux pumps in Staphylococcus aureus. J. Antimicrob. Chemoth. 2003, 51, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Konaté, K.; Mavoungou, J.F.; Lepengué, A.N.; Aworet-Samseny, R.R.R.; Hilou, A.; Souza, A.; Dicko, M.H.; M’Batchi, B. Antibacterial activity against β-lactamase producing methicillin and ampicillin-resistants Staphylococcus aureus: Fractional Inhibitory Concentration Index (FICI) determination. Ann. Clin. Microb. Antimicrob. 2012, 11, 18. [Google Scholar] [CrossRef]
- Suga, T.; Ishii, T.; Iwatsuki, M.; Yamamoto, T.; Nonaka, K.; Masuma, R.; Matsui, H.; Hanaki, H.; Omura, S.; Shiomi, K. Aranorosin circumvents arbekacin-resistance in MRSA by inhibiting the bifunctional enzyme AAC(6′)/APH(2″). J. Antibiot. 2012, 65, 527–529. [Google Scholar] [CrossRef]
- Chan, B.C.; Ip, M.; Lau, C.B.; Lui, S.L.; Jolivalt, C.; Ganem-Elbaz, C.; Litaudon, M.; Reiner, N.E.; Gong, H.; See, R.H.; et al. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011, 137, 767–773. [Google Scholar] [CrossRef]
- Qian, M.Y.; Tang, S.S.; Wu, C.M.; Wang, Y.; He, T.; Chen, T.T.; Xiao, X.L. Synergy between baicalein and penicillins against penicillinase-producing Staphylococcus aureus. Int. J. Med. Microbiol. 2015, 305, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Aghayan, S.S.; Kalalian Mogadam, H.; Fazli, M.; Darban-Sarokhalil, D.; Khoramrooz, S.S.; Jabalameli, F.; Yaslianifard, S.; Mirzaii, M. The effects of berberine and palmatine on efflux pumps inhibition with different gene patterns in Pseudomonas aeruginosa isolated from burn infections. Avicenna J. Med. Biotechnol. 2017, 9, 2–7. [Google Scholar]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.J.J.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against Gram-positive pathogenic bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef]
- Kalia, N.P.; Mahajan, P.; Mehra, R.; Nargotra, A.; Sharma, J.P.; Koul, S.; Khan, I.A. Capsaicin, a novel inhibitor of the NorA efflux pump, reduces the intracellular invasion of Staphylococcus aureus. J. Antimicrob. Chemother. 2012, 67, 2401–2408. [Google Scholar] [CrossRef]
- Dwivedi, G.R.; Tyagi, R.; Sanchita; Tripathi, S.; Pati, S.; Srivastava, S.K.; Darokar, M.P.; Sharma, A. Antibiotics potentiating potential of catharanthine against superbug Pseudomonas aeruginosa. J. Biomol. Struct. Dyn. 2018, 36, 4270–4284. [Google Scholar] [CrossRef]
- Siriyong, T.; Srimanote, P.; Chusri, S.; Yingyongnarongkul, B.E.; Suaisom, C.; Tipmanee, V.; Voravuthikunchai, S.P. Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2017, 17, 405. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Kumari, N.; Pahwa, S.; Agrahari, U.C.; Bhutani, K.K.; Jachak, S.M.; Nandanwar, H. NorA efflux pump inhibitory activity of coumarins from Mesua ferrea L. Fitoterapia 2013, 90, 140–150. Fitoterapia 2013, 90, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Stermitz, F.R.; Scriven, L.N.; Tegos, G.; Lewis, K. Two flavonols from Artemisa annua which potentiate the activity of berberine and norfloxacin against a resistant strain of Staphylococcus aureus. Planta Medica 2002, 68, 1140–1141. [Google Scholar] [CrossRef]
