The Anatomical and Evolutionary Impact of Pain, Pleasure, Motivation, and Cognition: Integrating Energy Metabolism and the Mind–Body BERN (Behavior, Exercise, Relaxation, and Nutrition) Framework
Abstract
1. Introduction
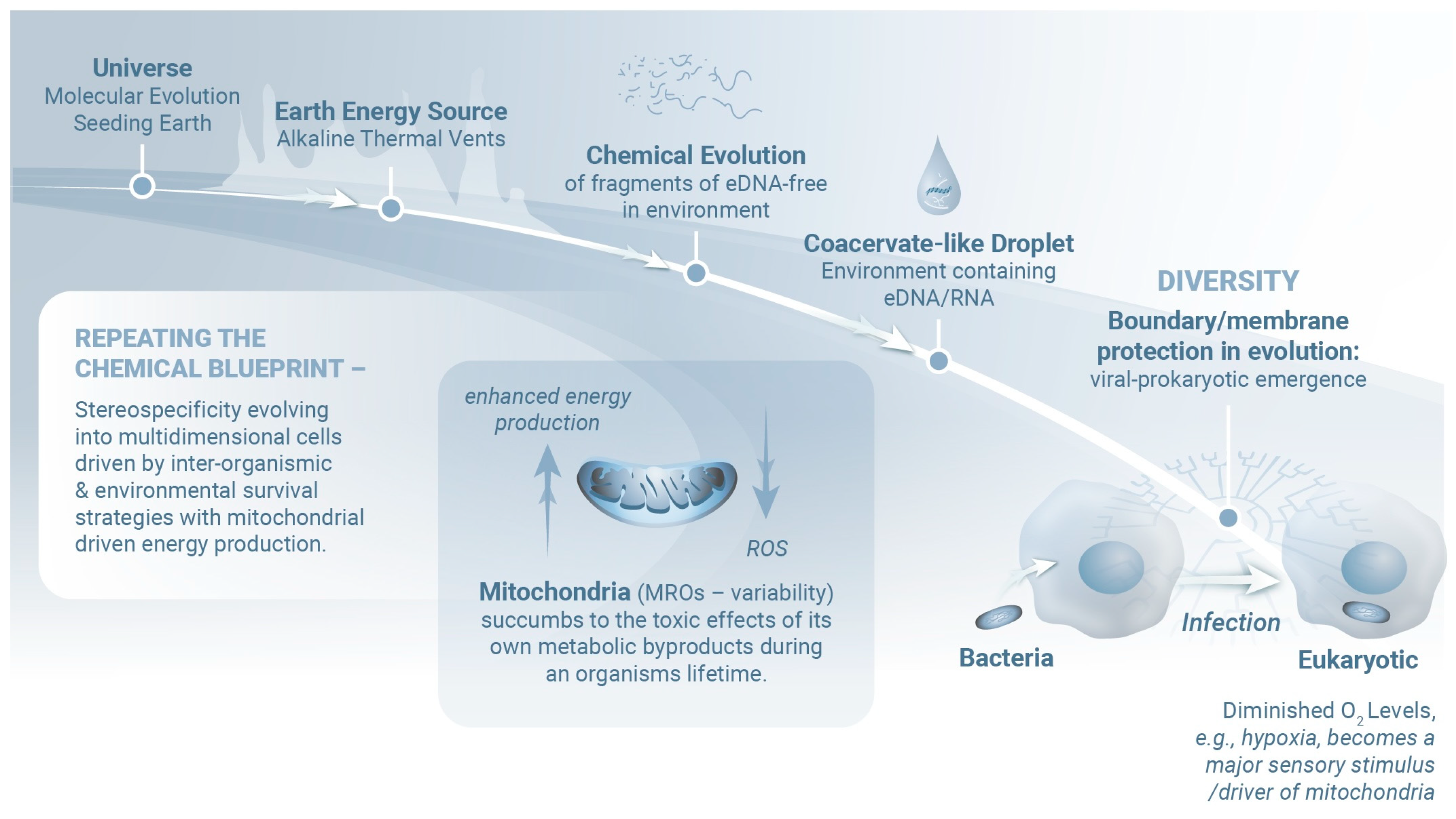
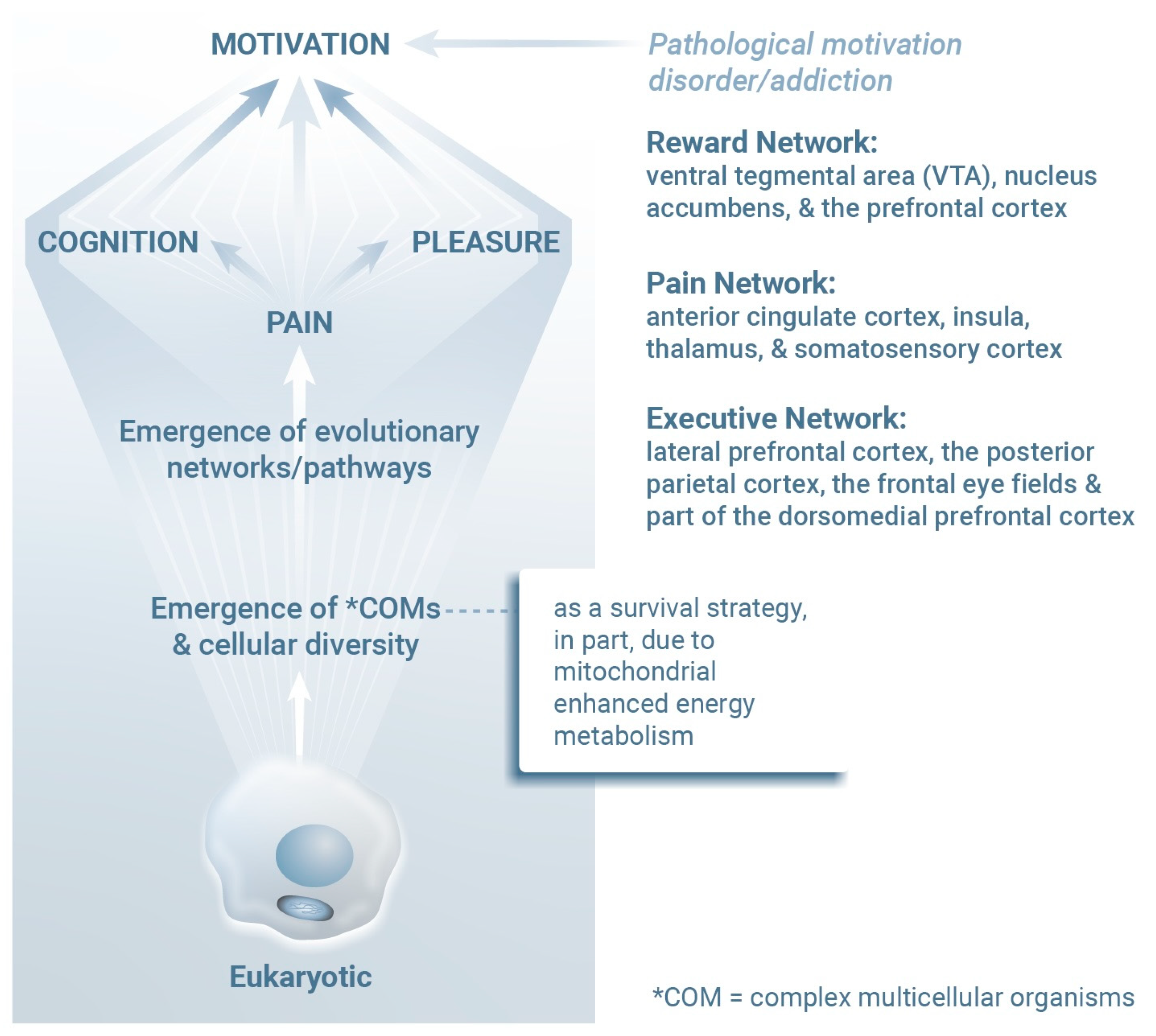
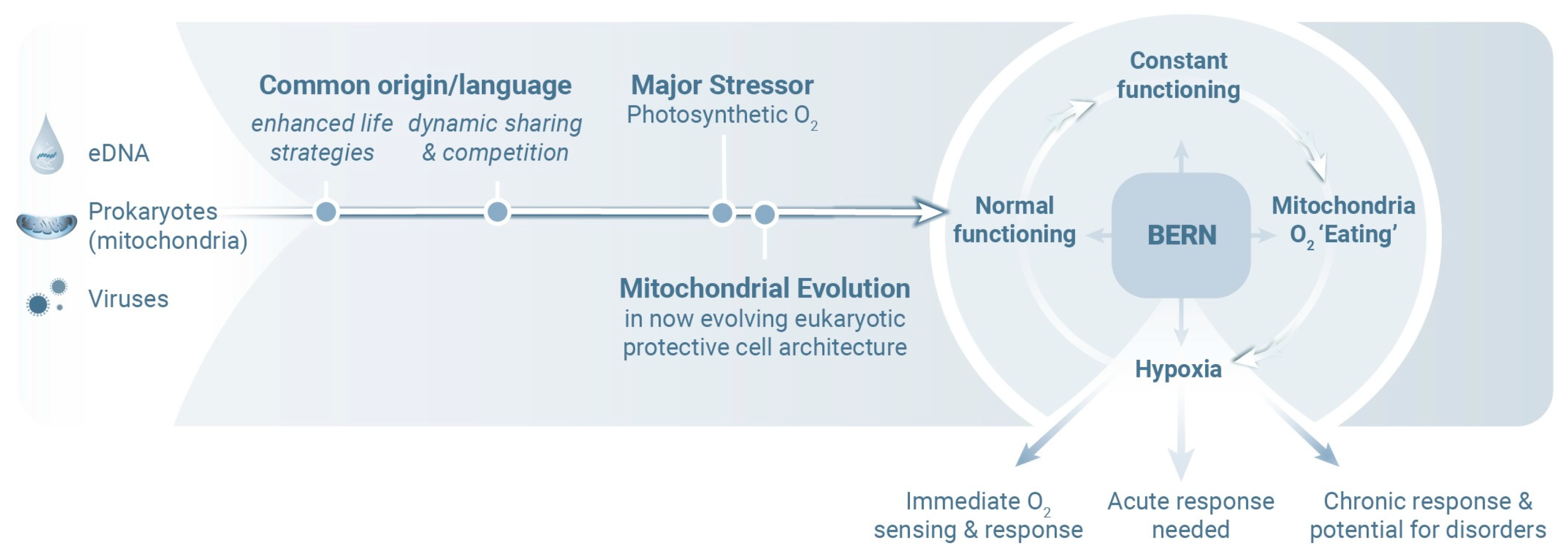
2. Molecular Mind–Body Platform
3. Psychiatric Ramifications
4. Evolutionary Efficiency
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADP | adenosine diphosphate |
| ATP | adenosine triphosphate |
| BERN | Behavior, Exercise, Relaxation, and Nutrition |
| DNA | deoxyribonucleic acid |
| eDNARNA | environmental DNAribonucleic acid |
| MROs | mitochondrion-related organelles |
| CNS | central nervous system |
| VTA | ventral tegmental area |
| NAc | nucleus accumbens |
| PFC | prefrontal cortex |
| ACC | anterior cingulate cortex |
| LPFC | lateral prefrontal cortex |
| PCC | posterior cingulate cortex |
| PPC | posterior parietal cortex |
| dmPFC | dorsomedial prefrontal cortex |
| HIF | hypoxia-inducible factor |
| ROSs | reactive oxygen species |
References
- Stefano, G.B.; Kream, R.M. Hypoxia defined as a common culprit/initiation factor in mitochondrial-mediated proinflammatory processes. Med. Sci. Monit. 2015, 21, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- McCoy, T.J.; Russell, S.S.; Zega, T.J.; Thomas-Keprta, K.L.; Singerling, S.A.; Brenker, F.E.; Timms, N.E.; Rickard, W.D.A.; Barnes, J.J.; Libourel, G.; et al. An evaporite sequence from ancient brine recorded in Bennu samples. Nature 2025, 637, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Dworkin, J.P.; Alexander, C.M.O.D.; Aponte, J.C.; Baczynski, A.A.; Barnes, J.J.; Bechtel, H.A.; Berger, E.L.; Burton, A.S.; Caselli, P.; et al. Abundant ammonia and nitrogen-rich soluble organic matter in samples from asteroid (101955) Bennu. Nat. Astron. 2025, 9, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2022, 23, 159–173. [Google Scholar] [CrossRef] [PubMed]
- López-Armada, M.J.; Riveiro-Naveira, R.R.; Vaamonde-García, C.; Valcárcel-Ares, M.N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion 2013, 13, 106–118. [Google Scholar] [CrossRef]
- Van Horssen, J.; Van Schaik, P.; Witte, M. Inflammation and mitochondrial dysfunction: A vicious circle in neurodegenerative disorders? Neurosci. Lett. 2017, 710, 132931. [Google Scholar] [CrossRef]
- Stefano, G.B.; Esch, T.; Kream, R.M. Behaviorally-mediated entrainment of whole-body metabolic processes: Conservation and evolutionary development of mitochondrial respiratory complexes. Med. Sci. Monit. 2019, 25, 9306–9309. [Google Scholar] [CrossRef]
- Stefano, G.B.; Esch, T.; Kream, R.M. Augmentation of Whole-Body Metabolic Status by Mind-Body Training: Synchronous Integration of Tissue- and Organ-Specific Mitochondrial Function. Med. Sci. Monit. Basic Res. 2019, 25, 8–14. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Hartmann, G.; Tschöp, M.; Fischer, R.; Bidlingmaier, C.; Riepl, R.; Tschöp, K.; Hautmann, H.; Endres, S.; Toepfer, M. High Altitude Increases Circulating Interleukin-6, Interleukin-1 Receptor Antagonist and C-Reactive Protein. Cytokine 2000, 12, 246–252. [Google Scholar] [CrossRef]
