Understanding the Immune System’s Intricate Balance: Activation, Tolerance, and Self-Protection
Abstract
1. Overview of Host Immunities
2. The Eradicable Immune Response
3. The Tolerable Immune Response
4. Immune Activation and Deactivation Mechanisms
4.1. The Spleen and Liver: Opposing Roles in Immune Regulation
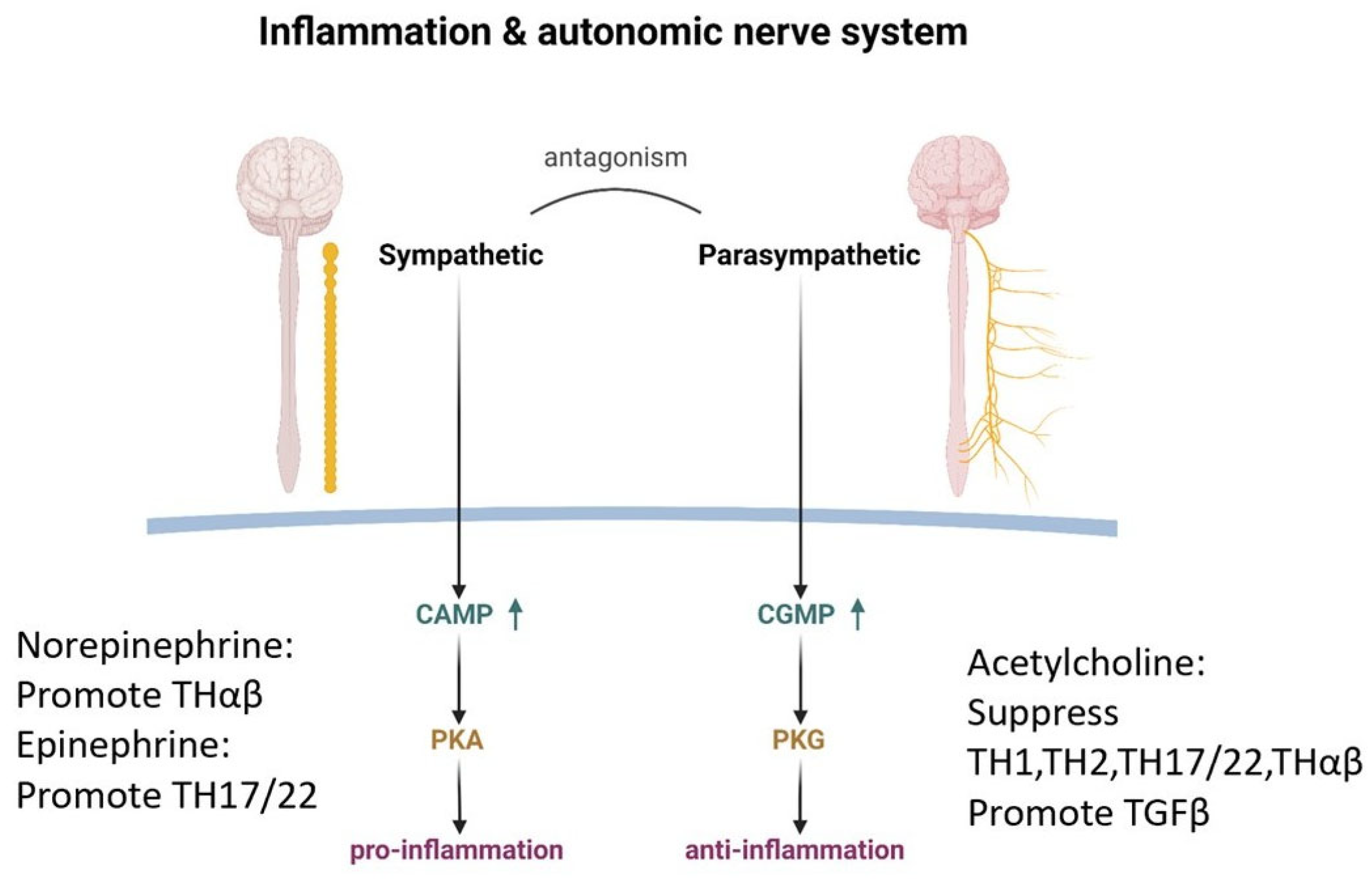
4.2. The Autonomic Nervous System: The Balance of Inflammation and Anti-Inflammation
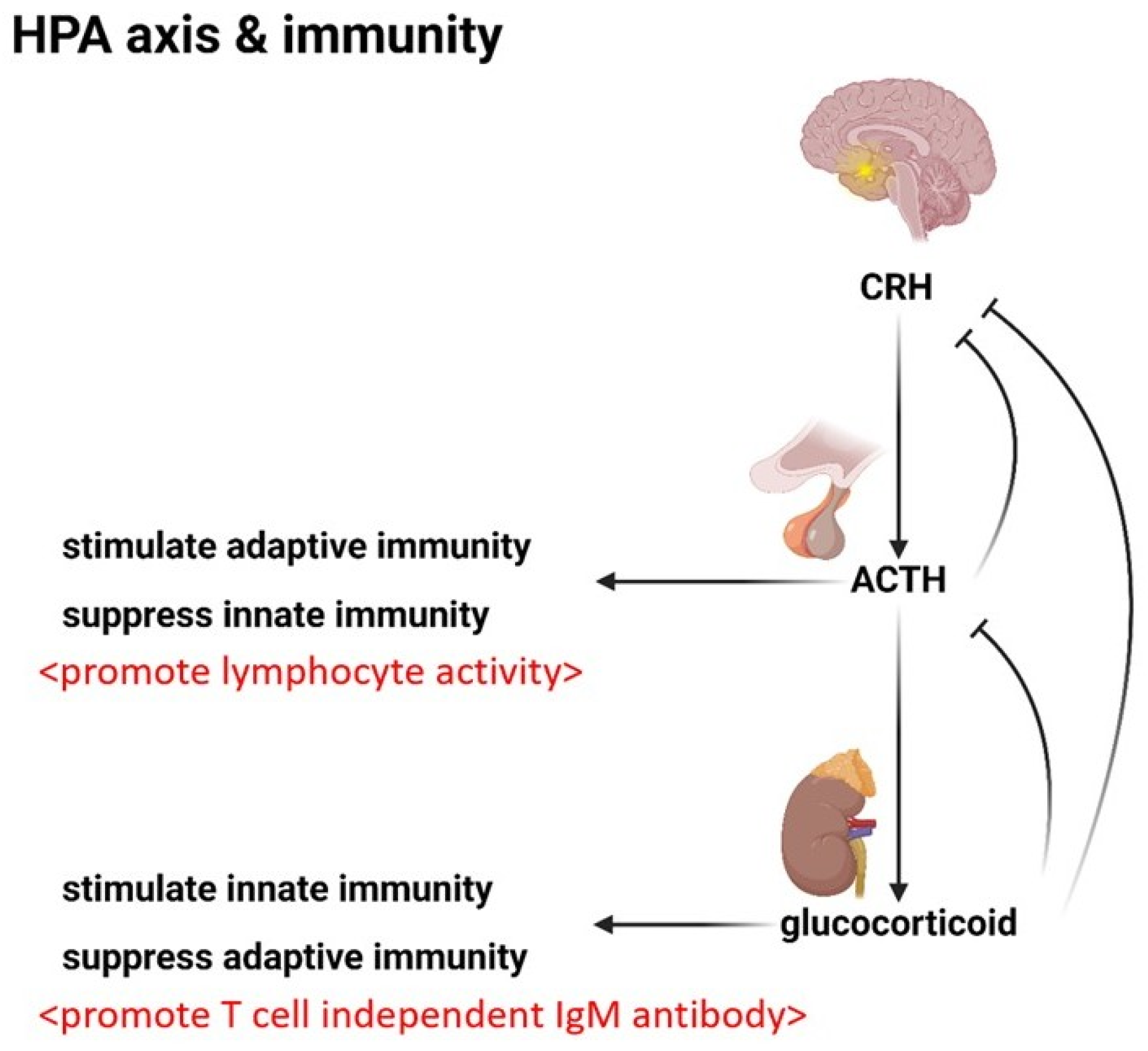
4.3. The Endocrine System: Modulating the Immunity
4.4. Immune Activation: The Mechanism
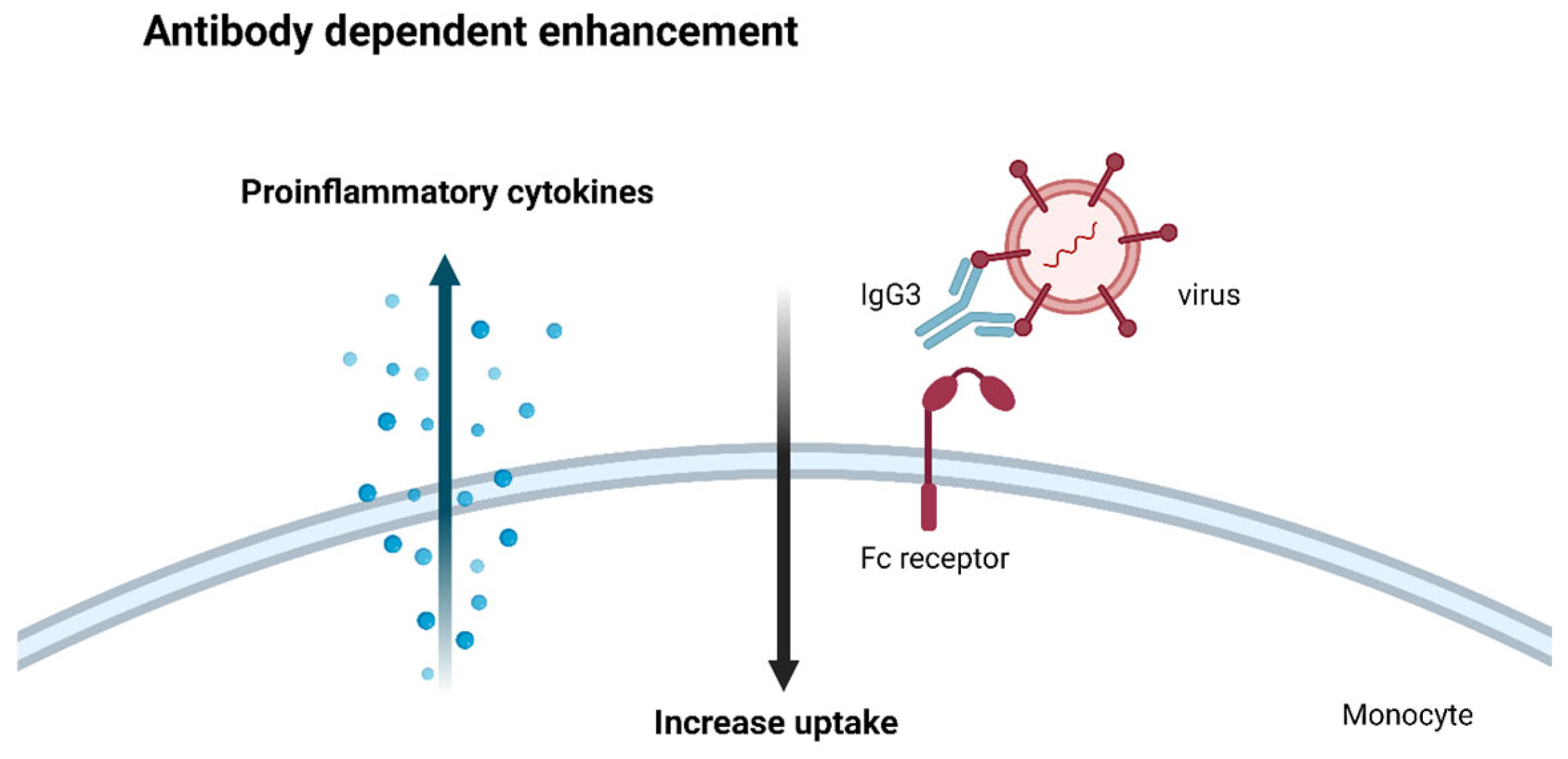
4.5. Antibody-Dependent Enhancement: An Adverse Effect
4.6. Clonal Anergy: The Art of Self-Tolerance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, W.C. A Framework of All Discovered Immunological Pathways and Their Roles for Four Specific Types of Pathogens and Hypersensitivities. Front. Immunol. 2020, 11, 1992. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Tsai, K.W.; Lu, K.C.; Shih, L.J.; Hu, W.C. Cancer as a Dysfunctional Immune Disorder: Pro-Tumor TH1-like Immune Response and Anti-Tumor THαβ Immune Response Based on the Complete Updated Framework of Host Immunological Pathways. Biomedicines 2022, 10, 2497. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.C. Human immune responses to Plasmodium falciparum infection: Molecular evidence for a suboptimal THαβ and TH17 bias over ideal and effective traditional TH1 immune response. Malar. J. 2013, 12, 392. [Google Scholar] [CrossRef]
- Chu, Y.T.; Liao, M.T.; Tsai, K.W.; Lu, K.C.; Hu, W.C. Interplay of Chemokines Receptors, Toll-like Receptors, and Host Immunological Pathways. Biomedicines 2023, 11, 2384. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, J. CD4 T Helper Cell Subsets and Related Human Immunological Disorders. Int. J. Mol. Sci. 2020, 21, 8011. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, C.E. T helper cell subsets: Diversification of the field. Eur. J. Immunol. 2023, 53, e2250218. [Google Scholar] [CrossRef] [PubMed]
- Clottu, A.S.; Humbel, M.; Fluder, N.; Karampetsou, M.P.; Comte, D. Innate Lymphoid Cells in Autoimmune Diseases. Front. Immunol. 2021, 12, 789788. [Google Scholar] [CrossRef]
- Yin, X.; Chen, S.; Eisenbarth, S.C. Dendritic Cell Regulation of T Helper Cells. Annu. Rev. Immunol. 2021, 39, 759–790. [Google Scholar] [CrossRef]
- Dong, C. Cytokine Regulation and Function in T Cells. Annu. Rev. Immunol. 2021, 39, 51–76. [Google Scholar] [CrossRef]
- Siracusa, F.; Muscate, F.; Perez, L.G. Murine T-Helper Cell Differentiation and Plasticity. Methods Mol. Biol. 2021, 2285, 65–75. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, L.; Zhu, C.; Zhang, M.; Liang, C. Current Knowledge of Th22 Cell and IL-22 Functions in Infectious Diseases. Pathogens 2023, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yang, G.; Xiao, F.; Xie, J.; Wang, S.; Lu, L.; Cui, D. Role of Th22 Cells in the Pathogenesis of Autoimmune Diseases. Front. Immunol. 2021, 12, 688066. [Google Scholar] [CrossRef]
- Hu, W.C. The Central THαβ Immunity Associated Cytokine: IL-10 Has a Strong Anti-Tumor Ability Toward Established Cancer Models In Vivo and Toward Cancer Cells In Vitro. Front. Oncol. 2021, 11, 655554. [Google Scholar] [CrossRef]
- Laudisi, F.; Stolfi, C.; Monteleone, I.; Monteleone, G. TGF-β1 signaling and Smad7 control T-cell responses in health and immune-mediated disorders. Eur. J. Immunol. 2023, 53, e2350460. [Google Scholar] [CrossRef]
- Chen, W. TGF-β Regulation of T Cells. Annu. Rev. Immunol. 2023, 41, 483–512. [Google Scholar] [CrossRef] [PubMed]