- Ramalhete, C.; Spengler, G.; Martins, A.; Martins, M.; Viveiros, M.; Mulhovo, S.; Ferreira, M.-J.U.; Amaral, L. Inhibition of efflux pumps in methicillin-resistant Staphylococcus aureus and Enterococcus faecalis resistant strains by triterpenoids from Momordica balsamina. Int. J. Antimicrob. Agents 2011, 37, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Kakarla, P.; Floyd, J.; Mukherjee, M.; Devireddy, A.R.; Inupakutika, M.A.; Ranweera, I.; Ranjana, K.C.; Shrestha, U.; Cheeti, U.R.; Willmon, T.M.; et al. Inhibition of the multidrug efflux pump LmrS from Staphylococcus aureus by cumin spice Cuminum cyminum. Arch. Microbiol. 2017, 199, 465–474. [Google Scholar] [CrossRef]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef]
- Smith, E.C.J.; Williamson, E.M.; Wareham, N.; Kaatz, G.W.; Gibbons, S. Antibacterials and modulators of bacterial resistance from the immature cones of Chamaecyparis lawsoniana. Phytochemistry 2007, 68, 210–217. [Google Scholar] [CrossRef]
- Bag, A.; Chattopadhyay, R.R. Efflux-pump inhibitory activity of a gallotannin from Terminalia chebula fruit against multidrug-resistant uropathogenic Escherichia coli. Nat. Prod. Res. 2014, 28, 1280–1283. [Google Scholar] [CrossRef]
- Ponnusamy, K.; Ramasamy, M.; Savarimuthu, I.; Paulraj, M.G. Indirubin potentiates ciprofloxacin activity in the NorA efflux pump of Staphylococcus aureus. Scand. J. Infect. Dis. 2010, 42, 500–505. [Google Scholar] [CrossRef]
- Holler, J.G.; Christensen, S.B.; Slotved, H.C.; Rasmussen, H.B.; Gúzman, A.; Olsen, C.E.; Petersen, B.; Molgaard, P. Novel inhibitory activity of the NorA efflux pump by a kaempferol rhamnoside isolated from Persea lingue Nees. J. Antimicrob. Chemother. 2012, 67, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Fakudze, N.T.; Sarbadhikary, P.; George, B.P.; Abrahamse, H. Ethnomedicinal Uses, phytochemistry, and anticancer potentials of African medicinal fruits: A Comprehensive Review. Pharmaceuticals 2023, 16, 1117. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Singh, S.; Wani, A.; Sharma, S.; Jain, S.K.; Singh, B.; Gupta, B.D.; Satti, N.K.; Koul, S.; Khan, I.A.; et al. Osthol and curcumin as inhibitors of human Pgp and multidrug efflux pumps of Staphylococcus aureus: Reversing the resistance against frontline antibacterial drugs. MedChemComm 2014, 5, 1540–1547. [Google Scholar] [CrossRef]
- Negi, N.; Prakash, P.; Gupta, M.L.; Mohapatra, T.M. Possible role of curcumin as an efflux pump inhibitor in multi drug resistant clinical isolates of Pseudomonas aeruginosa. J. Clin. Diagn. Res. 2014, 8, DC04–DC07. [Google Scholar] [CrossRef] [PubMed]
- Cabral, V.; Luo, X.; Junqueira, E.; Costa, S.S.; Mulhovo, S.; Duarte, A.; Couto, I.; Viveiros, M.; Ferreira, M.J. Enhancing activity of antibiotics against Staphylococcus aureus: Zanthoxylum capense constituents and derivatives. Phytomedicine 2015, 22, 469–476. [Google Scholar] [CrossRef]
- Mahamoud, A.; Chevalier, J.; Alibert-Franco, S.; Kern, W.V.; Pagès, J.M. Antibiotic efflux pumps in Gram-negative bacteria: The inhibitor response strategy. J. Antimicrob. Chemother. 2007, 59, 1223–1229. [Google Scholar] [CrossRef]
- Coates, N.J.; Gilpin, M.L.; Gwynn, M.N.; Lewis, D.E.; Milner, P.H.; Spear, S.R.; Tyler, J.W. SB-202742, a novel beta-lactamase inhibitor isolated from Spondias mombin. J. Nat. Prod. 1994, 57, 654–657. [Google Scholar] [CrossRef]
- Wang, D.; Xie, K.; Zou, D.; Meng, M.; Xie, M. Inhibitory effects of silybin on the efflux pump of methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 2018, 18, 827–833. [Google Scholar]
