Clinical and Genetic Characteristics of Parkinson’s Disease Patients with Substantia Nigra Hyperechogenicity
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Clinical and Ultrasonography Analysis
4.2. Genetic Analysis
4.3. Statistical Analysis and Data Visualization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s Disease |
| SNCA | α-synuclein |
| LRRK2 | Leucine-rich repeat kinase family 2 |
| VPS35 | Vacuolar protein sorting ortholog 35 |
| PRKN | Parkin |
| PINK1 | PTEN-induced kinase 1 |
| RBD | REM sleep behavior disorder |
| HGMD | Human Gene Mutation Database |
| HGVS | Human Genome Variation Society |
| ATP13A2 | ATPase cation transporting 13A2 |
| SN | Substantia nigra |
| TCS | Transcranial ultrasonography |
References
- Marsden, C.D. Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1994, 57, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Lill, C.M. Genetics of Parkinson’s disease. Mol. Cell. Probes 2016, 30, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Blauwendraat, C.; Nalls, M.A.; Singleton, A.B. The genetic architecture of Parkinson’s disease. Lancet Neurol. 2020, 19, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Rocca, W.A.; Bower, J.H.; McDonnell, S.K.; Peterson, B.J.; Maraganore, D.M. Time trends in the incidence of parkinsonism in Olmsted County, Minnesota. Neurology 2001, 57, 462–467. [Google Scholar] [CrossRef]
- Di Monte, D.A.; Lavasani, M.; Manning-Bog, A.B. Environmental factors in Parkinson’s disease. Neurotoxicology 2002, 23, 487–502. [Google Scholar] [CrossRef]
- Hernán, M.A.; Takkouche, B.; Caamaño-Isorna, F.; Gestal-Otero, J.J. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann. Neurol. 2002, 52, 276–284. [Google Scholar] [CrossRef]
- Dulski, J.; Ross, O.A.; Wszolek, Z.K. Genetics of Parkinson’s Disease: State-of-the-art and role in clinical settings. Neurol. Neurochir. Pol. 2024, 58, 38–46. [Google Scholar] [CrossRef]
- Berg, D. Hyperechogenicity of the substantia nigra: Pitfalls in assessment and specificity for Parkinson’s disease. J. Neural Transm. 2011, 118, 453–461. [Google Scholar] [CrossRef]
