Modulation of Abortiporus biennis Response to Oxidative Stress by Light as a New Eco-Friendly Approach with a Biotechnological Perspective
Abstract
1. Introduction
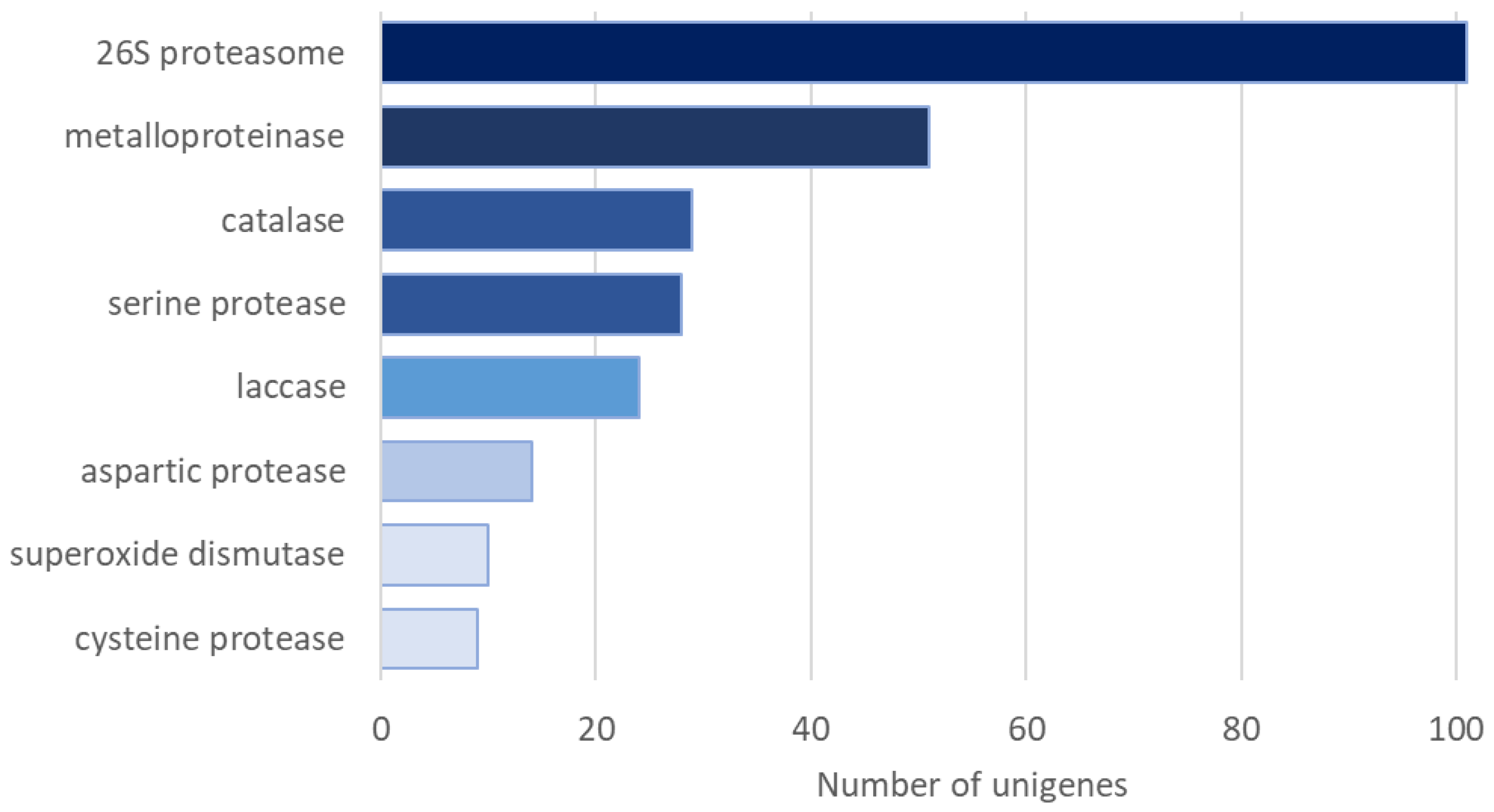
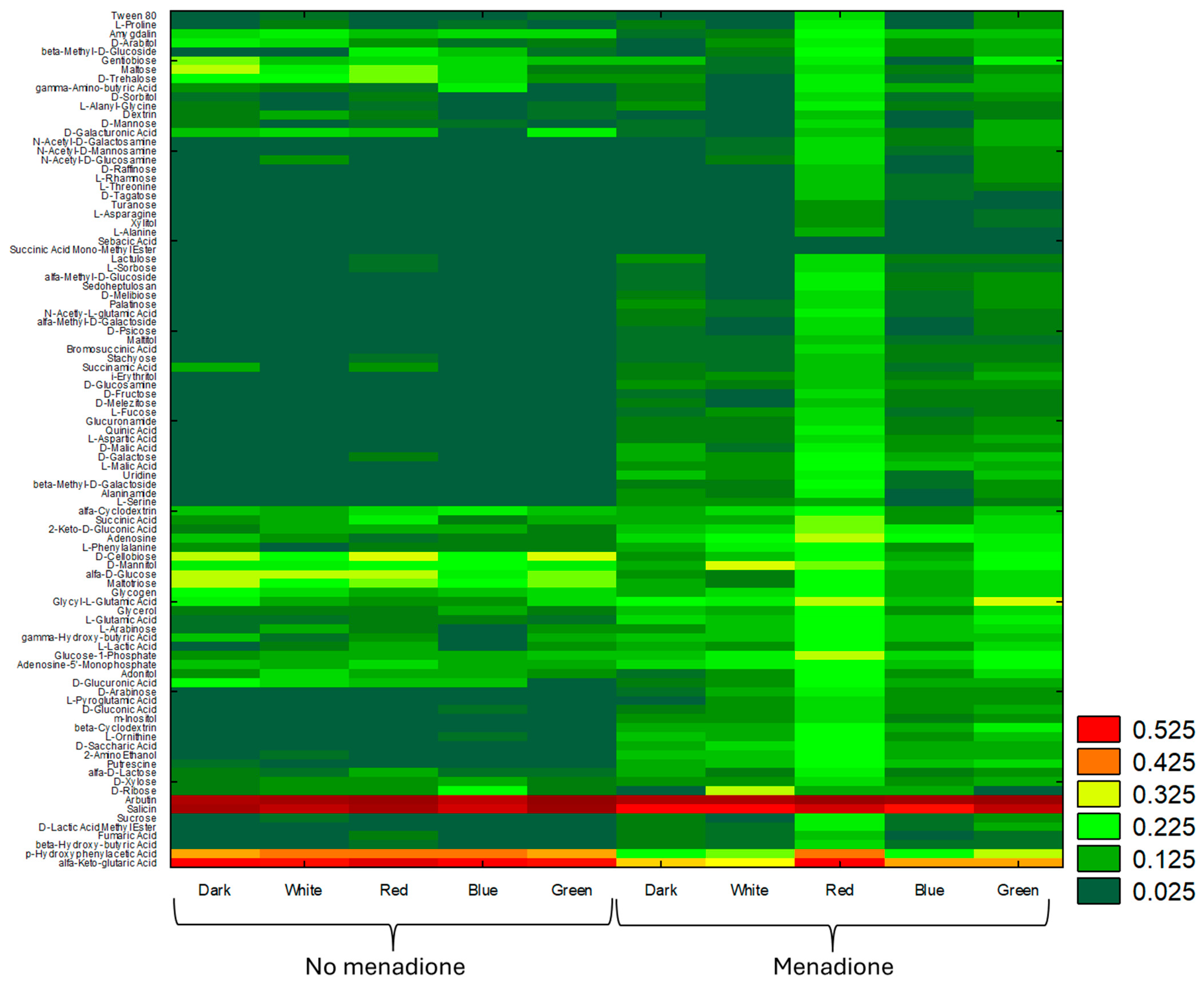
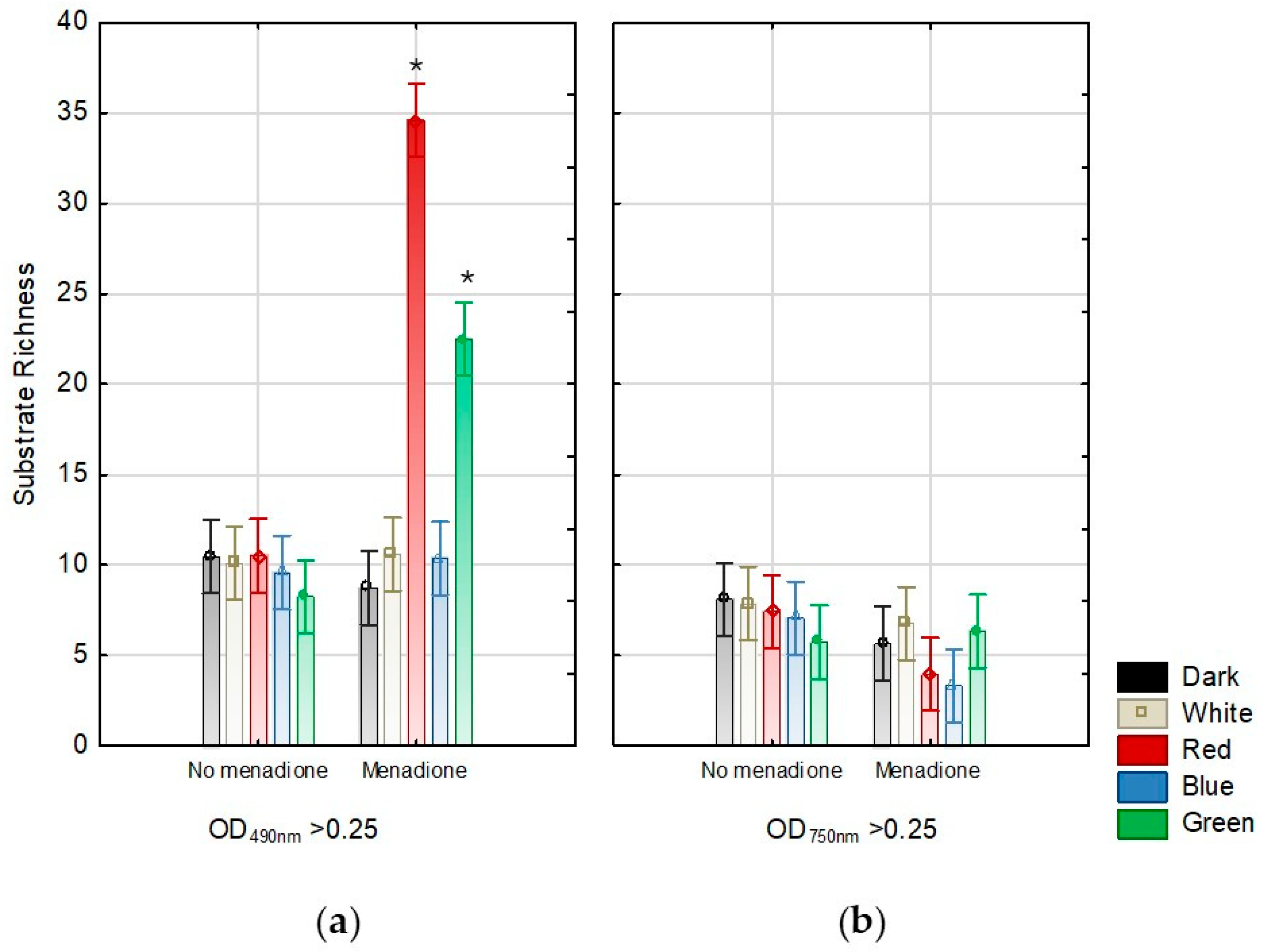
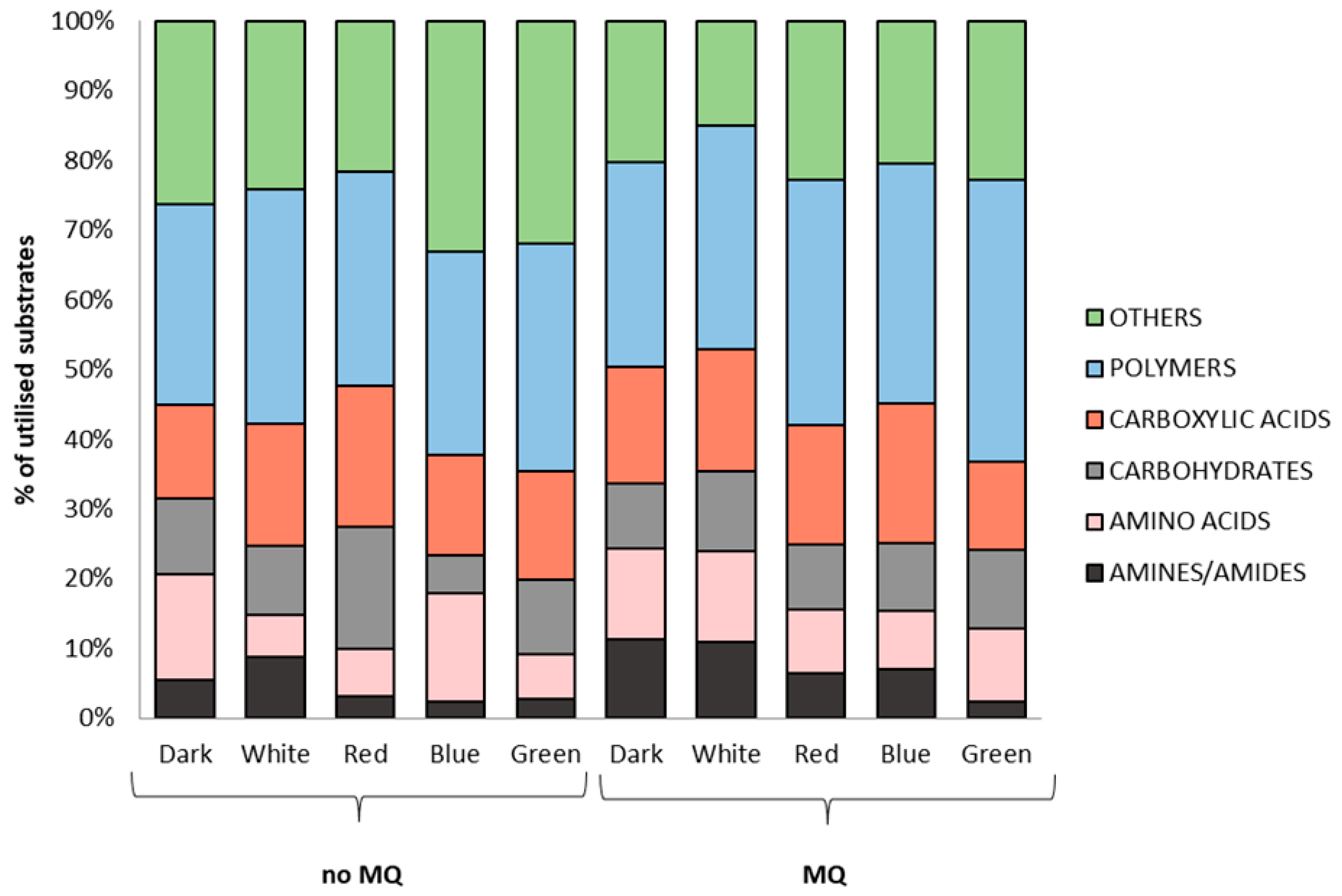
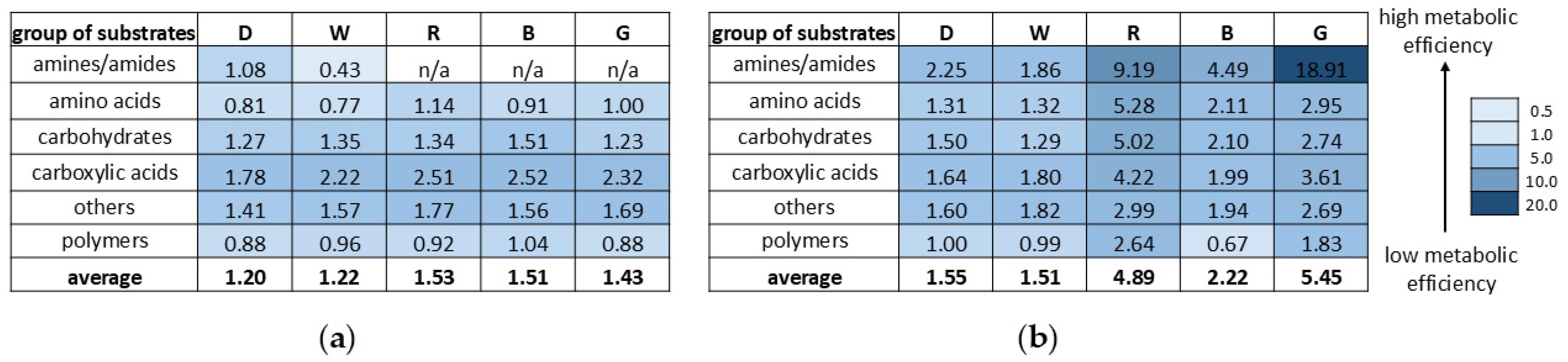
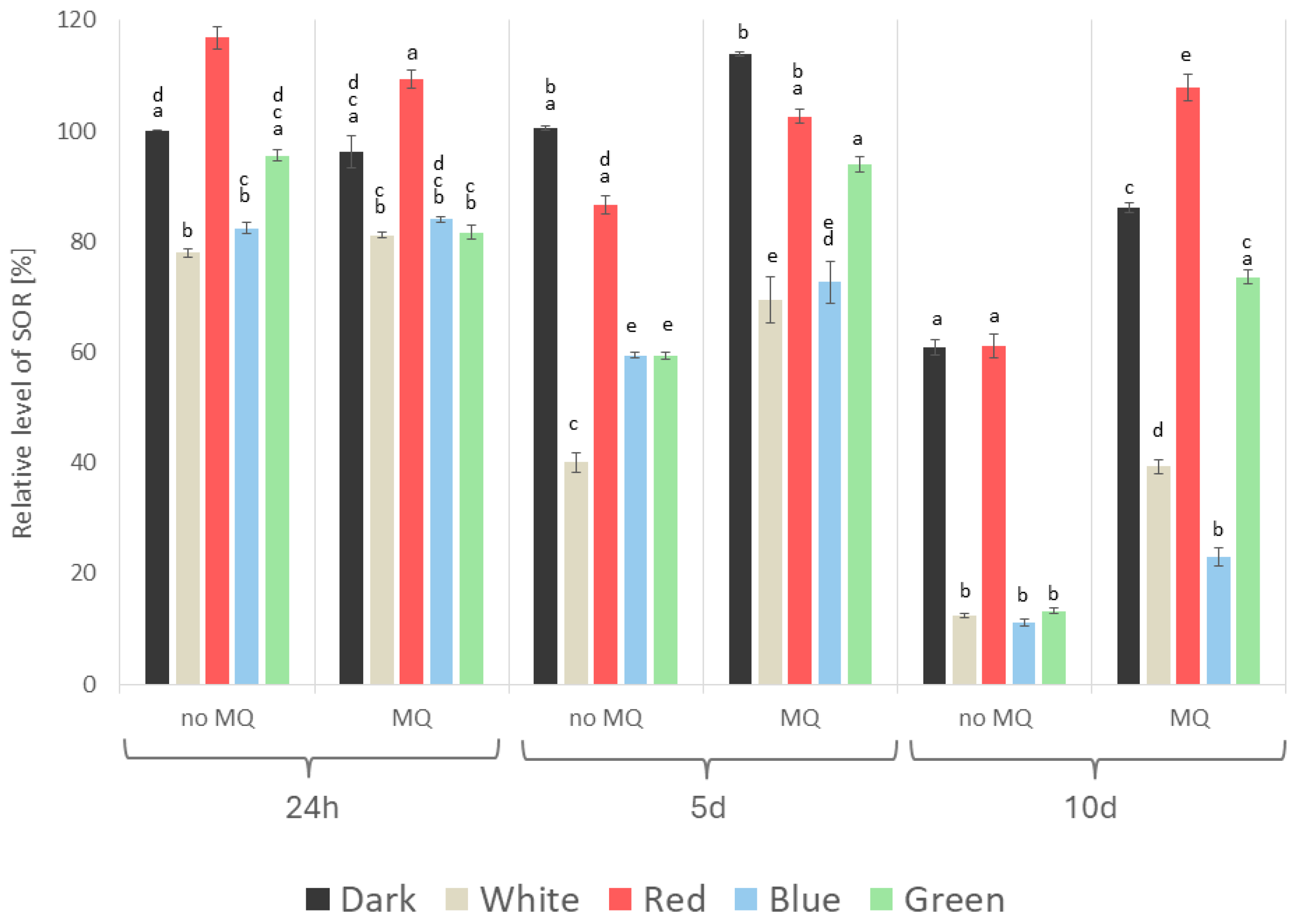
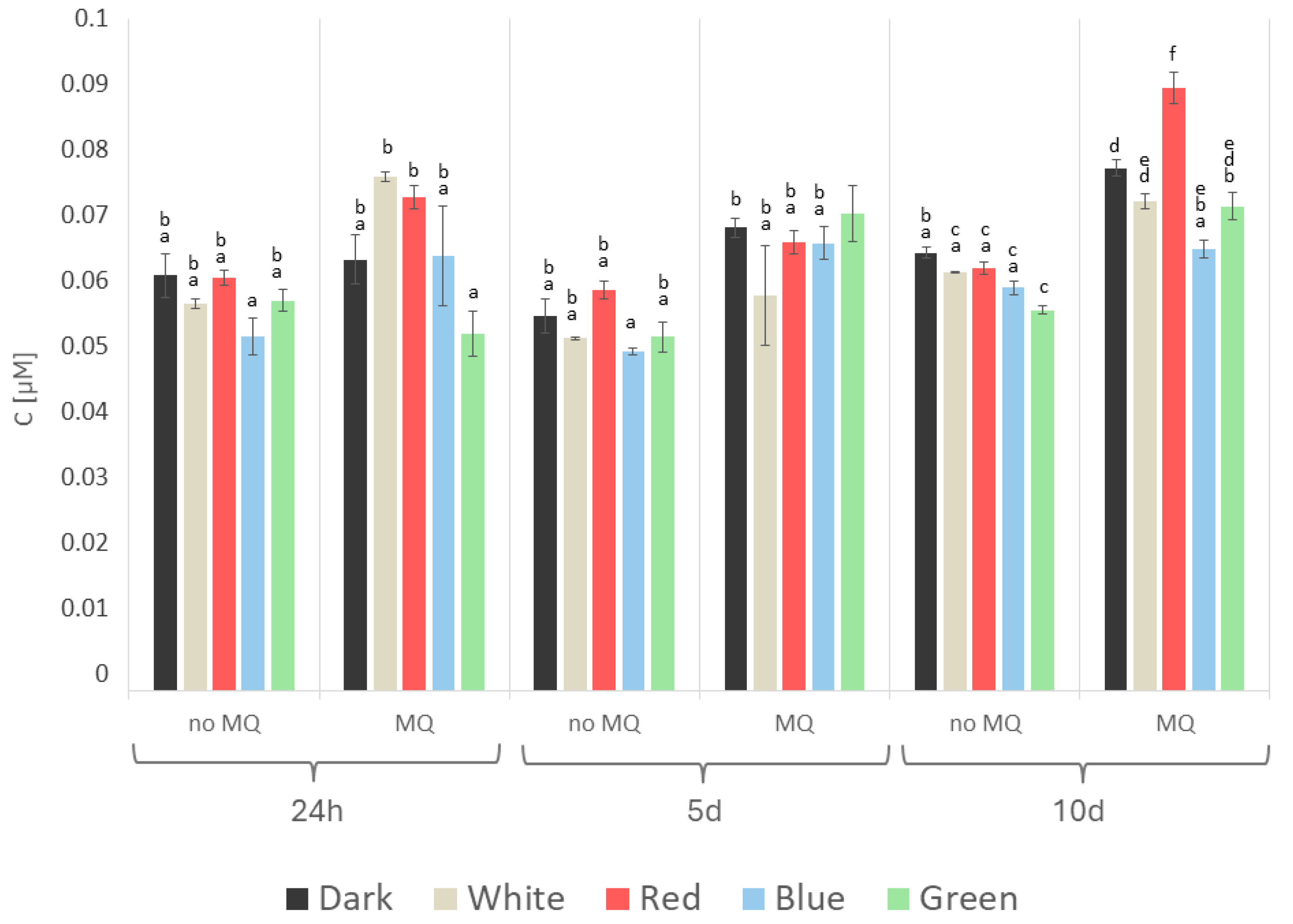
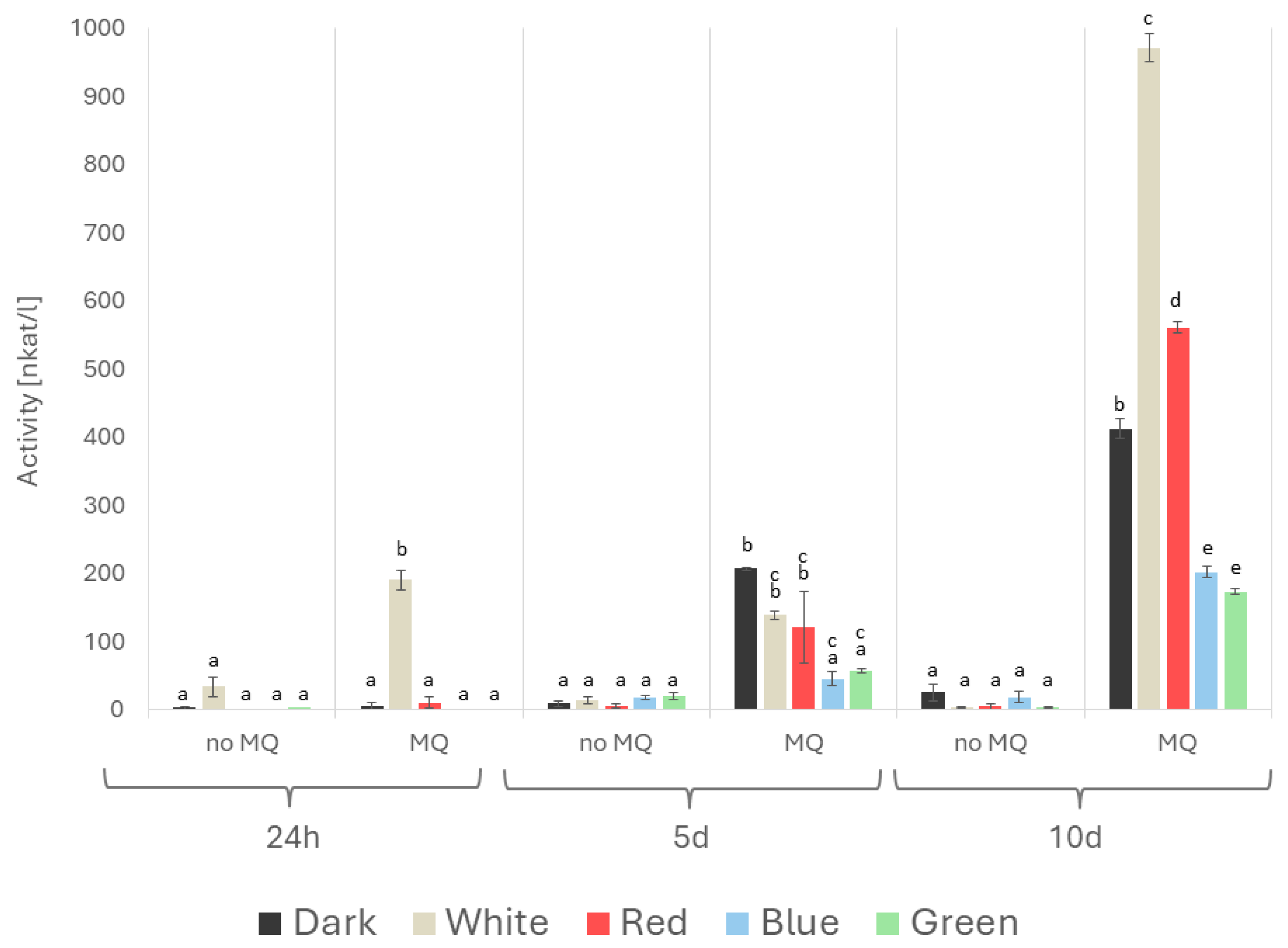
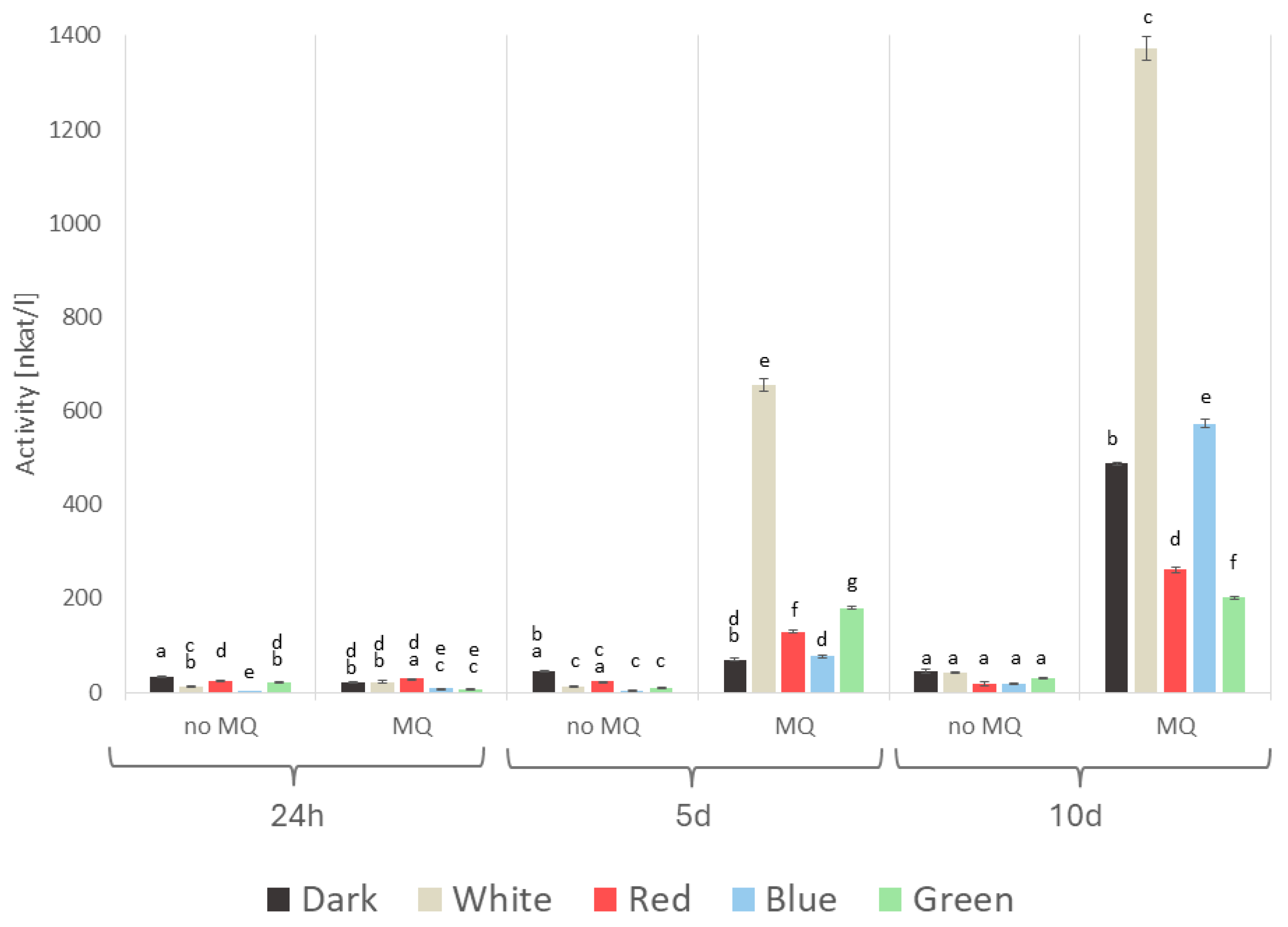
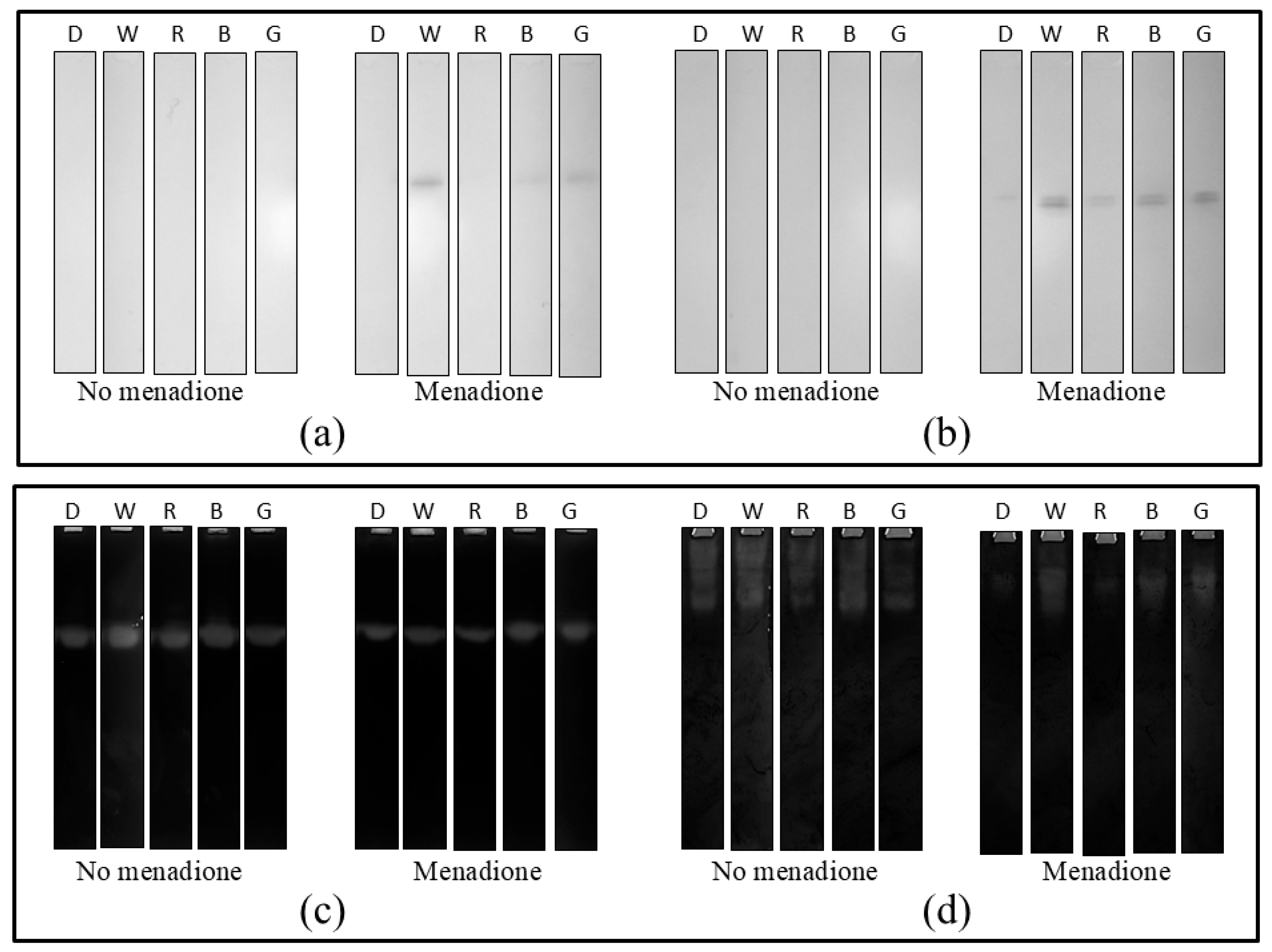
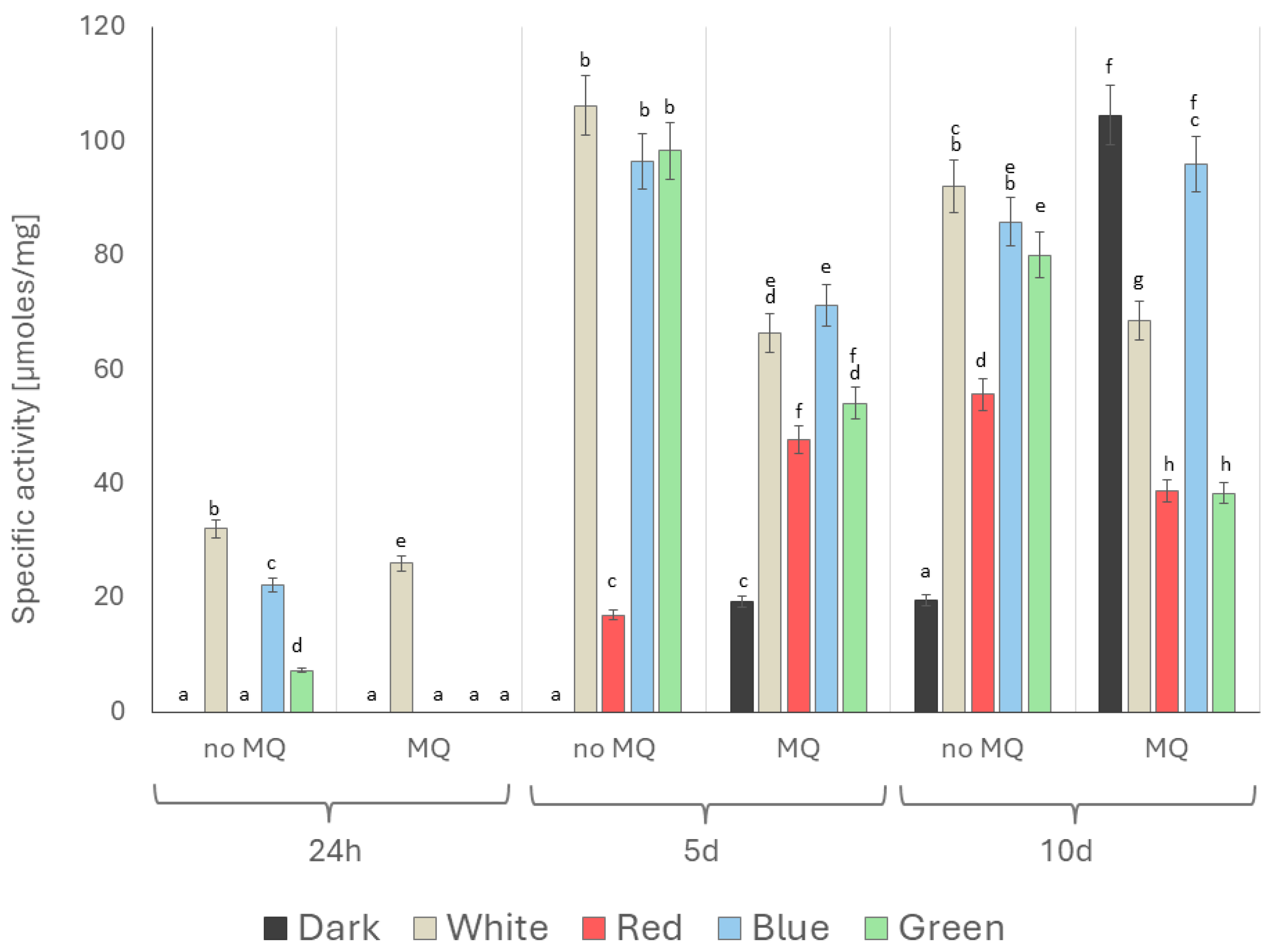
2. Results
3. Discussion
4. Materials and Methods
4.1. Fungal Strain and Cultivation
4.2. Analysis of the Expression Profile of Genes Encoding Putative Oxidative Stress Response Proteins
4.3. Evaluation of the Effect of Lighting Conditions and Menadione-Induced Oxidative Stress on the Growth and Metabolic Profiles of A. biennis Based on Phenotypic Analysis Using Biolog FF Panels
4.4. Preparation of Experimental Cultures, Induction of Stress Conditions, and Biochemical Analysis of A. biennis
4.4.1. Preparation of Crude Extracts
4.4.2. Detection of the Relative Level of Superoxide Anion Radical (SOR)
4.4.3. Determination of Phenolic Substances (PHCs)
4.4.4. Determination of Antioxidative Properties
4.4.5. Laccase (LAC) Assay
4.4.6. Zymographic Analysis of Catalase (CAT), Superoxide Dismutase (SOD), and Laccase (LAC) Activity
4.4.7. Measurement of Proteasome Peptidase Activity
4.4.8. Protein Determination
4.4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaakoub, H.; Mina, S.; Calenda, A.; Bouchara, J.-P.; Papon, N. Oxidative stress response pathways in fungi. Cell. Mol. Life Sci. 2022, 79, 333. [Google Scholar] [CrossRef] [PubMed]
- Skoneczny, M. (Ed.) Stress Response Mechanisms in Fungi: Theoretical and Practical Aspects; Springer: Cham, Switzerland, 2018; pp. 1–259. [Google Scholar]
- Breitenbach, M.; Weber, M.; Rinnerthaler, M.; Karl, T.; Breitenbach-Koller, L. Oxidative stress in fungi: Its function in signal transduction, interaction with plant hosts, and lignocellulose degradation. Biomolecules 2015, 5, 318–342. [Google Scholar] [CrossRef] [PubMed]