- Shiota, S.; Shimizu, M.; Sugiyama, J.i.; Morita, Y.; Mizushima, T.; Tsuchiya, T. Mechanisms of action of corilagin and tellimagrandin I that remarkably potentiate the activity of β-lactams against methicillin-resistant Staphylococcus aureus. Microbiol. Immunol. 2004, 48, 67–73. [Google Scholar] [CrossRef]
- Koche, D.; Shirsat, R.; Kawale, M. An overview of major classes of phytochemicals: Their types and role in disease prevention. Hislopia J. 2016, 9, 1–11. [Google Scholar]
- Hegnauer, R. Biochemistry, Distribution and Taxonomic Relevance of Higher-Plant Alkaloids. Phytochemistry 1988, 27, 2423–2427. [Google Scholar] [CrossRef]
- Atawodi, S.E.; Atawodi, J.C. Azadirachta indica (neem): A plant of multiple biological and pharmacological activities. Phytochem. Rev. 2009, 8, 601–620. [Google Scholar] [CrossRef]
- Kubo, I.; Muroi, H.; Kubo, A. Structural Functions of Antimicrobial Long-Chain Alcohols and Phenols. Bioorg. Med. Chem. 1995, 3, 873–880. [Google Scholar] [CrossRef]
- Merkl, R.; Hrádková, I.; Filip, V.; Smidrkal, J. Antimicrobial and Antioxidant Properties of Phenolic Acids Alkyl Esters. Czech J. Food Sci. 2010, 28, 275–279. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Alvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Stapleton, P.D.; Shah, S.; Anderson, J.C.; Hara, Y.; Hamilton-Miller, J.M.T.; Taylor, P.W. Modulation of β-lactam resistance in Staphylococcus aureus by catechins and gallates. Int. J. Antimicrob. Agents 2004, 23, 462–467. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Marquez, B.; Neuville, L.; Moreau, N.J.; Genet, J.P.; dos Santos, A.F.; de Andrade, M.C.C.; Sant’Ana, A.E.G. Multidrug resistance reversal agent from Jatropha elliptica. Phytochemistry 2005, 66, 1804–1811. [Google Scholar] [CrossRef]
- Obiang-Obounou, B.W.; Jang, Y.P. Enriching modern pharmacotherapy through synergy assessment for the combination of natural products and synthetic drugs. Arch. Pharm. Res. 2011, 34, 1579–1581. [Google Scholar] [CrossRef]
- Freitas, P.R.; de Araújo, A.C.J.; Barbosa, C.R.; Muniz, D.F.; Tintino, S.R.; Ribeiro, J.; Siqueira, J.P.; Filho, J.M.B.; de Sousa, G.R.; Coutinho, H.D.M. Inhibition of efflux pumps by monoterpene (α-Pinene) and impact on Staphylococcus aureus resistance to tetracycline and erythromycin. Curr. Drug Metab. 2021, 22, 123–126. [Google Scholar] [CrossRef]
- Liu, I.X.; Durham, D.G.; Richards, R.M.E. Baicalin synergy with β-lactam antibiotics against methicillin-resistant Staphylococcus aureus and other β-lactam-resistant strains of S. aureus. J. Pharm. Pharmacol. 2000, 52, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Stermitz, F.R.; Lorenz, P.; Tawara, J.N.; Zenewicz, L.A.; Lewis, K. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. USA 2000, 97, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Magnini, R.D.; Pedinielli, F.; Vergalli, J.; Ouedraogo, N.; Remy, S.; Hilou, A.; Brunel, J.M.; Pagés, J.M.; Davin-Regli, A. Acacia senegal Budmunchiamines as a potential adjuvant for rejuvenating phenicol activities towards Escherichia coli-resistant strains. Int. J. Mol. Sci. 2023, 24, 8790. [Google Scholar] [CrossRef]
