Triterpenoid CDDO-EA Protects from Hyperglycemia, Hyperinsulinemia, and Obesity by Decreasing Energy Intake
Abstract
1. Introduction
2. Results
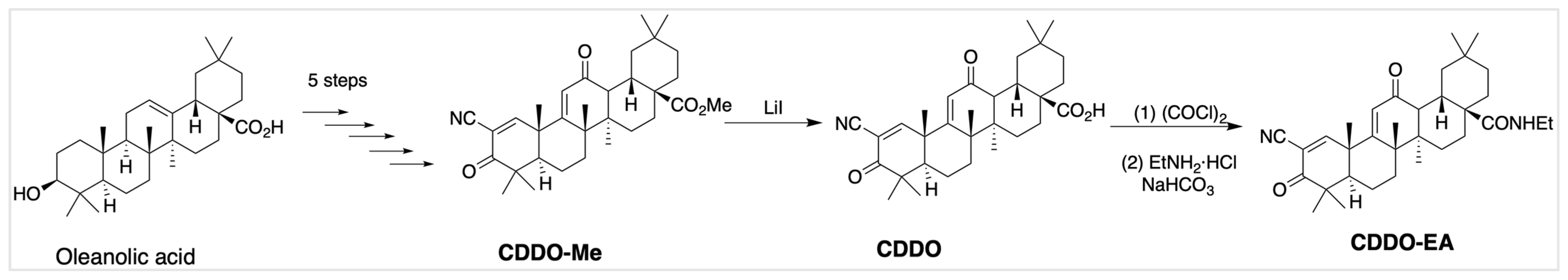
2.1. Validation of CDDO-EA Synthesis
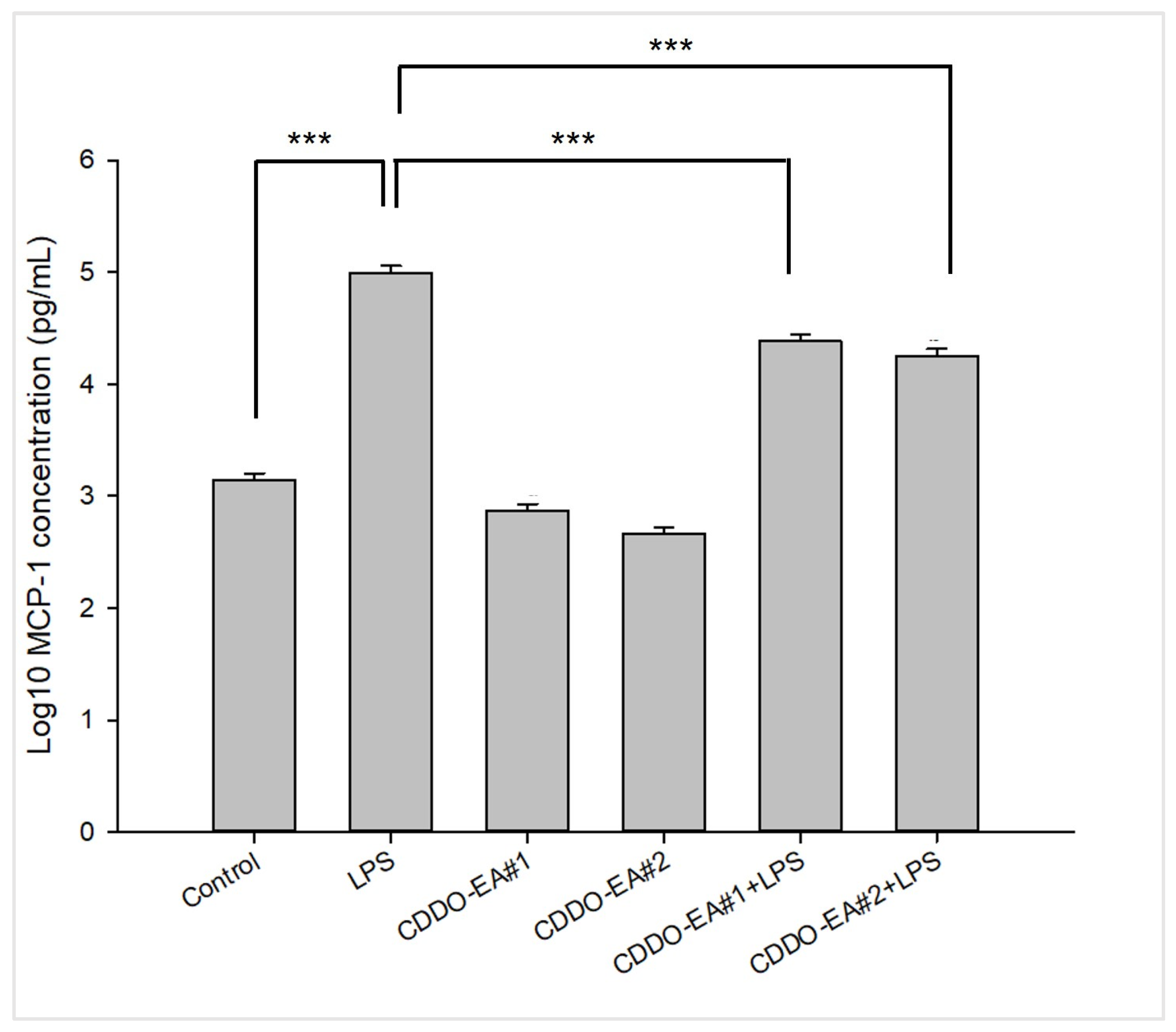
2.2. CDDO-EA Suppresses LPS-Induced MCP-1 Production in Macrophages
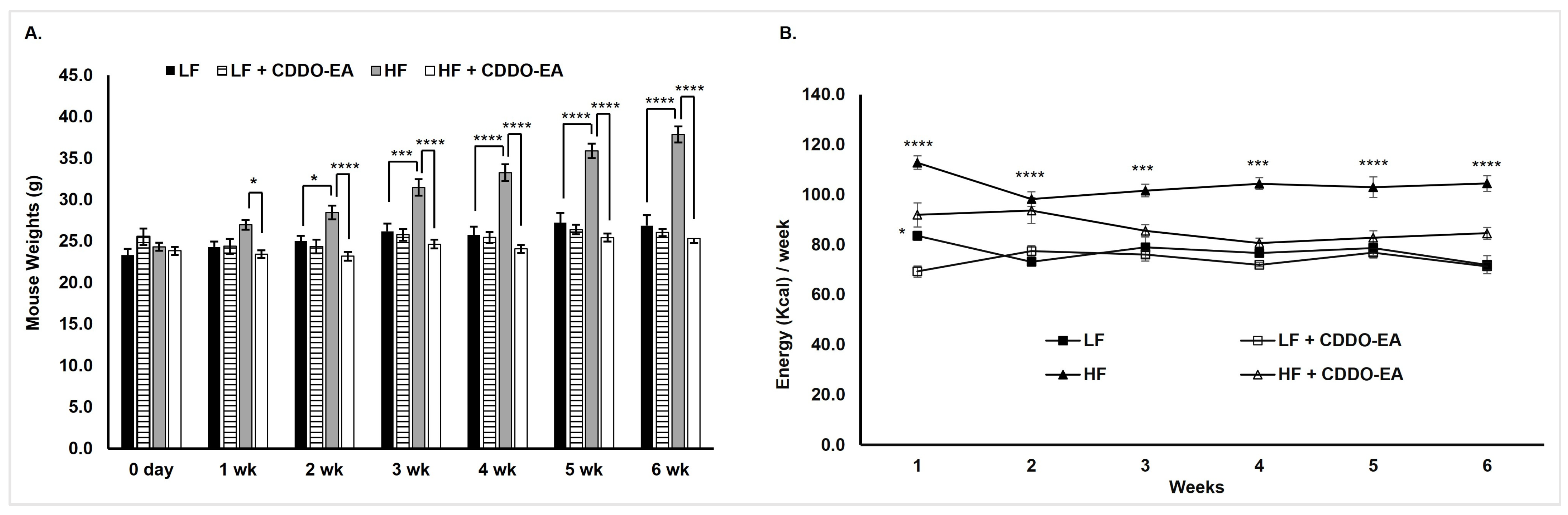
2.3. CDDO-EA Protects from High-Fat Diet-Induced Obesity
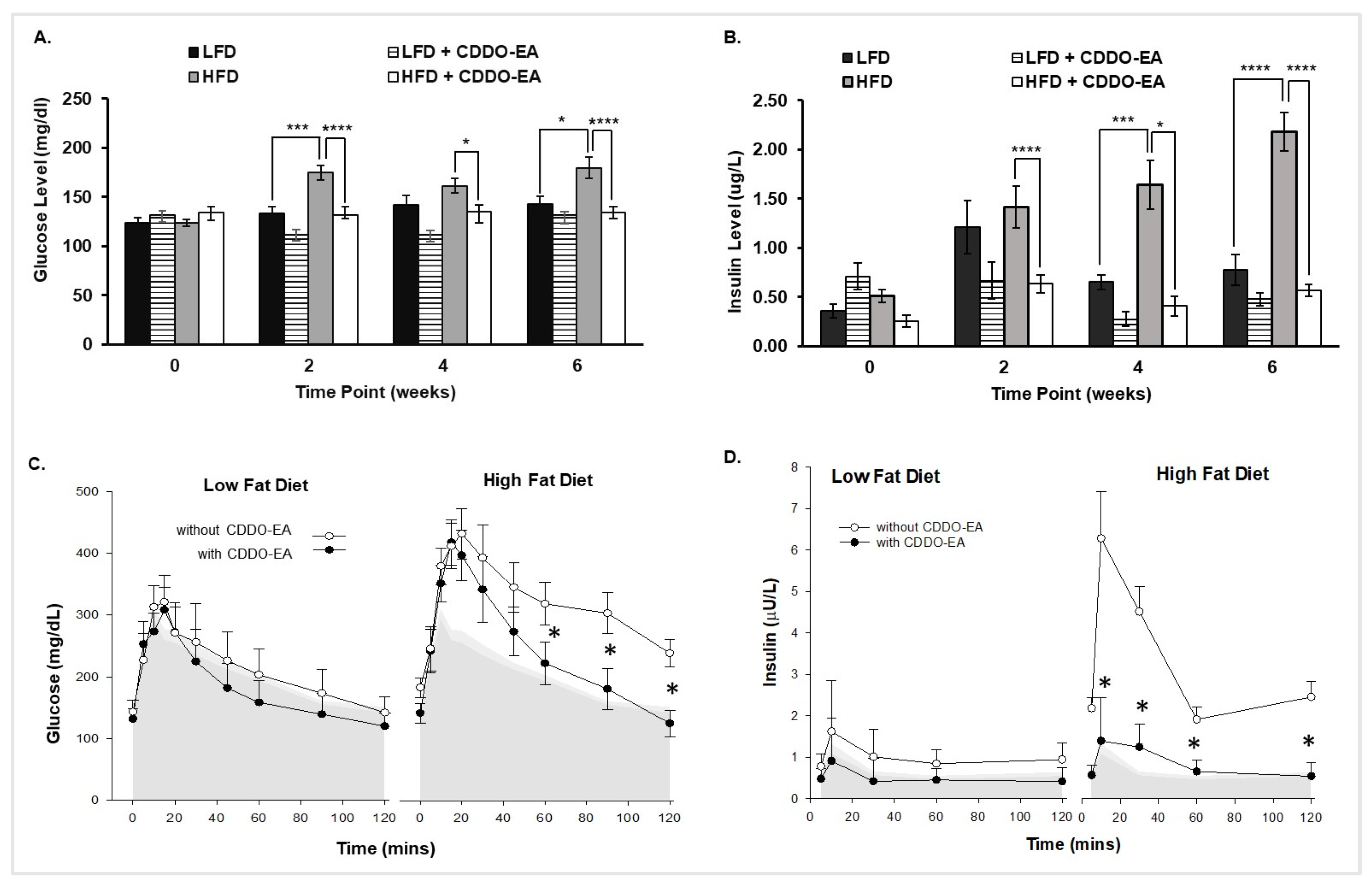
2.4. CDDO-EA Prevents Hyperglycemia and Hyperinsulinemia
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Synthesis of CDDO-EA
4.3. RAW264.7 Cells
4.4. MCP-1 Detection
4.5. Oral Glucose Tolerance Tests and Glucose Measurements
4.6. Insulin ELISA
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDDO | 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid |
| CDDO-EA | 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid-ethyl amide |
| CDDO-Cl | 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid-chloride |
| CDDO-Im | 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid-imidazole |
| CDDO-Me | 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid-methyl ester |
| EDTA | ethylenediaminetetraacetic acid |
| ELISA | enzyme-linked immunosorbent assay |
| GLP-1 | glucagon-like peptide 1 |
| GLUT4 | glucose transporter 4 |
| GTT | glucose tolerance test |
| HFD | high-fat diet |
| HRMS | high-resolution mass spectrometry |
| HPLC | high-performance liquid chromatography |
| LFD | low-fat diet |
| MCP-1 | monocyte chemotactic protein-1 |
| MeOH | methanol |
| NF-κB | nuclear factor-kappa B |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NMR | nuclear magnetic resonance |