- Jaszek, M.; Kos, K.; Matuszewska, A.; Grąz, M.; Stefaniuk, D.; Osińska-Jaroszuk, M.; Prendecka, M.; Jóźwik, E.; Grzywnowicz, K. Effective stimulation of the biotechnological potential of the medicinal white rot fungus: Phellinus pini by menadione-mediated oxidative stress. Appl. Biochem. Biotechnol. 2014, 174, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Tisch, D.; Schmoll, M. Light regulation of metabolic pathways in fungi. Appl. Microbiol. Biotechnol. 2010, 85, 1259–1277. [Google Scholar] [CrossRef]
- Yu, W.; Pei, R.; Zhang, Y.; Tu, Y.; He, B. Light regulation of secondary metabolism in fungi. J. Biol. Eng. 2023, 17, 57. [Google Scholar] [CrossRef]
- Fuller, K.K.; Loros, J.J.; Dunlap, J.C. Fungal photobiology: Visible light as a signal for stress, space and time. Curr. Genet. 2015, 61, 275–288. [Google Scholar] [CrossRef]
- Pawlik, A.; Ruminowicz-Stefaniuk, M.; Frąc, M.; Mazur, A.; Wielbo, J.; Janusz, G. The wood decay fungus Cerrena unicolor adjusts its metabolism to grow on various types of wood and light conditions. PLoS ONE 2019, 14, e0211744. [Google Scholar] [CrossRef]
- Bayram, O.; Feussner, K.; Dumkow, M.; Herrfurth, C.; Feussner, I.; Braus, G.H. Changes of global gene expression and secondary metabolite accumulation during light-dependent Aspergillus nidulans development. Fungal Genet. Biol. 2016, 87, 30–53. [Google Scholar] [CrossRef]
- Pawlik, A.; Mazur, A.; Wielbo, J.; Koper, P.; Żebracki, K.; Kubik-Komar, A.; Janusz, G. RNA sequencing reveals differential gene expression of Cerrena unicolor in response to variable lighting conditions. Int. J. Mol. Sci. 2019, 20, 290. [Google Scholar] [CrossRef]
- Montibus, M.; Laëtitia, P.-G.; Florence, R.-F.; Christian, B.; Ponts, N. Coupling of transcriptional response to oxidative stress and secondary metabolism regulation in filamentous fungi. Crit. Rev. Microbiol. 2015, 41, 295–308. [Google Scholar] [CrossRef]
- Mattila, H.; Österman-Udd, J.; Mali, T.; Lundell, T. Basidiomycota fungi and ROS: Genomic perspective on key enzymes involved in generation and mitigation of reactive oxygen species. Front. Fungal Biol. 2022, 3, 837605. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.; Hansberg, W.; Navarro, R. Fungal responses to reactive oxygen species. Med. Mycol. 2006, 44, S101–S107. [Google Scholar] [CrossRef] [PubMed]
- Jaszek, M.; Żuchowski, J.; Dajczak, E.; Cimek, K.; Gra̢z, M.; Grzywnowicz, K. Ligninolytic enzymes can participate in a multiple response system to oxidative stress in white-rot basidiomycetes: Fomes fomentarius and Tyromyces pubescens. Int. Biodeterior. Biodegrad. 2006, 58, 168–175. [Google Scholar] [CrossRef]
