Human Papillomavirus and Other Relevant Issues in Cervical Cancer Pathogenesis
Abstract
1. Introduction
2. Risk Factors
3. Screening Methods and Some Related Issues
4. HPV-Negative Cervical Cancer
5. Low-Risk HPV in Cervical Lesions
6. Influence of HIV and HSV-2
7. Cervical Screening and Associated Complexities
8. Smoking and Cervical Cancer Risk
9. Hormonal Influences in Cervical Cancer
10. The Role of Vaginal Bacterial Population
10.1. Lactobacillus Species in Vaginal Bacterial Flora
10.2. Diminished Vaginal Lactobacillus Dominance and Cervical Lesions
11. Conclusions
Funding
Conflicts of Interest
References
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020, A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, Q.; Cai, W.; Ruan, W. Trends of cervical cancer at global, regional, and national level: Data from the Global Burden of Disease study 2019. BMC Public Health 2021, 21, 894. [Google Scholar] [CrossRef]
- Muslin, C. Addressing the burden of cervical cancer for Indigenous women in Latin America and the Caribbean: A call for action. Front. Public Health 2024, 12, 1376748. [Google Scholar] [CrossRef]
- de Sanjosé, S.; Brotons, M.; Pavón, M.A. The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Veldhuijzen, N.J.; Snijders, P.J.; Reiss, P.; Meijer, C.J.; van de Wijgert, J.H. Factors affecting transmission of mucosal human papillomavirus. Lancet Infect. Dis. 2010, 10, 862–874. [Google Scholar] [CrossRef]
- Münger, K.; Baldwin, A.; Edwards, K.M.; Hayakawa, H.; Nguyen, C.L.; Owens, M.; Grace, M.; Huh, K. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 2004, 78, 11451–11460. [Google Scholar] [CrossRef]
- Lehoux, M.; D’Abramo, C.M.; Archambault, J. Molecular mechanisms of human papillomavirus-induced carcinogenesis. Public Health Genom. 2009, 12, 268–280. [Google Scholar] [CrossRef]
- Barreto, S.C.; Uppalapati, M.; Ray, A. Small circular DNAs in human pathology. Malays. J. Med. Sci. 2014, 21, 4–18. [Google Scholar]
- Balasubramaniam, S.D.; Balakrishnan, V.; Oon, C.E.; Kaur, G. Key molecular events in cervical cancer development. Medicina 2019, 55, 384. [Google Scholar] [CrossRef]
- Luvián-Morales, J.; Gutiérrez-Enríquez, S.O.; Granados-García, V.; Torres-Poveda, K. Risk factors for the development of cervical cancer: Analysis of the evidence. Front. Oncol. 2024, 14, 1378549. [Google Scholar] [CrossRef]
- Huang, J.; Deng, Y.; Boakye, D.; Tin, M.S.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E., 3rd; Xu, W.; Zheng, Z.J.; Elcarte, E.; et al. Global distribution, risk factors, and recent trends for cervical cancer: A worldwide country-level analysis. Gynecol. Oncol. 2022, 164, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Hellberg, D.; Stendahl, U. The biological role of smoking, oral contraceptive use and endogenous sexual steroid hormones in invasive squamous epithelial cervical cancer. Anticancer Res. 2005, 25, 3041–3046. [Google Scholar] [PubMed]
- Mukherjee, S.; Koner, B.C.; Ray, S.; Ray, A. Environmental contaminants in pathogenesis of breast cancer. Indian J. Exp. Biol. 2006, 44, 597–617. [Google Scholar]
- Toliman, P.J.; Kaldor, J.M.; Tabrizi, S.N.; Vallely, A.J. Innovative approaches to cervical cancer screening in low- and middle-income countries. Climacteric 2018, 21, 235–238. [Google Scholar] [CrossRef]
- Berman, T.A.; Schiller, J.T. Human papillomavirus in cervical cancer and oropharyngeal cancer: One cause, two diseases. Cancer 2017, 123, 2219–2229. [Google Scholar] [CrossRef]
- Timbang, M.R.; Sim, M.W.; Bewley, A.F.; Farwell, D.G.; Mantravadi, A.; Moore, M.G. HPV-related oropharyngeal cancer: A review on burden of the disease and opportunities for prevention and early detection. Hum. Vaccin. Immunother. 2019, 15, 1920–1928. [Google Scholar] [CrossRef]
- Tanaka, T.I.; Alawi, F. Human papillomavirus and oropharyngeal cancer. Dent. Clin. N. Am. 2018, 62, 111–120. [Google Scholar] [CrossRef]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef]
- Lewis, A.; Kang, R.; Levine, A.; Maghami, E. The new face of head and neck cancer: The HPV epidemic. Oncology 2015, 29, 616–626. [Google Scholar]
- Serra, S.; Chetty, R. p16. J. Clin. Pathol. 2018, 71, 853–858. [Google Scholar] [CrossRef]
- Rahimi, S. HPV-related squamous cell carcinoma of oropharynx: A review. J. Clin. Pathol. 2020, 73, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Poli, U.R.; Bidinger, P.D.; Gowrishankar, S. Visual inspection with acetic acid (VIA) screening program: 7 years experience in early detection of cervical cancer and pre-cancers in rural South India. Indian J. Community Med. 2015, 40, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Marina, O.C.; Sanders, C.K.; Mourant, J.R. Effects of acetic acid on light scattering from cells. J. Biomed. Opt. 2012, 17, 085002-1. [Google Scholar] [CrossRef] [PubMed]
- Sellors, J.W.; Sankaranarayanan, R. Colposcopy and Treatment of Cervical Intraepithelial Neoplasia: A Beginners’ Manual; International Agency for Research on Cancer (World Health Organization): Lyon, France, 2003. [Google Scholar]
- Raju, K. Evolution of Pap stain. Biomed. Res. Ther. 2016, 3, 490–500. [Google Scholar] [CrossRef]
- Gago, J.; Paolino, M.; Arrossi, S. Factors associated with low adherence to cervical cancer follow-up retest among HPV+/ cytology negative women: A study in programmatic context in a low-income population in Argentina. BMC Cancer 2019, 19, 367. [Google Scholar] [CrossRef]
- Del Mistro, A.; Frayle, H.; Ferro, A.; Callegaro, S.; Del Sole, A.; Stomeo, A.; Cirillo, E.; Fedato, C.; Pagni, S.; Barzon, L.; et al. Cervical cancer screening by high risk HPV testing in routine practice: Results at one year recall of high risk HPV-positive and cytology-negative women. J. Med. Screen. 2014, 21, 30–37. [Google Scholar] [CrossRef]
- Paengchit, K.; Kietpeerakool, C.; Wangchai, W.; Pouraeng, S.; Lalitwongsa, S. Cervical pathology in cytology-negative/HPV-positive women: Results from Lampang Cancer Hospital, Thailand. Asian Pac. J. Cancer Prev. 2014, 15, 7951–7954. [Google Scholar] [CrossRef]
- Lee, J.E.; Chung, Y.; Rhee, S.; Kim, T.H. Untold story of human cervical cancers: HPV-negative cervical cancer. BMB Rep. 2022, 55, 429–438. [Google Scholar] [CrossRef]
- Tjalma, W. HPV negative cervical cancers and primary HPV screening. Facts Views Vis. Obgyn. 2018, 10, 107–113. [Google Scholar]
- Lo, K.W.; Cheung, T.H.; Chung, T.K.; Wang, V.W.; Poon, J.S.; Li, J.C.; Lam, P.; Wong, Y.F. Clinical and prognostic significance of human papillomavirus in a Chinese population of cervical cancers. Gynecol. Obstet. Investig. 2001, 51, 202–207. [Google Scholar] [CrossRef]
- Kaliff, M.; Karlsson, M.G.; Sorbe, B.; Bohr Mordhorst, L.; Helenius, G.; Lillsunde-Larsson, G. HPV-negative tumors in a Swedish cohort of cervical cancer. Int. J. Gynecol. Pathol. 2020, 39, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Arezzo, F.; Cormio, G.; Loizzi, V.; Cazzato, G.; Cataldo, V.; Lombardi, C.; Ingravallo, G.; Resta, L.; Cicinelli, E. HPV-negative cervical cancer: A narrative review. Diagnostics 2021, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Franceschi, S.; Howell-Jones, R.; Snijders, P.J.; Clifford, G.M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int. J. Cancer 2011, 128, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Katki, H.A.; Schiffman, M.; Castle, P.E.; Fetterman, B.; Poitras, N.E.; Lorey, T.; Cheung, L.C.; Raine-Bennett, T.; Gage, J.C.; Kinney, W.K. Five-year risks of CIN 3+ and cervical cancer among women with HPV-positive and HPV-negative high-grade Pap results. J. Low. Genit. Tract Dis. 2013, 17 (Suppl. S1), S50–S55. [Google Scholar] [CrossRef]
- Inturrisi, F.; Rozendaal, L.; Veldhuijzen, N.J.; Heideman, D.A.M.; Meijer, C.J.L.M.; Berkhof, J. Risk of cervical precancer among HPV-negative women in the Netherlands and its association with previous HPV and cytology results: A follow-up analysis of a randomized screening study. PLoS Med. 2022, 19, e1004115. [Google Scholar] [CrossRef]
- Xing, B.; Guo, J.; Sheng, Y.; Wu, G.; Zhao, Y. Human papillomavirus-negative cervical cancer: A comprehensive review. Front. Oncol. 2021, 10, 606335. [Google Scholar] [CrossRef]
- Nicolás, I.; Marimon, L.; Barnadas, E.; Saco, A.; Rodríguez-Carunchio, L.; Fusté, P.; Martí, C.; Rodriguez-Trujillo, A.; Torne, A.; Del Pino, M.; et al. HPV-negative tumors of the uterine cervix. Mod. Pathol. 2019, 32, 1189–1196. [Google Scholar] [CrossRef]
- Jhingran, A.; Eifel, P.J.; Wharton, J.T.; Tortolero-Luna, G. Histologic classification of epithelial tumors (Neoplasms of the Cervix). In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Bast, R.C., Gansler, T.S., Holland, J.F., Frei, E., Eds.; BC Decker: Hamilton, ON, USA, 2003; Chapter 115. [Google Scholar]
- Yordanov, A.; Kostov, S.; Slavchev, S.; Strashilov, S.; Konsoulova, A.; Calleja-Agius, J.; Di Fiore, R.; Suleiman, S.; Kubelac, P.; Vlad, C.; et al. Adenosquamous carcinoma of the uterine cervix-Impact of histology on clinical management. Cancer Manag. Res. 2021, 13, 4979–4986. [Google Scholar] [CrossRef]
- Virarkar, M.; Vulasala, S.S.; Morani, A.C.; Waters, R.; Gopireddy, D.R.; Kumar, S.; Bhosale, P.; Lall, C. Neuroendocrine neoplasms of the gynecologic tract. Cancers 2022, 14, 1835. [Google Scholar] [CrossRef]
- Tempfer, C.B.; Tischoff, I.; Dogan, A.; Hilal, Z.; Schultheis, B.; Kern, P.; Rezniczek, G.A. Neuroendocrine carcinoma of the cervix: A systematic review of the literature. BMC Cancer 2018, 18, 530. [Google Scholar] [CrossRef]
- Chao, A.; Wu, R.C.; Lin, C.Y.; Chang, T.C.; Lai, C.H. Small cell neuroendocrine carcinoma of the cervix: From molecular basis to therapeutic advances. Biomed. J. 2023, 46, 100633. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, V.; Shalini, C.N.; Priya, S.; Rani, U.; Rajendiran, S.; Joseph, L.D. Small cell neuroendocrine carcinoma of the cervix: A rare entity. J. Clin. Diagn. Res. 2014, 8, 147–148. [Google Scholar] [CrossRef]
- Aletti, G.; Laffi, A. Neuroendocrine tumors of the cervix: An urgent call for joining forces. Int. J. Gynecol. Cancer 2019, 29, 985. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, F.A.; Devilee, P. Pathology and Genetics: Tumours of the Breast and Female Genital Organs; WHO Classification of Tumours series; Volume IV, IARC Press: Lyon, France, 2003. [Google Scholar]
- Iyoda, A.; Azuma, Y.; Sano, A. Neuroendocrine tumors of the lung: Clinicopathological and molecular features. Surg. Today 2020, 50, 1578–1584. [Google Scholar] [CrossRef]
- Shen, T.; Dong, T.; Wang, H.; Ding, Y.; Zhang, J.; Zhu, X.; Ding, Y.; Cai, W.; Wei, Y.; Wang, Q.; et al. Integrative machine learning frameworks to uncover specific protein signature in neuroendocrine cervical carcinoma. BMC Cancer 2025, 25, 57. [Google Scholar] [CrossRef]
- Park, S.; Park, J.W.; Rha, S.H.; Kim, S. Primary malignant melanoma of the vagina: A case report of a rare disease that is difficult to diagnose. Medicine 2025, 104, e41259. [Google Scholar] [CrossRef]
- Wei, K.N.; Fu, X.D.; Wang, M.Y.; Wang, L.X. Two cases of mixed large cell neuroendocrine carcinoma and adenocarcinoma of the cervix: Case report and review of the literature. Diagn. Pathol. 2024, 19, 166. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Liu, Y.; Wan, Y.; Yang, B.; Li, D.; Wang, S. Liquid-based cytology of small cell carcinoma of the cervix: A multicenter retrospective study. Onco. Targets Ther. 2024, 17, 557–565. [Google Scholar] [CrossRef]
- Gordhandas, S.; Schlappe, B.A.; Zhou, Q.; Iasonos, A.; Leitao, M.M., Jr.; Park, K.J.; de Brot, L.; Alektiar, K.M.; Sabbatini, P.J.; Aghajanian, C.A.; et al. Small cell neuroendocrine carcinoma of the cervix: Analysis of prognostic factors and patterns of metastasis. Gynecol. Oncol. Rep. 2022, 43, 101058. [Google Scholar] [CrossRef]
- Schultheis, A.M.; de Bruijn, I.; Selenica, P.; Macedo, G.S.; da Silva, E.M.; Piscuoglio, S.; Jungbluth, A.A.; Park, K.J.; Klimstra, D.S.; Wardelmann, E.; et al. Genomic characterization of small cell carcinomas of the uterine cervix. Mol. Oncol. 2022, 16, 833–845. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Wang, J. Small cell (neuroendocrine) carcinoma of the cervix: An analysis for 19 cases and literature review. Front. Cell. Infect. Microbiol. 2022, 12, 916506. [Google Scholar] [CrossRef] [PubMed]
- Ordulu, Z.; Mino-Kenudson, M.; Young, R.H.; Van de Vijver, K.; Zannoni, G.F.; Félix, A.; Burandt, E.; Wong, A.; Nardi, V.; Oliva, E. Morphologic and molecular heterogeneity of cervical neuroendocrine neoplasia: A report of 14 cases. Am. J. Surg. Pathol. 2022, 46, 1670–1681. [Google Scholar] [CrossRef]
- Pei, X.; Xiang, L.; Chen, W.; Jiang, W.; Yin, L.; Shen, X.; Zhou, X.; Yang, H. The next generation sequencing of cancer-related genes in small cell neuroendocrine carcinoma of the cervix. Gynecol. Oncol. 2021, 161, 779–786. [Google Scholar] [CrossRef]
- Van Ta, T.; Nguyen, Q.N.; Truong, V.L.; Tran, T.T.; Nguyen, H.P.; Vuong, L.D. Human papillomavirus infection, p16INK4a expression and genetic alterations in Vietnamese cervical neuroendocrine cancer. Malays. J. Med. Sci. 2019, 26, 151–157. [Google Scholar] [CrossRef]
- Feng, M.; Zou, J.; Zhang, Y.; Sun, L. Neuroendocrine carcinoma of cervix: A clinicopathologic study of 82 cases. China J. Pathol. 2018, 47, 328–333. [Google Scholar] [CrossRef]
- Siriaunkgul, S.; Utaipat, U.; Settakorn, J.; Sukpan, K.; Srisomboon, J.; Khunamornpong, S. HPV genotyping in neuroendocrine carcinoma of the uterine cervix in northern Thailand. Int. J. Gynaecol. Obstet. 2011, 115, 175–179. [Google Scholar] [CrossRef]
- Wang, K.L.; Yang, Y.C.; Wang, T.Y.; Chen, J.R.; Chen, T.C.; Chen, H.S.; Su, T.H.; Wang, K.G. Neuroendocrine carcinoma of the uterine cervix: A clinicopathologic retrospective study of 31 cases with prognostic implications. J. Chemother. 2006, 18, 209–216. [Google Scholar] [CrossRef]
- Ishida, G.M.; Kato, N.; Hayasaka, T.; Saito, M.; Kobayashi, H.; Katayama, Y.; Sasou, S.; Yaegashi, N.; Kurachi, H.; Motoyama, T. Small cell neuroendocrine carcinomas of the uterine cervix: A histological, immunohistochemical, and molecular genetic study. Int. J. Gynecol. Pathol. 2004, 23, 366–372. [Google Scholar] [CrossRef]
- Han, Z.; Aizezi, A.; Ma, L.; Su, Y.; Fan, L.; Liu, J. The association between human papillomavirus and lung cancer: A Mendelian randomization study. Infect. Genet. Evol. 2024, 123, 105646. [Google Scholar] [CrossRef]
- Sirera, G.; Videla, S.; Saludes, V.; Castellà, E.; Sanz, C.; Ariza, A.; Clotet, B.; Martró, E. Prevalence of HPV-DNA and E6 mRNA in lung cancer of HIV-infected patients. Sci. Rep. 2022, 12, 13196. [Google Scholar] [CrossRef]
- Zou, D.J.; Zhao, Y.B.; Yang, J.H.; Xu, H.T.; Li, Q.C.; Wu, G.P. Expression and significance of HPV16 E6/E7 mRNAs in the bronchial brush and TBNA cells of patients with small cell lung cancer. Technol. Cancer Res. Treat. 2021, 20, 15330338211019505. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.H.A.; do Amaral, C.M.; de França São Marcos, B.; Nascimento, K.C.G.; de Miranda Rios, A.C.; Quixabeira, D.C.A.; Muniz, M.T.C.; Silva Neto, J.D.C.; de Freitas, A.C. Presence and activity of HPV in primary lung cancer. J. Cancer Res. Clin. Oncol. 2018, 144, 2367–2376. [Google Scholar] [CrossRef]
- Shikova, E.; Ivanova, Z.; Alexandrova, D.; Shindov, M.; Lekov, A. Human papillomavirus prevalence in lung carcinomas in Bulgaria. Microbiol. Immunol. 2017, 61, 427–432. [Google Scholar] [CrossRef]
- Hartley, C.P.; Steinmetz, H.B.; Memoli, V.A.; Tafe, L.J. Small cell neuroendocrine carcinomas of the lung do not harbor high-risk human papillomavirus. Hum. Pathol. 2015, 46, 577–582. [Google Scholar] [CrossRef]