| OGTT | oral glucose tolerance test |
| PBS | phosphate-buffered saline |
| T2D | type 2 diabetes |
References
- Klein, S.; Gastaldelli, A.; Yki-Jarvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Anandhanarayanan, A.; Teh, K.; Goonoo, M.; Tesfaye, S.; Selvarajah, D. Diabetic Neuropathies. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Joharatnam-Hogan, N.; Carter, T.J.; Reynolds, N.; Ho, J.H.; Adam, S.; Board, R. Diabetes Mellitus in People with Cancer. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Solis-Herrera, C.; Triplitt, C.; Cersosimo, E.; DeFronzo, R.A. Pathogenesis of Type 2 Diabetes Mellitus. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Liby, K.T.; Sporn, M.B. Synthetic oleanane triterpenoids: Multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol. Rev. 2012, 64, 972–1003. [Google Scholar] [CrossRef]
- Shin, S.; Wakabayashi, J.; Yates, M.S.; Wakabayashi, N.; Dolan, P.M.; Aja, S.; Liby, K.T.; Sporn, M.B.; Yamamoto, M.; Kensler, T.W. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur. J. Pharmacol. 2009, 620, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.K.; Reddy, V.T.; Konopleva, M.; Andreeff, M.; Chan, L. The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Lepr(db/db) mice. J. Biol. Chem. 2010, 285, 40581–40592. [Google Scholar] [CrossRef]
- Camer, D.; Yu, Y.; Szabo, A.; Dinh, C.H.; Wang, H.; Cheng, L.; Huang, X.F. Bardoxolone methyl prevents insulin resistance and the development of hepatic steatosis in mice fed a high-fat diet. Mol. Cell Endocrinol. 2015, 412, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Camer, D.; Yu, Y.; Szabo, A.; Wang, H.; Dinh, C.H.; Huang, X.F. Bardoxolone methyl prevents obesity and hypothalamic dysfunction. Chem. Biol. Interact. 2016, 256, 178–187. [Google Scholar] [CrossRef]