- Son, A.; Meylan, F.; Gomez-Rodriguez, J.; Kaul, Z.; Sylvester, M.; Falduto, G.H.; Vazquez, E.; Haque, T.; Kitakule, M.M.; Wang, C.; et al. Dynamic chromatin accessibility licenses STAT5- and STAT6-dependent innate-like function of T(H)9 cells to promote allergic inflammation. Nat. Immunol. 2023, 24, 1036–1048. [Google Scholar] [CrossRef]
- Kharwadkar, R.; Ulrich, B.J.; Chu, M.; Koh, B.; Hufford, M.M.; Fu, Y.; Birdsey, G.M.; Porse, B.T.; Randi, A.M.; Kaplan, M.H. ERG Functionally Overlaps with Other Ets Proteins in Promoting TH9 Cell Expression of Il9 during Allergic Lung Inflammation. J. Immunol. 2023, 210, 537–546. [Google Scholar] [CrossRef]
- Damasceno, L.E.A.; Prado, D.S.; Veras, F.P.; Fonseca, M.M.; Toller-Kawahisa, J.E.; Rosa, M.H.; Públio, G.A.; Martins, T.V.; Ramalho, F.S.; Waisman, A.; et al. PKM2 promotes Th17 cell differentiation and autoimmune inflammation by fine-tuning STAT3 activation. J. Exp. Med. 2020, 217, e20190613. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Yang, N.; Zheng, Z.L.; Liu, D.; Xu, Q.L. T helper 17 (Th17) cell responses to the gut microbiota in human diseases. Biomed. Pharmacother. 2023, 161, 114483. [Google Scholar] [CrossRef]
- Schinocca, C.; Rizzo, C.; Fasano, S.; Grasso, G.; La Barbera, L.; Ciccia, F.; Guggino, G. Role of the IL-23/IL-17 Pathway in Rheumatic Diseases: An Overview. Front. Immunol. 2021, 12, 637829. [Google Scholar] [CrossRef]
- Hedblom, A.; Hejazi, S.M.; Canesin, G.; Choudhury, R.; Hanafy, K.A.; Csizmadia, E.; Persson, J.L.; Wegiel, B. Heme detoxification by heme oxygenase-1 reinstates proliferative and immune balances upon genotoxic tissue injury. Cell Death Dis. 2019, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Montecinos, L.; Eskew, J.D.; Smith, A. What Is Next in This “Age” of Heme-Driven Pathology and Protection by Hemopexin? An Update and Links with Iron. Pharmaceuticals 2019, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wu, Y.; Tang, W. Heme Catabolic Pathway in Inflammation and Immune Disorders. Front. Pharmacol. 2019, 10, 825. [Google Scholar] [CrossRef]
- Brusko, T.M.; Wasserfall, C.H.; Agarwal, A.; Kapturczak, M.H.; Atkinson, M.A. An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells. J. Immunol. 2005, 174, 5181–5186. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, M.; Ishimura, Y. The heme oxygenase-carbon monoxide system: A regulator of hepatobiliary function. Hepatology 2000, 31, 3–6. [Google Scholar] [CrossRef]
- Mackern-Oberti, J.P.; Obreque, J.; Mendez, G.P.; Llanos, C.; Kalergis, A.M. Carbon monoxide inhibits T cell activation in target organs during systemic lupus erythematosus. Clin. Exp. Immunol. 2015, 182, 1–13. [Google Scholar] [CrossRef]
- Elkhatib, S.K.; Case, A.J. Autonomic regulation of T-lymphocytes: Implications in cardiovascular disease. Pharmacol. Res. 2019, 146, 104293. [Google Scholar] [CrossRef]
- Bucsek, M.J.; Giridharan, T.; MacDonald, C.R.; Hylander, B.L.; Repasky, E.A. An overview of the role of sympathetic regulation of immune responses in infectious disease and autoimmunity. Int. J. Hyperth. 2018, 34, 135–143. [Google Scholar] [CrossRef]
- Contreras, F.; Prado, C.; Gonzalez, H.; Franz, D.; Osorio-Barrios, F.; Osorio, F.; Ugalde, V.; Lopez, E.; Elgueta, D.; Figueroa, A.; et al. Dopamine Receptor D3 Signaling on CD4+ T Cells Favors Th1- and Th17-Mediated Immunity. J. Immunol. 2016, 196, 4143–4149. [Google Scholar] [CrossRef]