- Hagenah, J.M.; Becker, B.; Bruggemann, N.; Djarmati, A.; Lohmann, K.; Sprenger, A.; Klein, C.; Seidel, G. Transcranial sonography findings in a large family with homozygous and heterozygous PINK1 mutations. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1071–1074. [Google Scholar] [CrossRef]
- Arkadir, D.; Dinur, T.; Becker Cohen, M.; Revel-Vilk, S.; Tiomkin, M.; Brüggemann, N.; Cozma, C.; Rolfs, A.; Zimran, A. Prodromal substantia nigra sonography undermines suggested association between substrate accumulation and the risk for GBA-related Parkinson’s disease. Eur. J. Neurol. 2019, 26, 1013–1018. [Google Scholar] [CrossRef]
- Cui, S.-S.; Fu, R.; Du, J.-J.; Lin, Y.-Q.; Huang, P.; Gao, C.; Zhou, H.-Y.; Chen, S.-D. Sex effects on clinical features in LRRK2 G2385R carriers and non-carriers in Parkinson’s disease. BMC Neurosci. 2021, 22, 22. [Google Scholar] [CrossRef] [PubMed]
- Sierra, M.; Sánchez-Juan, P.; Martínez-Rodríguez, M.I.; González-Aramburu, I.; García-Gorostiaga, I.; Quirce, M.R.; Palacio, E.; Carril, J.M.; Berciano, J.; Combarros, O.; et al. Olfaction and imaging biomarkers in premotor LRRK2 G2019S-associated parkinson disease. Neurology 2013, 80, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Svetel, M.; Tomić, A.; Mijajlović, M.; Dobričić, V.; Novaković, I.; Pekmezović, T.; Brajković, L.; Kostić, V.S. Transcranial sonography in dopa-responsive dystonia. Eur. J. Neurol. 2017, 24, 161–166. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Jiang, X.H.; Zhu, P.P.; Zhuo, W.Y.; Liu, L.B. Advancements in understanding substantia nigra hyperechogenicity via transcranial sonography in Parkinson’s disease and its clinical implications. Front. Neurol. 2024, 15, 1407860. [Google Scholar] [CrossRef]
- Jia, F.; Fellner, A.; Kumar, K.R. Monogenic Parkinson’s Disease: Genotype, Phenotype, Pathophysiology, and Genetic Testing. Genes 2022, 13, 471. [Google Scholar] [CrossRef]
- Brüggemann, N.; Hagenah, J.; Stanley, K.; Klein, C.; Wang, C.; Raymond, D.; Ozelius, L.; Bressman, S.; Saunders-Pullman, R. Substantia nigra hyperechogenicity with LRRK2 G2019S mutations. Mov. Disord. 2011, 26, 885–888. [Google Scholar] [CrossRef]
- Brüggemann, N.; Hagenah, J.; Reetz, K.; Schmidt, A.; Kasten, M.; Buchmann, I.; Eckerle, S.; Bähre, M.; Münchau, A.; Djarmati, A.; et al. Recessively inherited parkinsonism: Effect of ATP13A2 mutations on the clinical and neuroimaging phenotype. Arch. Neurol. 2010, 67, 1357–1363. [Google Scholar] [CrossRef]
