BAP31 Promotes Epithelial–Mesenchymal Transition Progression Through the Exosomal miR-423-3p/Bim Axis in Colorectal Cancer
Abstract
1. Introduction
2. Results
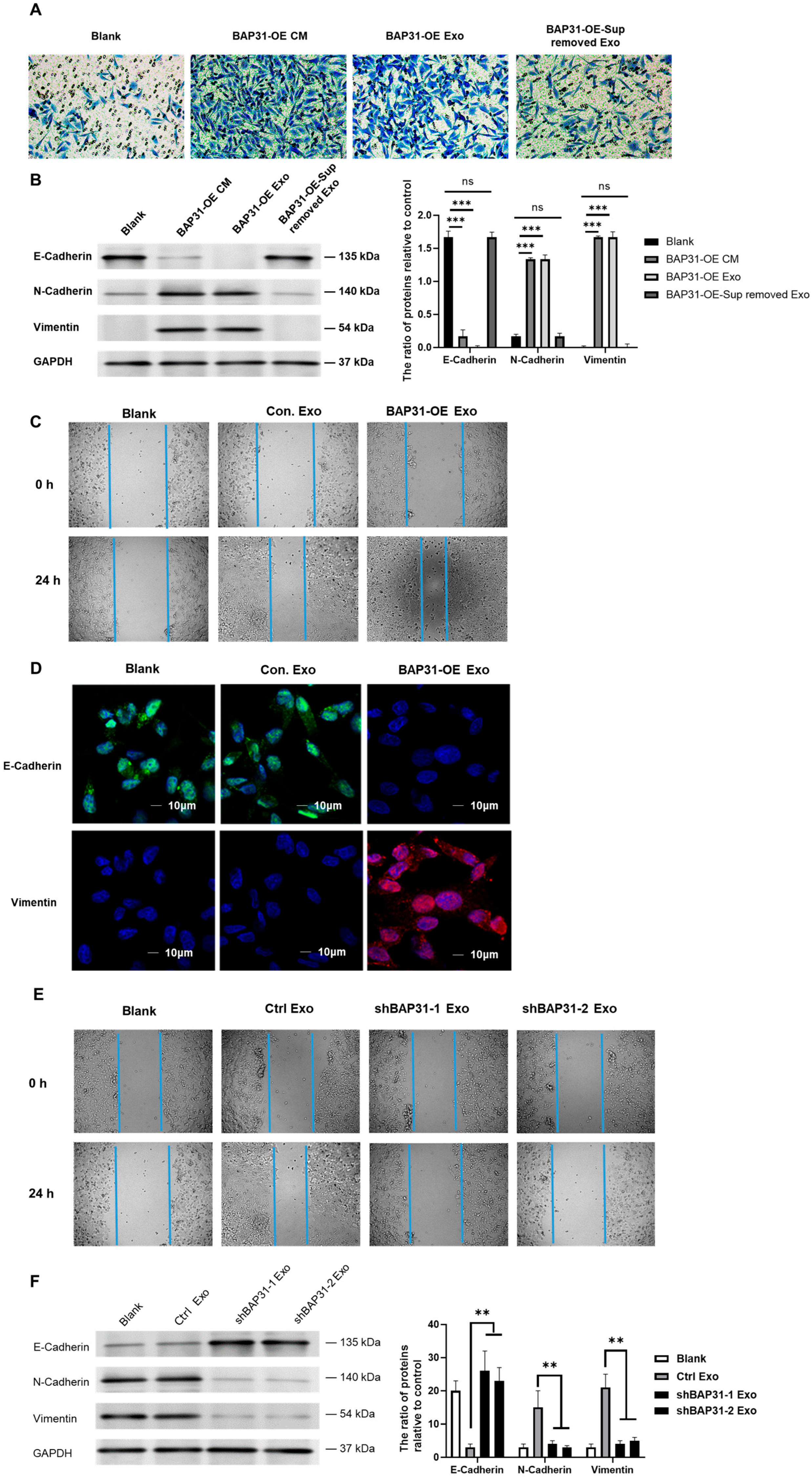
2.1. Exosomes from Cells with Elevated BAP31 Expression Enhance EMT in Recipient Cells
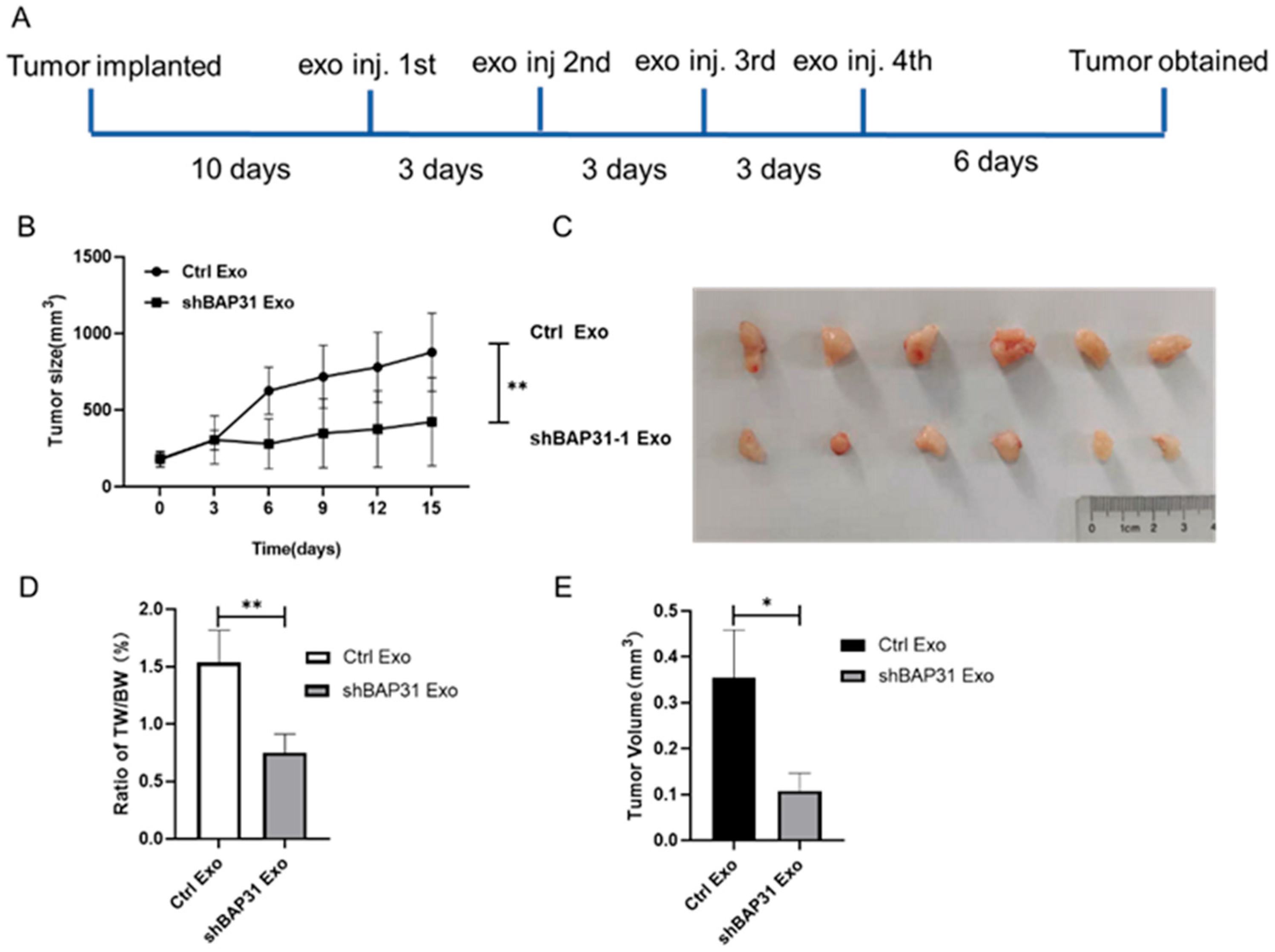
2.2. Exosomes from shBAP31 Cells Suppress Colorectal Cancer Progression
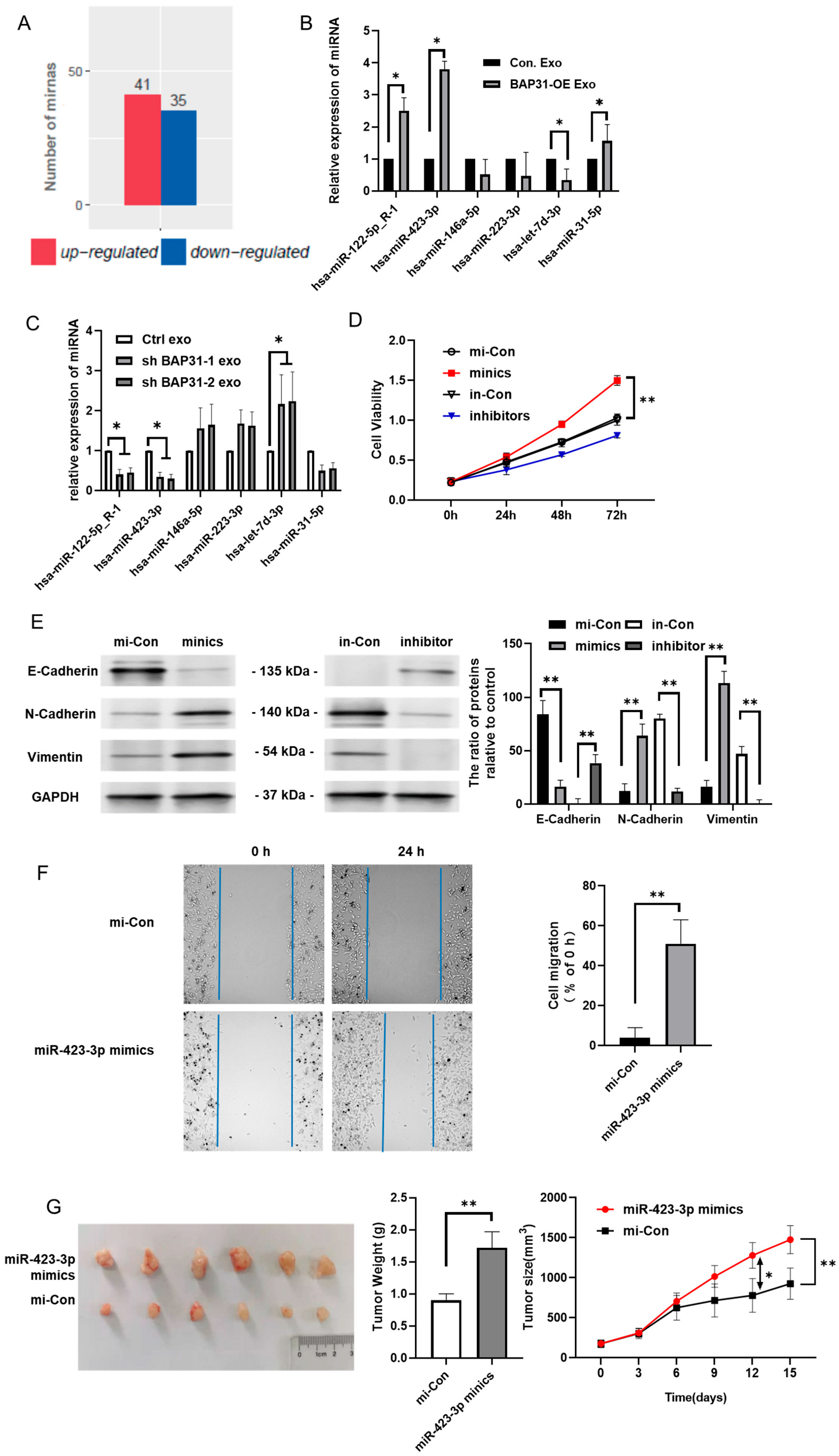
2.3. BAP31 Modulates miR-423-3p Within Exosomes Correlating with EMT
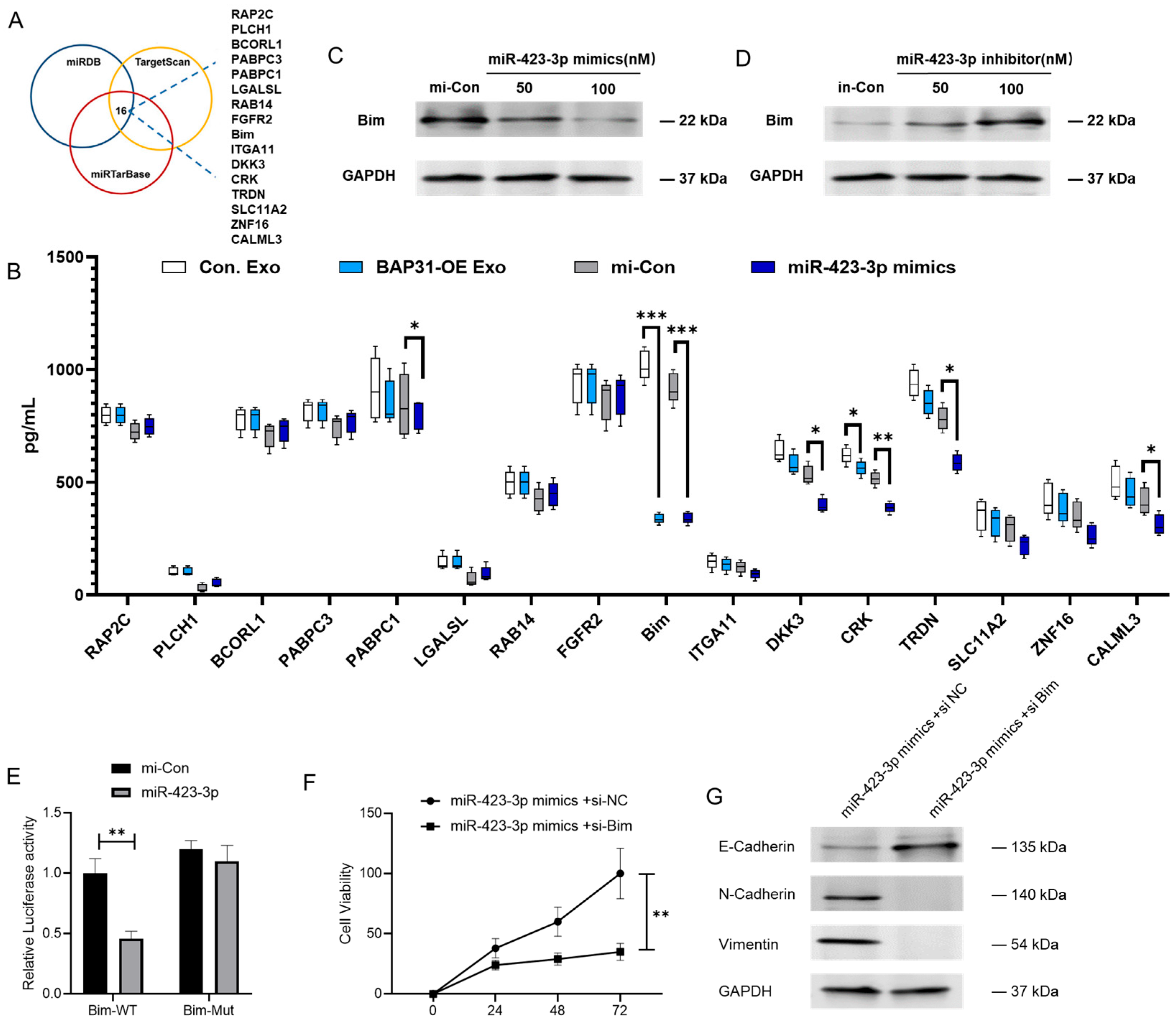
2.4. miR-423-3p Enhances EMT Through Its Interaction with Bim
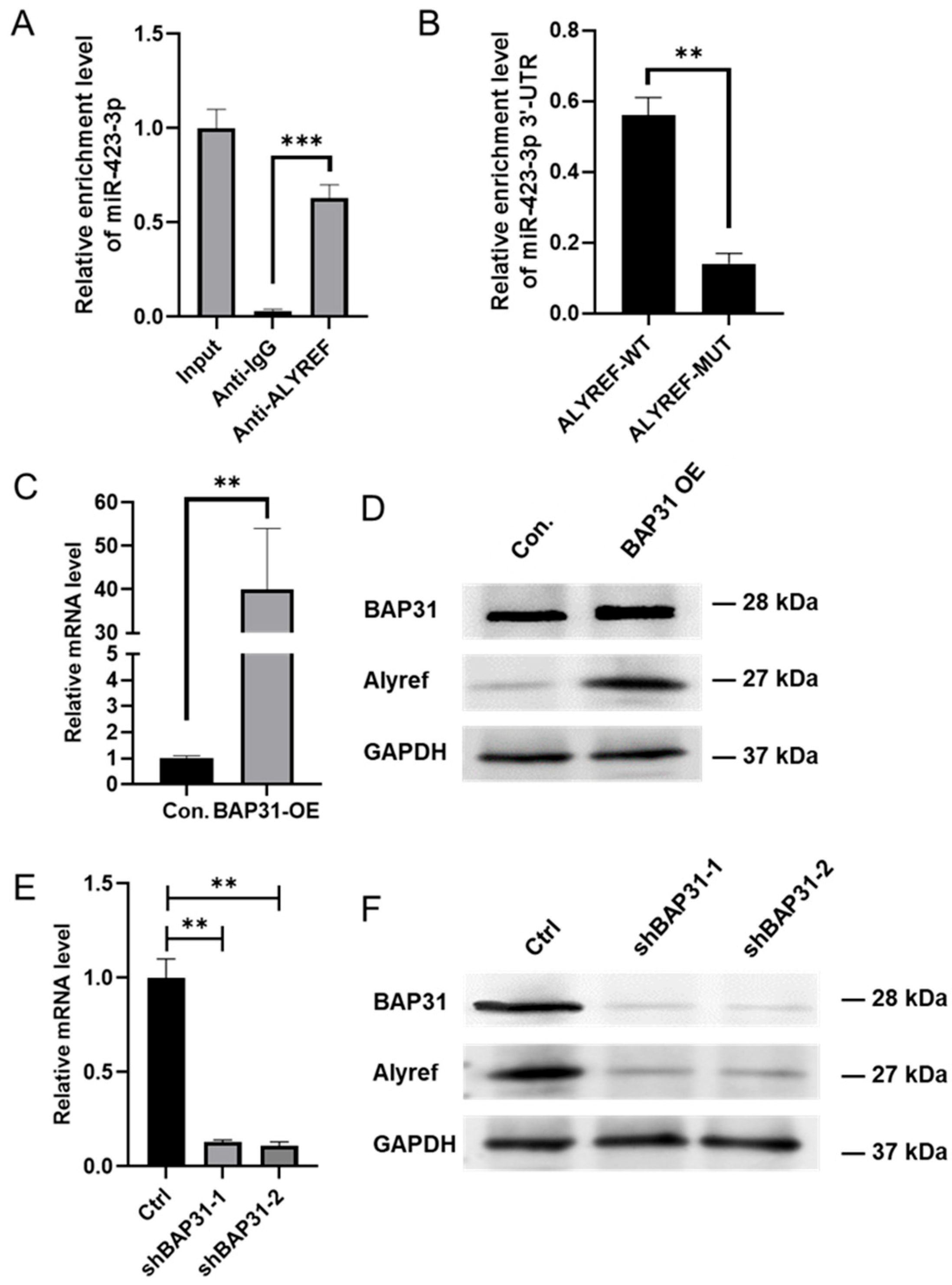
2.5. BAP31 Promotes the Selective Enrichment of miR-423-3p in Exosomes by Regulating Alyref
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Transfections
4.2. Cell Counting Assay
4.3. Confocal Laser Scanning
4.4. Exosome Isolation
4.5. Nanoparticle Tracking Analysis
4.6. Transmission Electron Microscopy
4.7. Transwell Assay
4.8. Wound Healing Assay
4.9. RT-PCR Analysis
4.10. Western Blot Analysis
4.11. ELISA
4.12. Dual-Luciferase Activity Assay
4.13. RNA Immunoprecipitation Assay
4.14. Animal Experiments