- Crary, B.; Hauser, S.L.; Borysenko, M.; Kutz, I.; Hoban, C.; Ault, K.A.; Weiner, H.L.; Benson, H. Epinephrine-induced changes in the distribution of lymphocyte subsets in peripheral blood of humans. J. Immunol. 1983, 131, 1178–1181. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Nadeau, B.A.; Chen, A.J.; Sarma, J.V.; Zetoune, F.S.; McGuire, S.R.; List, R.P.; Day, D.E.; Hoesel, L.M.; et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature 2007, 449, 721–725. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Nadeau, B.A.; Sarma, J.V.; Day, D.E.; Lentsch, A.B.; Huber-Lang, M.S.; Ward, P.A. Upregulation of phagocyte-derived catecholamines augments the acute inflammatory response. PLoS ONE 2009, 4, e4414. [Google Scholar] [CrossRef] [PubMed]
- Scanzano, A.; Cosentino, M. Adrenergic regulation of innate immunity: A review. Front. Pharmacol. 2015, 6, 171. [Google Scholar] [CrossRef]
- Sharma, D.; Farrar, J.D. Adrenergic regulation of immune cell function and inflammation. Semin. Immunopathol. 2020, 42, 709–717. [Google Scholar] [CrossRef]
- Papa, I.; Saliba, D.; Ponzoni, M.; Bustamante, S.; Canete, P.F.; Gonzalez-Figueroa, P.; McNamara, H.A.; Valvo, S.; Grimbaldeston, M.; Sweet, R.A.; et al. T(FH)-derived dopamine accelerates productive synapses in germinal centres. Nature 2017, 547, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Frohman, E.M.; Vayuvegula, B.; Gupta, S.; van den Noort, S. Norepinephrine inhibits gamma-interferon-induced major histocompatibility class II (Ia) antigen expression on cultured astrocytes via beta-2-adrenergic signal transduction mechanisms. Proc. Natl. Acad. Sci. USA 1988, 85, 1292–1296. [Google Scholar] [CrossRef]
- Melnikov, M.; Rogovskii, V.; Sviridova, A.; Lopatina, A.; Pashenkov, M.; Boyko, A. The Dual Role of the beta(2)-Adrenoreceptor in the Modulation of IL-17 and IFN-gamma Production by T Cells in Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 668. [Google Scholar] [CrossRef] [PubMed]
- Moriuchi, M.; Yoshimine, H.; Oishi, K.; Moriuchi, H. Norepinephrine inhibits human immunodeficiency virus type-1 infection through the NF-kappaB inactivation. Virology 2006, 345, 167–173. [Google Scholar] [CrossRef]
- Koch-Weser, J. Beta adrenergic blockade and circulating eosinophils. Arch. Intern. Med. 1968, 121, 255–258. [Google Scholar] [CrossRef]
- Engstad, C.S.; Lund, T.; Osterud, B. Epinephrine promotes IL-8 production in human leukocytes via an effect on platelets. Thromb. Haemost. 1999, 81, 139–145. [Google Scholar]
- Carvajal Gonczi, C.M.; Tabatabaei Shafiei, M.; East, A.; Martire, E.; Maurice-Ventouris, M.H.I.; Darlington, P.J. Reciprocal modulation of helper Th1 and Th17 cells by the beta2-adrenergic receptor agonist drug terbutaline. FEBS J. 2017, 284, 3018–3028. [Google Scholar] [CrossRef] [PubMed]
- Kesici, S.; Demirci, M.; Kesici, U. Antibacterial effects of lidocaine and adrenaline. Int. Wound J. 2019, 16, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.; Osterud, B. The promoting effect of epinephrine on lipopolysaccharide-induced interleukin-8 production in whole blood may be mediated by thromboxane A2. J. Thromb. Haemost. 2003, 1, 1042–1047. [Google Scholar] [CrossRef][Green Version]
- Mattingly, A.J.; Laitano, O.; Clanton, T.L. Epinephrine stimulates CXCL1 IL-1alpha, IL-6 secretion in isolated mouse limb muscle. Physiol. Rep. 2017, 5, e13519. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yan, J.; Liang, H.; Jiang, J. Epinephrine enhances the response of macrophages under LPS stimulation. Biomed. Res. Int. 2014, 2014, 254686. [Google Scholar] [CrossRef]
- Kradin, R.; Rodberg, G.; Zhao, L.H.; Leary, C. Epinephrine yields translocation of lymphocytes to the lung. Exp. Mol. Pathol. 2001, 70, 1–6. [Google Scholar] [CrossRef]
- Platzer, C.; Döcke, W.; Volk, H.; Prösch, S. Catecholamines trigger IL-10 release in acute systemic stress reaction by direct stimulation of its promoter/enhancer activity in monocytic cells. J. Neuroimmunol. 2000, 105, 31–38. [Google Scholar] [CrossRef]