- Castillo, A.; Aguayo, F.; Koriyama, C.; Shuyama, K.; Akiba, S.; Herrera-Goepfert, R.; Carrascal, E.; Klinge, G.; Sánchez, J.; Eizuru, Y. Human papillomavirus in lung carcinomas among three Latin American countries. Oncol. Rep. 2006, 15, 883–888. [Google Scholar] [CrossRef][Green Version]
- Brouchet, L.; Valmary, S.; Dahan, M.; Didier, A.; Galateau-Salle, F.; Brousset, P.; Degano, B. Detection of oncogenic virus genomes and gene products in lung carcinoma. Br. J. Cancer 2005, 92, 743–746. [Google Scholar] [CrossRef][Green Version]
- Thomas, P.; De Lamballerie, X.; Garbe, L.; Castelnau, O.; Kleisbauer, J.P. Detection of human papillomavirus by polymerase chain reaction in primary lung carcinoma. Bull. Cancer 1996, 83, 842–846. [Google Scholar]
- Schmitt, M.; Höfler, D.; Koleganova, N.; Pawlita, M. Human polyomaviruses and other human viruses in neuroendocrine tumors. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1558–1561. [Google Scholar] [CrossRef]
- Oraibi, O.H.; Wharry, L.I.; Lynn, A.A.; Chaudhry, F.; Jaume, J.C.; Jun, J.Y. Locally invasive pheochromocytoma combined with primary malignant adrenal lymphoma. AACE Clin. Case Rep. 2018, 5, e124–e128. [Google Scholar] [CrossRef]
- Badani, H.; White, T.; Schulick, N.; Raeburn, C.D.; Topkaya, I.; Gilden, D.; Nagel, M.A. Frequency of varicella zoster virus DNA in human adrenal glands. J. Neurovirol. 2016, 22, 400–402. [Google Scholar] [CrossRef]
- Sathe, P.A.; Shah, H.U.; Kothari, K.S.; Ranganathan, S.; Kandalkar, B.M. Bilateral Epstein-Barr virus-associated adrenal leiomyomas in a child without an established immunodeficiency. Pediatr. Dev. Pathol. 2012, 15, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, K.R.; Balcerek, M.I.; McGowan, T.E.; Love, A.; Chapman, P.R. VIPoma: An unsuspecting culprit of severe secretory diarrhoea in a human immunodeficiency virus-infected patient. Intern. Med. J. 2022, 52, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, S.; de Biase, D.; Fornelli, A.; Masetti, M.; Cuppini, A.; Bondi, A.; Tallini, G.; Jovine, E.; Pession, A. Hepatitis B virus infection and pancreatic neuroendocrine tumor: A case report. Pancreas 2015, 44, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Alejo, M.; Alemany, L.; Clavero, O.; Quiros, B.; Vighi, S.; Seoud, M.; Cheng-Yang, C.; Garland, S.M.; Juanpere, N.; Lloreta, J.; et al. Contribution of human papillomavirus in neuroendocrine tumors from a series of 10,575 invasive cervical cancer cases. Papillomavirus Res. 2018, 5, 134–142. [Google Scholar] [CrossRef]
- Onyema, M.C.; Drakou, E.E.; Dimitriadis, G.K. Endocrine abnormality in paraneoplastic syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101621. [Google Scholar] [CrossRef]
- Ray, A.; Barreto, S.C.; Armstrong, E.; Dogan, S. Pathobiology of cancer and clinical biochemistry. J. Pediatr. Biochem. 2013, 3, 187–201. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, D.; Fallon, J.T.; Zhong, M. Distinct genetic alterations in small cell carcinoma from different anatomic sites. Exp. Hematol. Oncol. 2015, 4, 2. [Google Scholar] [CrossRef]
- Pathak, S.; Starr, J.S.; Halfdanarson, T.; Sonbol, M.B. Understanding the increasing incidence of neuroendocrine tumors. Expert Rev. Endocrinol. Metab. 2023, 18, 377–385. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Jiang, W.; Ji, H.; Wang, Z.W.; Zhu, X. Discovery of key genes as novel biomarkers specifically associated with HPV-negative cervical cancer. Mol. Ther. Methods Clin. Dev. 2021, 21, 492–506. [Google Scholar] [CrossRef]
- Fernandes, A.; Viveros-Carreño, D.; Hoegl, J.; Ávila, M.; Pareja, R. Human papillomavirus-independent cervical cancer. Int. J. Gynecol. Cancer 2022, 32, 1–7. [Google Scholar] [CrossRef]
- Szymonowicz, K.A.; Chen, J. Biological and clinical aspects of HPV-related cancers. Cancer Biol. Med. 2020, 17, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Gheit, T. Mucosal and cutaneous human papillomavirus infections and cancer biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Várnai, A.D.; Bollmann, M.; Bánkfalvi, A.; Griefingholt, H.; Pfening, N.; Schmitt, C.; Pajor, L.; Bollmann, R. The spectrum of cervical diseases induced by low-risk and undefined-risk HPVS: Implications for patient management. Anticancer Res. 2007, 27, 563–570. [Google Scholar] [PubMed]
- Khieu, M.; Butler, S.L. High-Grade Squamous Intraepithelial Lesion of the Cervix; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Učakar, V.; Poljak, M.; Oštrbenk, A.; Klavs, I. Pre-vaccination prevalence of infections with 25 non-high-risk human papillomavirus types among 1,000 Slovenian women in cervical cancer screening. J. Med. Virol. 2014, 86, 1772–1779. [Google Scholar] [CrossRef]
- Bleeker, M.C.; Hogewoning, C.J.; Berkhof, J.; Voorhorst, F.J.; Hesselink, A.T.; van Diemen, P.M.; van den Brule, A.J.; Snijders, P.J.; Meijer, C.J. Concordance of specific human papillomavirus types in sex partners is more prevalent than would be expected by chance and is associated with increased viral loads. Clin. Infect. Dis. 2005, 41, 612–620. [Google Scholar] [CrossRef]
- Castle, P.E.; Cox, J.T.; Jeronimo, J.; Solomon, D.; Wheeler, C.M.; Gravitt, P.E.; Schiffman, M. An analysis of high-risk human papillomavirus DNA-negative cervical precancers in the ASCUS-LSIL Triage Study (ALTS). Obstet. Gynecol. 2008, 111, 847–856. [Google Scholar] [CrossRef]
- Regauer, S.; Reich, O.; Kashofer, K. Thin variant of high-grade squamous intraepithelial lesion-relationship with high-risk and possibly carcinogenic human papilloma virus subtypes and somatic cancer gene mutations. Histopathology 2019, 75, 405–412. [Google Scholar] [CrossRef]
- Guimerà, N.; Lloveras, B.; Alemany, L.; Iljazovic, E.; Shin, H.R.; Jung-Il, S.; de Sanjose, S.; Jenkins, D.; Bosch, F.X.; Quint, W. Laser capture microdissection shows HPV11 as both a causal and a coincidental infection in cervical cancer specimens with multiple HPV types. Histopathology 2013, 63, 287–292. [Google Scholar] [CrossRef]
- Zappacosta, R.; Lattanzio, G.; Viola, P.; Ianieri, M.M.; Gatta, D.M.; Rosini, S. A very rare case of HPV-53-related cervical cancer, in a 79-year-old woman with a previous history of negative Pap cytology. Clin. Interv. Aging 2014, 9, 683–688. [Google Scholar] [CrossRef]
- Petry, K.U.; Liebrich, C.; Luyten, A.; Zander, M.; Iftner, T. Surgical staging identified false HPV-negative cases in a large series of invasive cervical cancers. Papillomavirus Res. 2017, 4, 85–89. [Google Scholar] [CrossRef]
- González-Bosquet, E.; Muñoz, A.; Suñol, M.; Lailla, J.M. Cervical cancer and low-risk HPV; A case report. Eur. J. Gynaecol. Oncol. 2006, 27, 193–194. [Google Scholar] [PubMed]
- Pim, D.; Banks, L. Interaction of viral oncoproteins with cellular target molecules: Infection with high-risk vs low-risk human papillomaviruses. APMIS 2010, 118, 471–493. [Google Scholar] [CrossRef] [PubMed]
- Cornall, A.M.; Roberts, J.M.; Garland, S.M.; Hillman, R.J.; Grulich, A.E.; Tabrizi, S.N. Anal and perianal squamous carcinomas and high-grade intraepithelial lesions exclusively associated with “low-risk” HPV genotypes 6 and 11. Int. J. Cancer 2013, 133, 2253–2258. [Google Scholar] [CrossRef] [PubMed]
- Raybould, R.; Fiander, A.; Hibbitts, S. Human papillomavirus integration and its role in cervical malignant progression. Open Clin. Cancer J. 2011, 5, 1–7. [Google Scholar] [CrossRef]
- Silva, L.L.D.; Teles, A.M.; Santos, J.M.O.; Souza de Andrade, M.; Medeiros, R.; Faustino-Rocha, A.I.; Oliveira, P.A.; Dos Santos, A.P.A.; Ferreira Lopes, F.; Braz, G.; et al. Malignancy associated with low-risk HPV6 and HPV11, A systematic review and implications for cancer prevention. Cancers 2023, 15, 4068. [Google Scholar] [CrossRef]
- Turazza, E.; Lapeña, A.; Sprovieri, O.; Torres, C.P.; Gurucharri, C.; Maciel, A.; Lema, B.; Grinstein, S.; Kahn, T. Low-risk human papillomavirus types 6 and 11 associated with carcinomas of the genital and upper aero-digestive tract. Acta Obstet. Gynecol. Scand. 1997, 76, 271–276. [Google Scholar]
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Ibrahim Khalil, A.; Baussano, I.; Shah, A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health 2021, 9, e161–e169. [Google Scholar] [CrossRef]
- Mboumba Bouassa, R.S.; Prazuck, T.; Lethu, T.; Jenabian, M.A.; Meye, J.F.; Bélec, L. Cervical cancer in sub-Saharan Africa: A preventable noncommunicable disease. Expert Rev. Anti-Infect. Ther. 2017, 15, 613–627. [Google Scholar] [CrossRef]
- Liu, G.; Sharma, M.; Tan, N.; Barnabas, R.V. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS 2018, 32, 795–808. [Google Scholar] [CrossRef]
- Trejo, M.J.; Lishimpi, K.; Kalima, M.; Mwaba, C.K.; Banda, L.; Chuba, A.; Chama, E.; Msadabwe, S.C.; Bell, M.L.; Harris, R.B.; et al. Effects of HIV status on non-metastatic cervical cancer progression among patients in Lusaka, Zambia. Int. J. Gynecol. Cancer 2020, 30, 613–618. [Google Scholar] [CrossRef]
- Anastos, K.; Hoover, D.R.; Burk, R.D.; Cajigas, A.; Shi, Q.; Singh, D.K.; Cohen, M.H.; Mutimura, E.; Sturgis, C.; Banzhaf, W.C.; et al. Risk factors for cervical precancer and cancer in HIV-infected, HPV-positive Rwandan women. PLoS ONE 2010, 5, e13525. [Google Scholar] [CrossRef] [PubMed]
- Mane, A.; Nirmalkar, A.; Risbud, A.R.; Vermund, S.H.; Mehendale, S.M.; Sahasrabuddhe, V.V. HPV genotype distribution in cervical intraepithelial neoplasia among HIV-infected women in Pune, India. PLoS ONE 2012, 7, e38731. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.O.; Ahmed, N.U. The role of HIV in the progression through the stages of the human papillomavirus to cervical cancer pathway. AIDS Rev. 2018, 20, 94–1043. [Google Scholar] [CrossRef] [PubMed]
- Lovane, L.; Larsson, G.L.; Tulsidás, S.; Carrilho, C.; Andersson, S.; Karlsson, C. Endocervical adenocarcinomas and HPV genotyping in an HIV endemic milieu-a retrospective study. BMC Womens Health 2025, 25, 20. [Google Scholar] [CrossRef]
- Debeaudrap, P.; Kabore, F.N.; Setha, L.; Tegbe, J.; Doukoure, B.; Sotheara, M.; Segeral, O.; Aun, K.; Messou, E.; Bitolog, P.; et al. Performance of visual inspection, partial genotyping, and their combination for the triage of women living with HIV who are screen positive for human papillomavirus: Results from the AIMA-CC ANRS 12375 multicentric screening study. Int. J. Cancer 2025, 156, 598–607. [Google Scholar] [CrossRef]
- Pillay, P.; Galappaththi-Arachchige, H.N.; Taylor, M.; Roald, B.; Kjetland, E.F. Urinary human papillomavirus DNA as an indicator of gynaecological infection in young women in Schistosoma and HIV endemic South Africa. Front. Glob. Womens Health 2025, 5, 1436064. [Google Scholar] [CrossRef]
- Strickler, H.D.; Usyk, M.; Eltoum, I.E.; Bachman, N.; Hessol, N.A.; Flowers, L.; Rahangdale, L.; Atrio, J.M.; Ramirez, C.; Minkoff, H.; et al. Case-control study of cervicovaginal beta-/gamma-HPV infection in women with HIV and its relation with incident cervical precancer. J. Infect. Dis. 2025, 231, 595–599. [Google Scholar] [CrossRef]
- Naicker, N.; Osman, F.; Naidoo, K.; Bodley, N.; Mbambo, N.; Madlala, S.; Mhlongo, T.; Mbatha, N.; Maphumulo, A.; Munatsi, P.; et al. High burden of human papillomavirus and premalignant cervical lesions among women starting HIV treatment in KwaZulu-Natal, South Africa. Sex. Transm. Infect. 2025, 101, 187–190. [Google Scholar] [CrossRef]
- Mbulawa, Z.Z.A.; Kondlo, S.; Toni, S.; Faye, L.M.; Businge, C.B. Prevalence, characteristics, and distribution of human papillomavirus according to age and HIV status in women of Eastern Cape Province, South Africa. Viruses 2024, 16, 1751. [Google Scholar] [CrossRef]
- Rantshabeng, P.S.; Tsima, B.M.; Ndlovu, A.K.; Motlhatlhedi, K.; Sharma, K.; Masole, C.B.; Moraka, N.O.; Motsumi, K.; Maoto-Mokote, A.K.T.; Eshetu, A.B.; et al. High-risk human papillomavirus diversity among indigenous women of western Botswana with normal cervical cytology and dysplasia. BMC Infect. Dis. 2024, 24, 1163. [Google Scholar] [CrossRef]
- Murenzi, G.; Vuhahula, E.; Kimambo, A.; Matiku, S.; Tuyishime, O.; Liwa, E.; Habanabakize, T.; Rugengamanzi, E.; Malango, A.; Kubwimana, G.; et al. High-risk human papillomavirus genotyping in cervical cancers in Tanzania. Infect. Agents Cancer 2024, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Muchaili, L.; Simushi, P.; Mweene, B.C.; Mwakyoma, T.; Masenga, S.K.; Hamooya, B.M. Prevalence and correlates of human papillomavirus infection in females from Southern Province, Zambia: A cross-sectional study. PLoS ONE 2024, 19, e0299963. [Google Scholar] [CrossRef] [PubMed]
- Worku, E.; Yigizaw, G.; Admassu, R.; Mekonnen, D.; Gessessa, W.; Tessema, Z.; Walle, T. Prevalence and risk factors associated with precancerous and cancerous cervical lesions among HIV-infected women in University of Gondar specialized comprehensive referral hospital, Northwest Ethiopia: Cross-sectional study design. BMC Womens Health 2024, 24, 322. [Google Scholar] [CrossRef]
- Manga, S.M.; Ye, Y.; Nulah, K.L.; Manjuh, F.; Fokom-Domgue, J.; Scarinci, I.; Tita, A.N. Human papillomavirus types and cervical cancer screening among female sex workers in Cameroon. Cancers 2024, 16, 243. [Google Scholar] [CrossRef]
- Akakpo, P.K.; Ken-Amoah, S.; Enyan, N.I.E.; Agyare, E.; Salia, E.; Baidoo, I.; Derkyi-Kwarteng, L.; Asare, M.; Adjei, G.; Addo, S.A.; et al. High-risk human papillomavirus genotype distribution among women living with HIV; implication for cervical cancer prevention in a resource limited setting. Infect. Agents Cancer 2023, 18, 33. [Google Scholar] [CrossRef]
- Grover, S.; Bhatia, R.; Friebel-Klingner, T.M.; Mathoma, A.; Vuylsteke, P.; Khan, S.; Ralefala, T.; Tawe, L.; Bazzett-Matabele, L.; Monare, B.; et al. Cervical cancer screening in HIV-endemic countries: An urgent call for guideline change. Cancer Treat. Res. Commun. 2023, 34, 100682. [Google Scholar] [CrossRef]
- Kangethe, J.M.; Gichuhi, S.; Odari, E.; Pintye, J.; Mutai, K.; Abdullahi, L.; Maiyo, A.; Mureithi, M.W. Confronting the human papillomavirus-HIV intersection: Cervical cytology implications for Kenyan women living with HIV. South Afr. J. HIV Med. 2023, 24, 1508. [Google Scholar] [CrossRef]
- Megersa, T.; Dango, S.; Kumsa, K.; Lemma, K.; Lencha, B. Prevalence of high-risk human papillomavirus infections and associated factors among women living with HIV in Shashemene town public health facilities, Southern Ethiopia. BMC Womens Health 2023, 23, 125. [Google Scholar] [CrossRef]
- Sørbye, S.W.; Falang, B.M.; Botha, M.H.; Snyman, L.C.; van der Merwe, H.; Visser, C.; Richter, K.; Dreyer, G. Enhancing cervical cancer prevention in South African women: Primary HPV mRNA screening with different genotype combinations. Cancers 2023, 15, 5453. [Google Scholar] [CrossRef]
- Gupta, R.; Hussain, S.; Hariprasad, R.; Dhanasekaran, K.; Verma, S.; Agarwal, V.; Sandeep; Parveen, S.; Kaur, A.; Verma, C.P.; et al. High prevalence of cervical high-grade lesions and high-risk human papillomavirus infections in women living with HIV: A case for prioritizing cervical screening in this vulnerable group. Acta Cytol. 2022, 66, 496–506. [Google Scholar] [CrossRef]
- Lewis, S.; Mphande, M.; Chibwana, F.; Gumbo, T.; Banda, B.A.; Sigauke, H.; Moses, A.; Gupta, S.; Hoffman, R.M.; Moucheraud, C. Association of HIV status and treatment characteristics with VIA screening outcomes in Malawi: A retrospective analysis. PLoS ONE 2022, 17, e0262904. [Google Scholar] [CrossRef] [PubMed]