- Janik, P. Recent advances in Parkinson’s Disease research. Neurol. Neurochir. Pol. 2025, 59, 93–96. [Google Scholar] [CrossRef]
- Cardoso, F.; Goetz, C.G.; Mestre, T.A.; Sampaio, C.; Adler, C.H.; Berg, D.; Bloem, B.R.; Burn, D.J.; Fitts, M.S.; Gasser, T.; et al. A Statement of the MDS on Biological Definition, Staging, and Classification of Parkinson’s Disease. Mov. Disord. 2024, 39, 259–266. [Google Scholar] [CrossRef]
- Vlaar, A.M.M.; Bouwmans, A.; Mess, W.H.; Tromp, S.C.; Weber, W.E.J. Transcranial duplex in the differential diagnosis of parkinsonian syndromes: A systematic review. J. Neurol. 2009, 256, 530–538. [Google Scholar] [CrossRef]
- Shafieesabet, A.; Fereshtehnejad, S.-M.; Shafieesabet, A.; Delbari, A.; Baradaran, H.R.; Postuma, R.B.; Lökk, J. Hyperechogenicity of substantia nigra for differential diagnosis of Parkinson’s disease: A meta-analysis. Park. Relat. Disord. 2017, 42, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, B.; Tao, K.; Yang, H.; Wang, Y.; Zhou, T.; Yang, Y.; Yuan, L.; Liu, X.; Duan, Y. Iron accumulation and microglia activation contribute to substantia nigra hyperechogenicity in the 6-OHDA-induced rat model of Parkinson’s disease. Park. Relat. Disord. 2017, 36, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Merz, B.; Reiners, K.; Naumann, M.; Becker, G. Five-year follow-up study of hyperechogenicity of the substantia nigra in Parkinson’s disease. Mov. Disord. 2005, 20, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, K.; Szlachta, K.; Serafin-Król, M.; Gałązka-Friedman, J.; Friedman, A. Brain tissue echogenicity-implications for substantia nigra studies in parkinsonian patients. J. Neural Transm. 2012, 119, 363–367. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Huang, P.; Sun, Q.; Du, J.-J.; Cui, S.-S.; Tan, Y.-Y.; Hu, Y.-Y.; Zhan, W.-W.; Wang, Y.; Xiao, Q.; et al. Substantia nigra echogenicity associated with clinical subtypes of Parkinson’s disease. J. Park. Dis. 2018, 8, 333–340. [Google Scholar] [CrossRef]