- Blanchette, R.A.; Nilsson, T.; Daniel, G.; Abad, A. Biological degradation of wood. In Archaeological Wood; American Chemical Society: Washington, DC, USA, 1989; Volume 225, pp. 141–174. [Google Scholar]
- Kiviniemi, E.; Mikkola, A.; Mattila, H.; Wahlsten, M.; Lundell, T. Oxidative stress and culture atmosphere effects on bioactive compounds and laccase activity in the white rot fungus Phlebia radiata on birch wood substrate. Curr. Res. Microb. Sci. 2024, 7, 100280. [Google Scholar] [CrossRef]
- Borges, J.; Cardoso, P.; Lopes, I.; Figueira, E.; Venâncio, C. Exploring the potential of white-rot fungi exudates on the amelioration of salinized soils. Agriculture 2023, 13, 382. [Google Scholar] [CrossRef]
- Jaszek, M.; Grzywnowicz, K.; Malarczyk, E.; Leonowicz, A. Enhanced extracellular laccase activity as a part of the response system of white rot fungi: Trametes versicolor and Abortiporus biennis to paraquat-caused oxidative stress conditions. Pestic. Biochem. Physiol. 2006, 85, 147–154. [Google Scholar] [CrossRef]
- Leonowicz, A.; Cho, N.S.; Luterek, J.; Wilkolazka, A.; Wojtas-Wasilewska, M.; Matuszewska, A.; Hofrichter, M.; Wesenberg, D.; Rogalski, J. Fungal laccase: Properties and activity on lignin. J. Basic Microbiol. 2001, 41, 185–227. [Google Scholar] [CrossRef]
- Jaszek, M.; Miłek, J.; Żuchowski, J.; Stefaniuk, D.; Prendecka, M. Effective and complex stimulation of the biodegradation system of fungus Cerrena unicolor by rapeseed meal fermentation. Acta Biochim. Pol. 2016, 63, 549–554. [Google Scholar] [CrossRef]
- Grąz, M.; Jarosz-Wilkołazka, A.; Janusz, G.; Mazur, A.; Wielbo, J.; Koper, P.; Żebracki, K.; Kubik-Komar, A. Transcriptome-based analysis of the saprophytic fungus Abortiporus biennis—Response to oxalic acid. Microbiol. Res. 2017, 199, 79–88. [Google Scholar] [CrossRef]
- Rodríguez-Couto, S. Industrial and environmental applications of white-rot fungi. Mycosphere 2017, 8, 456–466. [Google Scholar] [CrossRef]
- Jarosz-Wilkołazka, A.; Grąz, M.; Braha, B.; Menge, S.; Schlosser, D.; Krauss, G.-J. Species-specific Cd-stress response in the white rot basidiomycetes Abortiporus biennis and Cerrena unicolor. Biometals 2006, 19, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Neish, B.; Trett, M.W.; Best, J.G.; Weightman, A.J.; Morgan, P.; Fry, J.C. Comparison of microbial and meiofaunal community analyses for determining impact of heavy metal contamination. J. Microbiol. Methods 2001, 45, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.G.; Paton, G.I.; Campbell, C.D. Combined microbial community level and single species biosensor responses to monitor recovery of oil polluted soil. Soil Biol. Biochem. 2004, 36, 1149–1159. [Google Scholar] [CrossRef]