- Palazon, A.; Goldrath, A.W.; Nizet, V.; Johnson, R.S. HIF transcription factors, inflammation, and immunity. Immunity 2014, 41, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-T.; Huang, L.-T.; Chen, C.-C.; Chen, C.-M. Molecular mechanisms underlying hyperoxia-induced lung fibrosis. Pediatr. Neonatol. 2022, 63, 109–116. [Google Scholar] [CrossRef]
- Bakare, A.B.; Lesnefsky, E.J.; Iyer, S. Leigh Syndrome: A tale of two genomes. Front. Physiol. 2021, 12, 693734. [Google Scholar] [CrossRef]
- van de Wal, M.A.E.; Adjobo-Hermans, M.J.W.; Keijer, J.; Schirris, T.J.J.; Homberg, J.R.; Wieckowski, M.R.; Grefte, S.; Van Schothorst, E.M.; Van Karnebeek, C.; Quintana, A.; et al. Ndufs4 knockout mouse models of Leigh syndrome: Pathophysiology and intervention. Brain 2021, 145, 45–63. [Google Scholar] [CrossRef]
- Jain, I.H.; Zazzeron, L.; Goli, R.; Alexa, K.; Schatzman-Bone, S.; Dhillon, H.; Goldberger, O.; Peng, J.; Shalem, O.; Sanjana, N.E.; et al. Hypoxia as a therapy for mitochondrial disease. Science 2016, 352, 54–61. [Google Scholar] [CrossRef]
- Ferrari, M.; Jain, I.H.; Goldberger, O.; Rezoagli, E.; Thoonen, R.; Cheng, K.-H.; Sosnovik, D.E.; Scherrer-Crosbie, M.; Mootha, V.K.; Zapol, W.M. Hypoxia treatment reverses neurodegenerative disease in a mouse model of Leigh syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, E4241–E4250. [Google Scholar] [CrossRef]
- Finsterer, J.; Zarrouk-Mahjoub, S. Psychosis in Leigh syndrome. Asian J. Psychiatry 2019, 41, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Jaballah, F.; Nouira, R.B.S.; Mallouli, S.; Boussaid, H.; Younes, S.; Zarrouk, L.; Younes, S. Schizophrenia-Like psychotic symptoms associated to Leigh Syndrome. Case Rep. Psychiatry 2023, 2023, 8886555. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B.; Kream, R.M. Primordial biochemicals within coacervate-Like droplets and the origins of life. Viruses 2025, 17, 146. [Google Scholar] [CrossRef]
- Záhonová, K.; Treitli, S.C.; Le, T.; Škodová-Sveráková, I.; Hanousková, P.; Čepička, I.; Tachezy, J.; Hampl, V. Anaerobic derivates of mitochondria and peroxisomes in the free-living amoeba Pelomyxa schiedti revealed by single-cell genomics. BMC Biol. 2022, 20, 56. [Google Scholar] [CrossRef]
- Schavemaker, P.E.; Muñoz-Gómez, S.A. The role of mitochondrial energetics in the origin and diversification of eukaryotes. Nat. Ecol. Evol. 2022, 6, 1307–1317. [Google Scholar] [CrossRef]
- Muñoz-Gómez, S.A.; Susko, E.; Williamson, K.; Eme, L.; Slamovits, C.H.; Moreira, D.; López-García, P.; Roger, A.J. Site-and-branch-heterogeneous analyses of an expanded dataset favour mitochondria as sister to known Alphaproteobacteria. Nat. Ecol. Evol. 2022, 6, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Stephens, O.R.; Grant, D.; Frimel, M.; Wanner, N.; Yin, M.; Willard, B.; Erzurum, S.C.; Asosingh, K. Characterization and origins of cell-free mitochondria in healthy murine and human blood. Mitochondrion 2020, 54, 102–112. [Google Scholar] [CrossRef]