- Qiu, Y.H.; Cheng, C.; Dai, L.; Peng, Y.P. Effect of endogenous catecholamines in lymphocytes on lymphocyte function. J. Neuroimmunol. 2005, 167, 45–52. [Google Scholar] [CrossRef]
- Bergquist, J.; Tarkowski, A.; Ekman, R.; Ewing, A. Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc. Natl. Acad. Sci. USA 1994, 91, 12912–12916. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, X.; Ma, X.; Liu, Y.; Ma, L.; Chen, Z.; Chen, H.; Si, L.; Chen, X. Acetylcholine regulates the development of experimental autoimmune encephalomyelitis via the CD4+ cells proliferation and differentiation. Int. J. Neurosci. 2020, 130, 788–803. [Google Scholar] [CrossRef]
- Malin, S.G.; Shavva, V.S.; Tarnawski, L.; Olofsson, P.S. Functions of acetylcholine-producing lymphocytes in immunobiology. Curr. Opin. Neurobiol. 2020, 62, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, Y.; Yamamoto, T.; Yoshida, M.; Zhang, Y.; Lee, J.; Hayashi, S.; Kadowaki, M. Cholinergic anti-inflammatory pathway ameliorates murine experimental Th2-type colitis by suppressing the migration of plasmacytoid dendritic cells. Sci. Rep. 2022, 12, 54. [Google Scholar] [CrossRef]
- Nizri, E.; Irony-Tur-Sinai, M.; Lory, O.; Orr-Urtreger, A.; Lavi, E.; Brenner, T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J. Immunol. 2009, 183, 6681–6688. [Google Scholar] [CrossRef]
- Oenema, T.A.; Smit, M.; Smedinga, L.; Racke, K.; Halayko, A.J.; Meurs, H.; Gosens, R. Muscarinic receptor stimulation augments TGF-beta1-induced contractile protein expression by airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L589–L597. [Google Scholar] [CrossRef] [PubMed]
- Fong, G.; Backman, L.J.; Alfredson, H.; Scott, A.; Danielson, P. The effects of substance P and acetylcholine on human tenocyte proliferation converge mechanistically via TGF-beta1. PLoS ONE 2017, 12, e0174101. [Google Scholar] [CrossRef]
- Ahluwalia, A.; Foster, P.; Scotland, R.S.; McLean, P.G.; Mathur, A.; Perretti, M.; Moncada, S.; Hobbs, A.J. Antiinflammatory activity of soluble guanylate cyclase: cGMP-dependent down-regulation of P-selectin expression and leukocyte recruitment. Proc. Natl. Acad. Sci. USA 2004, 101, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Flores-Costa, R.; Duran-Guell, M.; Casulleras, M.; Lopez-Vicario, C.; Alcaraz-Quiles, J.; Diaz, A.; Lozano, J.J.; Titos, E.; Hall, K.; Sarno, R.; et al. Stimulation of soluble guanylate cyclase exerts antiinflammatory actions in the liver through a VASP/NF-kappaB/NLRP3 inflammasome circuit. Proc. Natl. Acad. Sci. USA 2020, 117, 28263–28274. [Google Scholar] [CrossRef]
- Wollberg, P.; Söderqvist, H.; Nelson, B.D. Mitogen activation of human peripheral T lymphocytes induces the formation of new cyclic AMP response element-binding protein nuclear complexes. J. Biol. Chem. 1994, 269, 19719–19724. [Google Scholar] [CrossRef]
- Aley, K.O.; Levine, J.D. Role of protein kinase A in the maintenance of inflammatory pain. J. Neurosci. 1999, 19, 2181–2186. [Google Scholar] [CrossRef]
- Perez-Perez, D.; Santos-Argumedo, L.; Rodriguez-Alba, J.C.; Lopez-Herrera, G. Role of Protein Kinase A Activation in the Immune System with an Emphasis on Lipopolysaccharide-Responsive and Beige-like Anchor Protein in B Cells. Int. J. Mol. Sci. 2023, 24, 3098. [Google Scholar] [CrossRef]
- Torgersen, K.M.; Vang, T.; Abrahamsen, H.; Yaqub, S.; Taskén, K. Molecular mechanisms for protein kinase A-mediated modulation of immune function. Cell. Signal. 2002, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Busillo, J.M.; Azzam, K.M.; Cidlowski, J.A. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J. Biol. Chem. 2011, 286, 38703–38713. [Google Scholar] [CrossRef] [PubMed]