- Oluwatuyi, M.; Kaatz, G.W.; Gibbons, S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry 2004, 65, 3249–3254. [Google Scholar] [CrossRef]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, G.R.; Maurya, A.; Yadav, D.K.; Singh, V.; Khan, F.; Gupta, M.K.; Singh, M.; Darokar, M.P.; Srivastava, S.K. Synergy of clavine alkaloid ‘chanoclavine’ with tetracycline against multi-drug-resistant E. coli. J. Biomol. Struct. Dyn. 2019, 37, 1307–1325. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Lv, X.H.; Chen, M.S.; Guo, Y.; Ding, R.; Liu, B.; Deng, X.M.; Wang, J.F. Characterization of corosolic acid as a KPC-2 inhibitor that increases the susceptibility of KPC-2-positive bacteria to carbapenems. Front. Pharmacol. 2020, 11, 1047. [Google Scholar] [CrossRef]
- Sundaramoorthy, N.S.; Sivasubramanian, A.; Nagarajan, S. Simultaneous inhibition of MarR by salicylate and efflux pumps by curcumin sensitizes colistin resistant clinical isolates of Enterobacteriaceae. Microb. Pathog. 2020, 148, 104445. [Google Scholar] [CrossRef]
- Roccaro, A.S.; Blanco, A.R.; Giuliano, F.; Rusciano, D.; Enea, V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob. Agents Chemother. 2004, 48, 1968–1973. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Zhao, W.H.; Hara, Y.; Shimamura, T. Epigallocatechin gallate synergy with ampicillin/sulbactam against 28 clinical isolates of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 361–364. [Google Scholar] [CrossRef]
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 2017, 12, e0172273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Guo, Y.; Wen, Z.M.; Ci, X.X.; Xia, L.N.; Wang, Y.L.; Deng, X.M.; Wang, J.F. Isoalantolactone enhances the antimicrobial activity of penicillin G against Staphylococcus aureus by inactivating β-lactamase during protein translation. Pathogens 2020, 9, 161. [Google Scholar] [CrossRef]
- Gibbons, S.; Oluwatuyi, M.; Veitch, N.C.; Gray, A.I. Bacterial resistance modifying agents from Lycopus europaeus. Phytochemistry 2003, 62, 83–87. [Google Scholar] [CrossRef]
- Gallique, M.; Wei, K.; Maisuria, V.B.; Okshevsky, M.; McKay, G.; Nguyen, D.; Tufenkji, N. Cranberry-derived proanthocyanidins potentiate β-lactam antibiotics against resistant bacteria. Appl. Environ. Microb. 2021, 87, e00127-21. [Google Scholar] [CrossRef]
- Pal, A.; Tripathi, A. Quercetin inhibits carbapenemase and efflux pump activities among carbapenem-resistant Gram-negative bacteria. APMIS 2020, 128, 251–259. [Google Scholar] [CrossRef]
- Yi, K.F.; Liu, S.B.; Liu, P.Y.; Luo, X.W.; Zhao, J.F.; Yan, F.B.; Pan, Y.S.; Liu, J.H.; Zhai, Y.J.; Hu, G.Z. Synergistic antibacterial activity of tetrandrine combined with colistin against MCR-mediated colistin-resistant Salmonella. Biomed. Pharmacother. 2022, 149, 112873. [Google Scholar] [CrossRef] [PubMed]
- Kilic, I.; Ayar, Y.; Ceylan, I.; Kaya, P.K.; Caliskan, G. Nephrotoxicity caused by colistin use in ICU: A single centre experience. BMC Nephrol. 2023, 24, 302. [Google Scholar] [CrossRef]
- Barbosa, C.R.D.; Scherf, J.R.; de Freitas, T.S.; de Menezes, I.R.A.; Pereira, R.L.S.; dos Santos, J.F.S.; de Jesus, S.S.P.; Lopes, T.P.; Silveira, Z.D.; Oliveira-Tintino, C.D.D.; et al. Effect of Carvacrol and Thymol on NorA efflux pump inhibition in multidrug-resistant (MDR) Staphylococcus strains. J. Bioenerg. Biomembr. 2021, 53, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med. Res. 2019, 149, 129–145. [Google Scholar] [CrossRef]