- Miliotis, S.; Nicolalde, B.; Ortega, M.; Yepez, J.; Caicedo, A. Forms of extracellular mitochondria and their impact in health. Mitochondrion 2019, 48, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Dache, Z.A.A.; Thierry, A.R. Mitochondria-derived cell-to-cell communication. Cell Rep. 2023, 42, 112728. [Google Scholar] [CrossRef] [PubMed]
- Dache, Z.A.A.; Otandault, A.; Tanos, R.; Pastor, B.; Meddeb, R.; Sanchez, C.; Arena, G.; Lasorsa, L.; Bennett, A.; Grange, T.; et al. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 2020, 34, 3616–3630. [Google Scholar] [CrossRef]
- Park, J.-H.; Hayakawa, K. Extracellular mitochondria signals in CNS disorders. Front. Cell Dev. Biol. 2021, 9, 642853. [Google Scholar] [CrossRef]
- Raval, P.K.; Garg, S.G.; Gould, S.B. Endosymbiotic selective pressure at the origin of eukaryotic cell biology. eLife 2022, 11, e81033. [Google Scholar] [CrossRef]
- Mattson, M.P.; Gleichmann, M.; Cheng, A. Mitochondria in neuroplasticity and neurological disorders. Neuron 2008, 60, 748–766. [Google Scholar] [CrossRef]
- Johri, A.; Beal, M.F. Mitochondrial dysfunction in neurodegenerative diseases. J. Pharmacol. Exp. Ther. 2012, 342, 619–630. [Google Scholar] [CrossRef]
- Harris, J.J.; Jolivet, R.; Attwell, D. Synaptic energy use and supply. Neuron 2012, 75, 762–777. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; McEwen, B.S.; Epel, E.S.; Sandi, C. An energetic view of stress: Focus on mitochondria. Front. Neuroendocrinol. 2018, 49, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Ma, Y.; Chung, S. Mitochondrial dysfunction in psychiatric disorders. Schizophr. Res. 2024, 273, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Wallace, D.C. Mitochondrial etiology of Neuropsychiatric disorders. Biol. Psychiatry 2017, 83, 722–730. [Google Scholar] [CrossRef]
- Wei, W.; Cheng, B.; Zhao, Y.; He, D.; Chu, X.; Qin, X.; Zhang, N.; Shi, S.; Cai, Q.; Hui, J.; et al. Exploring the Interplay between Mitochondrial DNA and Lifestyle Factors in the Pathogenesis of Psychiatric Disorders. Depress. Anxiety 2024, 2024, 4914777. [Google Scholar] [CrossRef]
- Esch, T.; Fricchione, G.L.; Stefano, G.B. The therapeutic use of the relaxation response in stress-related diseases. Med. Sci. Monit. 2003, 9, RA23–RA34. [Google Scholar]
- Balban, M.Y.; Neri, E.; Kogon, M.M.; Weed, L.; Nouriani, B.; Jo, B.; Holl, G.; Zeitzer, J.M.; Spiegel, D.; Huberman, A.D. Brief structured respiration practices enhance mood and reduce physiological arousal. Cell Rep. Med. 2023, 4, 100895. [Google Scholar] [CrossRef]
- Cavanagh, M.; Cope, T.; Smith, D.; Tolley, I.; Orrock, P.; Vaughan, B. The effectiveness of an osteopathic manual technique compared with a breathing exercise on vagal tone as indicated by heart rate variability, a crossover study. J. Bodyw. Mov. Ther. 2024, 38, 449–453. [Google Scholar] [CrossRef]
- Herawati, I.; Ludin, A.F.M.; M, M.; Ishak, I.; Farah, N.M.F. Breathing exercise for hypertensive patients: A scoping review. Front. Physiol. 2023, 14, 1048338. [Google Scholar] [CrossRef]
- Leknes, S.; Tracey, I. A common neurobiology for pain and pleasure. Nat. Rev. Neurosci. 2008, 9, 314–320. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Ferguson, S.J. Bioenergetics, 4th ed.; University of Oxford: Oxford, UK, 2013. [Google Scholar] [CrossRef]