- de Castro Kroner, J.; Knoke, K.; Kofler, D.M.; Steiger, J.; Fabri, M. Glucocorticoids promote intrinsic human T(H)17 differentiation. J. Allergy Clin. Immunol. 2018, 142, 1669–1673.e11. [Google Scholar] [CrossRef] [PubMed]
- Schleimer, R.P. Glucocorticoids suppress inflammation but spare innate immune responses in airway epithelium. Proc. Am. Thorac. Soc. 2004, 1, 222–230. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Gurewich, Y.; Gallant, L.A.; Pinkhas, J. Prednisone-induced leukocytosis. Influence of dosage, method and duration of administration on the degree of leukocytosis. Am. J. Med. 1981, 71, 773–778. [Google Scholar] [CrossRef]
- Gonsalkorale, W.M.; Dascombe, M.J.; Hutchinson, I.V. Adrenocorticotropic hormone as a potential enhancer of T-lymphocyte function in the rat mixed lymphocyte reaction. Int. J. Immunopharmacol. 1995, 17, 197–206. [Google Scholar] [CrossRef]
- Johnson, E.W.; Hughes, T.K., Jr.; Smith, E.M. ACTH enhancement of T-lymphocyte cytotoxic responses. Cell. Mol. Neurobiol. 2005, 25, 743–757. [Google Scholar] [CrossRef]
- Dittel, L.J.; Dittel, B.N.; Brod, S.A. Ingested ACTH blocks Th17 production by inhibiting GALT IL-6. J. Neurol. Sci. 2020, 409, 116602. [Google Scholar] [CrossRef]
- Dittel, L.J.; Dittel, B.N.; Brod, S.A. Ingested (Oral) Adrenocorticotropic Hormone Inhibits IL-17 in the Central Nervous System in the Mouse Model of Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Immunohorizons 2022, 6, 497–506. [Google Scholar] [CrossRef]
- Sun, Z.; Cai, D.; Yang, X.; Shang, Y.; Li, X.; Jia, Y.; Yin, C.; Zou, H.; Xu, Y.; Sun, Q.; et al. Stress Response Simulated by Continuous Injection of ACTH Attenuates Lipopolysaccharide-Induced Inflammation in Porcine Adrenal Gland. Front. Vet. Sci. 2020, 7, 315. [Google Scholar] [CrossRef]
- Xu, R.; Zhao, L.; Liu, J.; Cao, L.; Zhao, T.; Yu, Y.; Xuan, D.; Wan, W.; Xue, Y.; Zou, H. Natural Adrenocorticotropic Hormone (ACTH) Relieves Acute Inflammation in Gout Patients by Changing the Function of Macrophages. J. Healthc. Eng. 2022, 2022, 9241835. [Google Scholar] [CrossRef] [PubMed]
- Agelaki, S.; Tsatsanis, C.; Gravanis, A.; Margioris, A.N. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect. Immun. 2002, 70, 6068–6074. [Google Scholar] [CrossRef] [PubMed]
- Benou, C.; Wang, Y.; Imitola, J.; VanVlerken, L.; Chandras, C.; Karalis, K.P.; Khoury, S.J. Corticotropin-releasing hormone contributes to the peripheral inflammatory response in experimental autoimmune encephalomyelitis. J. Immunol. 2005, 174, 5407–5413. [Google Scholar] [CrossRef]
- O’Kane, M.; Murphy, E.P.; Kirby, B. The role of corticotropin-releasing hormone in immune-mediated cutaneous inflammatory disease. Exp. Dermatol. 2006, 15, 143–153. [Google Scholar] [CrossRef]
- Wang, W.; Ji, P.; Dow, K.E. Corticotropin-releasing hormone induces proliferation and TNF-alpha release in cultured rat microglia via MAP kinase signalling pathways. J. Neurochem. 2003, 84, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, S.; Craig, E.A. The heat-shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Qiu, X.B.; Shao, Y.M.; Miao, S.; Wang, L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell. Mol. Life Sci. 2006, 63, 2560–2570. [Google Scholar] [CrossRef]
- Taylor, A.; Foo, S.S.; Bruzzone, R.; Dinh, L.V.; King, N.J.; Mahalingam, S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol. Rev. 2015, 268, 340–364. [Google Scholar] [CrossRef]