- Pankhurst, C.E.; Yu, S.; Hawke, B.G.; Harch, B.D. Capacity of fatty acid profiles and substrate utilization patterns to describe differences in soil microbial communities associated with increased salinity or alkalinity at three locations in South Australia. Biol. Fertil. Soils 2001, 33, 204–217. [Google Scholar] [CrossRef]
- Chou, H.-Y.; Chiang, M.W.-L.; Lin, W.-R.; Hsieh, S.-Y.; Jones, E.B.G.; Guo, S.-Y.; Pang, K.-L. Metabolic activity on Biolog FF MicroPlate suggests organic substrate decomposition by Aspergillus terreus NTOU4989 isolated from Kueishan Island Hydrothermal Vent Field, Taiwan. Fungal Ecol. 2022, 60, 101157. [Google Scholar] [CrossRef]
- Liu, S.; He, L.; Yao, K. The Antioxidative function of alpha-ketoglutarate and its applications. BioMed Res. Int. 2018, 2018, 3408467. [Google Scholar] [CrossRef]
- Emri, T.; Antal, K.; Varga, K.; Gila, B.C.; Pócsi, I. The Oxidative stress response highly depends on glucose and iron availability in Aspergillus fumigatus. J. Fungi 2024, 10, 221. [Google Scholar] [CrossRef]
- Pawlik, A.; Jaszek, M.; Swatek, A.; Ruminowicz-Stefaniuk, M.; Ciołek, B.; Mazur, A.; Janusz, G. Lighting conditions influence the dynamics of protease synthesis and proteasomal activity in the white rot fungus Cerrena unicolor. Biomolecules 2020, 10, 1322. [Google Scholar] [CrossRef]
- Simaan, H.; Lev, S.; Horwitz, B.A. Oxidant-sensing pathways in the responses of fungal pathogens to chemical stress signals. Front. Microbiol. 2019, 10, 567. [Google Scholar] [CrossRef]
- Saini, N.; Anmol, A.; Kumar, S.; Wani, A.W.; Bakshi, M.; Dhiman, Z. Exploring phenolic compounds as natural stress alleviators in plants- a comprehensive review. Physiol. Mol. Plant Pathol. 2024, 133, 102383. [Google Scholar] [CrossRef]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Shalaby, S.; Horwitz, B.A. Plant phenolic compounds and oxidative stress: Integrated signals in fungal–plant interactions. Curr. Genet. 2015, 61, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Abdelshafy, A.M.; Tarun, B.; Ze, L.; Lei, W.; Dong, L.; Zisheng, L.; Li, L. A comprehensive review on phenolic compounds from edible mushrooms: Occurrence, biological activity, application and future prospective. Crit. Rev. Food Sci. Nutr. 2022, 62, 6204–6224. [Google Scholar] [CrossRef]