- Nakisige, C.; Adams, S.V.; Namirembe, C.; Okoche, L.; Ferrenberg, J.; Towlerton, A.; Larsen, A.; Orem, J.; Casper, C.; Frenkel, L.; et al. Multiple high-risk HPV types contribute to cervical dysplasia in Ugandan women living with HIV on antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2022, 90, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Mcharo, R.; Lennemann, T.; France, J.; Torres, L.; Garí, M.; Mbuya, W.; Mwalongo, W.; Mahenge, A.; Bauer, A.; Mnkai, J.; et al. HPV type distribution in HIV positive and negative women with or without cervical dysplasia or cancer in East Africa. Front. Oncol. 2021, 11, 763717. [Google Scholar] [CrossRef]
- Jary, A.; Teguete, I.; Sidibé, Y.; Kodio, A.; Dolo, O.; Burrel, S.; Boutolleau, D.; Beauvais-Remigereau, L.; Sayon, S.; Kampo, M.; et al. Prevalence of cervical HPV infection, sexually transmitted infections and associated antimicrobial resistance in women attending cervical cancer screening in Mali. Int. J. Infect. Dis. 2021, 108, 610–616. [Google Scholar] [CrossRef]
- Taku, O.; Brink, A.; Meiring, T.L.; Phohlo, K.; Businge, C.B.; Mbulawa, Z.Z.A.; Williamson, A.L. Detection of sexually transmitted pathogens and co-infection with human papillomavirus in women residing in rural Eastern Cape, South Africa. PeerJ 2021, 9, e10793. [Google Scholar] [CrossRef]
- Peng, H.Q.; Liu, S.L.; Mann, V.; Rohan, T.; Rawls, W. Human papillomavirus types 16 and 33, herpes simplex virus type 2 and other risk factors for cervical cancer in Sichuan Province, China. Int. J. Cancer 1991, 47, 711–716. [Google Scholar] [CrossRef]
- Lehtinen, M.; Koskela, P.; Jellum, E.; Bloigu, A.; Anttila, T.; Hallmans, G.; Luukkaala, T.; Thoresen, S.; Youngman, L.; Dillner, J.; et al. Herpes simplex virus and risk of cervical cancer: A longitudinal, nested case-control study in the Nordic countries. Am. J. Epidemiol. 2002, 156, 687–692. [Google Scholar] [CrossRef]
- Pérez, L.O.; Barbisan, G.; Abba, M.C.; Laguens, R.M.; Dulout, F.N.; Golijow, C.D. Herpes simplex virus and human papillomavirus infection in cervical disease in Argentine women. Int. J. Gynecol. Pathol. 2006, 25, 42–47. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, S.; Xia, Y.; Lin, Y.; Zhou, G.; Yu, Y.; Feng, M. Association between human herpesvirus infection and cervical carcinoma: A systematic review and meta-analysis. Virol. J. 2023, 20, 288. [Google Scholar] [CrossRef]
- Freeman, E.E.; Weiss, H.A.; Glynn, J.R.; Cross, P.L.; Whitworth, J.A.; Hayes, R.J. Herpes simplex virus 2 infection increases HIV acquisition in men and women: Systematic review and meta-analysis of longitudinal studies. AIDS 2006, 20, 73–83. [Google Scholar] [CrossRef]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wen, X. Seropositivity to herpes simplex virus type 2, but not type 1 is associated with cervical cancer: NHANES (1999–2014). BMC Cancer 2017, 17, 726. [Google Scholar] [CrossRef] [PubMed]
- Bahena-Román, M.; Sánchez-Alemán, M.A.; Contreras-Ochoa, C.O.; Lagunas-Martínez, A.; Olamendi-Portugal, M.; López-Estrada, G.; Delgado-Romero, K.; Guzmán-Olea, E.; Madrid-Marina, V.; Torres-Poveda, K. Prevalence of active infection by herpes simplex virus type 2 in patients with high-risk human papillomavirus infection: A cross-sectional study. J. Med. Virol. 2020, 92, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Herrero, R.; Bosetti, C.; Muñoz, N.; Bosch, F.X.; Eluf-Neto, J.; Castellsagué, X.; Meijer, C.J.; Van den Brule, A.J.; Franceschi, S.; et al. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J. Natl. Cancer Inst. 2002, 94, 1604–1613. [Google Scholar] [CrossRef]
- Shanehsazzadeh, M.; Sharifi-Rad, J.; Behbahani, M.; Pourazar, A. Analysis of human papillomavirus and herpes simplex virus genus -2 from patients with cervical cancer in Isfahan, Iran. Mater. Sociomed. 2014, 26, 234–236. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, X.; Zheng, Y.; Tang, J.; Cai, W.; Wang, H.; Gao, Y.; Wang, Y. Relationship between cervical disease and infection with human papillomavirus types 16 and 18, and herpes simplex virus 1 and 2. J. Med. Virol. 2012, 84, 1920–1927. [Google Scholar] [CrossRef]
- El-All, H.S.; Refaat, A.; Dandash, K. Prevalence of cervical neoplastic lesions and human papilloma virus infection in Egypt: National Cervical Cancer Screening Project. Infect. Agents Cancer 2007, 2, 12. [Google Scholar] [CrossRef]
- Paba, P.; Bonifacio, D.; Di Bonito, L.; Ombres, D.; Favalli, C.; Syrjänen, K.; Ciotti, M. Co-expression of HSV2 and Chlamydia trachomatis in HPV-positive cervical cancer and cervical intraepithelial neoplasia lesions is associated with aberrations in key intracellular pathways. Intervirology 2008, 51, 230–234. [Google Scholar] [CrossRef]
- Wohlmeister, D.; Vianna, D.R.; Helfer, V.E.; Gimenes, F.; Consolaro, M.E.; Barcellos, R.B.; Rossetti, M.L.; Calil, L.N.; Buffon, A.; Pilger, D.A. Association of human papillomavirus and Chlamydia trachomatis with intraepithelial alterations in cervix samples. Mem. Inst. Oswaldo Cruz 2016, 111, 106–113. [Google Scholar] [CrossRef]
- Kwaśniewska, A.; Korobowicz, E.; Zdunek, M.; Skoczyński, M.; Kwaśniewski, W.; Daniłoś, J.; Goździcka-Józefiak, A. Prevalence of Chlamydia trachomatis and herpes simplex virus 2 in cervical carcinoma associated with human papillomavirus detected in paraffin-sectioned samples. Eur. J. Gynaecol. Oncol. 2009, 30, 65–70. [Google Scholar]
- Moharreri, M.; Sohrabi, A. Characteristics of HSV-2, M. genitalium and, C. trachomatis in HPV genotypes associated with cervical intraepithelial neoplasia and genital infections. Infect. Disord. Drug Targets 2021, 21, 112–118. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, A.L.; Malaguti, N.; Souza, R.P.; Uchimura, N.S.; Ferreira, É.C.; Pereira, M.W.; Carvalho, M.D.; Pelloso, S.M.; Bonini, M.G.; Gimenes, F.; et al. Association of human papillomavirus, Neisseria gonorrhoeae and Chlamydia trachomatis co-infections on the risk of high-grade squamous intraepithelial cervical lesion. Am. J. Cancer Res. 2016, 6, 1371–1383. [Google Scholar] [PubMed]
- Galloway, D.A.; McDougall, J.K. The oncogenic potential of herpes simplex viruses: Evidence for a ‘hit-and-run’ mechanism. Nature 1983, 302, 21–24. [Google Scholar] [CrossRef]
- Skeate, J.G.; Porras, T.B.; Woodham, A.W.; Jang, J.K.; Taylor, J.R.; Brand, H.E.; Kelly, T.J.; Jung, J.U.; Da Silva, D.M.; Yuan, W.; et al. Herpes simplex virus downregulation of secretory leukocyte protease inhibitor enhances human papillomavirus type 16 infection. J. Gen. Virol. 2016, 97, 422–434. [Google Scholar] [CrossRef]
- Hemminki, K.; Kanerva, A.; Försti, A.; Hemminki, A. Cervical, vaginal and vulvar cancer incidence and survival trends in Denmark, Finland, Norway and Sweden with implications to treatment. BMC Cancer 2022, 22, 456. [Google Scholar] [CrossRef]
- van der Aa, M.A.; Pukkala, E.; Coebergh, J.W.; Anttila, A.; Siesling, S. Mass screening programmes and trends in cervical cancer in Finland and the Netherlands. Int. J. Cancer 2008, 122, 1854–1858. [Google Scholar] [CrossRef]
- Idehen, E.E.; Virtanen, A.; Lilja, E.; Tuomainen, T.P.; Korhonen, T.; Koponen, P. Cervical cancer screening participation among women of Russian, Somali, and Kurdish origin compared with the general Finnish population: A register-based study. Int. J. Environ. Res. Public Health 2020, 17, 7899. [Google Scholar] [CrossRef]
- Sarkeala, T.; Lamminmäki, M.; Nygård, M.; Njor, S.H.; Virtanen, A.; Leivonen, A.; Hirvonen, E.; Toikkanen, S.; Campbell, S.; Stefansdóttir, H.; et al. Cervical, liver and stomach cancer incidence and mortality in non-Western immigrant women: A retrospective cohort study from four Nordic countries. Acta Oncol. 2023, 62, 977–987. [Google Scholar] [CrossRef]
- Bowden, S.J.; Doulgeraki, T.; Bouras, E.; Markozannes, G.; Athanasiou, A.; Grout-Smith, H.; Kechagias, K.S.; Ellis, L.B.; Zuber, V.; Chadeau-Hyam, M.; et al. Risk factors for human papillomavirus infection, cervical intraepithelial neoplasia and cervical cancer: An umbrella review and follow-up Mendelian randomisation studies. BMC Med. 2023, 21, 274. [Google Scholar] [CrossRef]
- Lynge, E.; Lönnberg, S.; Törnberg, S. Cervical cancer incidence in elderly women-biology or screening history? Eur. J. Cancer 2017, 74, 82–88. [Google Scholar] [CrossRef]
- Allott, E.H.; Shan, Y.; Chen, M.; Sun, X.; Garcia-Recio, S.; Kirk, E.L.; Olshan, A.F.; Geradts, J.; Earp, H.S.; Carey, L.A.; et al. Bimodal age distribution at diagnosis in breast cancer persists across molecular and genomic classifications. Breast Cancer Res. Treat. 2020, 179, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Lamminmäki, M.; Leivonen, A.; Sarkeala, T.; Virtanen, A.; Heinävaara, S. Health inequalities among Russian-born immigrant women in Finland: Longitudinal analysis on cervical cancer incidence and participation in screening. J. Migr. Health 2022, 6, 100117. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, L.; Kalliala, I.; Aro, K.; Auvinen, E.; Jakobsson, M.; Kiviharju, M.; Virtanen, S.; Dillner, J.; Nieminen, P.; Louvanto, K. Distribution of HPV genotypes differs depending on behavioural factors among young women. Microorganisms 2021, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Pulkkinen, J.; Huhtala, H.; Kholová, I. The role of Pap smear in the diagnostics of endocervical adenocarcinoma. APMIS 2021, 129, 195–203. [Google Scholar] [CrossRef]
- Bechini, A.; Moscadelli, A.; Velpini, B.; Bonito, B.; Orlando, P.; Putignano, P.; Posi, S.; Stacchini, L.; Bonanni, P.; Boccalini, S. Efficacy of HPV vaccination regarding vulvar and vaginal recurrences in previously treated women: The need for further evidence. Vaccines 2023, 11, 1084. [Google Scholar] [CrossRef]
- Williams, J.; Rakovac, I.; Victoria, J.; Tatarinova, T.; Corbex, M.; Barr, B.; Rose, T.; Sturua, L.; Obreja, G.; Andreasyan, D.; et al. Cervical cancer testing among women aged 30-49 years in the WHO European Region. Eur. J. Public Health 2021, 31, 884–889. [Google Scholar] [CrossRef]
- Stephens, E.S.; Dema, E.; McGee-Avila, J.K.; Shiels, M.S.; Kreimer, A.R.; Shing, J.Z. Human papillomavirus awareness by educational level and by race and ethnicity. JAMA. Netw. Open. 2023, 6, e2343325. [Google Scholar] [CrossRef]
- Patel, H.; Jeve, Y.B.; Sherman, S.M.; Moss, E.L. Knowledge of human papillomavirus and the human papillomavirus vaccine in European adolescents: A systematic review. Sex. Transm. Infect. 2016, 92, 474–479. [Google Scholar] [CrossRef]
- Ampofo, A.G.; Mackenzie, L.J.; Osei Asibey, S.; Oldmeadow, C.; Boyes, A.W. Prevalence and correlates of cervical cancer prevention knowledge among high school students in Ghana. Health Educ. Behav. 2024, 51, 185–196. [Google Scholar] [CrossRef]
- Aimiosior, M.O.; Omigbodun, A.O. Knowledge of cervical cancer and cervical cancer screening methods among female secondary school students in Ibadan, Nigeria. Afr. J. Biomed. Res. 2020, 23, 99–103. [Google Scholar]
- M Al Kindi, R.; H Al Sumri, H.; M Al Muhdhoori, T.; Al Mamari, K.; A Al Kalbani, M.; H Al-Azri, M. Knowledge of cervical cancer screening among Omani women attending a university teaching hospital: A cross-sectional study. BMC Womens Health 2024, 24, 40. [Google Scholar] [CrossRef] [PubMed]
- Thulaseedharan, J.V.; Frie, K.G.; Sankaranarayanan, R. Challenges of health promotion and education strategies to prevent cervical cancer in India: A systematic review. J. Educ. Health Promot. 2019, 8, 216. [Google Scholar] [CrossRef]
- Sanghvi, L.D. Cancer epidemiology: The Indian scene. J. Cancer Res. Clin. Oncol. 1981, 99, 1–14. [Google Scholar] [CrossRef]
- Prabhakar, A.K. Cervical cancer in India—Strategy for control. Indian J. Cancer 1992, 29, 104–113. [Google Scholar]
- Sharma, B.K.; Ray, A.; Murthy, N.S. Prevalence of serum antibodies to synthetic peptides to HPV16 epitopes among Indian women with cervical neoplasia. Eur. J. Cancer 1996, 32A, 872–876. [Google Scholar] [CrossRef]
- Sen, U.; Sankaranarayanan, R.; Mandal, S.; Ramanakumar, A.V.; Parkin, D.M.; Siddiqi, M. Cancer patterns in eastern India: The first report of the Kolkata cancer registry. Int. J. Cancer 2002, 100, 86–91. [Google Scholar] [CrossRef]
- Pal, S.K.; Mittal, B. Improving cancer care in India: Prospects and challenges. Asian Pac. J. Cancer Prev. 2004, 5, 226–228. [Google Scholar]
- Takiar, R.; Srivastav, A. Time trend in breast and cervix cancer of women in India-(1990–2003). Asian Pac. J. Cancer Prev. 2008, 9, 777–780. [Google Scholar]
- Mishra, G.A.; Pimple, S.A.; Shastri, S.S. Prevention of cervix cancer in India. Oncology 2016, 91 (Suppl. S1), 1–7. [Google Scholar] [CrossRef]
- India State-Level Disease Burden Initiative Cancer Collaborators. The burden of cancers and their variations across the states of India: The Global Burden of Disease Study 1990–2016. Lancet Oncol. 2018, 19, 1289–1306. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Sankarapillai, J.; Mathew, A.; Nair, R.A.; Gangane, N.; Khuraijam, S.; Barmon, D.; Pandya, S.; Majumdar, G.; Deshmane, V.; et al. Survival of patients with cervical cancer in India–findings from 11 population based cancer registries under National Cancer Registry Programme. Lancet Reg. Health Southeast Asia 2023, 24, 100296. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. More than a quarter of India’s youngsters have premarital sex. BMJ 2001, 322, 575. [Google Scholar] [CrossRef][Green Version]
- Maheswari, S.U.; Kalaivani, S. Pattern of sexual behavior in adolescents and young adults attending STD clinic in a tertiary care center in South India. Indian J. Sex. Transm. Dis. AIDS 2017, 38, 171–175. [Google Scholar] [CrossRef]
- Sharma, S.K.; Vishwakarma, D. Transitions in adolescent boys and young men’s high-risk sexual behaviour in India. BMC Public Health 2020, 20, 1089. [Google Scholar] [CrossRef]
- Dhawan, J.; Khandpur, S. Emerging trends in viral sexually transmitted infections in India. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 561–565. [Google Scholar] [CrossRef]
- Goel, S.; Chopra, D.; Choudhary, V.; Riyat, A.; Chopra, S. Changing trends of sexually transmitted infections and estimation of partner notification at a tertiary care center in North India. Indian J. Sex. Transm. Dis. AIDS 2020, 41, 176–180. [Google Scholar] [CrossRef]
- Parikh, P.M.; Mullapally, S.K.; Hingmire, S.; Kamal Uddin, A.F.M.; Thinn, M.M.; Shahi, A.; Tshomo, U.; Mohan, I.; Kaur, S.; Ghadyalpatil, N. Cervical cancer in SAARC countries. South Asian J. Cancer 2023, 12, 1–8. [Google Scholar] [CrossRef]
- Muthuramalingam, M.R.; Muraleedharan, V.R. Patterns in the prevalence and wealth-based inequality of cervical cancer screening in India. BMC Womens Health 2023, 23, 337. [Google Scholar] [CrossRef]
- Konno, R.; Sagae, S.; Yoshikawa, H.; Basu, P.S.; Hanley, S.J.; Tan, J.H.; Shin, H.R. Cervical Cancer Working Group report. Jpn. J. Clin. Oncol. 2010, 40 (Suppl. S1), i44–i50. [Google Scholar] [CrossRef]
- Hareesh, P.V.; Rajkumar, E.; Gopi, A.; Sri Lakshmi, K.N.V.; Romate, J. Prevalence and determinants of hand hygiene behavior among Indian population: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 2619. [Google Scholar] [CrossRef]
- Roe, K. A latent pathogen infection classification system that would significantly increase healthcare safety. Immunol. Res. 2023, 71, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Sabeena, S.; Bhat, P.; Kamath, V.; Arunkumar, G. Possible non-sexual modes of transmission of human papilloma virus. J. Obstet. Gynaecol. Res. 2017, 43, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Jha, R.P.; Shri, N.; Bhattacharyya, K.; Patel, P.; Dhamnetiya, D. Secular trends in incidence and mortality of cervical cancer in India and its states, 1990-2019, Data from the Global Burden of Disease 2019 Study. BMC Cancer 2022, 22, 149. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, Y.; Rajaa, S.; Giriyappa, D.K. Global pattern and trend of cervical cancer incidence from 1993 to 2012, Joinpoint regression and age-period-cohort analysis. Indian J. Cancer 2022, 59, 521–531. [Google Scholar] [CrossRef]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30 (Suppl. S5), F12–F23. [Google Scholar] [CrossRef]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and burden of human papillomavirus and related diseases, molecular pathogenesis, and vaccine evaluation. Front. Public Health 2021, 8, 552028. [Google Scholar] [CrossRef]