- Rothman, A.L.; Ennis, F.A. Immunopathogenesis of Dengue hemorrhagic fever. Virology 1999, 257, 1–6. [Google Scholar] [CrossRef]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef]
- Arvin, A.M.; Fink, K.; Schmid, M.A.; Cathcart, A.; Spreafico, R.; Havenar-Daughton, C.; Lanzavecchia, A.; Corti, D.; Virgin, H.W. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 2020, 584, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.Y.; Dong, D.Y.; Liu, R.J.; Han, J.F.; Wang, G.C.; Zhao, H.; Li, X.F.; Deng, Y.Q.; Zhu, S.Y.; Wang, X.Y.; et al. Human IgG subclasses against enterovirus Type 71: Neutralization versus antibody dependent enhancement of infection. PLoS ONE 2013, 8, e64024. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Dhama, N.; Gupta, R.D. Dengue virus neutralizing antibody: A review of targets, cross-reactivity, and antibody-dependent enhancement. Front. Immunol. 2023, 14, 1200195. [Google Scholar] [CrossRef] [PubMed]
- Fiala, G.J.; Gomes, A.Q.; Silva-Santos, B. From thymus to periphery: Molecular basis of effector gammadelta-T cell differentiation. Immunol. Rev. 2020, 298, 47–60. [Google Scholar] [CrossRef]
- Fujihashi, K.; Dohi, T.; Kweon, M.N.; McGhee, J.R.; Koga, T.; Cooper, M.D.; Tonegawa, S.; Kiyono, H. gammadelta T cells regulate mucosally induced tolerance in a dose-dependent fashion. Int. Immunol. 1999, 11, 1907–1916. [Google Scholar] [CrossRef]
- Gao, L.; Xuan, L.; Wu, X.; Fan, Z.; Huang, F.; Yi, Z.; Li, Y.; Liu, Q. Increase of Regulatory γδ T Cells Reduces the Incidence of Acute Graft-Versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation. Blood 2016, 128, 2230. [Google Scholar] [CrossRef]
- Guan, H.; Zu, G.; Slater, M.; Elmets, C.; Xu, H. GammadeltaT cells regulate the development of hapten-specific CD8+ effector T cells in contact hypersensitivity responses. J. Investig. Dermatol. 2002, 119, 137–142. [Google Scholar] [CrossRef]
- Inagaki-Ohara, K.; Chinen, T.; Matsuzaki, G.; Sasaki, A.; Sakamoto, Y.; Hiromatsu, K.; Nakamura-Uchiyama, F.; Nawa, Y.; Yoshimura, A. Mucosal T cells bearing TCRgammadelta play a protective role in intestinal inflammation. J. Immunol. 2004, 173, 1390–1398. [Google Scholar] [CrossRef]
- Mengel, J.; Cardillo, F.; Aroeira, L.S.; Williams, O.; Russo, M.; Vaz, N.M. Anti-gamma delta T cell antibody blocks the induction and maintenance of oral tolerance to ovalbumin in mice. Immunol. Lett. 1995, 48, 97–102. [Google Scholar] [CrossRef]
- Peng, G.; Wang, H.Y.; Peng, W.; Kiniwa, Y.; Seo, K.H.; Wang, R.F. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 2007, 27, 334–348. [Google Scholar] [CrossRef]
- Rezende, R.M.; Nakagaki, B.N.; Moreira, T.G.; Lopes, J.R.; Kuhn, C.; Tatematsu, B.K.; Boulenouar, S.; Maghzi, A.H.; Rubino, S.; Menezes, G.B.; et al. gammadelta T Cell-Secreted XCL1 Mediates Anti-CD3-Induced Oral Tolerance. J. Immunol. 2019, 203, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Schilbach, K.; Krickeberg, N.; Kaisser, C.; Mingram, S.; Kind, J.; Siegers, G.M.; Hashimoto, H. Suppressive activity of Vdelta2(+) gammadelta T cells on alphabeta T cells is licensed by TCR signaling and correlates with signal strength. Cancer Immunol. Immunother. 2020, 69, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Hayday, A.C.; Barber, D.F.; Douglas, N.; Hoffman, E.S. Signals involved in gamma/delta T cell versus alpha/beta T cell lineage commitment. Semin. Immunol. 1999, 11, 239–249. [Google Scholar] [CrossRef]
- Dopfer, E.P.; Hartl, F.A.; Oberg, H.H.; Siegers, G.M.; Yousefi, O.S.; Kock, S.; Fiala, G.J.; Garcillan, B.; Sandstrom, A.; Alarcon, B.; et al. The CD3 conformational change in the gammadelta T cell receptor is not triggered by antigens but can be enforced to enhance tumor killing. Cell Rep. 2014, 7, 1704–1715. [Google Scholar] [CrossRef]