- Halabura, M.I.W.; Avelino, K.V.; Araújo, N.L.; Kassem, A.S.S.; Seixas, F.A.V.; Barros, L.; Fernandes, Â.; Liberal, Â.; Ivanov, M.; Soković, M.; et al. Light conditions affect the growth, chemical composition, antioxidant and antimicrobial activities of the white-rot fungus Lentinus crinitus mycelial biomass. Photochem. Photobiol. Sci. 2023, 22, 669–686. [Google Scholar] [CrossRef]
- Gómez-Toribio, V.; García-Martín, A.B.; Martínez, M.J.; Martínez, Á.T.; Guillén, F. Induction of extracellular hydroxyl radical production by white-rot fungi through quinone redox cycling. Appl. Environ. Microbiol. 2009, 75, 3944–3953. [Google Scholar] [CrossRef]
- Yu, Z.; Streng, C.; Seibeld, R.F.; Igbalajobi, O.A.; Leister, K.; Ingelfinger, J.; Fischer, R. Genome-wide analyses of light-regulated genes in Aspergillus nidulans reveal a complex interplay between different photoreceptors and novel photoreceptor functions. PLoS Genet. 2021, 17, e1009845. [Google Scholar] [CrossRef]
- Corrochano, L.M. Light in the fungal world: From photoreception to gene transcription and beyond. Annu. Rev. Genet. 2019, 53, 149–170. [Google Scholar] [CrossRef]
- Adam, A.; Deimel, S.; Pardo-Medina, J.; García-Martínez, J.; Konte, T.; Limón, M.C.; Avalos, J.; Terpitz, U. Protein activity of the Fusarium fujikuroi rhodopsins CarO and OpsA and their relation to fungus–plant interaction. Int. J. Mol. Sci. 2018, 19, 215. [Google Scholar] [CrossRef]
- García-Martínez, J.; Brunk, M.; Avalos, J.; Terpitz, U. The CarO rhodopsin of the fungus Fusarium fujikuroi is a light-driven proton pump that retards spore germination. Sci. Rep. 2015, 5, 7798. [Google Scholar] [CrossRef]
- Verde-Yáñez, L.; Usall, J.; Teixidó, N.; Vall-llaura, N.; Torres, R. Deciphering the effect of light wavelengths in Monilinia spp. DHN-melanin production and their interplay with ROS metabolism in M. fructicola. J. Fungi 2023, 9, 653. [Google Scholar] [CrossRef]
- Yu, Z.; Fischer, R. Light sensing and responses in fungi. Nat. Rev. Microbiol. 2019, 17, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Z.; Armant, O.; Fischer, R. Fungi use the SakA (HogA) pathway for phytochrome-dependent light signalling. Nat. Microbiol. 2016, 1, 16019. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Aguirre, J.; Herrera-Estrella, A.; Corrochano, L.M. The complexity of fungal vision. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Dantas, A.D.S.; Day, A.; Ikeh, M.; Kos, I.; Achan, B.; Quinn, J. Oxidative stress responses in the human fungal pathogen, Candida albicans. Biomolecules 2015, 5, 142–165. [Google Scholar] [CrossRef]