- Chang, P.F.; Acevedo, D.; Mandarino, L.J.; Reyna, S.M. Triterpenoid CDDO-EA inhibits lipopolysaccharide-induced inflammatory responses in skeletal muscle cells through suppression of NF-kappaB. Exp. Biol. Med. 2023, 248, 175–185. [Google Scholar] [CrossRef]
- Fu, L.; Gribble, G.W. Efficient and Scalable Synthesis of Bardoxolone Methyl (CDDO-methyl Ester). Org. Lett. 2013, 15, 1622–1625. [Google Scholar] [CrossRef]
- Everitt, J.I.; Shapiro, S.J. The art and science of introducing animals to the research environment. ILAR J. 2006, 47, 281–282. [Google Scholar] [CrossRef][Green Version]
- Schapiro, S.A.; Everitt, J.I. Preparation of animals for use in the laboratory: Issues and challenges for the Institutional Animal Care and Use Committee (IACUC). ILAR J. 2006, 47, 370–375. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yates, M.S.; Tauchi, M.; Katsuoka, F.; Flanders, K.C.; Liby, K.T.; Honda, T.; Gribble, G.W.; Johnson, D.A.; Johnson, J.A.; Burton, N.C.; et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol. Cancer Ther. 2007, 6, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Chartoumpekis, D.V.; Kensler, T.W. New player on an old field; the keap1/Nrf2 pathway as a target for treatment of type 2 diabetes and metabolic syndrome. Curr. Diabetes Rev. 2013, 9, 137–145. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Yang, W.; Hu, S.; Wu, Y.; Zhao, B.; Hu, H.; Du, S. Focus on Notoginsenoside R1 in Metabolism and Prevention Against Human Diseases. Drug Des. Devel Ther. 2020, 14, 551–565. [Google Scholar] [CrossRef]