- Dave, V.P.; Cao, Z.; Browne, C.; Alarcon, B.; Fernandez-Miguel, G.; Lafaille, J.; de la Hera, A.; Tonegawa, S.; Kappes, D.J. CD3 delta deficiency arrests development of the alpha beta but not the gamma delta T cell lineage. EMBO J. 1997, 16, 1360–1370. [Google Scholar] [CrossRef]
- Kadlecek, T.A.; van Oers, N.S.C.; Lefrancois, L.; Olson, S.; Finlay, D.; Chu, D.H.; Connolly, K.; Killeen, N.; Weiss, A. Differential Requirements for ZAP-70 in TCR Signaling and T Cell Development. J. Immunol. 1998, 161, 4688–4694. [Google Scholar] [CrossRef]
- Laird, R.M.; Hayes, S.M. Roles of the Src tyrosine kinases Lck and Fyn in regulating gammadeltaTCR signal strength. PLoS ONE 2010, 5, e8899. [Google Scholar] [CrossRef]
- Van Neerven, J.; Coligan, J.E.; Koning, F. Structural comparison of alpha/beta and gamma/delta T cell receptor-CD3 complexes reveals identical subunit interactions but distinct cross-linking patterns of T cell receptor chains. Eur. J. Immunol. 1990, 20, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.; Mezouar, S.; Cano, C.; Frohna, P.; Madakamutil, L.; Mège, J.L.; Olive, D. Role of Vγ9vδ2 T lymphocytes in infectious diseases. Front. Immunol. 2022, 13, 928441. [Google Scholar] [CrossRef]
- Sumaria, N.; Fiala, G.J.; Inácio, D.; Curado-Avelar, M.; Cachucho, A.; Pinheiro, R.; Wiesheu, R.; Kimura, S.; Courtois, L.; Blankenhaus, B.; et al. Perinatal thymic-derived CD8αβ-expressing γδ T cells are innate IFN-γ producers that expand in IL-7R-STAT5B-driven neoplasms. Nat. Immunol. 2024, 25, 1207–1217. [Google Scholar] [CrossRef]
- Goodnow, C.C.; Crosbie, J.; Jorgensen, H.; Brink, R.A.; Basten, A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature 1989, 342, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, L.; Kosco, M.H.; Köhler, G.; Lamers, M.C. Immunoglobulin D-deficient mice can mount normal immune responses to thymus-independent and -dependent antigens. Proc. Natl. Acad. Sci. USA 1993, 90, 1887–1891. [Google Scholar] [CrossRef] [PubMed]
- Duty, J.A.; Szodoray, P.; Zheng, N.Y.; Koelsch, K.A.; Zhang, Q.; Swiatkowski, M.; Mathias, M.; Garman, L.; Helms, C.; Nakken, B.; et al. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J. Exp. Med. 2009, 206, 139–151. [Google Scholar] [CrossRef]
- Quach, T.D.; Manjarrez-Orduno, N.; Adlowitz, D.G.; Silver, L.; Yang, H.; Wei, C.; Milner, E.C.; Sanz, I. Anergic responses characterize a large fraction of human autoreactive naive B cells expressing low levels of surface IgM. J. Immunol. 2011, 186, 4640–4648. [Google Scholar] [CrossRef]
- Soulas, P.; Koenig-Marrony, S.; Julien, S.; Knapp, A.M.; Garaud, J.C.; Pasquali, J.L.; Martin, T. A role for membrane IgD in the tolerance of pathological human rheumatoid factor B cells. Eur. J. Immunol. 2002, 32, 2623–2634. [Google Scholar] [CrossRef]
- Nguyen, T.G.; Little, C.B.; Yenson, V.M.; Jackson, C.J.; McCracken, S.A.; Warning, J.; Stevens, V.; Gallery, E.G.; Morris, J.M. Anti-IgD antibody attenuates collagen-induced arthritis by selectively depleting mature B-cells and promoting immune tolerance. J. Autoimmun. 2010, 35, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.; Klaus, G.G. Cross-linking of surface IgM, but not surface IgD receptors, by soluble monoclonal antibodies primes murine B cells to secrete immunoglobulin in response to lymphokines. Eur. J. Immunol. 1993, 23, 574–577. [Google Scholar] [CrossRef]
- Huang, Y.; Heiser, R.A.; Detanico, T.O.; Getahun, A.; Kirchenbaum, G.A.; Casper, T.L.; Aydintug, M.K.; Carding, S.R.; Ikuta, K.; Huang, H.; et al. gammadelta T cells affect IL-4 production and B-cell tolerance. Proc. Natl. Acad. Sci. USA 2015, 112, E39–E48. [Google Scholar] [CrossRef]