- Hall, J.M.; Georgiades, M.J.; Hammond, D.A.; Feng, X.; Moustafa, A.A.; Lewis, S.J.G.; Todd, G. Hyperechogenicity of the Substantia Nigra in Parkinson’s Disease: Insights from Two Brothers with Markedly Different Disease Durations. Case Rep. Neurol. Med. 2017, 2017, 3673159. [Google Scholar] [CrossRef]
- Gjerstad, M.D.; Boeve, B.; Wentzel-Larsen, T.; Aarsland, D.; Larsen, J.P. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson’s disease over time. J. Neurol. Neurosurg. Psychiatry 2008, 79, 387–391. [Google Scholar] [CrossRef]
- Baumann-Vogel, H.; Hor, H.; Poryazova, R.; Valko, P.; Werth, E.; Baumann, C.R. REM sleep behavior in Parkinson disease: Frequent, particularly with higher age. PLoS ONE 2020, 15, e0243454. [Google Scholar] [CrossRef]
- Mašková, J.; Školoudík, D.; Štofaniková, P.; Ibarburu, V.; Kemlink, D.; Zogala, D.; Trnka, J.; Krupička, R.; Šonka, K.; Růžička, E.; et al. Comparative study of the substantia nigra echogenicity and 123I-Ioflupane SPECT in patients with synucleinopathies with and without REM sleep behavior disorder. Sleep Med. 2020, 70, 116–123. [Google Scholar] [CrossRef]
- Iwanami, M.; Miyamoto, T.; Miyamoto, M.; Hirata, K.; Takada, E. Relevance of substantia nigra hyperechogenicity and reduced odor identification in idiopathic REM sleep behavior disorder. Sleep Med. 2010, 11, 361–365. [Google Scholar] [CrossRef]
- Iranzo, A.; Lomeña, F.; Stockner, H.; Valldeoriola, F.; Vilaseca, I.; Salamero, M.; Molinuevo, J.L.; Serradell, M.; Duch, J.; Pavía, J.; et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: A prospective study. Lancet Neurol. 2010, 9, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Siefker, C.; Becker, G. Echogenicity of the substantia nigra in Parkinson’s disease and its relation to clinical findings. J. Neurol. 2001, 248, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, K.J.; Brüssel, T.; Leitner, P.; Krüger, R.; Bauer, P.; Woitalla, D.; Tomiuk, J.; Gasser, T.; Berg, D. Transcranial ultrasound in different monogenetic subtypes of Parkinson’s disease. J. Neurol. 2007, 254, 613–616. [Google Scholar] [CrossRef]
- Berg, D. Disturbance of iron metabolism as a contributing factor to SN hyperechogenicity in Parkinson’s disease: Implications for idiopathic and monogenetic forms. Neurochem. Res. 2007, 32, 1646–1654. [Google Scholar] [CrossRef]