- Shen, W.; Wu, J.; Shi, L.; Feng, H.; Yang, X.; Zhang, Y. Explore the mechanisms of triterpenoids from Ganoderma lucidum in the protection against Alzheimer’s disease via microbiota-gut-brain axis with the aid of network pharmacology. Fitoterapia 2024, 178, 106150. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef]
- Cuadrado, A.; Cazalla, E.; Bach, A.; Bathish, B.; Naidu, S.D.; DeNicola, G.M.; Dinkova-Kostova, A.T.; Fernandez-Gines, R.; Grochot-Przeczek, A.; Hayes, J.D.; et al. Health position paper and redox perspectives—Bench to bedside transition for pharmacological regulation of NRF2 in noncommunicable diseases. Redox Biol. 2025, 81, 103569. [Google Scholar] [CrossRef]
- Catrysse, L.; van Loo, G. Inflammation and the Metabolic Syndrome: The Tissue-Specific Functions of NF-kappaB. Trends Cell Biol. 2017, 27, 417–429. [Google Scholar] [CrossRef]
- Fink, L.N.; Costford, S.R.; Lee, Y.S.; Jensen, T.E.; Bilan, P.J.; Oberbach, A.; Bluher, M.; Olefsky, J.M.; Sams, A.; Klip, A. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity 2014, 22, 747–757. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, L.; Zhang, M.; Wang, P.; Zhang, L.; Yuan, C.; Qi, J.; Qiao, Y.; Kuo, P.C.; Gao, C. NF-kappaB- and AP-1-mediated DNA looping regulates osteopontin transcription in endotoxin-stimulated murine macrophages. J. Immunol. 2011, 186, 3173–3179. [Google Scholar] [CrossRef] [PubMed]