- Wagh, P.; Kulkarni, P.; Kerkar, S.; Tongaonkar, H.; Deodhar, K.; Rekhi, B.; Salvi, V.; Chaudhari, H.; Warke, H.; Mania-Pramanik, J. Types of human papillomavirus observed in hospital-based population. Indian J. Med. Microbiol. 2019, 37, 557–562. [Google Scholar] [CrossRef]
- Dutta, S.; Begum, R.; Mazumder Indra, D.; Mandal, S.S.; Mondal, R.; Biswas, J.; Dey, B.; Panda, C.K.; Basu, P. Prevalence of human papillomavirus in women without cervical cancer: A population-based study in Eastern India. Int. J. Gynecol. Pathol. 2012, 31, 178–183. [Google Scholar] [CrossRef]
- Datta, P.; Bhatla, N.; Pandey, R.M.; Dar, L.; Patro, A.R.; Vasisht, S.; Kriplani, A.; Singh, N. Type-specific incidence and persistence of HPV infection among young women: A prospective study in North India. Asian Pac. J. Cancer Prev. 2012, 13, 1019–1024. [Google Scholar] [CrossRef]
- Misra, J.S.; Srivastava, A.N.; Zaidi, Z.H. Cervical cytopathological changes associated with onset of menopause. J. Midlife Health 2018, 9, 180–184. [Google Scholar] [CrossRef]
- Kalavathy, M.C.; Mathew, A.; Jagathnath Krishna, K.M.; Saritha, V.N.; Sujathan, K. Risk factors and prevalence of cervical squamous intraepithelial lesions among women in south India: A community-based cross-sectional study. Indian J. Cancer 2022, 59, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Kalantri, S.; Raphael, V.; Dey, B.; Khonglah, Y.; Das, A. Prevalence of human papillomavirus infection in abnormal pap smears. Cytojournal 2023, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Moscicki, A.B.; Hills, N.; Shiboski, S.; Powell, K.; Jay, N.; Hanson, E.; Miller, S.; Clayton, L.; Farhat, S.; Broering, J.; et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA 2001, 285, 2995–3002. [Google Scholar] [CrossRef]
- Gopalani, S.V.; Saraiya, M.; Huang, B.; Tucker, T.C.; Mix, J.M.; Chaturvedi, A.K. Population-level incidence of human papillomavirus-positive oropharyngeal, cervical, and anal cancers, by smoking status. J. Natl. Cancer Inst. 2024, 116, 1173–1177. [Google Scholar] [CrossRef]
- Malevolti, M.C.; Lugo, A.; Scala, M.; Gallus, S.; Gorini, G.; Lachi, A.; Carreras, G. Dose-risk relationships between cigarette smoking and cervical cancer: A systematic review and meta-analysis. Eur. J. Cancer Prev. 2023, 32, 171–183. [Google Scholar] [CrossRef]
- Sugawara, Y.; Tsuji, I.; Mizoue, T.; Inoue, M.; Sawada, N.; Matsuo, K.; Ito, H.; Naito, M.; Nagata, C.; Kitamura, Y.; et al. Cigarette smoking and cervical cancer risk: An evaluation based on a systematic review and meta-analysis among Japanese women. Jpn. J. Clin. Oncol. 2019, 49, 77–86. [Google Scholar] [CrossRef]
- Aguayo, F.; Muñoz, J.P.; Perez-Dominguez, F.; Carrillo-Beltrán, D.; Oliva, C.; Calaf, G.M.; Blanco, R.; Nuñez-Acurio, D. High-risk human papillomavirus and tobacco smoke interactions in epithelial carcinogenesis. Cancers 2020, 12, 2201. [Google Scholar] [CrossRef]
- Muñoz, J.P.; Carrillo-Beltrán, D.; Aedo-Aguilera, V.; Calaf, G.M.; León, O.; Maldonado, E.; Tapia, J.C.; Boccardo, E.; Ozbun, M.A.; Aguayo, F. Tobacco exposure enhances human papillomavirus 16 oncogene expression via EGFR/PI3K/Akt/c-Jun signaling pathway in cervical cancer cells. Front. Microbiol. 2018, 9, 3022. [Google Scholar] [CrossRef]
- White, C.M.; Bakhiet, S.; Bates, M.; Ruttle, C.; Pilkington, L.J.; Keegan, H.; O’Toole, S.A.; Sharp, L.; O’Kelly, R.; Tewari, P.; et al. Exposure to tobacco smoke measured by urinary nicotine metabolites increases risk of p16/Ki-67 co-expression and high-grade cervical neoplasia in HPV positive women: A two year prospective study. Cancer Epidemiol. 2020, 68, 101793. [Google Scholar] [CrossRef]
- Yuan, R.; Ren, F.; Xie, Y.; Li, K.; Tong, Z. The global, regional, and national burdens of cervical cancer attributable to smoking from 1990 to 2019, Population-based study. JMIR Public Health Surveill. 2022, 8, e40657. [Google Scholar] [CrossRef]
- Joseph, N.; Nelliyanil, M.; Supriya, K.; Babu, Y.; Naik, R.; Purushothama, K.; Kotian, S.M.; Angeline, R.; Sharavathi, K.; Saralaya, V.; et al. Association between occupational history of exposure to tobacco dust and risk of carcinoma cervix: A case-control study. Indian J. Cancer 2016, 53, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Siokos, A.G.; Siokou-Siova, O.; Tzafetas, I. Correlation between cervical carcinogenesis and tobacco use by sexual partners. Hell. J. Nucl. Med. 2019, 22 (Suppl. S2), 184–190. [Google Scholar] [PubMed]
- Brown, D.C.; Pereira, L.; Garner, J.B. Cancer of the cervix and the smoking husband. Can. Fam. Physician 1982, 28, 499–502. [Google Scholar]
- Tokudome, S. Semen of smokers and cervical cancer risk. J. Natl. Cancer Inst. 1997, 89, 96–97. [Google Scholar] [CrossRef]
- Malevolti, M.C.; Maci, C.; Lugo, A.; Possenti, I.; Gallus, S.; Gorini, G.; Carreras, G. Second-hand smoke exposure and cervical cancer: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2023, 149, 14353–14363. [Google Scholar] [CrossRef]
- Su, B.; Qin, W.; Xue, F.; Wei, X.; Guan, Q.; Jiang, W.; Wang, S.; Xu, M.; Yu, S. The relation of passive smoking with cervical cancer: A systematic review and meta-analysis. Medicine 2018, 97, e13061. [Google Scholar] [CrossRef]
- Du, X.; Li, M.; Zhou, Y.; Yang, H.; Isachenko, V.; Takagi, T.; Meng, Y. Evidence of passive smoking as a risk factor of high-grade squamous intraepithelial lesion: A case-control study. Biol. Pharm. Bull. 2020, 43, 1061–1066. [Google Scholar] [CrossRef]
- Chatzistamatiou, K.; Moysiadis, T.; Vryzas, D.; Chatzaki, E.; Kaufmann, A.M.; Koch, I.; Soutschek, E.; Boecher, O.; Tsertanidou, A.; Maglaveras, N.; et al. Cigarette smoking promotes infection of cervical cells by high-risk human papillomaviruses, but not subsequent E7 oncoprotein expression. Int. J. Mol. Sci. 2018, 19, 422. [Google Scholar] [CrossRef]
- Eldridge, R.C.; Pawlita, M.; Wilson, L.; Castle, P.E.; Waterboer, T.; Gravitt, P.E.; Schiffman, M.; Wentzensen, N. Smoking and subsequent human papillomavirus infection: A mediation analysis. Ann. Epidemiol. 2017, 27, 724–730.e1. [Google Scholar] [CrossRef]
- Feng, R.M.; Hu, S.Y.; Zhao, F.H.; Zhang, R.; Zhang, X.; Wallach, A.I.; Qiao, Y.L. Role of active and passive smoking in high-risk human papillomavirus infection and cervical intraepithelial neoplasia grade 2 or worse. J. Gynecol. Oncol. 2017, 28, e47. [Google Scholar] [CrossRef]
- Ma, K.; Li, S.; Wu, S.; Zhu, J.; Yang, Y. Impact of smoking exposure on human papillomavirus clearance among Chinese women: A follow-up propensity score matching study. Tob. Induc. Dis. 2023, 21, 42. [Google Scholar] [CrossRef]
- Wierzbicka, M.; Klussmann, J.P.; San Giorgi, M.R.; Wuerdemann, N.; Dikkers, F.G. Oral and laryngeal HPV infection: Incidence, prevalence and risk factors, with special regard to concurrent infection in head, neck and genitals. Vaccine 2021, 39, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Eggersmann, T.K.; Sharaf, K.; Baumeister, P.; Thaler, C.; Dannecker, C.J.; Jeschke, U.; Mahner, S.; Weyerstahl, K.; Weyerstahl, T.; Bergauer, F.; et al. Prevalence of oral HPV infection in cervical HPV positive women and their sexual partners. Arch. Gynecol. Obstet. 2019, 299, 1659–1665. [Google Scholar] [CrossRef]
- McBride, A.A. Oncogenic human papillomaviruses. Philos. Trans. R Soc. Lond. B Biol. Sci. 2017, 372, 20160273. [Google Scholar] [CrossRef]
- Tumban, E. A current update on human papillomavirus-associated head and neck cancers. Viruses 2019, 11, 922. [Google Scholar] [CrossRef]
- Nagelhout, G.; Ebisch, R.M.; Van Der Hel, O.; Meerkerk, G.J.; Magnée, T.; De Bruijn, T.; Van Straaten, B. Is smoking an independent risk factor for developing cervical intra-epithelial neoplasia and cervical cancer? A systematic review and meta-analysis. Expert Rev. Anticancer. Ther. 2021, 21, 781–794. [Google Scholar] [CrossRef]
- Fang, J.H.; Yu, X.M.; Zhang, S.H.; Yang, Y. Effect of smoking on high-grade cervical cancer in women on the basis of human papillomavirus infection studies. J. Cancer Res. Ther. 2018, 14, S184–S189. [Google Scholar] [CrossRef]
- Hikari, T.; Honda, A.; Hashiguchi, M.; Okuma, R.; Kurihara, M.; Fukuda, A.; Okuma, E.; Nakao, Y.; Yokoyama, M. The difference in the effectiveness of human papillomavirus vaccine based on smoking status. J. Obstet. Gynaecol. Res. 2022, 48, 1859–1866. [Google Scholar] [CrossRef]
- Koeneman, M.M.; Hendriks, N.; Kooreman, L.F.; Winkens, B.; Kruitwagen, R.F.; Kruse, A.J. Prognostic factors for spontaneous regression of high-risk human papillomavirus-positive cervical intra-epithelial neoplasia grade 2. Int. J. Gynecol. Cancer 2019, 29, 1003–1009. [Google Scholar] [CrossRef]
- Xu, H.; Egger, S.; Velentzis, L.S.; O’Connell, D.L.; Banks, E.; Darlington-Brown, J.; Canfell, K.; Sitas, F. Hormonal contraceptive use and smoking as risk factors for high-grade cervical intraepithelial neoplasia in unvaccinated women aged 30-44 years: A case-control study in New South Wales, Australia. Cancer Epidemiol. 2018, 55, 162–169. [Google Scholar] [CrossRef]
- Zidi, S.; Sahli, M.; Mezlini, A.; Yacoubli-Loueslati, B. Association of combined tobacco smoking, hormonal contraceptive use and status matrimonial with cervical cancer evolution in Tunisian women. Pathol. Oncol. Res. 2020, 26, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Torres-Poveda, K.; Ruiz-Fraga, I.; Madrid-Marina, V.; Chavez, M.; Richardson, V. High risk HPV infection prevalence and associated cofactors: A population-based study in female ISSSTE beneficiaries attending the HPV screening and early detection of cervical cancer program. BMC Cancer 2019, 19, 1205. [Google Scholar] [CrossRef] [PubMed]
- Bovo, A.C.; Pedrão, P.G.; Guimarães, Y.M.; Godoy, L.R.; Resende, J.C.P.; Longatto-Filho, A.; Reis, R.D. Combined oral contraceptive use and the risk of cervical cancer: Literature review. Rev. Bras. Ginecol. Obstet. 2023, 45, e818–e824. [Google Scholar] [CrossRef] [PubMed]
- Kamani, M.; Akgor, U.; Gültekin, M. Review of the literature on combined oral contraceptives and cancer. Ecancermedicalscience 2022, 16, 1416. [Google Scholar] [CrossRef]
- Anastasiou, E.; McCarthy, K.J.; Gollub, E.L.; Ralph, L.; van de Wijgert, J.H.H.M.; Jones, H.E. The relationship between hormonal contraception and cervical dysplasia/cancer controlling for human papillomavirus infection: A systematic review. Contraception 2022, 107, 1–9. [Google Scholar] [CrossRef]
- Roura, E.; Travier, N.; Waterboer, T.; de Sanjosé, S.; Bosch, F.X.; Pawlita, M.; Pala, V.; Weiderpass, E.; Margall, N.; Dillner, J.; et al. The influence of hormonal factors on the risk of developing cervical cancer and pre-cancer: Results from the EPIC cohort. PLoS ONE 2016, 11, e0147029. [Google Scholar] [CrossRef]
- Loopik, D.L.; IntHout, J.; Melchers, W.J.G.; Massuger, L.F.A.G.; Bekkers, R.L.M.; Siebers, A.G. Oral contraceptive and intrauterine device use and the risk of cervical intraepithelial neoplasia grade III or worse: A population-based study. Eur. J. Cancer 2020, 124, 102–109. [Google Scholar] [CrossRef]
- Skorstengaard, M.; Lynge, E.; Napolitano, G.; Blaakær, J.; Bor, P. Risk of precancerous cervical lesions in women using a hormone-containing intrauterine device and other contraceptives: A register-based cohort study from Denmark. Hum. Reprod. 2021, 36, 1796–1807. [Google Scholar] [CrossRef]
- Iversen, L.; Fielding, S.; Lidegaard, Ø.; Hannaford, P.C. Contemporary hormonal contraception and cervical cancer in women of reproductive age. Int. J. Cancer 2021, 149, 769–777. [Google Scholar] [CrossRef]
- Fischer, S.; Kuebler, U.; Abbruzzese, E.; Breymann, C.; Mernone, L.; Ehlert, U. Endogenous oestradiol and progesterone as predictors of oncogenic human papillomavirus (HPV) persistence. BMC Cancer 2022, 22, 145. [Google Scholar] [CrossRef]
- Hsieh, C.J.; Hong, M.K.; Chen, P.C.; Wang, J.H.; Chu, T.Y. Antiestrogen use reduces risk of cervical neoplasia in breast cancer patients: A population-based study. Oncotarget 2017, 8, 29361–29369. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.K.; Chiang, C.H.; Cheng, C.H.; Chu, T.Y. Anti-estrogen therapy achieves complete remission and stability in recurrent cervical cancer: A case study. Am. J. Case Rep. 2025, 26, e946296. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Shiozawa, T.; Xin, L.; Nikaido, T.; Fujii, S. Immunohistochemical detection of sex steroid receptors, cyclins, and cyclin-dependent kinases in the normal and neoplastic squamous epithelia of the uterine cervix. Cancer 1998, 82, 1709–1719. [Google Scholar] [CrossRef]
- Nikolaou, M.; Koumoundourou, D.; Ravazoula, P.; Papadopoulou, M.; Michail, G.; Decavalas, G. An immunohistochemical analysis of sex-steroid receptors, tumor suppressor gene p53 and Ki-67 in the normal and neoplastic uterine cervix squamous epithelium. Med. Pregl. 2014, 67, 202–207. [Google Scholar] [CrossRef]
- Hong, M.K.; Wang, J.H.; Su, C.C.; Li, M.H.; Hsu, Y.H.; Chu, T.Y. Expression of estrogen and progesterone receptor in tumor stroma predicts favorable prognosis of cervical squamous cell carcinoma. Int. J. Gynecol. Cancer 2017, 27, 1247–1255. [Google Scholar] [CrossRef]
- den Boon, J.A.; Pyeon, D.; Wang, S.S.; Horswill, M.; Schiffman, M.; Sherman, M.; Zuna, R.E.; Wang, Z.; Hewitt, S.M.; Pearson, R.; et al. Molecular transitions from papillomavirus infection to cervical precancer and cancer: Role of stromal estrogen receptor signaling. Proc. Natl. Acad. Sci. USA 2015, 112, E3255–E3264. [Google Scholar] [CrossRef]
- López-Romero, R.; Garrido-Guerrero, E.; Rangel-López, A.; Manuel-Apolinar, L.; Piña-Sánchez, P.; Lazos-Ochoa, M.; Mantilla-Morales, A.; Bandala, C.; Salcedo, M. The cervical malignant cells display a down regulation of ER-alpha but retain the ER-beta expression. Int. J. Clin. Exp. Pathol. 2013, 6, 1594–1602. [Google Scholar]
- Fan, D.M.; Tian, X.Y.; Wang, R.F.; Yu, J.J. The prognosis significance of TGF-beta1 and ER protein in cervical adenocarcinoma patients with stage Ib~IIa. Tumour. Biol. 2014, 35, 11237–11242. [Google Scholar] [CrossRef]
- Hong, M.K.; Wang, J.H.; Li, M.H.; Su, C.C.; Chu, T.Y. Progesterone receptor isoform B in the stroma of squamous cervical carcinoma: An independent favorable prognostic marker correlating with hematogenous metastasis. Taiwan J. Obstet. Gynecol. 2024, 63, 853–860. [Google Scholar] [CrossRef]
- Yuan, J.; Wen, M.; Matnuri, A.; Zhao, S.; Jian, N.; Shen, G. The expression of lnc-CCDC170-4:1, ESR1, lncRNA SRA, and CYP19A1 in cervical squamous cell carcinoma and their relationship with the clinical characteristics. Front. Oncol. 2024, 14, 1430826. [Google Scholar] [CrossRef]
- Bartl, T.; Grimm, C.; Mader, R.M.; Zielinski, C.; Prager, G.; Unseld, M.; Herac-Kornauth, M. Interactions of EGFR/PTEN/mTOR-pathway activation and estrogen receptor expression in cervical cancer. J. Pers. Med. 2023, 13, 1186. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Zheng, P.S. ESRRB inhibits the TGFbeta signaling pathway to drive cell proliferation in cervical cancer. Cancer Res. 2023, 83, 3095–3114. [Google Scholar] [CrossRef]