- Ullah, I.; Zhao, L.; Hai, Y.; Fahim, M.; Alwayli, D.; Wang, X.; Li, H. Metal elements and pesticides as risk factors for Parkinson’s disease—A review. Toxicol. Rep. 2021, 8, 607–616. [Google Scholar] [CrossRef]
- Yıldırım, İ.; Altunç, A.T.; Gür, E.; Hacikurteş, G.; Usta Sağlam, N.G.; Kızıltan, G.; Turan, Ş. C19orf12 gene mutation with neuropsychiatric symptoms: A case report. Neurocase 2024, 30, 156–158. [Google Scholar] [CrossRef]
- Garcia-Malo, C.; Wanner, V.; Miranda, C.; Romero Peralta, S.; Agudelo, L.; Cano-Pumarega, I.; Granizo, J.J.; Garcia-Borreguero, D. Quantitative transcranial sonography of the substantia nigra as a predictor of therapeutic response to intravenous iron therapy in restless legs syndrome. Sleep. Med. 2020, 66, 123–129. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Milanowski, Ł.M.; Lindemann, J.A.; Hoffman-Zacharska, D.; Soto-Beasley, A.I.; Barcikowska, M.; Boczarska-Jedynak, M.; Deutschlander, A.; Kłodowska, G.; Dulski, J.; Fedoryshyn, L.; et al. Frequency of mutations in PRKN, PINK1, and DJ1 in Patients With Early-Onset Parkinson Disease from neighboring countries in Central Europe. Park. Relat. Disord. 2021, 86, 48–51. [Google Scholar] [CrossRef]
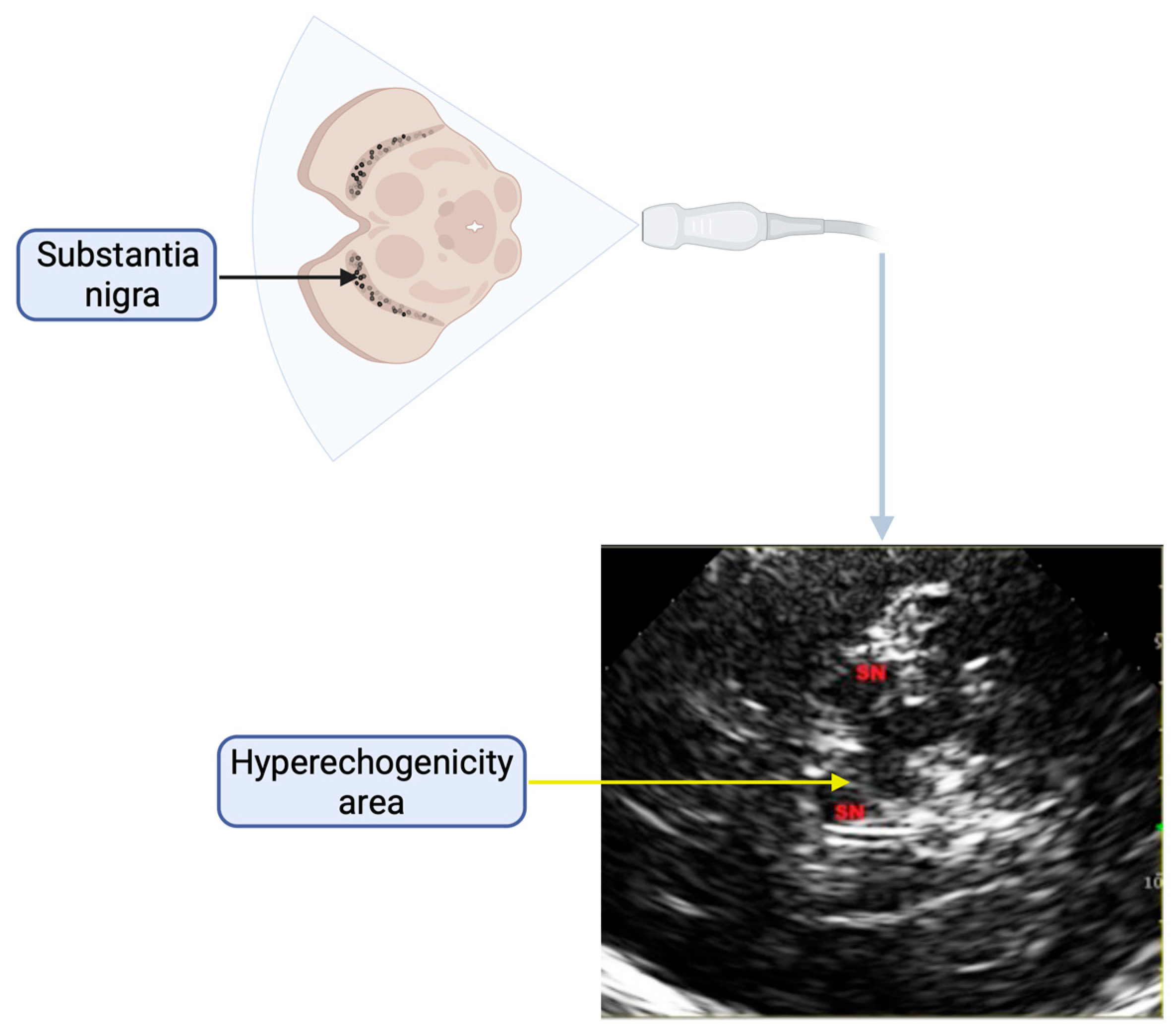
| Variable | Hyperechogenicity (N = 48) | No Hyperechogenicity (N = 31) | p Value |
|---|---|---|---|
| Pathogenic mutations (LRRK2, DJ1, PRKN, PINK1, SNCA) | 1 (2.1%) | 2 (6.5%) | 0.558 |
| Gender (males) | 33 (67.4%) | 15 (50%) | 0.157 |
| Family history | 11 (73.3%) | 8 (57.1%) | 0.450 |
| Dyskinesia | 5 (33.3%) | 4 (28.6%) | 1.000 |
| Fluctuations | 6 (40%) | 4 (28.6%) | 1.000 |
| Tobacco smoking | 6 (40%) | 7 (50%) | 0.819 |
| Alcohol | 3 (20%) | 2 (14.3%) | 1.000 |
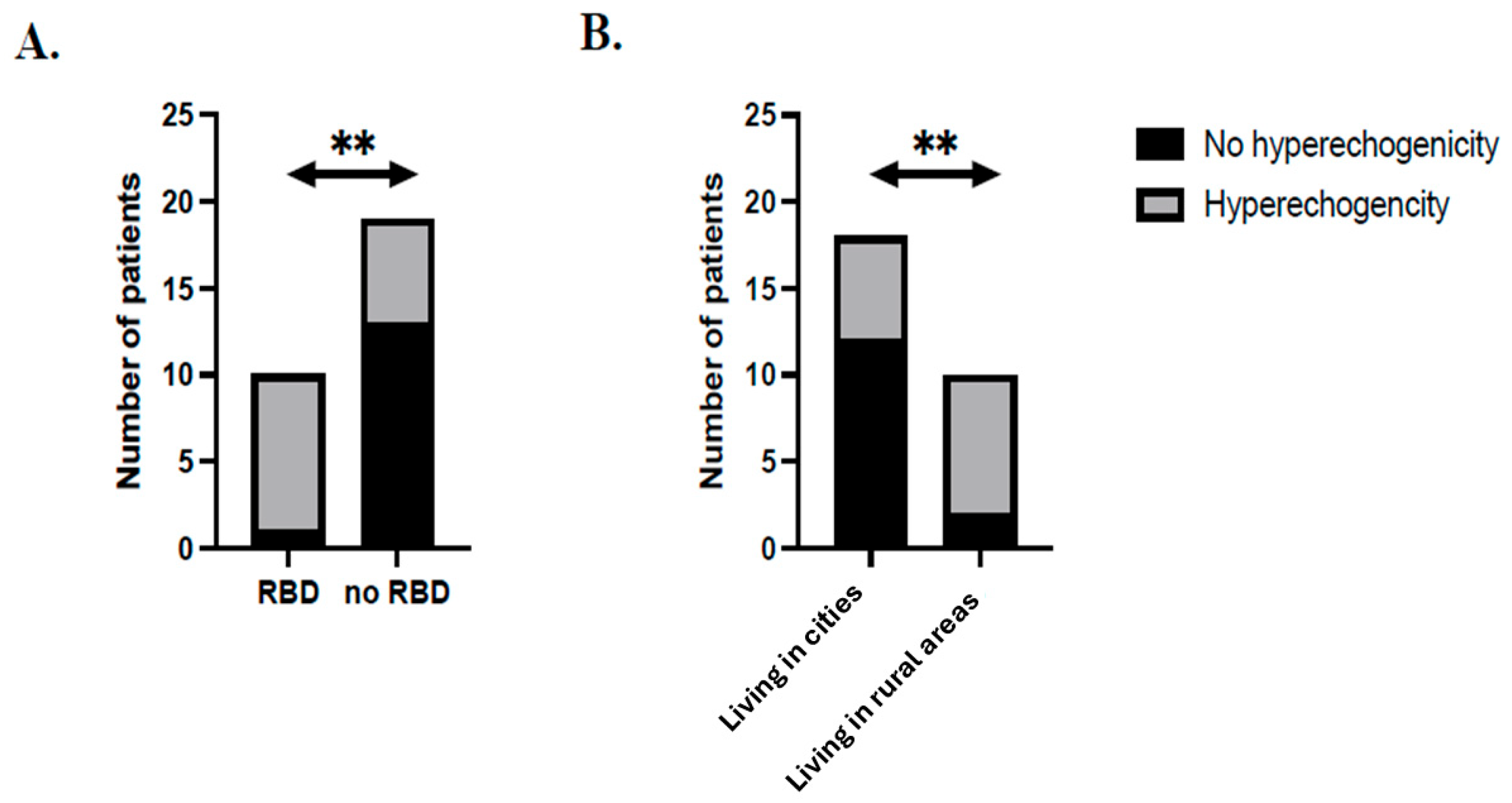
| Place of residence (city) | 6 (42.9%) | 12 (85.7%) | 0.020 |
| RLS | 5 (33.3%) | 4 (28.6%) | 1.000 |
| Depression | 3 (20%) | 5 (35.7%) | 0.678 |
| Dementia | 1 (6.7%) | 2 (14.3%) | 1.000 |
| Dysarthria | 5 (33.3%) | 7 (50%) | 0.704 |
| Dysphagia | 4 (26.7%) | 2 (14.3%) | 0.420 |
| RBD | 9 (60%) | 1 (7.1%) | 0.004 |
| First symptom: | |||
| -Bradykinesia | 8 (53.3%) | 3 (21.4%) | 0.677 |
| -Rigidity | 11 (73.3%) | 4 (28.6%) | 0.656 |
| -Rest tremor | 9 (60%) | 6 (42.9%) | 0.435 |
| First treatment: | |||
| -Levodopa | 13 (86.7%) | 11 (78.6%) | 0.480 |