- Aza, P.; Camarero, S. Fungal laccases: Fundamentals, engineering and classification update. Biomolecules 2023, 13, 1716. [Google Scholar] [CrossRef]
- Angelova, M.B.; Pashova, S.B.; Spasova, B.K.; Vassilev, S.V.; Slokoska, L.S. Oxidative stress response of filamentous fungi induced by hydrogen peroxide and paraquat. Mycol. Res. 2005, 109, 150–158. [Google Scholar] [CrossRef]
- Pawlik, A.; Jaszek, M.; Sulej, J.; Janusz, G. Light-regulated synthesis of extra—and intracellular enzymes related to wood degradation by the white rot fungus Cerrena unicolor during solid-state fermentation on ash sawdust-based medium. Acta. Biochim. Pol. 2019, 66, 419–425. [Google Scholar] [CrossRef]
- Ikehata, K.; Buchanan, I.D.; Smith, D.W. Recent developments in the production of extracellular fungal peroxidases and laccases for waste treatment. J. Environ. Eng. Sci. 2004, 3, 1–19. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Zhao, L.; Ding, Z.; Ma, H.; Terry, N. Fungal laccase production from lignocellulosic agricultural wastes by solid-state fermentation: A review. Microorganisms 2019, 7, 665. [Google Scholar] [CrossRef]
- Kavitha, S.; Chandra, T.S. Oxidative stress protection and glutathione metabolism in response to hydrogen peroxide and menadione in riboflavinogenic fungus Ashbya gossypii. Appl. Biochem. Biotechnol. 2014, 174, 2307–2325. [Google Scholar] [CrossRef]
- Krsmanović, N.; Rašeta, M.; Mišković, J.; Bekvalac, K.; Bogavac, M.; Karaman, M.; Isikhuemhen, O.S. Effects of UV stress in promoting antioxidant activities in fungal species Trametes versicolor (L.) Lloyd and Flammulina velutipes (Curtis) Singer. Antioxidants 2023, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Grzywnowicz, K.; Leonowicz, A. Fungal culture collection [FCL—current list]. Biotechnologia 1995, 1, 202–214. [Google Scholar]
- Lindeberg, G.; Holm, G. Occurrence of tyrosinase and laccase in fruit bodies and mycelia of some Hymenomycetes. Physiol. Plant. 1952, 5, 100–114. [Google Scholar] [CrossRef]
- Oszust, K.; Panek, J.; Pertile, G.; Siczek, A.; Oleszek, M.; Frąc, M. Metabolic and genetic properties of Petriella setifera precultured on waste. Front. Microbiol. 2018, 9, 115. [Google Scholar] [CrossRef]