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nieto, M.A.; Huang, R.Y.-J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef]
- Krishnamachary, B.; Zagzag, D.; Nagasawa, H.; Rainey, K.; Okuyama, H.; Baek, J.H.; Semenza, G.L. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006, 66, 2725–2731. [Google Scholar] [CrossRef] [PubMed]
- Schamel, W.W.; Kuppig, S.; Becker, B.; Gimborn, K.; Hauri, H.P.; Reth, M. A high-molecular-weight complex of membrane proteins BAP29/BAP31 is involved in the retention of membrane-bound IgD in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2003, 100, 9861–9866. [Google Scholar] [CrossRef]
- Wang, B.; Heath-Engel, H.; Zhang, D.; Nguyen, N.; Thomas, D.Y.; Hanrahan, J.W.; Shore, G.C. BAP31 interacts with Sec61 translocons and promotes retrotranslocation of CFTRDeltaF508 via the derlin-1 complex. Cell 2008, 133, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Namusamba, M.; Wu, Y.; Yang, J.; Zhang, Q.; Wang, C.; Wang, T.; Wang, B. BAP31 Promotes Angiogenesis via Galectin-3 Upregulation in Neuroblastoma. Int. J. Mol. Sci. 2024, 25, 2946. [Google Scholar] [CrossRef]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef]