| -Other | 12 (80%) | 12 (85.7%) | 1.000 |
| Agonist usage (now) | 1 (6.7%) | 4 (28.6%) | 0.327 |
| Age of onset (years) | 51.5 ± 13.7 | 40.9 ± 13.1 | 0.040 |
| Age at study (years) | 58.2 ± 13.4 | 48.3 ± 11.4 | 0.040 |
| Disease duration (years) | 6.8 ± 3.8 | 7.5 ± 7.7 | 0.730 |
| Gene | Variant | cDNA HGVS # | HGVS Protein | HGMD Path. Class. | ClinVar * Path. Class. | ACMG Class. |
|---|---|---|---|---|---|---|
| PRKN | Ex2 del | c.(?_40)_(109_?)del | p.? | DM | pathogenic | - |
| Ex4 del | c.(?_440)_(534_?)del | p.? | DM | pathogenic | - | |
| p.Glu79Ter | c.235G>T | p.Glu79Ter | DM | - | LPath | |
| LRRK2 | p.Asn1437His | c.4309A>C | p.Asn1437His | DM | not provided | VUS/LPath |
| Patients’ genotypes | ||||||
| PRKN | c.[(?-40)_(109_?)del];[(?-440_534_?)del]; p.[?];[?] | |||||
| PRKN | c.[(?-40)_(109_?)del];[235G>T]; p.[?];[(Glu79Ter)] | |||||
| LRRK2 | c.[4309A>C];[=]; p.[(Asn1437His)];[=] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milanowski, Ł.; Szukało, P.; Kowalska, M.; Sikorska, A.; Hoffman-Zacharska, D.; Koziorowski, D. Clinical and Genetic Characteristics of Parkinson’s Disease Patients with Substantia Nigra Hyperechogenicity. Int. J. Mol. Sci. 2025, 26, 5492. https://doi.org/10.3390/ijms26125492
Milanowski Ł, Szukało P, Kowalska M, Sikorska A, Hoffman-Zacharska D, Koziorowski D. Clinical and Genetic Characteristics of Parkinson’s Disease Patients with Substantia Nigra Hyperechogenicity. International Journal of Molecular Sciences. 2025; 26(12):5492. https://doi.org/10.3390/ijms26125492
Chicago/Turabian StyleMilanowski, Łukasz, Piotr Szukało, Małgorzata Kowalska, Alicja Sikorska, Dorota Hoffman-Zacharska, and Dariusz Koziorowski. 2025. "Clinical and Genetic Characteristics of Parkinson’s Disease Patients with Substantia Nigra Hyperechogenicity" International Journal of Molecular Sciences 26, no. 12: 5492. https://doi.org/10.3390/ijms26125492
APA StyleMilanowski, Ł., Szukało, P., Kowalska, M., Sikorska, A., Hoffman-Zacharska, D., & Koziorowski, D. (2025). Clinical and Genetic Characteristics of Parkinson’s Disease Patients with Substantia Nigra Hyperechogenicity. International Journal of Molecular Sciences, 26(12), 5492. https://doi.org/10.3390/ijms26125492