- Cheng, A.; Hou, Y.; Mattson, M.P. Mitochondria and neuroplasticity. ASN Neuro 2010, 2, e00045. [Google Scholar] [CrossRef] [PubMed]
- Manji, H.; Kato, T.; Di Prospero, N.A.; Ness, S.; Beal, M.F.; Krams, M.; Chen, G. Impaired mitochondrial function in psychiatric disorders. Nat. Rev. Neurosci. 2012, 13, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and Cancer: A Dawn for Evolutionary Medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.I.; Neumeister, M.W. Pain: Pathways and Physiology. Clin. Plast. Surg. 2020, 47, 173–180. [Google Scholar] [CrossRef]
- Geuter, S.; Reynolds Losin, E.A.; Roy, M.; Atlas, L.Y.; Schmidt, L.; Krishnan, A.; Koban, L.; Wager, T.D.; Lindquist, M.A. Multiple Brain Networks Mediating Stimulus-Pain Relationships in Humans. Cereb. Cortex 2020, 30, 4204–4219. [Google Scholar] [CrossRef]
- Lewis, R.G.; Florio, E.; Punzo, D.; Borrelli, E. The Brain’s Reward System in Health and Disease. In Circadian Clock in Brain Health and Disease; Engmann, O., Brancaccio, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 1344, pp. 57–69. [Google Scholar]
- Park, H.J.; Friston, K. Structural and functional brain networks: From connections to cognition. Science 2013, 342, 1238411. [Google Scholar] [CrossRef]
- Cohen, J.R.; D’Esposito, M. The Segregation and Integration of Distinct Brain Networks and Their Relationship to Cognition. J. Neurosci. 2016, 36, 12083–12094. [Google Scholar] [CrossRef]
- Baik, J.-H. Stress and the dopaminergic reward system. Exp. Mol. Med. 2020, 52, 1879–1890. [Google Scholar] [CrossRef]
- Seeley, W.W. Behavioral Variant Frontotemporal Dementia. Continuum 2019, 25, 76–100. [Google Scholar] [CrossRef]
- Lewis, R.G.; Florio, E.; Punzo, D.; Borrelli, E. The brain’s reward system in health and disease. Adv. Exp. Med. Biol. 2021, 1344, 57–69. [Google Scholar] [CrossRef]
- Horsburgh, A.; Summers, S.J.; Lewis, A.; Keegan, R.J.; Flood, A. The Relationship Between Pain and Interoception: A Systematic Review and Meta-Analysis. J. Pain. 2024, 25, 104476. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Čeko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Kummer, K.; Sheets, P.L. Targeting PFC dysfunction in pain. J. Pharmacol. Exp. Ther. 2024, 389, 268–276. [Google Scholar] [CrossRef]
- Yun, A.J.; Lee, P.Y.; Doux, J.D.; Conley, B.R. A general theory of evolution based on energy efficiency: Its implications for diseases. Med. Hypotheses 2005, 66, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M. The bioenergetic cost of building a metazoan. Proc. Natl. Acad. Sci. USA 2024, 121, e2414742121. [Google Scholar] [CrossRef]
- Attwell, D.; Laughlin, S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001, 21, 1133–1145. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. A cellular perspective on brain energy metabolism and functional imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef]
- Lennie, P. The cost of cortical computation. Curr. Biol. 2003, 13, 493–497. [Google Scholar] [CrossRef]