- Ali, H.; Traj, P.; Szebeni, G.J.; Gémes, N.; Resch, V.; Paragi, G.; Mernyák, E.; Minorics, R.; Zupkó, I. Investigation of the antineoplastic effects of 2-(4-chlorophenyl)-13 alpha-estrone sulfamate against the HPV16-positive human invasive cervical carcinoma cell line SiHa. Int. J. Mol. Sci. 2023, 24, 6625. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Mendoza, M.; Mirzaei, E.; Prado-Garcia, H.; Miranda, L.D.; Figueroa, A.; Lemini, C. The interplay of GPER1 with 17beta-aminoestrogens in the regulation of the proliferation of cervical and breast cancer cells: A pharmacological approach. Int. J. Environ. Res. Public Health 2022, 19, 12361. [Google Scholar] [CrossRef] [PubMed]
- Friese, K.; Kost, B.; Vattai, A.; Marmé, F.; Kuhn, C.; Mahner, S.; Dannecker, C.; Jeschke, U.; Heublein, S. The G protein-coupled estrogen receptor (GPER/GPR30) may serve as a prognostic marker in early-stage cervical cancer. J. Cancer Res. Clin. Oncol. 2018, 144, 13–19. [Google Scholar] [CrossRef]
- Hernandez-Silva, C.D.; Riera-Leal, A.; Ortiz-Lazareno, P.C.; Jave-Suárez, L.F.; Ramírez De Arellano, A.; Lopez-Pulido, E.I.; Macías-Barragan, J.G.; Montoya-Buelna, M.; Dávila-Rodríguez, J.R.; Chabay, P.; et al. GPER overexpression in cervical cancer versus premalignant lesions: Its activation induces different forms of cell death. Anticancer Agents Med. Chem. 2019, 19, 783–791. [Google Scholar] [CrossRef]
- Rörig, L.; Ruckriegl, S.; Gallwas, J.; Gründker, C. G protein-coupled estrogen receptor 1 (GPER1) regulates expression of SERPINE1/PAI-1 and inhibits tumorigenic potential of cervical squamous cell carcinoma cells in vitro. Cancer Genom. Proteom. 2025, 22, 13–23. [Google Scholar] [CrossRef]
- Gaxiola-Rubio, A.; Jave-Suárez, L.F.; Hernández-Silva, C.D.; Ramírez-de-Arellano, A.; Villegas-Pineda, J.C.; Lizárraga-Ledesma, M.J.; Ramos-Solano, M.; Diaz-Palomera, C.D.; Pereira-Suárez, A.L. The G-protein-coupled estrogen receptor agonist G-1 mediates antitumor effects by activating apoptosis pathways and regulating migration and invasion in cervical cancer cells. Cancers 2024, 16, 3292. [Google Scholar] [CrossRef]
- Ogawa, M.; Hashimoto, K.; Kitano, S.; Yamashita, S.; Toda, A.; Nakamura, K.; Kinose, Y.; Kodama, M.; Sawada, K.; Kimura, T. Estrogen induces genomic instability in high-risk HPV-infected cervix and promotes the carcinogenesis of cervical adenocarcinoma. Biochem. Biophys. Res. Commun. 2023, 659, 80–90. [Google Scholar] [CrossRef]
- Zhu, Z.; Nie, X.; Deng, L.; Ding, J.; Chen, J.; Zhu, J.; Yin, X.; Guo, B.; Zhang, F. Regulation of cervical cancer via G15-mediated inhibition of G protein-coupled estrogen receptor. Anticancer Drugs 2024, 35, 817–829. [Google Scholar] [CrossRef]
- Ruckriegl, S.; Loris, J.; Wert, K.; Bauerschmitz, G.; Gallwas, J.; Gründker, C. Knockdown of G protein-coupled estrogen receptor 1 (GPER1) enhances tumor-supportive properties in cervical carcinoma cells. Cancer Genom. Proteom. 2023, 20, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, T.; Wang, K.; Zhang, S.; Sun, Q.; Yang, X. ER-alpha36 promotes the malignant progression of cervical cancer mediated by estrogen via HMGA2. Front. Oncol. 2021, 11, 712849. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, A.; Zhang, X.; Hu, S.; Bao, Z.; Zhang, Y.; Jiang, X.; He, H.; Zhang, T.C. ERa-36 instead of ERa mediates the stimulatory effects of estrogen on the expression of viral oncogenes HPV E6/E7 and the malignant phenotypes in cervical cancer cells. Virus Res. 2021, 306, 198602. [Google Scholar] [CrossRef]
- Ino, Y.; Akimoto, T.; Takasawa, A.; Takasawa, K.; Aoyama, T.; Ueda, A.; Ota, M.; Magara, K.; Tagami, Y.; Murata, M.; et al. Elevated expression of G protein-coupled receptor 30 (GPR30) is associated with poor prognosis in patients with uterine cervical adenocarcinoma. Histol. Histopathol. 2020, 35, 351–359. [Google Scholar] [CrossRef]
- Lee, S.A.; Baik, S.; Chung, S.H. Functional roles of female sex hormones and their nuclear receptors in cervical cancer. Essays Biochem. 2021, 65, 941–950. [Google Scholar] [CrossRef]
- Spurgeon, M.E.; den Boon, J.A.; Horswill, M.; Barthakur, S.; Forouzan, O.; Rader, J.S.; Beebe, D.J.; Roopra, A.; Ahlquist, P.; Lambert, P.F. Human papillomavirus oncogenes reprogram the cervical cancer microenvironment independently of and synergistically with estrogen. Proc. Natl. Acad. Sci. USA 2017, 114, E9076–E9085. [Google Scholar] [CrossRef]
- Ramachandran, B. Functional association of oestrogen receptors with HPV infection in cervical carcinogenesis. Endocr. Relat. Cancer 2017, 24, R99–R108. [Google Scholar] [CrossRef]
- De Nola, R.; Loizzi, V.; Cicinelli, E.; Cormio, G. Dynamic crosstalk within the tumor microenvironment of uterine cervical carcinoma: Baseline network, iatrogenic alterations, and translational implications. Crit. Rev. Oncol. Hematol. 2021, 162, 103343. [Google Scholar] [CrossRef]
- Rojo-León, V.; García, C.; Valencia, C.; Méndez, M.A.; Wood, C.; Covarrubias, L. The E6/E7 oncogenes of human papilloma virus and estradiol regulate hedgehog signaling activity in a murine model of cervical cancer. Exp. Cell Res. 2019, 381, 311–322. [Google Scholar] [CrossRef]
- Wang, W.; Spurgeon, M.E.; Pope, A.; McGregor, S.; Ward-Shaw, E.; Gronski, E.; Lambert, P.F. Stress keratin 17 and estrogen support viral persistence and modulate the immune environment during cervicovaginal murine papillomavirus infection. Proc. Natl. Acad. Sci. USA 2023, 120, e2214225120. [Google Scholar] [CrossRef]
- Srinivasan, S.; Fredricks, D.N. The human vaginal bacterial biota and bacterial vaginosis. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 750479. [Google Scholar] [CrossRef] [PubMed]
- Balkus, J.E.; Richardson, B.A.; Rabe, L.K.; Taha, T.E.; Mgodi, N.; Kasaro, M.P.; Ramjee, G.; Hoffman, I.F.; Abdool Karim, S.S. Bacterial vaginosis and the risk of trichomonas vaginalis acquisition among HIV-1-negative women. Sex. Transm. Dis. 2014, 41, 123–128. [Google Scholar] [CrossRef]
- Wei, Q.; Fu, B.; Liu, J.; Zhang, Z.; Zhao, T. Candida albicans and bacterial vaginosis can coexist on Pap smears. Acta Cytol. 2012, 56, 515–519. [Google Scholar] [CrossRef]
- Koumans, E.H.; Sternberg, M.; Bruce, C.; McQuillan, G.; Kendrick, J.; Sutton, M.; Markowitz, L.E. The prevalence of bacterial vaginosis in the United States, 2001-2004, associations with symptoms, sexual behaviors, and reproductive health. Sex. Transm. Dis. 2007, 34, 864–869. [Google Scholar] [CrossRef]
- Gillet, E.; Meys, J.F.; Verstraelen, H.; Verhelst, R.; De Sutter, P.; Temmerman, M.; Vanden Broeck, D. Association between bacterial vaginosis and cervical intraepithelial neoplasia: Systematic review and meta-analysis. PLoS ONE 2012, 7, e45201. [Google Scholar] [CrossRef]
- Suehiro, T.T.; Malaguti, N.; Damke, E.; Uchimura, N.S.; Gimenes, F.; Souza, R.P.; Sela da Silva, V.R.; Lopes Consolaro, M.E. Association of human papillomavirus and bacterial vaginosis with increased risk of high-grade squamous intraepithelial cervical lesions. Int. J. Gynecol. Cancer 2019, 29, 242–249. [Google Scholar] [CrossRef]
- Anton, L.; Sierra, L.J.; DeVine, A.; Barila, G.; Heiser, L.; Brown, A.G.; Elovitz, M.A. Common cervicovaginal microbial supernatants alter cervical epithelial function: Mechanisms by which Lactobacillus crispatus contributes to cervical health. Front. Microbiol. 2018, 9, 2181. [Google Scholar] [CrossRef]
- George Onyango, C.; Ogonda, L.; Guyah, B. The role of co-infections and hormonal contraceptives in cervical intraepithelial neoplasia prevalence among women referred to a tertiary hospital in Western Kenya. Infect. Agent. Cancer 2025, 20, 11. [Google Scholar] [CrossRef]
- Klein, J.M.A.; Runge, I.; Pannen, A.K.; Wakuma, T.; Abera, S.F.; Adissie, A.; Unverzagt, S.; Schmitt, M.; Waterboer, T.; Höfler, D.; et al. Prevalence of bacterial vaginosis, sexually transmitted infections and their association with HPV infections in asymptomatic women attending antenatal care in Ethiopia. Ecancermedicalscience 2024, 18, 1783. [Google Scholar] [CrossRef]
- Loonen, A.J.M.; Verhagen, F.; Luijten-de Vrije, I.; Lentjes-Beer, M.; Huijsmans, C.J.; van den Brule, A.J.C. Vaginal dysbiosis seems associated with hrHPV infection in women attending the Dutch Cervical Cancer Screening Program. Front. Cell. Infect. Microbiol. 2024, 14, 1330844. [Google Scholar] [CrossRef]
- Wang, H.; Nie, K.; Liu, Z.; Zhao, Y.; Ha, Y.; Zhang, H.; Mao, D. Analysis of risk factors associated with cervical HPV infection and their effects on female sexual function and anxiety: A multicenter cross-sectional study based on Chinese women. Front. Oncol. 2024, 14, 1468160. [Google Scholar] [CrossRef] [PubMed]
- Disi, A.; Li, J.; Zhang, D.; Xiao, B.; Bi, H. Status of common sexually transmitted infection in population referred for colposcopy and correlation with human papillomavirus infection. BMC Womens Health 2023, 23, 579. [Google Scholar] [CrossRef]
- Kaliterna, V.; Kaliterna, P.; Pejkovic, L.; Vulic, R.; Zanchi, L.; Cerskov, K. Prevalence of human papillomavirus (HPV) among females in the general population of the Split and Dalmatia County and Its association with genital microbiota and infections: A prospective study. Viruses 2023, 15, 443. [Google Scholar] [CrossRef] [PubMed]
- Ortiz Segarra, J.; Vega Crespo, B.; Campoverde Cisneros, A.; Salazar Torres, K.; Delgado López, D.; Ortiz, S. Human papillomavirus prevalence and associated factors in indigenous women in Ecuador: A cross-sectional analytical study. Infect. Dis. Rep. 2023, 15, 267–278. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, W.; Gao, W.; Zhao, K.; Pan, X.; Jiang, X.; Zhao, J. Correlation between vaginal flora and cervical immune function of human papilloma virus-infected patients with cervical cancer. Afr. Health Sci. 2023, 23, 179–185. [Google Scholar] [CrossRef]
- Adhikari, I.; Eriksson, T.; Harjula, K.; Hokkanen, M.; Apter, D.; Nieminen, P.; Luostarinen, T.; Lehtinen, M. Association of Chlamydia trachomatis infection with cervical atypia in adolescent women with short-term or long-term use of oral contraceptives: A longitudinal study in HPV vaccinated women. BMJ Open 2022, 12, e056824. [Google Scholar] [CrossRef]
- Yu, H.; Ma, L.; Bian, M.; Li, Q.; Liang, H. Association of abnormal vaginal microflora and HPV infection in cervical precancerous lesions: A retrospective study. J. Infect. Dev. Ctries 2022, 16, 1089–1095. [Google Scholar] [CrossRef]
- Mosmann, J.P.; Zayas, S.; Kiguen, A.X.; Venezuela, R.F.; Rosato, O.; Cuffini, C.G. Human papillomavirus and Chlamydia trachomatis in oral and genital mucosa of women with normal and abnormal cervical cytology. BMC Infect. Dis. 2021, 21, 422. [Google Scholar] [CrossRef]
- Xie, L.; Li, Q.; Dong, X.; Kong, Q.; Duan, Y.; Chen, X.; Li, X.; Hong, M.; Liu, T. Investigation of the association between ten pathogens causing sexually transmitted diseases and high-risk human papilloma virus infection in Shanghai. Mol. Clin. Oncol. 2021, 15, 132. [Google Scholar] [CrossRef]
- Chen, H.; Luo, L.; Wen, Y.; He, B.; Ling, H.; Shui, J.; He, P.; Hou, X.; Tang, S.; Li, Z. Chlamydia trachomatis and human papillomavirus infection in women from southern Hunan Province in China: A large observational study. Front. Microbiol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Escarcega-Tame, M.A.; López-Hurtado, M.; Escobedo-Guerra, M.R.; Reyes-Maldonado, E.; Castro-Escarpulli, G.; Guerra-Infante, F.M. Co-infection between genotypes of the human papillomavirus and Chlamydia trachomatis in Mexican women. Int. J. STD AIDS 2020, 31, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Osei Sekyere, J.; Oyenihi, A.B.; Trama, J.; Adelson, M.E. Species-specific analysis of bacterial vaginosis-associated bacteria. Microbiol. Spectr. 2023, 11, e0467622. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.J.; Tyrrell, K.L.; Citron, D.M. Lactobacillus species: Taxonomic complexity and controversial susceptibilities. Clin. Infect. Dis. 2015, 60 (Suppl. S2), S98–S107. [Google Scholar] [CrossRef] [PubMed]
- Jespers, V.; Menten, J.; Smet, H.; Poradosú, S.; Abdellati, S.; Verhelst, R.; Hardy, L.; Buvé, A.; Crucitti, T. Quantification of bacterial species of the vaginal microbiome in different groups of women, using nucleic acid amplification tests. BMC Microbiol. 2012, 12, 83. [Google Scholar] [CrossRef]
- Matsumoto, T.; Sugano, M. 16S rRNA gene sequence analysis for bacterial identification in the clinical laboratory. Rinsho Byori 2013, 61, 1107–1115. [Google Scholar]
- Patel, J.B. 16S rRNA gene sequencing for bacterial pathogen identification in the clinical laboratory. Mol. Diagn. 2001, 6, 313–321. [Google Scholar] [CrossRef]
- Church, D.L.; Cerutti, L.; Gürtler, A.; Griener, T.; Zelazny, A.; Emler, S. Performance and application of 16S rRNA gene cycle sequencing for routine identification of bacteria in the clinical microbiology laboratory. Clin. Microbiol. Rev. 2020, 33, e00053-e19. [Google Scholar] [CrossRef]
- Ames, N.J.; Ranucci, A.; Moriyama, B.; Wallen, G.R. The human microbiome and understanding the 16S rRNA gene in translational nursing science. Nurs. Res. 2017, 66, 184–197. [Google Scholar] [CrossRef]
- Pfister, P.; Risch, M.; Brodersen, D.E.; Böttger, E.C. Role of 16S rRNA helix 44 in ribosomal resistance to hygromycin, B. Antimicrob. Agents Chemother. 2003, 47, 1496–1502. [Google Scholar] [CrossRef]
- Clarridge, J.E., 3rd. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 2004, 17, 840–862. [Google Scholar] [CrossRef]
- Miyazaki, K.; Kitahara, K. Functional metagenomic approach to identify overlooked antibiotic resistance mutations in bacterial rRNA. Sci. Rep. 2018, 8, 5179. [Google Scholar] [CrossRef] [PubMed]
- Herath, S.; Balendran, T.; Herath, A.; Iddawela, D.; Wickramasinghe, S. Comparison of diagnostic methods and analysis of socio-demographic factors associated with Trichomonas vaginalis infection in Sri Lanka. PLoS ONE 2021, 16, e0258556. [Google Scholar] [CrossRef] [PubMed]
- Simpson, P.; Higgins, G.; Qiao, M.; Waddell, R.; Kok, T. Real-time PCRs for detection of Trichomonas vaginalis beta-tubulin and 18S rRNA genes in female genital specimens. J. Med. Microbiol. 2007, 56 Pt 6, 772–777. [Google Scholar] [CrossRef]
- Guo, R.; Zheng, N.; Lu, H.; Yin, H.; Yao, J.; Chen, Y. Increased diversity of fungal flora in the vagina of patients with recurrent vaginal candidiasis and allergic rhinitis. Microb. Ecol. 2012, 64, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Długosz, E.; Wiśniewski, M. Molecular diagnostic of parasites using rRNA gene sequence. Wiad. Parazytol. 2006, 52, 263–269. [Google Scholar]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.; Zhong, X.; Koenig, S.S.; Fu, L.; Ma, Z.S.; Zhou, X.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef]
- Roachford, O.S.E.; Alleyne, A.T.; Nelson, K.E. Insights into the vaginal microbiome in a diverse group of women of African, Asian and European ancestries. PeerJ 2022, 10, e14449. [Google Scholar] [CrossRef]
- Moosa, Y.; Kwon, D.; de Oliveira, T.; Wong, E.B. Determinants of vaginal microbiota composition. Front. Cell. Infect. Microbiol. 2020, 10, 467. [Google Scholar] [CrossRef]
- Marrazzo, J.M. Interpreting the epidemiology and natural history of bacterial vaginosis: Are we still confused? Anaerobe 2011, 17, 186–190. [Google Scholar] [CrossRef]
- France, M.T.; Mendes-Soares, H.; Forney, L.J. Genomic comparisons of Lactobacillus crispatus and Lactobacillus iners reveal potential ecological drivers of community composition in the vagina. Appl. Environ. Microbiol. 2016, 82, 7063–7073. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, S.; Lee, H.; Song, Y.M.; Lee, K.; Han, M.J.; Sung, J.; Ko, G. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS ONE 2013, 8, e63514. [Google Scholar] [CrossRef] [PubMed]