- Paździoch-Czochra, M.; Malarczyk, E.; Sielewiesiuk, J. Relationship of demethylation processes to veratric acid concentration and cell density in cultures of Rhodococcus erythropolis. Cell Biol. Int. 2003, 27, 325–336. [Google Scholar] [CrossRef]
- Malarczyk, E. Transformation of phenolic acids by Nocardia. Acta Microbiol. Pol. 1989, 38, 45–53. [Google Scholar]
- Paduch, R.; Matysik, G.; Wójciak–Kosior, M.; Kandefer–Szerszeń, M.; Skalska–Kamińska, A.; Nowak–Kryska, M.; Niedziela, P. Lamium album extracts express free radical scavenging and cytotoxic activities. Pol. J. Environ. Stud. 2008, 17, 569–580. [Google Scholar]
- Leonowicz, A.; Grzywnowicz, K. Quantitative estimation of laccase forms in some white-rot fungi using syringaldazine as a substrate. Enzym. Microb. Technol. 1981, 3, 55–58. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Wayne, L.G.; Diaz, G.A. A double staining method for differentiating between two classes of mycobacterial catalase in polyacrylamide electrophoresis gels. Anal. Biochem. 1986, 157, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Kirk, T.K.; Harkin, J.M.; Cowling, E.B. Degradation of the lignin model compount springgylglycol-β-guaiacyl ether by Polyporus versicolor and Stereum frustulatum. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1968, 165, 145–163. [Google Scholar] [CrossRef]
- Tanaka, K.; Yoshimura, T.; Ichihara, A. role of substrate in reversible activation of proteasomes (multi-protease complexes) by sodium dodecyl sulfate. J. Biochem. 1989, 106, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Skoda, B.; Malek, L. Dry pea seed proteasome: Purification and enzymic activities. Plant Physiol. 1992, 99, 1515–1519. [Google Scholar] [CrossRef]
- Staszczak, M. The 26S proteasome of the lignin-degrading Basidiomycete Phlebia radiata. Enzym. Microb. Technol. 2007, 40, 347–353. [Google Scholar] [CrossRef]
- Lang, M.; Stober, F.; Lichtenthaler, H.K. Fluorescence emission spectra of plant leaves and plant constituents. Radiat. Environ. Biophys. 1991, 30, 333–347. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
| Lighting Conditions | Time Since Induction with Menadione | No MQ | MQ | No MQ vs. MQ Statistical Difference p-Value and Statistical Significance | No MQ and MQ Combined Groups |
|---|---|---|---|---|---|
| 24 h | 29.48 ± 4.99 | 29.29 ± 3.61 | 0.9609 | 29.39 ± 3.90 c,d,e | |