- Wang, J.L.; Jiao, H.H.; Meng, J.W.; Qiao, M.Y.; Du, H.X.; He, M.; Ming, K.; Liu, J.G.; Wang, D.Y.; Wu, Y. Baicalin inhibits biofilm formation and the quorum-sensing system by regulating the MsrA drug efflux pump in Staphylococcus saprophyticus. Front. Microbiol. 2019, 10, 2800. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Zhou, Z.G.; Hu, Y.H.; Wu, J.H.; Li, Y.M.; Huang, W.L. HZ08 reverse p-glycoprotein mediated multidrug resistance in vitro and in vivo. PLoS ONE 2015, 10, e0116886. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Pastan, I. Biochemistry of multidrug-resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 1993, 62, 385–427. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.K. The contribution of P-glycoprotein to pharmacokinetic drug-drug interactions. J. Clin. Pharmacol. 1999, 39, 1203–1211. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.M.; Yoo, Y.S.; Yoo, J.S.; Yoo, J.I.; Kim, H.S.; Lee, Y.S.; Chung, G.T. Alterations of gyrA, gyrB, and parC and activity of efflux pump in fluoroquinolone-resistant Acinetobacter baumannii. Osong Public Health Res. Perspect. 2011, 2, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, D.; Schultze, N.; Borchardt, J.; Bottcher, I.; Schaufler, K.; Guenther, S. Synergistic antimicrobial activities of epigallocatechin gallate, myricetin, daidzein, gallic acid, epicatechin, 3-hydroxy-6-methoxyflavone and genistein combined with antibiotics against ESKAPE pathogens. J. Appl. Microbiol. 2022, 132, 949–963. [Google Scholar] [CrossRef]
- Nakayama, M.; Shimatani, K.; Ozawa, T.; Shigemune, N.; Tomiyama, D.; Yui, K.; Katsuki, M.; Ikeda, K.; Nonaka, A.; Miyamoto, T. Mechanism for the antibacterial action of epigallocatechin gallate (EGCg) on Bacillus subtilis. Biosci. Biotechnol. Biochem. 2015, 79, 845–854. [Google Scholar] [CrossRef]
- Singkham-In, U.; Higgins, P.G.; Wannigama, D.L.; Hongsing, P.; Chatsuwan, T. Rescued chlorhexidine activity by resveratrol against carbapenem-resistant Acinetobacter baumannii via down-regulation of AdeB efflux pump. PLoS ONE 2020, 15, e0243082. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. Beta-lactams and beta-lactamase inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Fleming, A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Br. J. Exp. Pathol. 1929, 10, 226–236. [Google Scholar] [CrossRef]
- Abraham, E.P.; Chain, E. An enzyme from bacteria able to destroy penicillin. Nature 1940, 146, 837. [Google Scholar] [CrossRef]
- Reading, C.; Cole, M. Clavulanic acid: A Beta-lactamase-inhibiting beta-lactam from Streptomyces-Clavuligerus. Antimicrob. Agents Chemother. 1977, 11, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Charnas, R.L.; Knowles, J.R. Kinetic studies on inactivation of Escherichia coli RTEM beta-lactamase by clavulanic acid. Biochemistry 1978, 17, 2180–2184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Hu, Z.Q.; Hara, Y.; Shimamura, T. Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2266. [Google Scholar] [CrossRef] [PubMed]
- Siriwong, S.; Teethaisong, Y.; Thumanu, K.; Dunkhunthod, B.; Eumkeb, G. The synergy and mode of action of quercetin plus amoxicillin against amoxicillin-resistant Staphylococcus epidermidis. BMC Pharmacol. Toxicol. 2016, 17, 39. [Google Scholar] [CrossRef]
- Ghosh, A.; Das, B.K.; Roy, A.; Mandal, B.; Chandra, G. Antibacterial activity of some medicinal plant extracts. J. Nat. Med. 2008, 62, 259–262. [Google Scholar] [CrossRef]
- Deutch, C.E. Limited effectiveness of over-the-counter plant preparations used for the treatment of urinary tract infections as inhibitors of the urease activity from Staphylococcus saprophyticus. J. Appl. Microbiol. 2017, 122, 1380–1388. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