- Erecińska, M.; Silver, I.A. Tissue oxygen tension and brain sensitivity to hypoxia. Respir. Physiol. 2001, 128, 263–276. [Google Scholar] [CrossRef]
- Esch, T.; Stefano, G.B.; Michaelsen, M.M. The foundations of mind-body medicine: Love, good relationships, and happiness modulate stress and promote health. Stress Health 2024, 40, e3387. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, A.L. Mind-Body Medicine: A Special Health Report; Harvard Health Publications: Boston, MA, USA, 2001. [Google Scholar]
- Esch, T. The Neurobiology of Meditation and Mindfulness. In Meditation—Neuroscientific Approaches and Philosophical Implications. Studies in Neuroscience, Consciousness and Spirituality; Schmidt, S., Walach, H., Eds.; Springer International Publishing: Cham, Switzerland, 2014; Volume 2, pp. 153–173. [Google Scholar] [CrossRef]
- Michaelsen, M.M.; Esch, T. Motivation and reward mechanisms in health behavior change processes. Brain Res. 2021, 1757, 147309. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, E.M. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006, 6, 318–328. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Chavan, S.S.; Tracey, K.J. Molecular and functional neuroscience in immunity. Annu. Rev. Immunol. 2018, 36, 783–812. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Choi, D.-K. Hypoxia Inducible factor pathway and physiological adaptation: A cell survival pathway? Mediat. Inflamm. 2015, 2015, 584758. [Google Scholar] [CrossRef]
- Burtscher, J.; Hohenauer, E.; Burtscher, M.; Millet, G.P.; Egg, M. Environmental and behavioral regulation of HIF-mitochondria crosstalk. Free. Radic. Biol. Med. 2023, 206, 63–73. [Google Scholar] [CrossRef]
- Esch, T.; Stefano, G.B. The BERN Framework of Mind-Body Medicine: Integrating Self-Care, Health Promotion, Resilience, and Applied Neuroscience. Front. Integr. Neurosci. 2022, 16, 913573. [Google Scholar] [CrossRef]
- Esch, T. Integrating OpenNotes and Promoting Self-Management in Primary Care in Germany: The Witten Model. The BMJ (Opinion) 2021. Available online: https://blogs.bmj.com/bmj/2021/04/01/tobias-esch-integrating-opennotes-and-promoting-self-management-in-primary-care-in-germany-the-witten-model (accessed on 1 June 2025).
- Esch, T. Der Nutzen von Selbstheilungspotenzialen in der professionellen Gesundheitsfürsorge am Beispiel der Mind-Body-Medizin. Bundesgesundheitsblatt–Gesundheitsforschung–Gesundheitsschutz 2020, 63, 577–585. [Google Scholar] [CrossRef]
- Esch, T.; Kream, R.M.; Stefano, G.B. Chromosomal processes in mind-body medicine: Chronic stress, cell aging, and telomere length. Med. Sci. Monit. Basic Res. 2018, 24, 134–140. [Google Scholar] [CrossRef]
- Esch, T.; Stefano, G.B. The neurobiology of stress management. Neuro Endocrinol. Lett. 2010, 31, 19–39. [Google Scholar]
- Esch, T. Gesund im Stress: Der Wandel des Stresskonzeptes und seine Bedeutung für Prävention, Gesundheit und Lebensstil. Das. Gesundheitswesen 2002, 64, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Uhl, J.; Schönfeld, S.; Meyer, L.; Reus, A.; Neumann, C.; Langer, L.; Michaelsen, M.M.; Esch, T. (Digital) Mind-Body Intervention to Promote Health and Subjective Well-Being of Residents in Nursing Homes: A Cluster-Randomized Controlled Pilot Study. Das. Gesundheitswesen 2025. [Google Scholar] [CrossRef] [PubMed]