| Dark | 5 days | 11.28 ± 1.92 | 20.89 ± 3.81 | 0.0175 | 16.09 ± 5.91 a,b,c,d |
| 10 days | 13.26 ± 2.18 | 1.40 ± 1.86 | 0.0020 * | 7.33 ± 3.94 a | |
| 24 h | 21.44 ± 6.08 | 26.32 ± 6.44 | 0.3941 | 23.88 ± 6.21 b,c,d,e | |
| White | 5 days | 7.00 ± 3.15 | 27.39 ± 3.60 | 0.0018 * | 17.19 ± 11.57 a,b,c,d |
| 10 days | 22.14 ± 15.19 | 5.73 ± 1.42 | 0.1360 | 13.93 ± 13.19 a,b,c | |
| 24 h | 26.90 ± 8.28 | 32.02 ± 0.23 | 0.3446 | 29.46 ± 5.94 d,e | |
| Red | 5 days | 7.92 ± 0.46 | 26.86 ± 6.25 | 0.0064 | 17.39 ± 11.10 a,b,c,d |
| 10 days | 14.21 ± 2.85 | 12.20 ± 3.20 | 0.4615 | 13.21 ± 2.93 a,b | |
| 24 h | 25.85 ± 2.65 | 17.73 ± 2.31 | 0.0162 | 21.79 ± 4.97 a,b,c,d,e | |
| Blue | 5 days | 25.14 ± 5.09 | 22.19 ± 6.11 | 0.5564 | 23.67 ± 5.28 b,c,d,e |
| 10 days | 12.24 ± 1.84 | 0.00 | 0.0003 * | 6.12 ± 6.81 a | |
| 24 h | 18.38 ± 6.72 | 22.17 ± 5.52 | 0.4931 | 20.28 ± 5.88 a,b,c,d,e | |
| Green | 5 days | 32.47 ± 4.32 | 39.55 ± 15.85 | 0.4968 | 36.01 ± 11.09 e |
| 10 days | 10.86 ± 3.13 | 4.67 ± 0.39 | 0.0273 | 7.77 ± 3.94 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlik, A.; Rudawska, A.; Swatek, A.; Janusz, G.; Frąc, M.; Grąz, M.; Matuła, P.; Jaszek, M. Modulation of Abortiporus biennis Response to Oxidative Stress by Light as a New Eco-Friendly Approach with a Biotechnological Perspective. Int. J. Mol. Sci. 2025, 26, 5482. https://doi.org/10.3390/ijms26125482
Pawlik A, Rudawska A, Swatek A, Janusz G, Frąc M, Grąz M, Matuła P, Jaszek M. Modulation of Abortiporus biennis Response to Oxidative Stress by Light as a New Eco-Friendly Approach with a Biotechnological Perspective. International Journal of Molecular Sciences. 2025; 26(12):5482. https://doi.org/10.3390/ijms26125482
Chicago/Turabian StylePawlik, Anna, Adrianna Rudawska, Anita Swatek, Grzegorz Janusz, Magdalena Frąc, Marcin Grąz, Przemysław Matuła, and Magdalena Jaszek. 2025. "Modulation of Abortiporus biennis Response to Oxidative Stress by Light as a New Eco-Friendly Approach with a Biotechnological Perspective" International Journal of Molecular Sciences 26, no. 12: 5482. https://doi.org/10.3390/ijms26125482
APA StylePawlik, A., Rudawska, A., Swatek, A., Janusz, G., Frąc, M., Grąz, M., Matuła, P., & Jaszek, M. (2025). Modulation of Abortiporus biennis Response to Oxidative Stress by Light as a New Eco-Friendly Approach with a Biotechnological Perspective. International Journal of Molecular Sciences, 26(12), 5482. https://doi.org/10.3390/ijms26125482