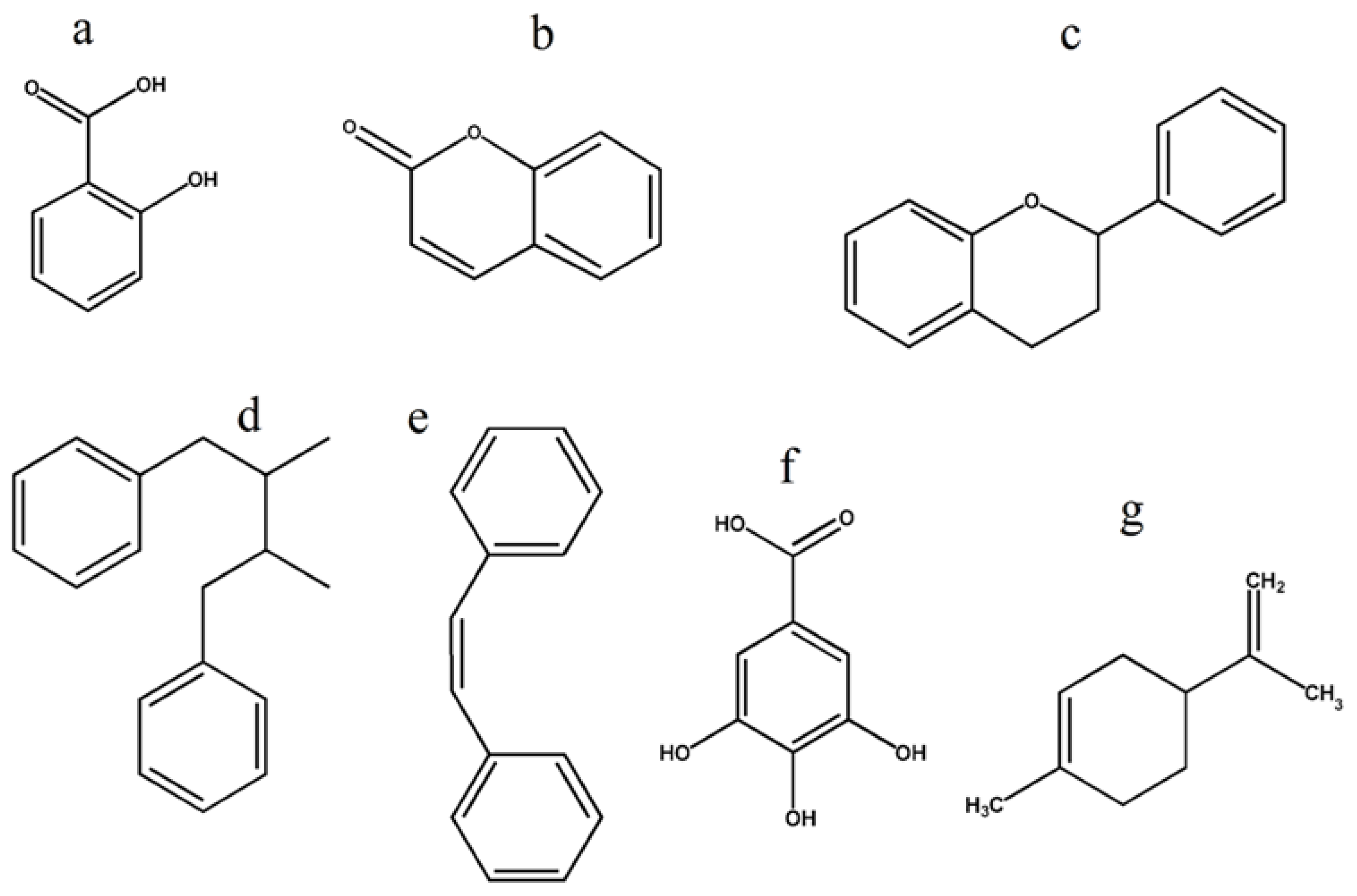
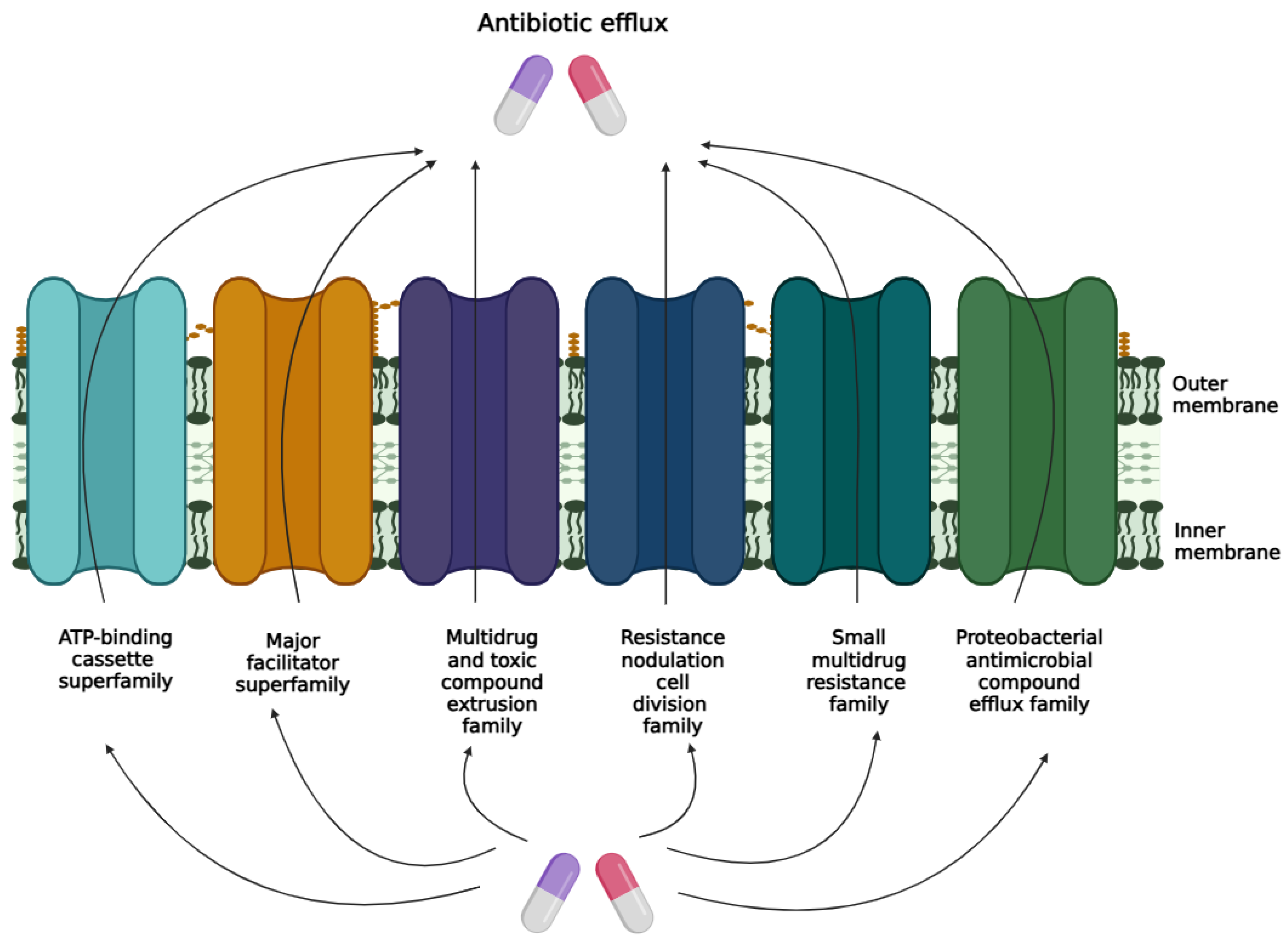

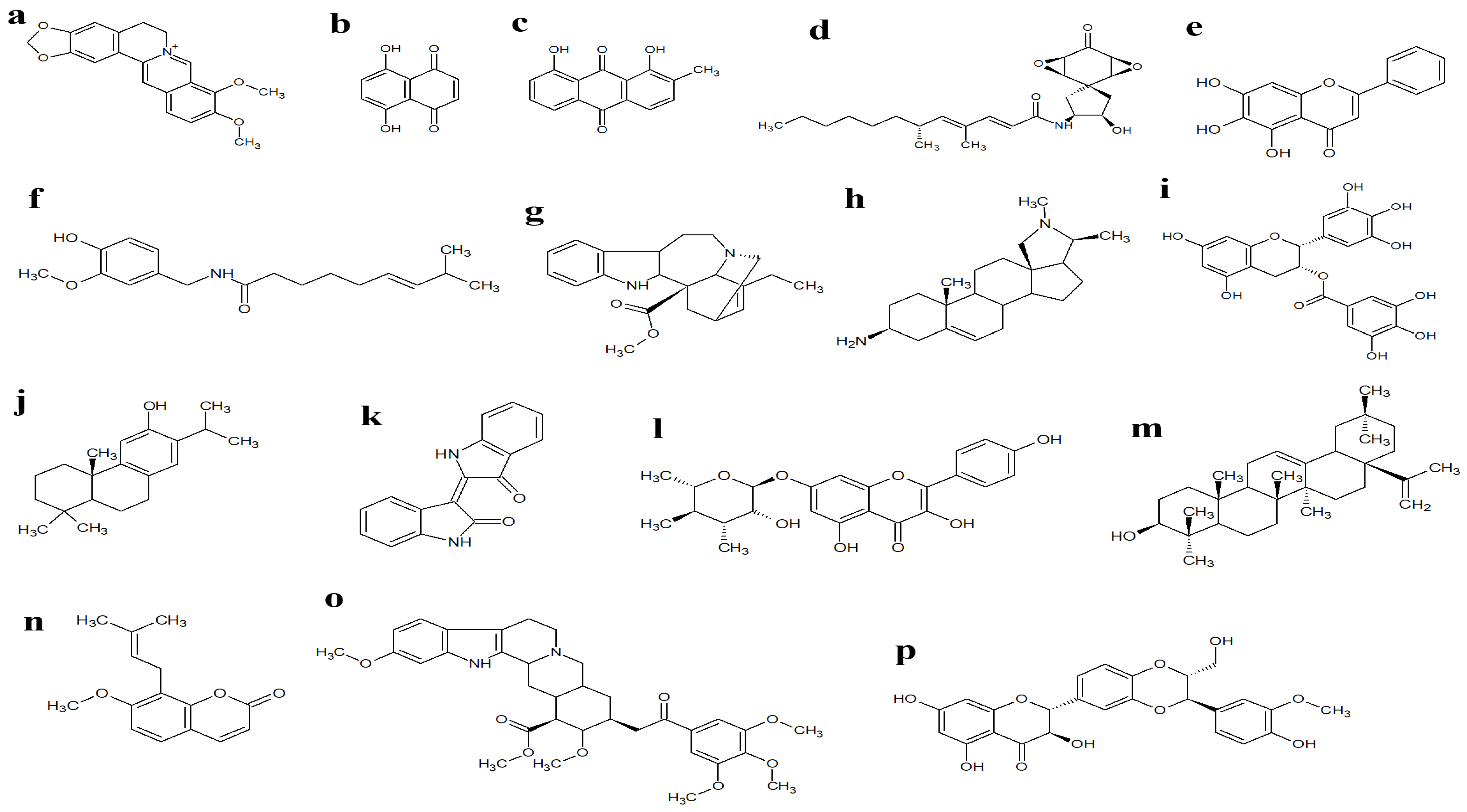


| Plant Name | Secondary Metabolites | Antimicrobial Activity Against | References |
|---|---|---|---|
| Arbutus unedo L. | Anthocyanins, flavonoids, Quinines, tannins | S. aureus, E. coli, P. aeruginosa | [27] |
| Artabotrys crassifolius Hook.f. & Thomson | Liridine | B. cereus | [28] |
| Bauhinia purpurea Linn. | Alkaloids, fatty acids, phytol esters Steroids, triterpenoids, | A. niger, B. subtilis, Claviceps purpurea, E. coli, Klebsiella, P. aeruginosa, S. aureus, S. typhimurium | [29] |
| Berberis vulgaris L., Berberis petiolaris Kunth., Berberis aristate DC., Berberis integerrima Bonge. | Berberine | Brucella abortus | [30] |
| Cananga odorata (Lam.) Hook. f. & Thomson. | Sampangine | M. intracellulare | [31] |
| Cannabis sativa L. | Cannabinoids | MRSA, E. faecalis, S. pneumoniae | [32] |
| Cassia auriculatalinn Linn. | Alkaloids, flavonoids, glycosides, phenols terpenoids, proteins, saponins, tannins, | B. subtilis, C. albicans, Aspergillus niger, E. coli, S. aureus | [33] |
| Cnidoscolus acontifolius (Mill) I.M.Johnst, Newbouldia laevis (P.Beauv.) Seem.ex Bureau, Adansonia digitata L., Alchornea laxiflorab (Benth.) Pax & K. Hoffm., | Alkaloids, anthraquinones, reducing sugars flavonoids, phenols, resins, Saponins, steroids tannins, terpenoids, carbohydrates, cardioactive glycosides | E. coli, S. aureus, B. subtilis, P. aeruginosa | [34] |
| Gloriosa superba Linn. | Terpenoids, tannin, steroids, glycosides, alkaloids | B. cereus, B. subtilis, E. coli, K. pneumonia, P. aeruginosa, P. vulgaris, S. aureus, S. faecalis, S. typhimurium, S. cremoris | [35] |
| Glycyrrhiza inflata Batalin. | Licochalcone A | S. aureus | [36] |
| Mammea americana L. | Mammea B/BA | MRSA, S. aureus | [37] |
| Momordica charantia L. | Alkaloids, flavonoids, glycosides, Saponins, steroids, tannins | B. subtilis, E. coli, P. aeruginosa, S. aureus | [38] |
| Ocimum sanctum L. | Alkaloids, Steroidal compounds, tannins | E. coli, S. aureus, P. mirabilis | [39] |
| Phyllanthus emblica L. | Alkaloids, fats, glyceroids, carbohydrates, phenolics, lignin, oil, tannins, flavonoids, saponins, terpenoids | E. coli, S. aureus, P. aeruginosa, B. subtilis | [40] |
| Psidium guajava L. | Alkaloid, polyphenols, saponins, tannins, terpenoids, | S. aureus, E. coli, P. aeruginosa | [41] |
| Punica granatum L. | Punicalagin | S. aureus, P. aeruginosa, Enterococcus mundtii | [42] |
| Rhodomyrtus tomentosa (Ait.) Hassk. | Rhodomyrtosone B | Vancomycin-resistant E. faecium, MRSA | [43] |
| Sanguinaria canadensis L. | Sanguinarine | B. subtilis, S. epidermidis, S. aureus, MRSA | [44] |
| Schinus lentiscifolius Marchand. | Moronic acid | S. pyogenes, S. aureus, B. subtilis | [45] |
| Phytochemicals | Plant Name | Efflux Pump Inhibitory Activity Against | β-Lactamase Inhibitory Activity Against | References |
|---|---|---|---|---|
| 1,4-Naphthalenedione (Figure 4b) | Holoptelea integrifolia (Roxb.) Planch. | Not reported | S. aureus | [60] |
| 2-Methoxy chrysophanol (Figure 4c) | Clutia myricoides Jaub. & Spach | Not reported | K. pneumoniae | [61] |
| 5-O-Methylglovanon | Glycosmis plants | Not reported | S. epidermidis (MIC = 25–50 µg/mL) and ampicillin-resistant S. aureus (MIC = 12.50–50 µg/mL) | [62] |