- Kwasniewski, W.; Wolun-Cholewa, M.; Kotarski, J.; Warchol, W.; Kuzma, D.; Kwasniewska, A.; Gozdzicka-Jozefiak, A. Microbiota dysbiosis is associated with HPV-induced cervical carcinogenesis. Oncol. Lett. 2018, 16, 7035–7047. [Google Scholar] [CrossRef]
- Brusselaers, N.; Shrestha, S.; van de Wijgert, J.; Verstraelen, H. Vaginal dysbiosis and the risk of human papillomavirus and cervical cancer: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2019, 221, 9–18.E8. [Google Scholar] [CrossRef]
- Guo, M.; Feng, X.; Ma, J.; Zhu, K.; Niyazi, M. Differential study on the relationship between HPV infection and vaginal microbiota composition in Uygur and Han women. Microb. Pathog. 2025, 198, 107149. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X. Vaginal microbiome distinction in women with HPV+, cervical intraepithelial neoplasia, and cervical cancer, a retrospective study. Front. Cell. Infect. Microbiol. 2025, 14, 1483544. [Google Scholar] [CrossRef]
- Parvez, R.; Vijayakumar, S.; Vins, A.; Ramaiah, S.; Anbarasu, A.; Biswas, L.; Beniwal, N.; Kaur, H.; Muruganandam, N. Understanding the vaginal microbiome among women with different genotypes of human papillomavirus infection in remote Andaman islands. Front. Cell. Infect. Microbiol. 2025, 14, 1486166. [Google Scholar] [CrossRef]
- Asensio-Puig, L.; de Andrés-Pablo, Á.; Khannous-Lleiffe, O.; Ibáñez, R.; Acera, A.; de Sanjosé, S.; Gabaldón, T.; Alemany, L.; Bruni, L.; Pavón, M.À. Proof of concept study: Comparability of microbiome diversity in self- and physician-collected HPV-positive and HPV-negative cervicovaginal samples. Int. J. Mol. Sci. 2024, 25, 5736. [Google Scholar] [CrossRef]
- Fan, Z.; Han, D.; Fan, X.; Zeng, Y.; Zhao, L. Analysis of the correlation between cervical HPV infection, cervical lesions and vaginal microecology. Front. Cell. Infect. Microbiol. 2024, 14, 1405789. [Google Scholar] [CrossRef]
- Jimenez, N.R.; Mancilla, V.; Łaniewski, P.; Herbst-Kralovetz, M.M. Immunometabolic contributions of Atopobiaceae family members in human papillomavirus infection, cervical dysplasia and cancer. J. Infect. Dis. 2024, jiae533. [Google Scholar] [CrossRef]
- Łaniewski, P.; Joe, T.R.; Jimenez, N.R.; Eddie, T.L.; Bordeaux, S.J.; Quiroz, V.; Peace, D.J.; Cui, H.; Roe, D.J.; Caporaso, J.G.; et al. Viewing native American cervical cancer disparities through the lens of the vaginal microbiome: A pilot study. Cancer Prev. Res. 2024, 17, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Zhang, F.; Cai, H.Y.; Lv, Y.M.; Pi, M.Y. Cross-sectional study on the correlation between vaginal microecology and high-risk human papillomavirus infection: Establishment of a clinical prediction model. Int. J. Womens Health 2024, 16, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Kang, Y.; Medlegeh Fu, K.; Zhang, Y.; Yang, W. An analysis of the vaginal microbiota and cervicovaginal metabolomics in cervical lesions and cervical carcinoma. Heliyon 2024, 10, e33383. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.; Feng, R.; Han, M.; Chen, F.; Hu, Y. Altered vaginal cervical microbiota diversity contributes to HPV-induced cervical cancer via inflammation regulation. PeerJ 2024, 12, e17415. [Google Scholar] [CrossRef]
- Yu, T.; Gao, S.; Jin, F.; Yan, B.; Wang, W.; Wang, Z. Characteristics of the vaginal microbiota and vaginal metabolites in women with cervical dysplasia. Front. Cell. Infect. Microbiol. 2024, 14, 1457216. [Google Scholar] [CrossRef]
- Wen, Q.; Wang, S.; Min, Y.; Liu, X.; Fang, J.; Lang, J.; Chen, M. Associations of the gut, cervical, and vaginal microbiota with cervical cancer: A systematic review and meta-analysis. BMC Womens Health 2025, 25, 65. [Google Scholar] [CrossRef]
- Ray, A.; Cleary, M.P. The potential role of leptin in tumor invasion and metastasis. Cytokine Growth Factor Rev. 2017, 38, 80–97. [Google Scholar] [CrossRef]
- Alizhan, D.; Ukybassova, T.; Bapayeva, G.; Aimagambetova, G.; Kongrtay, K.; Kamzayeva, N.; Terzic, M. Cervicovaginal microbiome: Physiology, age-related changes, and protective role against human papillomavirus infection. J. Clin. Med. 2025, 14, 1521. [Google Scholar] [CrossRef]
- Park, M.G.; Cho, S.; Oh, M.M. Menopausal changes in the microbiome-A review focused on the genitourinary microbiome. Diagnostics 2023, 13, 1193. [Google Scholar] [CrossRef]
- Chambers, L.M.; Bussies, P.; Vargas, R.; Esakov, E.; Tewari, S.; Reizes, O.; Michener, C. The microbiome and gynecologic cancer: Current evidence and future opportunities. Curr. Oncol. Rep. 2021, 23, 92. [Google Scholar] [CrossRef]
- Łaniewski, P.; Ilhan, Z.E.; Herbst-Kralovetz, M.M. The microbiome and gynaecological cancer development, prevention and therapy. Nat. Rev. Urol. 2020, 17, 232–250. [Google Scholar] [CrossRef] [PubMed]
- Norenhag, J.; Du, J.; Olovsson, M.; Verstraelen, H.; Engstrand, L.; Brusselaers, N. The vaginal microbiota, human papillomavirus and cervical dysplasia: A systematic review and network meta-analysis. BJOG 2020, 127, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.P.; Sun, T.T.; Wang, S.; Fan, Q.B.; Shi, H.H.; Zhu, L.; Lang, J.H. Correlation between the diversity of vaginal microbiota and the risk of high-risk human papillomavirus infection. Int. J. Gynecol. Cancer 2019, 29, 28–34. [Google Scholar] [CrossRef]
- Qingqing, B.; Jie, Z.; Songben, Q.; Juan, C.; Lei, Z.; Mu, X. Cervicovaginal microbiota dysbiosis correlates with HPV persistent infection. Microb. Pathog. 2021, 152, 104617. [Google Scholar] [CrossRef]
- Leon-Gomez, P.; Romero, V.I. Human papillomavirus, vaginal microbiota and metagenomics: The interplay between development and progression of cervical cancer. Front. Microbiol. 2025, 15, 1515258. [Google Scholar] [CrossRef]
- Wang, K.D.; Xu, D.J.; Wang, B.Y.; Yan, D.H.; Lv, Z.; Su, J.R. Inhibitory effect of vaginal Lactobacillus supernatants on cervical cancer cells. Probiotics Antimicrob. Proteins 2018, 10, 236–242. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Wu, F.; Chen, J.; Luo, J.; Wu, C.; Chen, T. Effectiveness of vaginal probiotics Lactobacillus crispatus chen-01 in women with high-risk HPV infection: A prospective controlled pilot study. Aging 2024, 16, 11446–11459. [Google Scholar] [CrossRef]
- Park, Y.C.; Ouh, Y.T.; Sung, M.H.; Park, H.G.; Kim, T.J.; Cho, C.H.; Park, J.S.; Lee, J.K. A phase 1/2a, dose-escalation, safety and preliminary efficacy study of oral therapeutic vaccine in subjects with cervical intraepithelial neoplasia 3. J. Gynecol. Oncol. 2019, 30, e88. [Google Scholar] [CrossRef]
- Xu, P.; Mageswaran, U.M.; Nisaa, A.A.; Balasubramaniam, S.D.; Rajendran, D.; Ismail, E.H.B.E.; Kadir, M.N.; Oon, C.E.; Tan, C.S.; Sany, S.B.; et al. Roles of probiotics against HPV through the gut-vaginal axis. Int. J. Gynaecol. Obstet. 2025, 169, 1–8. [Google Scholar] [CrossRef]
- Ang, X.Y.; Roslan, N.S.; Ahmad, N.; Yusof, S.M.; Abdullah, N.; Nik Ab Rahman, N.N.; Woon, J.J.; Teh, C.S.; Todorov, S.D.; Liu, G.; et al. Lactobacillus probiotics restore vaginal and gut microbiota of pregnant women with vaginal candidiasis. Benef. Microbes 2023, 14, 421–431. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Li, W. Deciphering the role of human gastrointestinal microbiota in the pathogenesis of vaginal infection and cervical cancer. J. Environ. Pathol. Toxicol. Oncol. 2020, 39, 365–373. [Google Scholar] [CrossRef] [PubMed]
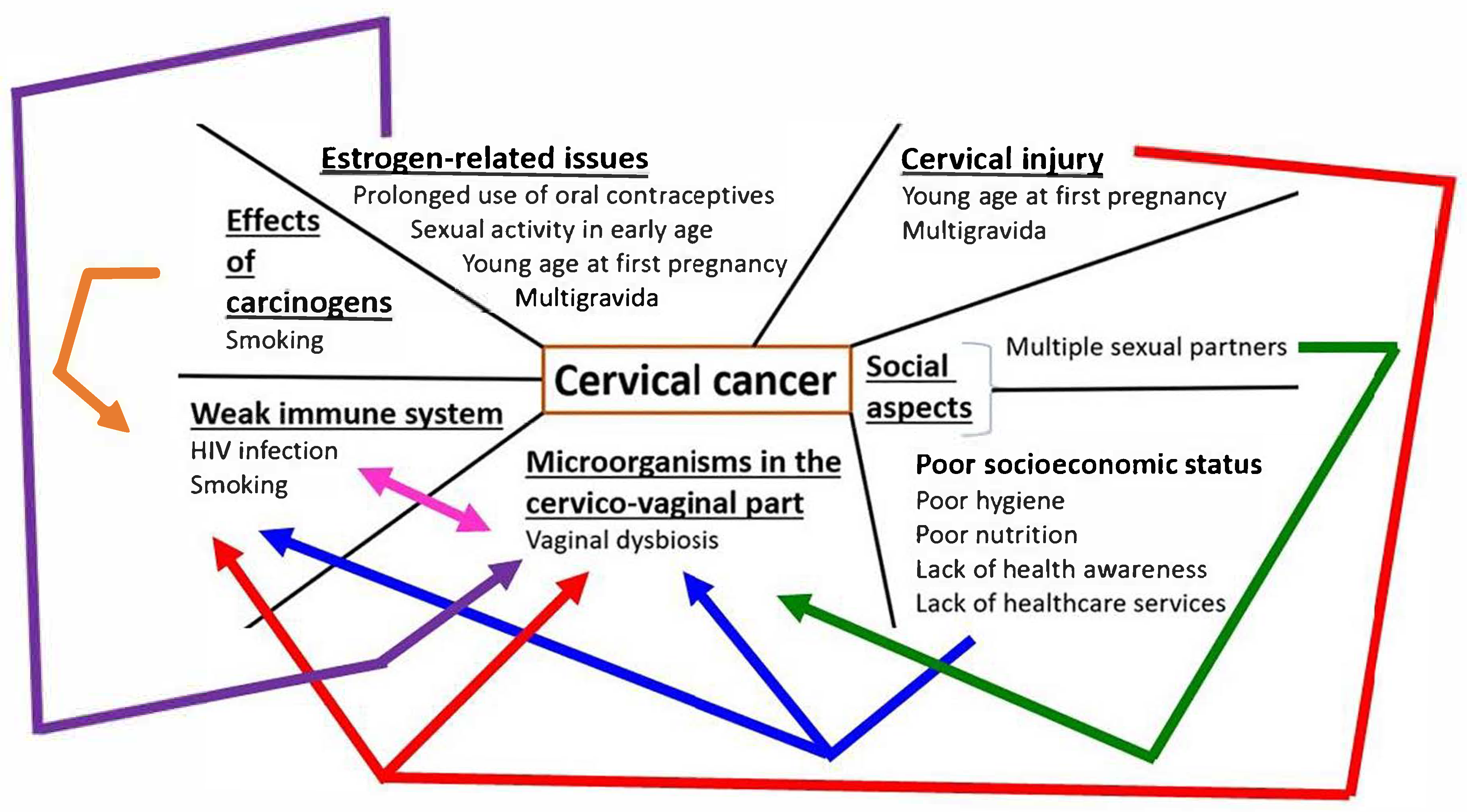
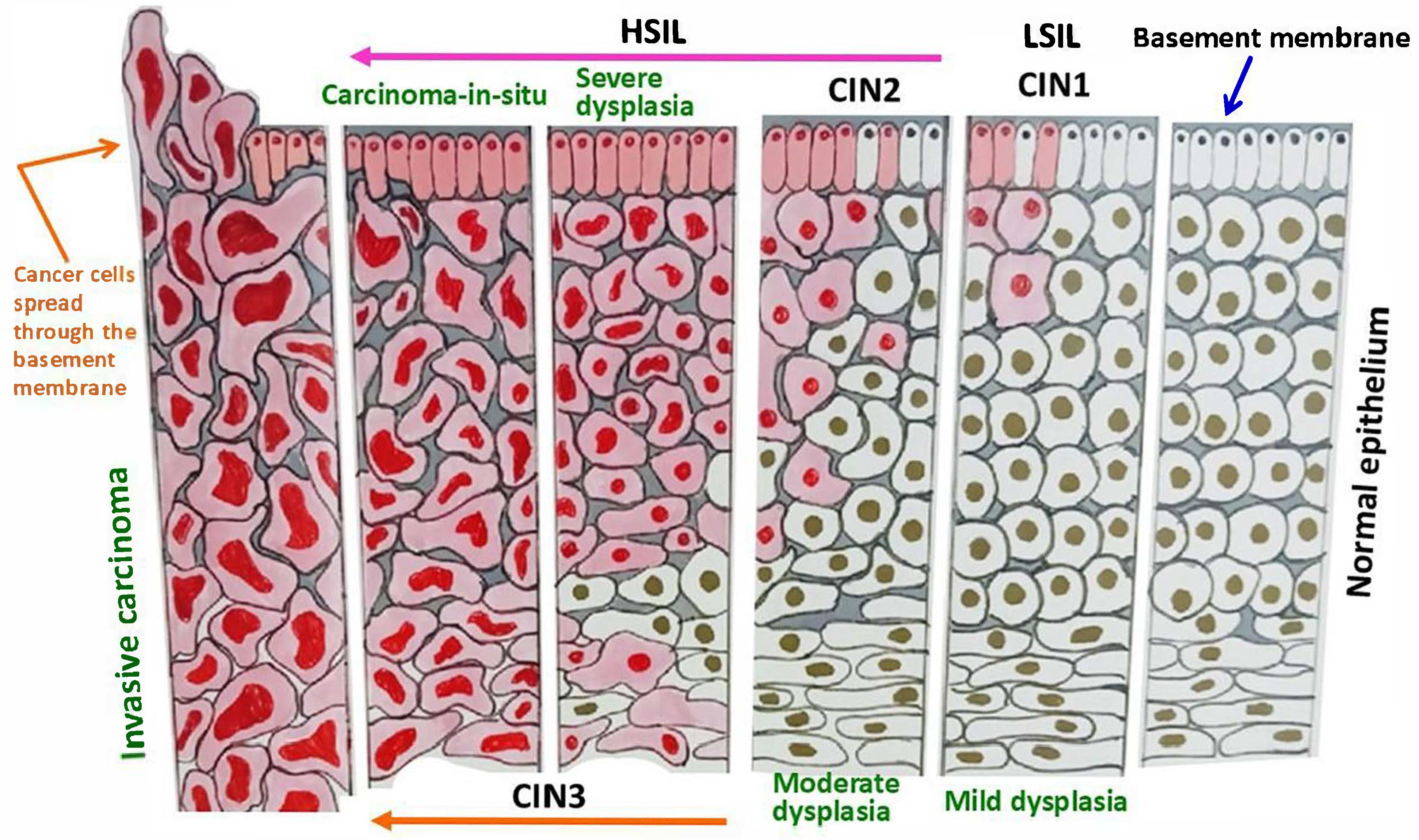

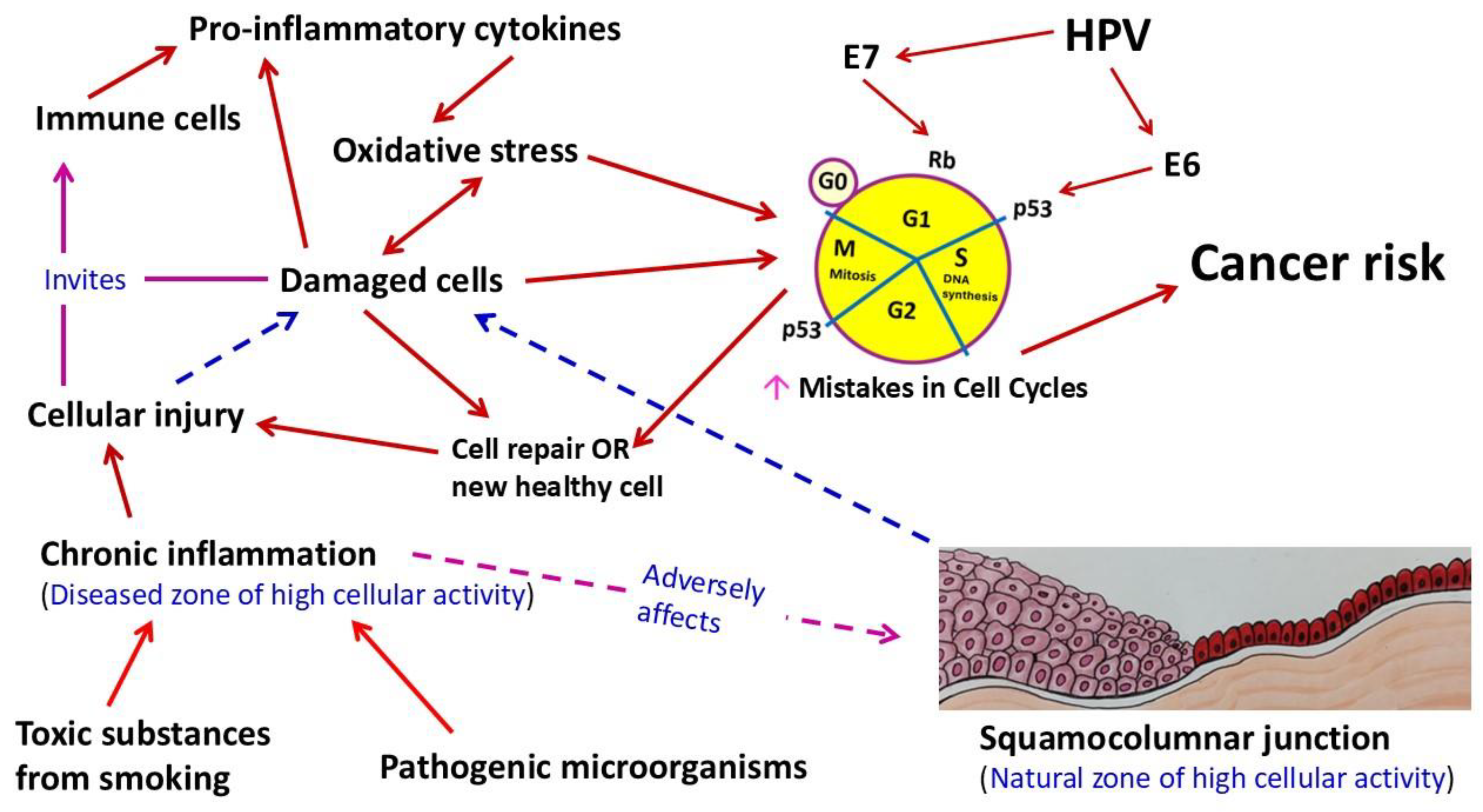
| Tumor Type | Authors, Place of Study, and Year of Publication | Study Designs | Findings in Brief |
|---|---|---|---|
| Cervical cancer (neuroendocrine tumors) | Shen et al., 2025 (China) [48] | Neuroendocrine cervical carcinoma samples from eight patients were analyzed | One patient had cancer of mixed types, which was excluded but HPV-16 positive. Out of seven cases, six were HPV-18 positive, and one was positive for HPV-16. |
| Park et al., 2025 (Korea) [49] | Case report: Primary malignant melanoma of the vagina from a 53-year-old female patient | The initial diagnosis was atypical squamous cells of undetermined significance, positive for HPV-39, -68, and -74; after 1 year, LSIL, positive for HPV-52 and -58; afterwards, ASC-H, positive for HPV-50 and -51. | |
| Wei et al., 2024 (China) [50] | Two cases of cervical mixed cancer of large-cell neuroendocrine carcinoma and adenocarcinoma | One case was HPV-18 positive. | |
| Liu et al., 2024 (China) [51] | Seven cases (out of eleven) with small-cell carcinoma of the cervix were tested for HPV before treatment—between 2017 and 2023 | All seven were positive for HPV; five cases were positive for HPV-18 and two cases were HPV-16. | |
| Gordhandas et al., 2022 (United States) [52] | A total of 63 patients with small-cell neuroendocrine carcinoma of the cervix (41 had limited-stage and 22 had extensive-stage); from January 1990 to June 2021 | Overall, HPV positive cases were 17 (34%, limited-stage: 13 or 38%). Missing cases: 13. | |
| Schultheis et al., 2022 (Germany and United States) [53] | Nine cases of uterine cervix small-cell carcinomas were detected over a 20-year period | Eight cases were HPV-18 and one case was HPV-16 positive. | |
| Lu et al., 2022 (China) [54] | Evaluation of HPV for 9 cases with small-cell carcinoma (out of 19); from 2012 to 2021 | HPV infection rate was 77.77% (7/9); HPV-18: 5, HPV-16: 1, and HPV-52: 1. | |
| Ordulu et al., 2022 (Multinational study) [55] | 14 cases (small-cell carcinoma: 6, large-cell carcinoma: 6, other neuroendocrine tumors: 2) | Except for three cases of small-cell carcinoma, all cases were positive for HPV. | |
| Pei et al., 2021 (China) [56] | 51 patients with small-cell carcinoma, between 2007 and 2020 | In all cases, HPV infections were detected (HPV-18 in 47 cases). | |
| Van Ta et al., 2019 (Vietnam) [57] | 30 neuroendocrine tumors | HPV was identified in 26 (86.7%) cases; HPV-18 in 15 cases. | |
| Feng et al., 2018 (China) [58] | 82 patients with neuroendocrine carcinoma; from 2008 to 2016 (small-cell carcinoma: 74, large-cell carcinoma: 7, atypical carcinoid: 1) | HPV was detected in 72 cases; high-risk types in 70 cases, and HPV-18 in 49 cases. | |
| Siriaunkgul et al., 2011 (Thailand) [59] | 97 cases with neuroendocrine carcinoma; between 1992 and 2009 | HPV was detected in 93 samples (95.87%), of which 76 were single HPV type infection; HPV-18: 70 cases. | |
| Wang et al., 2006 (Taiwan) [60] | 26 specimens were analyzed (out of 31 cases, between 1991 and 2003) | In 18 cases, HPV infection was present; HPV-negative cases: 8, HPV-18: 17, and HPV-16: 1. | |
| Ishida et al., 2004 (Japan) [61] | 10 cases of small-cell carcinoma, 3 were pure small-cell cancer | HPV was detected in 7/10; HPV-18 was detected in all pure tumors and four mixed tumors; no other HPV types were isolated. | |
| Small-cell lung cancer and other neuroendocrine tumors of the lung | Han et al., 2024 (China) [62] | 36 SNPs from 4 genome-wide association studies’ data were examined with HPV-16 E7 and HPV-18 E7 | No significant association was found between small-cell lung carcinoma and HPV-16 E7 or HPV-18 E7. |
| Sirera et al., 2022 (Spain) [63] | Specimens were collected from 41 patients with lung cancer, including 3 small-cell carcinoma cases (7%) | Two cases were positive for HPV: one case of HPV-16 infection, and in another case, HPV was not classified (low- or high-risk). | |
| Zou et al., 2021 (China) [64] * | E6 and E7 mRNA levels of HPV-16 were detected in the bronchial brushing and TBNA of 184 patients with lung cancer (44 cases of small-cell lung cancer) and 126 benign lung diseases | Both E6 and E7 mRNA expression levels in small-cell carcinoma and squamous cell carcinoma (n = 80) were significantly higher compared to benign cells. Among small-cell cancer, the expression levels in 38 central type cases were significantly higher than in six cases of peripheral type. | |
| de Oliveira et al., 2018 (Brazil) [65] | 63 samples of lung cancer from different histological types | HPV was found in 33 samples; small-cell carcinoma: 18.18%, and large-cell carcinoma: 9.1%. | |
| Shikova et al., 2017 (Bulgaria) [66] | 132 lung cancer specimens of different histological types including 24 small-cell carcinoma cases were analyzed for HPV | In the small-cell carcinoma group, HPV was present in seven cases (29.2%); HPV-16: two, HPV-18: three, HPV-16 + HPV-18 coinfection: two. | |
| Hartley et al., 2015 (United States) [67] | 19 patients’ specimens; from 2004 to 2013 | No HPV was found. | |
| Castillo et al., 2006 (Mexico, Colombia, and Peru) [68] | 36 lung carcinomas of different histological types including 9 cases of small-cell carcinoma | Among small-cell carcinomas, HPV was detected in three cases, which were positive for HPV-16. | |
| Brouchet et al., 2005 (France) [69] | 122 cases of lung cancer, including small-cell carcinoma (n = 9), large-cell neuroendocrine carcinoma (n = 13), typical carcinoid (n = 6), and atypical carcinoid (n = 3). In tissue sections, the presence of HPV, EBV, HHV-8, CMV, and SV40 was investigated. | None of the cases showed any positive results. | |
| Thomas et al., 1996 (France) [70] | 31 biopsies of lung cancer (different histological types), including 6 small-cell carcinoma cases | HPV was positive in 1 small-cell carcinoma (1/6) and in 1 other neuroendocrine tumor (1/1). | |
| Schmitt et al., 2011 (Germany) [71] | Neuroendocrine tumors from various sites, including lungs and skin, were analyzed (n = 43) for different viruses (e.g., HPV, EBV, HBV, MCV) | EBV was positive in 1 case (1/6) of pulmonary large cell carcinoma and in 1 case (1/5) of small-cell carcinoma; 2 cases (2/3) of Merkel cell carcinoma were positive for MCV. | |
| Pheochromocytoma | Oraibi et al., 2018 (United States) [72] | Case report: pheochromocytoma with primary adrenal lymphoma | Lymphoma was associated with EBV. |
| Badani et al., 2016 (United States) [73] | 63 specimens of adrenal gland tissue, including 21 cases of pheochromocytoma, were analyzed for VZV and HSV-1 | VZV was found in four normal adrenal gland tissue samples. No VZV or HSV-1 was detected in cases of pheochromocytoma. | |
| Sathe et al., 2012 (India) [74] | Case report: a pediatric case with bilateral adrenal neoplasms (without immunodeficiency) | Adrenal leiomyoma that was associated with EBV (initially, pheochromocytoma was suspected). | |
| Pancreatic neuroendocrine tumors | Vemuri et al., 2022 (Australia) [75] | Case report: a patient with HIV and HBV coinfection | VIPoma in the pancreatic tail was diagnosed. |
| Fiorino et al., 2015 (Italy) [76] | Case report: a patient with cirrhosis and two synchronous malignancies—in the liver (hepatocellular carcinoma) and in the pancreatic tail (neuroendocrine tumor) | In neuroendocrine tumor cells, the HBV genome was detected. |
| Classification | HPV Genotypes |
|---|---|
| High-risk | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 |
| Probable high-risk | 26, 30, 34 *, 53, 66, 67, 68, 69, 70, 71, 73, 82, 90 |
| Undetermined | 2, 3, 7, 10, 27, 57, 62, 85, 91 |
| Low-risk | 6, 11, 32, 40, 42, 43, 44 **, 54, 61, 72, 74, 81, 83, 84, 86, 87, 89 |
| Authors and Brief Study Outline | Findings |
|---|---|
| Lovane et al., 2025 [108]|40 cases of endocervical adenocarcinoma; Mozambique, 2017–2018. | A total of 14 were HIV-positive. Every case was positive for at least one of the HPV-16/-18/-45 genotypes; HPV-18 was the most common type. Multiple infections were exclusively detected in HIV-negative cases. |