| Immune Pathways | Driven Cytokines, ILCs, DC | Transcription Factors | Effector Cells | CD4 T Cells | B Cells | NKT Cells | Pathogen/Pathogenesis | Autoimmune |
|---|---|---|---|---|---|---|---|---|
| Initiatory | ||||||||
| Tfh | IL-21, FDC, LTi | STAT1, STAT3, STAT5B | IL-21 CD4 T cells | IgG B cells | iNKTfh | |||
| Eradicable immunities | ||||||||
| TH1 | IL-12, ILC1, mDC2 | STAT4 | Macrophages (M1), CTL (Tc1, EM4) | IFN-γCD4 T cells | IgG3 B cells | iNKT1 | Intracellular microorganisms (bacteria, fungi, and protozoa) | Type 4 DTH |
| TH2 (TH2a) | IL-4, iILC2, LC | STAT6, STAT1 | Eosinophils (iEOS), mast cells (MCt) | IL-4, IL-5 CD4 T cells | IgG4 B cells | iNKT2 | Endoparasites (helminths) | Type 1 allergy (IgG4) |
| TH2 (TH2b) | IL-4, nILC2, LC, Tfh13 | STAT6, STAT3 | Basophils, mast cells (MCct) | IL-4, IL-13 CD4 T cells | IgE B cells | iNKT2 | Ectoparasites (Insects) | Type 1 allergy (IgE) |
| TH22 | IL-1, mDC1, ILC3 NCR+ | STAT3 | Neutrophils (N1) | IL-1, TNFα, IL-22 CD4 T cells | IgG2 B cells | iNKT17 | Extracellular microorganisms (bacteria, fungi, and protozoa) | Type 3 Immune complex |
| THαβ | IL-10, pDC, IFNα, ILC10 | STAT1, STAT2 | NK cells (NK1), CTL (Tc2, EM1) | IL-10 CD4 T cells | IgG1 B cells | iNKT10 | Infectious particles (viruses/prions) | Type 2 ADCC |
| Immune pathways | Driven cytokines, ILCs | Transcription factors | Effector cells | CD4 T cells | B cells | NKT cells | Pathogen/pathogenesis | Autoimmune |
| Regulatory | ||||||||
| Treg | TGFβ, DCreg, ILCreg | STAT5A, STAT5B | TGF-β CD4 T cells | IgA B cells | iNKTreg | |||
| Tolerable immunities | ||||||||
| TH1like | IL-12, TGF-β, ILC1 | STAT4, STAT5 | Macrophages (M2), CD8 T cells (EM3) | IFN-γ/TGF-β CD4 T cells | IgA1 B cells | iNKT1 | Intracellular microorganisms (bacteria, fungi, and protozoa) | Type 4 DTH |
| TH9 | IL-4, TGF-β, TSLP ILC2 | STAT6, STAT5 | Eosinophils (rEOS), basophils, mast cells (MMC9) | IL-9 CD4 T cells | IgA2 B cells | iNKT2 | Parasites (helminths and insects) | Type 1 allergy |
| TH17 | IL-6, TGF-β, ILC3 NCR- | STAT3, STAT5 | Neutrophils (N2) | IL-17 CD4 T cells | IgA2 B cells | iNKT17 | Extracellular microorganisms (bacteria, fungi, and protozoa) | Type 3 immune complex |
| TH3 | IL-10, TGF-β, ILC10 | STAT1, STAT2, STAT5 | NK cells (NK2), CD8 T cells (EM2) | IL-10/TGF-β CD4 T cells | IgA1 B cells | iNKT10 | Infectious particles (viruses/prions) | Type 2 ADCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-Y.; Shih, L.-J.; Liao, M.-T.; Tsai, K.-W.; Lu, K.-C.; Hu, W.-C. Understanding the Immune System’s Intricate Balance: Activation, Tolerance, and Self-Protection. Int. J. Mol. Sci. 2025, 26, 5503. https://doi.org/10.3390/ijms26125503
Chen J-Y, Shih L-J, Liao M-T, Tsai K-W, Lu K-C, Hu W-C. Understanding the Immune System’s Intricate Balance: Activation, Tolerance, and Self-Protection. International Journal of Molecular Sciences. 2025; 26(12):5503. https://doi.org/10.3390/ijms26125503
Chicago/Turabian StyleChen, Jui-Yun, Li-Jane Shih, Min-Tser Liao, Kuo-Wang Tsai, Kuo-Cheng Lu, and Wan-Chung Hu. 2025. "Understanding the Immune System’s Intricate Balance: Activation, Tolerance, and Self-Protection" International Journal of Molecular Sciences 26, no. 12: 5503. https://doi.org/10.3390/ijms26125503
APA StyleChen, J.-Y., Shih, L.-J., Liao, M.-T., Tsai, K.-W., Lu, K.-C., & Hu, W.-C. (2025). Understanding the Immune System’s Intricate Balance: Activation, Tolerance, and Self-Protection. International Journal of Molecular Sciences, 26(12), 5503. https://doi.org/10.3390/ijms26125503