| Abietane diterpenes | Rosmarinus officinalis L. | S. aureus (MIC = 16–64 µg/mL) | Not reported | [63] |
| Alkaloid compounds | Cienfuegosia digitata Cav. | Not reported | Ampicillin and methicillin-resistant S. aureus (ZOI = 10–16 mm) | [64] |
| Aranorosin (Figure 4d) | Gymnascella aurantiaca. Peck | Not reported | MRSA (ZOI = 10 mm) | [65] |
| Baicalein (Figure 4e) | Scutellaria baicalensis Georgi | MRSA (MIC = 64–256 µg/mL) | S. aureus (MIC = 128 µg/mL) | [66,67] |
| Berberine (Figure 4a) | Berberis spp. | P. aeruginosa (MIC = 125–250 µg/mL) | Not reported | [68] |
| Caffeoylquinic acids | Artemisia absinthium L. | E. faecalis, S. aureus (MIC = 32–>256 µg/mL) | Not reported | [69] |
| Capsaicin (Figure 4f) | Capsicum spp. | S. aureus (MIC ≥ 100 µg/mL) | Not reported | [70] |
| Catharanthine (Figure 4g) | Catharanthus roseus (L.) G.Don | P. aeruginosa (MIC = 400 µg/mL) | Not reported | [71] |
| Conessine (Figure 4h) | Holarrhena antidysenterica (L.) Wall.ex A. DC. | P. aeruginosa (MIC = 40 µg/mL) | Not reported | [72] |
| Coumarins | Mesua ferrea L. | S. aureus (MIC = 3.12–100 µg/mL) | Not reported | [73] |
| Crysoplenol, Crysoplenetin | Artemissia annua L. | S. aureus (MIC = 250–500 µg/mL) | Not reported | [74] |
| Cucurbitane-type triterpenoids | Momordica balsamina L. (Cucurbitaceae) | E. faecalis (MIC = 100–>200 µg/mL), MRSA (MIC = 25–50 µg/mL) | Not reported | [75] |
| Cumin | Cuminum cyminum L. | S. aureus (MIC = 5–25 µg/mL) | Not reported | [76] |
| Epigallocatechin gallate (Figure 4i) | Camellia sinensis (L.) Kuntze | Not reported | MRSA (MIC ≤ 100 µg/mL) | [77] |
| Ferruginol (Figure 4j) | Chamaecyparis lawsoniana (A.Murray bis) Parl. | S. aureus (MIC = 4–128 µg/mL) | Not reported | [78] |
| Gallotannin glucopyranose | Terminalia chebula Retzius | E. coli (MIC = 12.1–97.5 µg/mL) | Not reported | [79] |
| Indirubin (Figure 4k) | Wrightia tinctoria (Roxb.) R. Br. | S. epidermidis (MIC = 25 µg/mL), S. aureus (MIC = 12.5 µg/mL) | Not reported | [80] |
| Kaempferol rhamnoside (Figure 4l) | Persea lingue (Ruiz & Pav.) Nees | S. aureus (IC50 = 2 µM) | Not reported | [81] |
| Oleanolic acid (Figure 4m) | Carpobrotus edulis (L.) L.Bolus | MRSA (MIC = 25–50 µg/mL) | Not reported | [82] |
| Osthol (Figure 4n) | Cnidii monnieri (L.) | S. aureus (MIC = 25 µg/mL), P. aeruginosa (MIC = 128 µg/mL) | Not reported | [83,84] |
| Phenylpropanoid ailanthoidiol | Zanthoxylum capense (Thunb.) Harv. | S. aureus (MIC = 50–100 µg/mL) | Not reported | [85] |
| Reserpine (Figure 4o) | Rauwolfia vomitoria Afzel., Rauwolfia serpentina (L.) Benth. ex Kurz | E. coli (ZOI = 15.5–16.5 mm) | Not reported | [86] |
| SB-202742 (Anacardic acid derivatives) | Spondias mombin L. | Not reported | E. coli (IC50 = 10.1 µg/mL), P. aeruginosa (IC50 = 40.5 µg/mL), P. mirabilis (IC50 = 111.3 µg/mL) | [87] |
| Silybin (Figure 4p) | Silybum marianum (L.) Gaertn | MRSA (ZOI = 29 mm) | Not reported | [88] |
| Tellimagrandin I | Rosa canina L. | Not reported | MRSA (MIC = 150 µg/mL) | [89] |
| Phytochemicals | Combination with Antibiotic | Bacterial Target | Inhibitory Activity Against | References |
|---|---|---|---|---|
| 2,6-dimethyl-4-phenyl-pyridine-3,5-dicarboxylic acid diethyl ester | Ciprofloxacin | S. aureus | Efflux pump (MIC = 3–8 µg/mL) | [98] |
| 5-O-Methylglovanon | Ampicillin | S. aureus, S. epidermidis | β-lactamase | [99] |
| α-pinene | Erythromycin, Tetracycline | S. aureus | Efflux pump (MIC = 128 µg/mL) | [100] |