- Kohls, N.; Esch, T.; Gerber, L.; Adrian, L.; Wittmann, M. Mindfulness meditation and fantasy relaxation in a group setting leads to a diminished sense of self and an increased present orientation. Behav. Sci. 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Möltner, H.; Leve, J.; Esch, T. Burnout-Prävention und mobile Achtsamkeit: Evaluation eines appbasierten Gesundheitstrainings bei Berufstätigen. Das. Gesundheitswesen 2017, 57, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Gimpel, C.; Von Scheidt, C.; Jose, G.; Sonntag, U.; Stefano, G.B.; Michalsen, A.; Esch, T. Changes and interactions of flourishing, mindfulness, sense of coherence, and quality of life in patients of a mind-body medicine outpatient clinic. Complement. Med. Res. 2014, 21, 154–162. [Google Scholar] [CrossRef]
- Schnieder, S.; Stappert, S.; Takahashi, M.; Fricchione, G.L.; Esch, T.; Krajewski, J. Sustainable reduction of sleepiness through salutogenic self-care procedure in lunch breaks: A pilot study. Evid.-Based Complement. Altern. Med. 2013, 2013, 387356. [Google Scholar] [CrossRef]
- Esch, T.; Jose, G.; Gimpel, C.; Von Scheidt, C.; Michalsen, A. Die Flourishing Scale (FS) von Diener et al. liegt jetzt in einer autorisierten deutschen Fassung (FS-D) vor: Einsatz bei einer Mind-Body-medizinischen Fragestellung. Complement. Med. Res. 2013, 20, 267–275. [Google Scholar] [CrossRef]
- Esch, T.; Sonntag, U.; Esch, S.M.; Thees, S. Stress Management and Mind-Body Medicine: A randomized controlled longitudinal evaluation of students’ health and effects of a behavioral group intervention at a middle-size German university (SM-MESH). Complement. Med. Res. 2013, 20, 129–137. [Google Scholar] [CrossRef]
- Feicht, T.; Wittmann, M.; Jose, G.; Mock, A.; Von Hirschhausen, E.; Esch, T. Evaluation of a Seven-Week Web-Based Happiness Training to Improve Psychological Well-Being, Reduce Stress, and Enhance Mindfulness and Flourishing: A Randomized Controlled Occupational Health Study. Evid.-Based Complement. Altern. Med. 2013, 2013, 676953. [Google Scholar] [CrossRef]
- Esch, T.; Duckstein, J.; Welke, J.; Braun, V. Mind/body techniques for physiological and psychological stress reduction: Stress management via Tai Chi training—A pilot study. Med. Sci. Monit. 2007, 13, CR488–CR497. [Google Scholar]
- Michalsen, A.; Grossman, P.; Acil, A.; Langhorst, J.; Lüdtke, R.; Esch, T.; Stefano, G.B.; Dobos, G.J. Rapid stress reduction and anxiolysis among distressed women as a consequence of a three-month intensive yoga program. Med. Sci. Monit. 2005, 11, CR555–CR561. [Google Scholar]
- Picard, M.; McEwen, B.S. Psychological Stress and Mitochondria: A Systematic Review. Psychosom. Med. 2018, 80, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S. Mitochondria as signaling organelles. BMC Biol. 2014, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, G. The mitochondrial integrated stress response: A novel approach to anti-aging and pro-longevity. Ageing Res. Rev. 2025, 103, 102603. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Daniels, T.E.; Olsen, E.M.; Tyrka, A.R. Stress and Psychiatric Disorders: The role of mitochondria. Annu. Rev. Clin. Psychol. 2020, 16, 165–186. [Google Scholar] [CrossRef]