| Debeaudrap et al., 2025 [109]|2253 WLHIV; Côte d’Ivoire, Burkina Faso and Cambodia, 2019–2021. | A total of 932 (41%) were HPV-positive. Of the 777 HPV-positive participants with histopathology results, 105 (13.5%) had CIN2+ lesions, and 75 (9.5%) had CIN3+ lesions. |
| Pillay et al., 2025 [110]|235 young sexually active women; South Africa | HIV was detected in 49 (20.4%) subjects. HPV DNA was found in 147 (62.6%) urinary and 177 (75.3%) cervico-vaginal lavage samples. HIV positivity is statistically associated with the presence of HPV DNA in urine and cervico-vaginal lavage. |
| Strickler et al., 2024 [111]|WIHS ongoing cohort (enrolled 2793, CIN2 and CIN3: 124, controls: 247); United States. | In women with HIV, cigarette smoking, parity, and CD4 cell count displayed a positive association with cervical precancer condition. However, there was a strong inverse (protective) relationship between beta-/gamma-HPV infection and the risk of cervical precancer. |
| Naicker et al., 2024 [112]|260 WLHIV; South Africa. | Approximately 67% of women were positive for high-risk HPV, and about one-third had abnormal cervical cytology. |
| Mbulawa et al., 2024 [113]|325 participants (HIV-positive: 208); South Africa. | HPV prevalence was 67.8% among HIV-positive women, and HPV infection was found to decrease with increasing age. HPV-58 was the most prevalent type. Among HPV-vaccinated women, 12.9–42.2% (depending on the vaccines) were infected with one or more HPV types. |
| Rantshabeng et al., 2024 [114]|171 non-HPV-vaccinated indigenous women; Botswana. | A total of 53/171 (31%) and 56/171 (32.7%) were positive for HIV and high-risk HPV, respectively, and 23/171 (13.5%) had cervical dysplasia. In women with cervical dysplasia, commonly detected HPV types were HPV-39, HPV-51, HPV-52, and HPV-56, while HPV-16 and -18 were not found. |
| Murenzi et al., 2024 [115]|Paraffin-embedded tissue sections from 227 cases (out of 440 cervical carcinomas) with valid HPV results; Tanzania, 2020. | A total of 65 (28.6%) were WLHIV. 19.8% (n = 45) were HIV negative, and 55.1% (n = 117) were of unknown HIV status. Interestingly, HPV-68 was associated with HIV positivity. |
| Muchaili et al., 2024 [116]|4612 female subjects screened for HPV infection; Zambia, 2021–2022. | Among the 2734 WLHIV, 1960 were referred to the cancer clinic by the ART facility. Of the participants, 1640 (35.56%) were HPV-positive, of which 986 (36.06%) were also positive for HIV. |
| Worku et al., 2024 [117]|915 HIV-positive patients at the ART clinic who were not screened for cervical cancer previously; Ethiopia, 2021. | The VIA test showed a positive result in 224 (24.48%) women. Among VIA-positive cases, the diagnostic assessment revealed that 72.4% had abnormal cervical pathology. |
| Manga et al., 2024 [118]|599 female sex workers aged 30 years and older; Cameroon, 2020. | Overall, 372 (62.1%) were positive for HPV, and 218 were positive for both HPV and HIV. HPV-51 and -53 were the most common types, whereas HPV-18 and -16 were the least prevalent. HIV-positive cases were 1.65 times more likely to be infected with HPV compared to the HIV-negative group. |
| Akakpo et al., 2023 [119]|330 WLHIV; Ghana, 2020–2021. | In total, 141 women had HIV/HPV co-infection. The most common high-risk HIV type was HPV-59 (50.3%, n = 71). |
| Grover et al., 2023 [120]|1131 cervical cancer patients; Botswana, 2015–2020. | More than 68% (n = 770) of these patients were HIV-positive. |
| Kangethe et al., 2023 [121]|647 WLHIV; Kenya, 2021–2022. | Out of 224 WLHIV with high-risk HPV infections, 21.4% had abnormal cervical cytology; HPV-52 was the most common genotype. |
| Megersa et al., 2023 [122]|406 WLHIV; Ethiopia, 2022. | More than one-third of WLHIV had high-risk HPV (35.2%, n = 173). The commonest type was HPV-16 (15.3%, n = 62), |
| Sørbye et al., 2023 [123]|Subjects n = 710: HIV-negative (373/52.5%), WLHIV (337/47.5%); South Africa, 2016–2020. | The HPV positivity rate and CIN3+ prevalence were significantly higher among WLHIV compared to HIV-negative women. |
| Gupta et al., 2022 [124]|Cross-sectional study: 135 WLHIV and 160 HIV-negative women; India, 2019–2021. | WLHIV had significantly higher cervical cytological abnormalities (14.1%, n = 19) and high-risk HPV infection (28.9%, n = 39) than HIV-negative women (cytological abnormalities: 3.1%, n = 5; HPV: 9.3%, n = 15). |
| Lewis et al., 2022 [125]|1405 women after first-time VIA screening (1305 WLHIV); Malawi, 2017–2019. | Among WLHIV, 65 cases had pre-cancerous lesions (5%), and suspected cancer cases were 13 (1%). In only three HIV-negative women (3%), pre-cancerous lesion was detected. There was no association between the screening outcome and the status of HIV. |
| Nakisige et al., 2022 [126]|188 WLHIV and 116 HIV-negative women; Uganda, 2017–2020. | High-risk HPV was detected in 67% of WLHIV and 52% of HIV-negative women. CIN3 was present in 41 WLHIV (22%) and 7 HIV-negative women (6%); however cervical cancer was diagnosed in 13 WLHIV (4%) and 24 HIV-negative women (21%). HPV-16 was the most common high-risk type. |
| Mcharo et al., 2021 [127]|Out of 2134 women, 804 were included (418 WLHIV and 386 HIV-negative); Tanzania, 2013–2020. | CIN1–61 WLHIV and 13 HIV-negative, HSIL (CIN 2 and 3)–52 WLHIV and 16 HIV-negative, cervical cancer–107 WLHIV and 129 HIV-negative. Roughly 80% of CIN1 and HSIL cases were WLHIV. |
| Jary et al., 2021 [128]|144 women screened for cervical cancer (WLHIV: n = 44, high-risk HPV: n = 90); Mali, 2018. | High-risk HPV infection was significantly higher in WLHIV compared with HIV-negative women. Moreover, the prevalence of HSV-2 was significantly higher in WLHIV than in HIV-negative women, and in women with high-risk HPV infection compared with those not infected with high-risk HPV. |
| Taku et al., 2021 [129]|Cross-sectional study: 205 cervical tissue specimens; South Africa, 2017–2018. | High-risk HPV, HIV, and HSV-2 were detected in 66 cases (32.2%), 79 cases (38.5%), and 12 cases (5.9%), respectively. Both high-risk HPV and HSV-2 infections were present in 8 subjects, and 34 subjects had both high-risk HPV and HIV infections. HIV and HSV-2 infections were significantly and positively associated with high-risk HPV infection. |
| Positive Correlation | No Correlation |
|---|---|
| Smith et al., 2002 [138]. Samples were collected from seven countries. Cervical exfoliated cells were collected from all subjects, and biopsy specimens from cancer patients (1158 squamous cell carcinoma and 105 adenocarcinoma or adenosquamous carcinoma). HSV-2 seroprevalence was significantly higher (p < 0.001) among cancer patients than control subjects. Similar significance was observed among the HPV DNA-positive women. Among patients (total- 1263), 560 were HSV-2 seropositive, and among control subjects (n = 1117), 286 were HSV-2 positive. Cervical samples from 1098 (94.8%) squamous cell carcinoma, 95 (90.5%) adenocarcinoma or adenosquamous carcinoma, and 164 (14.7%) controls were positive for HPV DNA. | El-All et al., 2007 [141]. The study was conducted in the Egyptian population and included complete data from 5453 women. Abnormal changes in the cervical epithelium were found in 424 women. Among them, invasive carcinoma was diagnosed in four cases. Other abnormalities are: low-grade lesions (41.0%), high-grade lesions (5.2%), atypical glandular cell of undetermined significance (15.3%), and atypical squamous cells of undetermined significance (ASCUS) (34.4%). Only one case from the latter category was positive for HSV-2. HPV was evaluated in 217 cases; 66% were positive, 29% were negative, and 5% had no definite diagnosis. |
| Paba et al., 2008 [142]. In this Italian study, 149 cervical cancer and CIN biopsies were examined. HPV DNA-positive cases were 136, Chlamydia trachomatis DNA was identified in 32 cases, and 29 were positive for HSV-2. C. trachomatis was significantly associated with multiple-type HPV infections (p = 0.0001). Whereas, there was a borderline significance (p = 0.053) for HSV-2 infection. Survivin, a member of the family of inhibitor-of-apoptosis proteins, was overexpressed in lesions that were positive for both HSV-2 and HPV (p = 0.027). | Wohlmeister et al., 2016 [143]. In this cross-sectional study in Brazil, cervical cytological samples were collected from 169 women during routine gynecologic examination. No cellular abnormalities were found in 75 women (HPV-positive: 9), reactive or inflammatory benign features were seen in 76 women (HPV-positive: 8), and atypia or cervical lesions were diagnosed in 18 cases (all were HPV-positive), which included 10 low-grade lesions, 3 high-grade lesions, and 4 cases of ASCUS. Only one subject was positive for HSV-2. |
| Kwaśniewska et al., 2009 [144]. This study was conducted in Poland, and the study groups consisted of 520 patients with squamous cell carcinoma of the cervix (HPV-positive: 468, 90%), 50 adenocarcinomas, and 50 controls. In patients with squamous cell carcinoma, HSV-2 was detected in 145 cases (28%) and C. trachomatis in 135 cases (26%), and in adenocarcinoma, HSV-2 was detected in 15 cases (30%) and C. trachomatis in 12 cases (24%); 4 controls were positive for HSV-2 as well as for C. trachomatis. Compared to the control group, a significantly higher occurrence of HSV-2 and C. trachomatis was noticed in specimens from patients with cervical cancer (p < 0.05). No correlation was seen between HPV and HSV-2. | Moharreri et al., 2021 [145]. The study was performed on 195 liquid-based cytology specimens collected from Iranian women; 50 samples were from CIN cases. Finally, 148 HPV-positive samples were analyzed for different sexually transmitted pathogens such as Mycoplasma genitalium, C. trachomatis, and HSV-2. C. trachomatis infection was present in 24 cases, M. genitalium infection in 3 cases, and only 1 case was positive for HSV-2. No statistically significant differences were found between these pathogens and CIN. |
| de Abreu et al., 2016 [146]. A total of 838 women were enrolled from basic health units of the Brazilian public health system for cervical screening. Among them, 614 women had no epithelial lesions (HPV: 101, high-risk HPV: 66), and 224 women had the following cervical abnormalities (HPV: 183, high-risk HPV: 164): 56 low-grade lesions, 71 high-grade lesions, 65 ASCUS, 27 atypical squamous cells- cannot exclude high-grade squamous intraepithelial lesion, and 5 atypical glandular cells. HSV-2 infection was present in 20 subjects who had no epithelial abnormalities, and in 13 cases who had cervical abnormalities. The coexistence of HPV-DNA and high-risk HPV infection with HSV-2 showed an elevated risk of ≥ ASCUS cytology, but no enhanced risk of high-grade lesions was noticed. | Jary et al., 2021 [128]. In this study in Mali, women infected with HIV (n = 44) and HIV-negative women (n = 96) who attended cervical cancer screening were included. The prevalence of high-risk HPV was higher in HIV-positive women (p = 0.014): high-risk HPV infection in HIV-positive cases was 34 (77%), and in HIV-negative women was 53 (55%). Overall, the prevalence rates of HPV and high-risk HPV were 74% (n = 104) and 63% (n = 90), respectively. VIA/VILI screening was positive for 20 subjects: 1 had normal histology, 6 had CIN1, 5 had CIN2 or higher, 5 had cervicitis, and no definite diagnosis for 3 women. Among patients with cervical lesions (n = 11), 7 were positive for high-risk HPV, and only 1 was HIV-positive. The prevalence of HSV-2 was higher in HIV-positive cases (n = 37, 84%) compared to HIV-negative women (n = 29, 32%) (p < 0.0001). Similarly, HSV-2 was higher in subjects who had high-risk HPV (n = 47, 56%) than in women without high-risk HPV infection (n = 19, 37%) (p = 0.035). |
| Study Details | Key Results |
|---|---|
| George Onyango et al., 2025 [272]. A cross-sectional study in Kenya, which screened coinfections with HIV, HSV-2, and C. trachomatis in women with cervical abnormalities (n = 517). | The prevalence of CIN was 18.4%. Coinfections with HIV or HSV-2 were two times more likely to test positive for CIN in comparison with uninfected subjects. Similarly, C. trachomatis infected women were three times more likely to test positive for CIN. |
| Klein et al., 2024 [273]. A cross-sectional study from seven centers in Ethiopia was conducted to examine asymptomatic pregnant women (n = 779). | C. trachomatis and HSV-2 were significantly more common among women who were positive for HPV (any, n = 257) and high-risk HPV (n = 172). |
| Loonen et al., 2024 [274]. This study from the Netherlands analyzed the prevalence of pathogenic organisms from 500 high-risk HPV-negative and 492 high-risk HPV-positive cervical smears. | C. trachomatis, M. genitalium, and bacterial vaginosis * had a significantly higher prevalence in high-risk HPV-positive smears in comparison to high-risk HPV-negative samples. |
| Wang et al., 2024 [275]. A multicenter cross-sectional study in China, which analyzed HPV-negative (n = 261) and HPV-positive (n = 270) women with normal or abnormal cervical histology. | As opposed to HPV-negative women, in the HPV-positive group, there were significantly higher incidence rates of lower genital tract infections with C. trachomatis, gonococcus, trichomonas, as well as mycotic infection. |
| Disi et al., 2023 [276]. The study examined cervical specimens from 719 women who were referred for colposcopy in China. Among them, 615 were high-risk HPV-positive, and 104 were high-risk HPV-negative. | U. parvum and HSV-2 had significantly higher prevalence in high-risk HPV-positive women. Among HPV-16 positive (n = 165) and HPV-18 positive (n = 54) women, the prevalence of N. gonorrhoeae infection was significantly higher compared to other patients. The infection rate of C. trachomatis was significantly higher in LSIL or milder cases. |
| Kaliterna et al., 2023 [277]. This study was conducted in Croatia on 1050 asymptomatic women and found 107 (10.2%) women were positive for high-risk HPV. | A total of 40 women had an abnormal PAP result; 16 of them were positive for high-risk HPV. Statistically significant associations were found between high-risk HPV positivity and infections with U. urealyticum, C. trachomatis, and G. vaginalis. |
| Ortiz Segarra et al., 2023 [278]. This cross-sectional study collected materials by endocervical brushes from 396 sexually active women from the indigenous communities of Ecuador. | An infection rate of 28.28% was recorded for any type of HPV, 23.48% for high-risk HPV, and 10.35% for low-risk HPV. A statistically significant association was detected between high-risk HPV and C. trachomatis infections. |
| Wang et al., 2023 [279]. This study from China managed genital tract infections of women with high-risk HPV (n = 246) and without high-risk HPV (control group, n = 354), and analyzed their cervical samples (secretion and exfoliated cells). | Multivariate logistic regression analysis showed that U. urealyticum and C. trachomatis were independent risk factors for high-risk HPV infection. |
| Adhikari et al., 2022 [280]. This was a community-randomized trial on HPV-vaccinated women at ages 18.5 and 22 years in Finland. The total number of participants at 18.5 years of age was 11,701, and at 22 years of age were 6618. | At the first visit, 940 had squamous intraepithelial lesions (HPV-16/18 negative: 886). Without lesions, 480 were positive for HPV-16/18. Out of 11,701 participants, the cytological results were missing for 781. On the second visit, 129 had lesions (HPV-16/18 negative: 106, missing: 21). Without lesions, 49 were positive for HPV-16/18, and the cytological results were missing for 1133 (out of 6489 participants). The risk of intraepithelial lesions among HPV vaccinated women was increased significantly with C. trachomatis infection and prolonged use of oral contraceptives. |