- de Zeeuw, D.; Akizawa, T.; Audhya, P.; Bakris, G.L.; Chin, M.; Christ-Schmidt, H.; Goldsberry, A.; Houser, M.; Krauth, M.; Lambers Heerspink, H.J.; et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 2013, 369, 2492–2503. [Google Scholar] [CrossRef] [PubMed]
- Neymotin, A.; Calingasan, N.Y.; Wille, E.; Naseri, N.; Petri, S.; Damiano, M.; Liby, K.T.; Risingsong, R.; Sporn, M.; Beal, M.F.; et al. Neuroprotective effect of Nrf2/ARE activators, CDDO ethylamide and CDDO trifluoroethylamide, in a mouse model of amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2011, 51, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Stack, C.; Ho, D.; Wille, E.; Calingasan, N.Y.; Williams, C.; Liby, K.; Sporn, M.; Dumont, M.; Beal, M.F. Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington’s disease. Free Radic. Biol. Med. 2010, 49, 147–158. [Google Scholar] [CrossRef]
- Honda, T.; Janosik, T.; Honda, Y.; Han, J.; Liby, K.T.; Williams, C.R.; Couch, R.D.; Anderson, A.C.; Sporn, M.B.; Gribble, G.W. Design, synthesis, and biological evaluation of biotin conjugates of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid for the isolation of the protein targets. J. Med. Chem. 2004, 47, 4923–4932. [Google Scholar] [CrossRef]
- Chang, F.M.; Reyna, S.M.; Granados, J.C.; Wei, S.J.; Innis-Whitehouse, W.; Maffi, S.K.; Rodriguez, E.; Slaga, T.J.; Short, J.D. Inhibition of neddylation represses lipopolysaccharide-induced proinflammatory cytokine production in macrophage cells. J. Biol. Chem. 2012, 287, 35756–35767. [Google Scholar] [CrossRef]