- Andreazza, A.C.; Shao, L.; Wang, J.-F.; Young, L.T. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch. Gen. Psychiatry 2010, 67, 360–368. [Google Scholar] [CrossRef]
- Scaini, G.; Mason, B.L.; Diaz, A.P.; Jha, M.K.; Soares, J.C.; Trivedi, M.H.; Quevedo, J. Dysregulation of mitochondrial dynamics, mitophagy and apoptosis in major depressive disorder: Does inflammation play a role? Mol. Psychiatry 2021, 27, 1095–1102. [Google Scholar] [CrossRef]
- Ye, J.; Duan, C.; Han, J.; Chen, J.; Sun, N.; Li, Y.; Yuan, T.; Peng, D. Peripheral mitochondrial DNA as a neuroinflammatory biomarker for major depressive disorder. Neural Regen. Res. 2024, 20, 1541–1554. [Google Scholar] [CrossRef]
- Roberts, R.C. Mitochondrial dysfunction in schizophrenia: With a focus on postmortem studies. Mitochondrion 2020, 56, 91–101. [Google Scholar] [CrossRef]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Kann, O.; Papageorgiou, I.E.; Draguhn, A. Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J. Cereb. Blood Flow Metab. 2014, 34, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sun, P.; Li, J.; Yang, Q.; Tian, Q.; Yuan, S.; Zhang, X.; Chen, P.; Li, C.; Zhang, X. Exploring genetic associations and drug targets for mitochondrial proteins and schizophrenia risk. Schizophrenia 2025, 11, 10. [Google Scholar] [CrossRef]
- Friston, K. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.; Kiebel, S. Predictive coding under the free-energy principle. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1211–1221. [Google Scholar] [CrossRef]
- Barlow, H.B. Possible principles underlying the transformation of sensory messages. In Sensory Communication; MIT Press: Cambridge, MA, USA, 1961; pp. 217–234. [Google Scholar]
- Rao, R.P.N.; Ballard, D.H. Predictive coding in the visual cortex: A functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 1999, 2, 79–87. [Google Scholar] [CrossRef]
- Keller, G.B.; Bonhoeffer, T.; Hübener, M. Sensorimotor mismatch signals in primary visual cortex of the behaving mouse. Neuron 2012, 74, 809–815. [Google Scholar] [CrossRef]
- Miall, R.C.; Wolpert, D.M. Forward models for physiological motor control. Neural Netw. 1996, 9, 1265–1279. [Google Scholar] [CrossRef]
- Shipp, S.; Adams, R.A.; Friston, K.J. Reflections on agranular architecture: Predictive coding in the motor cortex. Trends Neurosci. 2013, 36, 706–716. [Google Scholar] [CrossRef]
- Schultz, W.; Dayan, P.; Montague, P.R. A neural substrate of prediction and reward. Science 1997, 275, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Chanes, L.; Barrett, L.F. Redefining the role of limbic areas in cortical processing. Trends Cogn. Sci. 2015, 20, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, M.; Ploeger, A. Two different mismatches: Integrating the developmental and the Evolutionary-Mismatch hypothesis. Perspect. Psychol. Sci. 2022, 17, 1737–1745. [Google Scholar] [CrossRef]