- Yang, M.-H.; Wu, M.-Z.; Chiou, S.-H.; Chen, P.-M.; Chang, S.-Y.; Liu, C.-J.; Teng, S.-C.; Wu, K.-J. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat. Cell Biol. 2008, 10, 295–305. [Google Scholar] [CrossRef]
- Li, T.; Hao, Z.; Tang, Z.; Li, C.; Cheng, L.; Wang, T.; Zhu, X.; He, Y.; Huang, Y.; Wang, B. BAP31 Regulates Wnt Signaling to Modulate Cell Migration in Lung Cancer. Front. Oncol. 2022, 12, 859195. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Wang, H.; Min, J.; Xu, C.; Liu, Y.; Yu, Z.; Gong, A.; Xu, M. Hypoxia-elicited Exosomes Promote the Chemoresistance of Pancreatic Cancer Cells by Transferring LncROR via Hippo Signaling. J. Cancer 2023, 14, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Tsukasaki, Y.; Dasgupta, S.; Mukhopadhyay, N.; Ikebe, M.; Sauter, E.R. Exosomes in Human Breast Milk Promote EMT. Clin. Cancer Res. 2016, 22, 4517–4524. [Google Scholar] [CrossRef]
- Garcia-Martin, R.; Wang, G.; Brandão, B.B.; Zanotto, T.M.; Shah, S.; Kumar Patel, S.; Schilling, B.; Kahn, C.R. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature 2021, 601, 446–451. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Jung, S.C.; Nam, S.K.; Park, Y.; Seo, S.H.; Park, K.U.; Oh, H.-K.; Kim, D.W.; Kang, S.-B.; Lee, H.S. Tissue miR-200c-3p and circulating miR-1290 as potential prognostic biomarkers for colorectal cancer. Sci. Rep. 2022, 12, 2295. [Google Scholar] [CrossRef]
- Wong, V.C.-L.; Wong, M.-I.; Lam, C.-T.; Lung, M.L.; Lam, K.-O.; Lee, V.H.-F. Hallmark microRNA signature in liquid biopsy identifies hepatocellular carcinoma and differentiates it from liver metastasis. J. Cancer 2021, 12, 4585–4594. [Google Scholar] [CrossRef]
- Lin, J.; Huang, S.; Wu, S.; Ding, J.; Zhao, Y.; Liang, L.; Tian, Q.; Zha, R.; Zhan, R.; He, X. MicroRNA-423 promotes cell growth and regulates G1/S transition by targeting p21Cip1/Waf1 in hepatocellular carcinoma. Carcinogenesis 2011, 32, 1641–1647. [Google Scholar] [CrossRef]
- Merino, D.; Best, S.A.; Asselin-Labat, M.-L.; Vaillant, F.; Pal, B.; Dickins, R.A.; Anderson, R.L.; Strasser, A.; Bouillet, P.; Lindeman, G.J.; et al. Pro-apoptotic Bim suppresses breast tumor cell metastasis and is a target gene of SNAI2. Oncogene 2015, 34, 3926–3934. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Yamauchi, A.; Oda, H. EMT-inducing transcription factor ZEB1-associated resistance to the BCL-2/BCL-XL inhibitor is overcome by BIM upregulation in ovarian clear cell carcinoma cells. Biochem. Biophys. Res. Commun. 2020, 526, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Greenhough, A.; Wallam, C.A.; Hicks, D.J.; Moorghen, M.; Williams, A.C.; Paraskeva, C. The proapoptotic BH3-only protein Bim is downregulated in a subset of colorectal cancers and is repressed by antiapoptotic COX-2/PGE2 signalling in colorectal adenoma cells. Oncogene 2010, 29, 3398–3410. [Google Scholar] [CrossRef]
- Rosati, E.; Sabatini, R.; Rampino, G.; De Falco, F.; Di Ianni, M.; Falzetti, F.; Fettucciari, K.; Bartoli, A.; Screpanti, I.; Marconi, P. Novel targets for endoplasmic reticulum stress-induced apoptosis in B-CLL. Blood 2010, 116, 2713–2723. [Google Scholar] [CrossRef]
- Licatalosi, D.D.; Ye, X.; Jankowsky, E. Approaches for measuring the dynamics of RNA-protein interactions. Wiley Interdiscip. Rev. RNA 2020, 11, e1565. [Google Scholar] [CrossRef]