| Yu et al., 2022 [281]. This retrospective study included 132 patients from a hospital in China and found 104 were positive for HPV (high-risk type: n = 88). | The multivariable analysis showed that infections with HPV-16, C. trachomatis, and M. hominis were independent risk factors for cervical precancerous lesions. |
| Mosmann et al., 2021 [282]. This cross-sectional study from Argentina included 50 women with normal cervical cytology and 50 women with abnormal cervical cytology (from a maternity hospital) to assess the status of oral and genital HPV and C. trachomatis infection. In the abnormal group, 9 had HSIL, and 41 had LSIL. | HPV DNA was detected in 27% of women (27/100), 18 from genital samples and 14 from oral samples; both mucosal samples were positive in 5 women—3 from the normal group. The most common type was HPV-16 in both normal and abnormal cytology; it was the only type in oral mucosa. HPV was detected more frequently in the normal group (n = 18) than in the abnormal group (n = 9). Conversely, C. trachomatis DNA was detected more frequently in the abnormal group (n = 30) than the normal group (n = 19). From genital samples, C. trachomatis was detected in 35 women, and in 31 from oral samples; both mucosal sites were infected in 17 women—8 from the normal group. Both HPV and C. trachomatis significantly correlated with cytological status. However, HPV and C. trachomatis coinfection was seen in 14 women—7 had normal cytology. |
| Xie et al., 2021 [283]. A retrospective study in China, which included 668 patients from a gynecology department—of which 415 women were positive for HPV. | Logistic regression analysis showed that HPV-positive cases were significantly associated with C. trachomatis, U. parvum (serotypes 3 and 6), and M. hominis. Whereas M. genitalium and U. parvum (aforesaid serotypes) significantly correlated with CIN. |
| Chen et al., 2020 [284]. This study in China collected cervical swab samples from three groups of women: apparently healthy women who came for a routine checkup (n = 1006), women who came for assistance at the reproductive support center (n = 666), and women who visited gynecology outpatient clinics (n = 3334). The total number of participants was 5006. | Overall, the prevalence of infection with HPV was 15.5% (778/5006), C. trachomatis was 4.7% (236/5006), and coinfection was 1.2% (59/5006). For the apparently healthy women (asymptomatic), infection with HPV was 10.8% (109/1006), C. trachomatis was 3.8% (38/1006), and coinfection was 0.6% (6/1006). A higher prevalence of infection with C. trachomatis was found in HPV-positive women compared to HPV-negative women; a similar phenomenon was also noticed in the opposite way. |
| Escarcega-Tame et al., 2020 [285]. This cross-sectional study in Mexico was performed on endocervical samples obtained from 189 women with suspected infection. In fact, 184 had an infection with HPV, C. trachomatis, or both. | Fifty-six women were positive for HPV (one or more genotypes), 77 were positive for C. trachomatis, 51 had both infections, and 5 were not infected. There was an association between HPV and CIN1 (n = 22). HPV–C. trachomatis coinfection showed a correlation with the development of CIN1 (n = 31) and CIN3 (n = 7). However, only C. trachomatis infection was associated with cervicitis (n = 25). In this study, 60 women had CIN1, 11 had CIN3, and 40 women were suffering from cervicitis. |
| Prokaryotic system | Analysis of the 16S ribosomal RNA (rRNA) gene is an excellent method for the detection and proper identification of any bacteria, apart from the usefulness of this method in the determination of phylogenetic connections among bacteria. In infections, the isolate might be really responsible for the disease or could be present without causing any harm or as a contaminant. Therefore, precise pathogen identification is important for an appropriate treatment strategy. It may be worth mentioning that 16S rRNA gene analysis is a powerful method for the identification of new pathogenic bacteria, non-cultured bacteria, phenotypically aberrant or rarely isolated strains, slow-growing organisms, fastidious pathogens, and bacteria that are poorly described or poorly distinguished by conventional approaches [289,290]. Fortunately, 16S rRNA analysis methods are currently available in many clinical microbiology laboratories due to the growing accessibility of molecular biology techniques such as PCR and sequencing facilities. The bacterial rRNA genes precisely consist of 5S, 16S, and 23S (as well as intergenic regions). With regard to the 16S rRNA gene, which is a part of the ribosome’s small subunit, this gene is usually around 1.5 kb long (i.e., roughly 1500 nucleotides) and has a number of important functions such as protein synthesis, positioning of ribosomal proteins, and supporting the binding of the 50S and 30S subunits [291]. In addition, all bacteria contain at least one copy of the 16S gene, which encompasses different sequences that include highly conserved, variable, and hypervariable regions. For each bacterial species, the hypervariable regions are unique and thus used for bacterial classification. On the other hand, the conserved regions are utilized to develop universal primers, which can bind to known sequences (common in other bacteria) [292]. By and large, in our routine laboratory practice, these universal primers are designed to target the first ~500 base pairs of the 16S rRNA gene, since the analysis of this portion (V1–V3 regions) is usually thought satisfactory for precise detection of most bacterial species [291]. Nevertheless, the 16S rRNA gene can be a target for a number of antibacterial drugs, and mutations in this gene may influence bacterial susceptibility to these drugs; thus, phenotypic resistance to these drugs can be distinguished by the 16S rRNA gene sequencing [293,294,295]. Therefore, analysis of the 16S rRNA gene perhaps has a direct impact on therapeutic management. |
| Eukaryotic system | Like the utilization of 16S rRNA gene characteristics in the prokaryotic system, 18S rRNA in eukaryotic cells is a common molecular marker. Different studies reported that analysis of 18S rRNA is very useful in the detection of various protozoal infections/presence—for example, Trichomonas vaginalis and a range of vaginal fungal species such as Candida albicans, Candida glabrata, and Saccharomyces cerevisiae [296,297,298]. Like 16S rRNA of the small subunit, 18S rRNA (1.8 kb) is a part of the small 40S ribosomal subunit, while 5S, 5.8S, and 28S rRNAs constitute the large 60S subunit (complete ribosome with ribosomal proteins—80S in eukaryotes compared to 70S in prokaryotes). Both conserved and variable regions are present in the 18S rRNA gene. Sequencing of the 18S rRNA gene, particularly with the internal transcribed spacers (ITS: intervening noncoding regions), is advantageous in molecular diagnosis [299]. |
| Investigators | Study Design | Salient Observations |
|---|---|---|
| Guo et al., 2025 [309]. China | Between June 2021 and June 2022, vaginal DNA samples were collected from 151 Uygur and Han women—Han controls (n = 22), HPV transient infection (n = 26), and persistent infection group (n = 28), as well as Uygur controls (n = 28), transient infection (n = 17), and persistent infection group (n = 30). | Ethnic-specific differences in vaginal microbes were observed. In Han women, Sneathia increased significantly in persistent HPV cases. In Uygur women, Gardnerella, Streptococcus, Prevotella, and Shuttleworthia increased significantly in the transient infection group, with lower Lactobacillus in comparison with other groups. |
| Li and Wu 2025 [310] China | DNA was extracted from vaginal samples of normal controls (n = 16), HPV-positive women (n = 16), CIN1 (n = 14), CIN2 (n = 6), CIN3 (n = 15), and cervical cancer patients (n = 2) between December 2018 and September 2020. | Lactobacillus was the prevalent bacterium in all groups, and the prevalence rates are: 70.9% in normal controls, 60.2% in HPV-positive women, 63.9% in CIN1, 97.7% in CIN2, 52.0% in CIN3, and 36.9% in cervical cancer. An increased proportion of Gardnerella was detected with HPV infection, possibly associated with the progression of cervical lesions. |
| Parvez et al., 2025 [311] India | Between December 2018 and April 2022, vaginal swabs were collected, and DNA extraction was performed in 58 subjects—62% were HPV-positive and 38% were HPV-negative. Among the HPV-positive group, symptomatic cases were 57%, and 39% were asymptomatic cases. | Lactobacillus showed a high abundance in HPV-negative samples (76.7%), although lower in HPV-positive samples (60.3%). Gardnerella was significantly higher in HPV-positive (22.4%) than HPV-negative (10.0%). Similarly, Coriobacteriaceae, Prevotella, Aerococcus, and Clostridium had elevated levels in HPV-positive samples. Interestingly, L. iners was more prevalent in HPV-positive, while L. helveticus was dominant in HPV-negative samples. |
| Asensio-Puig et al., 2024 [312] Spain | Out of 22 pairs of cervicovaginal samples (liquid-based cytology samples and self-collected specimens), 14 samples met the sequencing quality criteria, and 8 were HPV-positive. | Compared to HPV-negative samples, HPV-positive samples documented a lower abundance of Lactobacillus, but a higher abundance of L. iners, and a high bacterial diversity that specifically included Atopobium, Parvimonas, Mageeibacillus, Sneathia, Megasphaera, Peptoniphilus, and Dialister. In the HPV-negative cases, a higher abundance of L. crispatus and L. gasseri were detected. |
| Fan et al., 2024 [313] China | From June to December 2020, enrolment of 125 subjects who underwent HPV test, cytology test, and colposcopy. There were 27 normal women, 40 with high-risk HPV infection, 40 with CIN and 18 cervical cancer patients. | The diversity of vaginal bacterial species in cervical cancer patients was primarily associated with a decrease in Lactobacillus and Cyanobacteria and was also correlated with an enhanced number of Dialister and Peptoniphilus. Compared to normal controls and the high-risk HPV group, the relative abundance of Cyanobacteria in the CIN group was significantly decreased. |
| Jimenez et al., 2024 [314] United States | The study included 100 primarily Hispanic and non-Hispanic White premenopausal women. There were 20 HPV-negative and 31 HPV-positive women without dysplasia, 12 with low-grade lesions (LSIL), 27 with high-grade lesions (HSIL), and 10 with invasive cervical cancer (samples were not available from one participant). | Atopobiacaeae colonization was associated with increased vaginal pH, vaginal dysbiosis, and decreased Lactobacillus dominance. A higher prevalence of Atopobiaceae was detected among Hispanic subjects as well as women with higher gravidity and parity. In cervical cancer, Atopobiaceae and particularly Fannyhessea vaginae had a higher prevalence. Atopobiacaeae were positively correlated with vaginal microbiome, such as Anaerococcus, Dialister, Prevotella, Sneathia, and Bifidobacterium/Gardnerella *, as well as with pro-inflammatory cytokines such as IL-1α, IL-1β, and TNFα. (* Gardnerella vaginalis belongs to the Bifidobacteriaceae family) |
| Łaniewski et al., 2024 [315] United States | This pilot study recruited 31 women (16 Native American and 15 non-Native women) between December 2020 and April 2022. | Women with vaginal dysbiosis had higher vaginal pH and were more frequently infected with high-risk HPV. There was also an association between high-risk HPV and Gardnerella vaginalis infections. Women with higher levels of Lactobacillus had normal vaginal pH and tended to have HPV-negative status. L. crispatus (not L. iners) was negatively associated with bacterial vaginosis-associated bacteria, e.g., Gardnerella, Fannyhessea, Prevotella, Sneathia, and Megasphaera. Interestingly, vaginal dysbiosis was linked with larger household size, lower education level, and high parity. |
| Liu et al., 2024 [316] China | Cases were collected from the Gynecology and Obstetrics department between Mar 2022 and Mar 2023. HPV-positive cases were 241, and HPV-negative cases were 1759. | A significant deficiency in Lactobacilli was noticed in HPV-positive cases compared to the HPV-negative group. Moreover, in comparison to HPV-negative subjects, the results showed that bacterial vaginitis and aerobic vaginitis were closely associated with HPV-positive cases. |
| Ou et al., 2024 [317] China | Based on the histological assessment (from January to October 2021), the subjects were categorized as follows: normal controls (without neoplasia, n = 10), CIN1 (n = 15), CIN2 (n = 25), CIN3 (n = 25), and invasive cancer (n = 25). | Among controls, L. crispatus was dominant. Compared to controls, the proportion of Lactobacillus was reduced with the progression of cervical dysplasia. In cancer patients, the abundance of L. johnsonii and L. iners was higher, and taxa such as Atopobiaceae, Prevotellaceae, and Streptococcaceae were dominant. |
| Yang et al., 2024 [318] China | The study subjects were married women who visited the gynecology outpatient clinic between September 2019 and February 2020. Participants were categorized into five groups: persistent high-risk HPV without cervical pathology (n = 20), with low-grade lesions (LSIL, n = 20), high-grade lesions (HSIL, n = 20), squamous cancer (n = 20), and HPV-negative controls (n = 19). | In the non-infected controls, the predominant bacteria were Lactobacillus, Gardnerella, Prevotella, and Streptococcus. On the other hand, the vaginal bacterial composition in subjects of all groups with HPV showed alterations that were predominantly associated with a reduction in Lactobacillus and an increase in Gardnerella when compared with the normal HPV-negative control group. The findings revealed a decrease in Lactobacillus and an increase in Gardenerella subsequent to persistent HPV infection. |
| Yu et al., 2024 [319] China | Subjects diagnosed with cervical lesions for the first time were included. There were 30 healthy controls, 29 with low-grade squamous intraepithelial lesions, 33 with high-grade lesions (HSIL), and 29 cervical cancer patients. | Compared to the other groups, decreased Lactobacillus abundance was documented in cancer patients. The abundance of Acinetobacter, Bacillus, Corynebacterium, Escherichia, Fenollaria, Peptoniphilus, Staphylococcus, and Streptococcus showed a rising trend with the degrees of cervical lesions and cancer. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ray, A. Human Papillomavirus and Other Relevant Issues in Cervical Cancer Pathogenesis. Int. J. Mol. Sci. 2025, 26, 5549. https://doi.org/10.3390/ijms26125549
Ray A. Human Papillomavirus and Other Relevant Issues in Cervical Cancer Pathogenesis. International Journal of Molecular Sciences. 2025; 26(12):5549. https://doi.org/10.3390/ijms26125549
Chicago/Turabian StyleRay, Amitabha. 2025. "Human Papillomavirus and Other Relevant Issues in Cervical Cancer Pathogenesis" International Journal of Molecular Sciences 26, no. 12: 5549. https://doi.org/10.3390/ijms26125549
APA StyleRay, A. (2025). Human Papillomavirus and Other Relevant Issues in Cervical Cancer Pathogenesis. International Journal of Molecular Sciences, 26(12), 5549. https://doi.org/10.3390/ijms26125549