- Bakhtiari, S. Energy efficiency as a normative account for predictive coding. Patterns 2022, 3, 100661. [Google Scholar] [CrossRef]
- Ficco, L.; Mancuso, L.; Manuello, J.; Teneggi, A.; Liloia, D.; Duca, S.; Costa, T.; Kovacs, G.Z.; Cauda, F. Disentangling predictive processing in the brain: A meta-analytic study in favour of a predictive network. Sci. Rep. 2021, 11, 16258. [Google Scholar] [CrossRef]
- Esch, T. The ABC model of happiness-neurobiological aspects of motivation and positive mood, and their dynamic changes through practice, the course of life. Biology 2022, 11, 843. [Google Scholar] [CrossRef]
- Mukandala, G.; Tynan, R.; Lanigan, S.; O’Connor, J. The effects of hypoxia and inflammation on synaptic signaling in the CNS. Brain Sci. 2016, 6, 6. [Google Scholar] [CrossRef]
- Fiskum, V.; Sandvig, A.; Sandvig, I. Silencing of Activity During Hypoxia Improves Functional Outcomes in Motor Neuron Networks in vitro. Front. Integr. Neurosci. 2021, 15, 792863. [Google Scholar] [CrossRef] [PubMed]
- Hencz, A.; Magony, A.; Thomas, C.; Kovacs, K.; Szilagyi, G.; Pal, J.; Sik, A. Mild hypoxia-induced structural and functional changes of the hippocampal network. Front. Cell. Neurosci. 2023, 17, 1277375. [Google Scholar] [CrossRef]
- Bustamante-Barrientos, F.A.; Luque-Campos, N.; Araya, M.J.; Lara-Barba, E.; De Solminihac, J.; Pradenas, C.; Molina, L.; Herrera-Luna, Y.; Utreras-Mendoza, Y.; Elizondo-Vega, R.; et al. Mitochondrial dysfunction in neurodegenerative disorders: Potential therapeutic application of mitochondrial transfer to central nervous system-residing cells. J. Transl. Med. 2023, 21, 613. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefano, G.B.; Buttiker, P.; Michaelsen, M.M.; Esch, T. The Anatomical and Evolutionary Impact of Pain, Pleasure, Motivation, and Cognition: Integrating Energy Metabolism and the Mind–Body BERN (Behavior, Exercise, Relaxation, and Nutrition) Framework. Int. J. Mol. Sci. 2025, 26, 5491. https://doi.org/10.3390/ijms26125491
Stefano GB, Buttiker P, Michaelsen MM, Esch T. The Anatomical and Evolutionary Impact of Pain, Pleasure, Motivation, and Cognition: Integrating Energy Metabolism and the Mind–Body BERN (Behavior, Exercise, Relaxation, and Nutrition) Framework. International Journal of Molecular Sciences. 2025; 26(12):5491. https://doi.org/10.3390/ijms26125491
Chicago/Turabian StyleStefano, George B., Pascal Buttiker, Maren M. Michaelsen, and Tobias Esch. 2025. "The Anatomical and Evolutionary Impact of Pain, Pleasure, Motivation, and Cognition: Integrating Energy Metabolism and the Mind–Body BERN (Behavior, Exercise, Relaxation, and Nutrition) Framework" International Journal of Molecular Sciences 26, no. 12: 5491. https://doi.org/10.3390/ijms26125491
APA StyleStefano, G. B., Buttiker, P., Michaelsen, M. M., & Esch, T. (2025). The Anatomical and Evolutionary Impact of Pain, Pleasure, Motivation, and Cognition: Integrating Energy Metabolism and the Mind–Body BERN (Behavior, Exercise, Relaxation, and Nutrition) Framework. International Journal of Molecular Sciences, 26(12), 5491. https://doi.org/10.3390/ijms26125491