- Lee, F.C.Y.; Ule, J. Advances in CLIP Technologies for Studies of Protein-RNA Interactions. Mol. Cell 2018, 69, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.S.; Schafer, D.M.; Rothamel, K.L.; Yeo, G.W. Decoding protein-RNA interactions using CLIP-based methodologies. Nat. Rev. Genet. 2024, 25, 879–895. [Google Scholar] [CrossRef]
- Chen, J.; Guo, H.; Jiang, H.; Namusamba, M.; Wang, C.; Lan, T.; Wang, T.; Wang, B. A BAP31 intrabody induces gastric cancer cell death by inhibiting p27 proteasome degradation. Int. J. Cancer 2019, 144, 2051–2062. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Sun, B.-F.; Chen, Y.-S.; Xu, J.-W.; Lai, W.-Y.; Li, A.; Wang, X.; Bhattarai, D.P.; Xiao, W.; et al. 5-methylcytosine promotes mRNA export—NSUN2 as the methyltransferase and ALYREF as an mC reader. Cell Res. 2017, 27, 606–625. [Google Scholar] [CrossRef]
- Chong, P.A.; Vernon, R.M.; Forman-Kay, J.D. RGG/RG motif regions in RNA binding and phase separation. Mol. Biol. 2018, 430, 4650–4665. [Google Scholar] [CrossRef]
- Bhandari, J.; Guillén-Mendoza, C.; Banks, K.; Eliaz, L.; Southwell, S.; Eyaa, D.; Luna, R.; Aguilera, A.; Xue, X. The molecular chaperone ALYREF promotes R-loop resolution and maintains genome stability. J. Biol. Chem. 2024, 300, 107996. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Liu, W.; Yang, S.; Wang, T.; Wang, B. BAP31 Promotes Epithelial–Mesenchymal Transition Progression Through the Exosomal miR-423-3p/Bim Axis in Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 5483. https://doi.org/10.3390/ijms26125483
Wang C, Liu W, Yang S, Wang T, Wang B. BAP31 Promotes Epithelial–Mesenchymal Transition Progression Through the Exosomal miR-423-3p/Bim Axis in Colorectal Cancer. International Journal of Molecular Sciences. 2025; 26(12):5483. https://doi.org/10.3390/ijms26125483
Chicago/Turabian StyleWang, Changli, Wanting Liu, Sheng Yang, Tianyi Wang, and Bing Wang. 2025. "BAP31 Promotes Epithelial–Mesenchymal Transition Progression Through the Exosomal miR-423-3p/Bim Axis in Colorectal Cancer" International Journal of Molecular Sciences 26, no. 12: 5483. https://doi.org/10.3390/ijms26125483
APA StyleWang, C., Liu, W., Yang, S., Wang, T., & Wang, B. (2025). BAP31 Promotes Epithelial–Mesenchymal Transition Progression Through the Exosomal miR-423-3p/Bim Axis in Colorectal Cancer. International Journal of Molecular Sciences, 26(12), 5483. https://doi.org/10.3390/ijms26125483