- Honda, T.; Rounds, B.V.; Bore, L.; Finlay, H.J.; Favaloro, F.G., Jr.; Suh, N.; Wang, Y.; Sporn, M.B.; Gribble, G.W. Synthetic oleanane and ursane triterpenoids with modified rings A and C: A series of highly active inhibitors of nitric oxide production in mouse macrophages. J. Med. Chem. 2000, 43, 4233–4246. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantu, A.E.; Rasa, C.; Mito, S.; Cantu, D.; Lopez-Alvarenga, J.C.; Rivera-Lopez, L.L.; Rios, I.; Abrego-Gonzalez, A.; Reyna, S.M. Triterpenoid CDDO-EA Protects from Hyperglycemia, Hyperinsulinemia, and Obesity by Decreasing Energy Intake. Int. J. Mol. Sci. 2025, 26, 5485. https://doi.org/10.3390/ijms26125485
Cantu AE, Rasa C, Mito S, Cantu D, Lopez-Alvarenga JC, Rivera-Lopez LL, Rios I, Abrego-Gonzalez A, Reyna SM. Triterpenoid CDDO-EA Protects from Hyperglycemia, Hyperinsulinemia, and Obesity by Decreasing Energy Intake. International Journal of Molecular Sciences. 2025; 26(12):5485. https://doi.org/10.3390/ijms26125485
Chicago/Turabian StyleCantu, Austin E., Cordelia Rasa, Shizue Mito, Denae Cantu, Juan Carlos Lopez-Alvarenga, Leslie L. Rivera-Lopez, Israel Rios, Ashley Abrego-Gonzalez, and Sara M. Reyna. 2025. "Triterpenoid CDDO-EA Protects from Hyperglycemia, Hyperinsulinemia, and Obesity by Decreasing Energy Intake" International Journal of Molecular Sciences 26, no. 12: 5485. https://doi.org/10.3390/ijms26125485
APA StyleCantu, A. E., Rasa, C., Mito, S., Cantu, D., Lopez-Alvarenga, J. C., Rivera-Lopez, L. L., Rios, I., Abrego-Gonzalez, A., & Reyna, S. M. (2025). Triterpenoid CDDO-EA Protects from Hyperglycemia, Hyperinsulinemia, and Obesity by Decreasing Energy Intake. International Journal of Molecular Sciences, 26(12), 5485. https://doi.org/10.3390/ijms26125485