| Baicalin | Cefotaxime, methicillin, benzylpenicillin, amoxicillin, ampicillin | MRSA | β-lactamase (MIC = 4–25 µg/mL) | [101] |
| Berberine | 5ʹ- Methoxyhydnocarpin | S. aureus | Efflux pump (MIC = 32 µg/mL) | [102] |
| Budmunchiamines | Chloramphenicol | E. coli | Efflux pump (MIC = 8–64 µg/mL) | [103] |
| Carnosol, carnosic acid | Erythromycin | S. aureus | Efflux pump (MIC = 32–256 µg/mL) | [104] |
| Carvacrol | Erythromycin | Erythromycin-resistant Group A Streptococci | Efflux pump (MIC = 8–64 µg/mL) | [105] |
| Chanoclavine | Tetracycline | MDE E. coli | Efflux pump (MIC = 0.78–100 µg/mL) | [106] |
| Corosolic acid | Carbapenems | E. coli | β-lactamase (MIC = 0.08–1.5 µg/mL) | [107] |
| Curcumin, salicylate | Colistin | Carbapenem resistant Enterobacteriaceae | Efflux pump (MIC = 0.01–4 µg/mL) | [108] |
| Epigallocatechin-gallate | Tetracycline | Staphylococcal isolates | Efflux pump (MIC = 0.06–32 µg/mL) | [109] |
| Epigallocatechin-gallate | Sulbactam/ampicillin | MRSA | β-Lactamase (MIC = 4 µg/mL) | [110] |
| Gallic acid, quercetin, luteolin, protocatechuic acid | Amphenicol, tetracycline, fluoroquinolone, quinolone, β lactams | E. coli, S. aureus | β-Lactamase (MIC = 0.02–47 µg/mL) for E. coli and (MIC = 0.02–7.8 µg/mL) for S. aureus | [111] |
| Isoalantolactone | Penicillin G | S. aureus | β-Lactamase (MIC = 0.008–16 µg/mL) | [112] |
| Methyl-1alpha-acetoxy-7alpha 14alpha-dihydroxy-8,15-isopimaradien-18-oate, Methyl-1alpha,14alpha-diacetoxy-7alpha-hydroxy-8,15-isopimaradien-18-oate | Erythromycin, tetracycline | S. aureus | Efflux pump (MIC = 32–256 µg/mL) | [113] |
| Proanthocyanidins | Ampicillin, meropenem, cefotaxime | E. coli, Staphylococci strains, Klebsiella | β-Lactamase (MIC = 37.5–2400 µg/mL) | [114] |
| Quercetin | Meropenem | Carbapenem resistant K. pneumoniae, E. coli, P. aeruginosa, A. baumannii | Efflux pump | [115] |
| Tellimagrandin I, corilagin, ellagic acid, gallic acid | Oxacillin | MRSA | β-Lactamase (MIC = 0.25–256 µg/mL) | [89] |
| Tetrandrine | Colistin (Toxicity has been reported) | Colistin-resistant Salmonella | Efflux pump (MIC = 0.004–4 µg/mL) | [116,117] |
| Thymol, Carvacrol | Norfloxacin | S. aureus | Efflux pump (MIC = 32 µg/mL) | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zai, M.J.; Cock, I.E.; Cheesman, M.J. Plant Metabolites as Potential Agents That Potentiate or Block Resistance Mechanisms Involving β-Lactamases and Efflux Pumps. Int. J. Mol. Sci. 2025, 26, 5550. https://doi.org/10.3390/ijms26125550
Zai MJ, Cock IE, Cheesman MJ. Plant Metabolites as Potential Agents That Potentiate or Block Resistance Mechanisms Involving β-Lactamases and Efflux Pumps. International Journal of Molecular Sciences. 2025; 26(12):5550. https://doi.org/10.3390/ijms26125550
Chicago/Turabian StyleZai, Muhammad Jawad, Ian Edwin Cock, and Matthew James Cheesman. 2025. "Plant Metabolites as Potential Agents That Potentiate or Block Resistance Mechanisms Involving β-Lactamases and Efflux Pumps" International Journal of Molecular Sciences 26, no. 12: 5550. https://doi.org/10.3390/ijms26125550
APA StyleZai, M. J., Cock, I. E., & Cheesman, M. J. (2025). Plant Metabolites as Potential Agents That Potentiate or Block Resistance Mechanisms Involving β-Lactamases and Efflux Pumps. International Journal of Molecular Sciences, 26(12), 5550. https://doi.org/10.3390/ijms26125550







