Abstract
Breast and endometrial cancer are prevalent and share both hormonal and environmental risk factors. This study aimed to identify shared germline genetic risk loci for these cancers. In total, 1116 endometrial cancer cases, 3200 breast cancer cases, and 5021 healthy controls were included in a merged sliding window haplotype genome-wide association study (GWAS). This analysis employed a logistic regression model in PLINK v1.07. The results from this merged analysis were compared with previous individual analyses of the same samples. The analysis identified three loci that influenced both the risk of breast and endometrial cancer: 8p21.1 (OR 2.1; p 1.6 × 10−8), 16q24.3 (OR 2.4; p 3.8 × 10−8) and 17q11.2 (OR 1.3; p 4.3 × 10−8). This combined haplotype GWAS of endometrial and breast cancers identified three loci associated with shared genetic risk, two of which were novel: 16q24.3 and 17q11.2. Further studies are warranted to replicate these findings and to determine its pathophysiological role and future clinical implications.
1. Introduction
Breast (BC) and endometrial cancer (EC) affect over 2.7 million women annually [1]. Both cancers are estrogen-dependent tumors and share hormonal and environmental risk factors. Key risk factors include early menarche, late menopause, delayed first birth, nulliparity, prolonged menopausal hormone therapy, and obesity, all of which contribute to an increased risk of these cancers [2,3,4,5,6,7].
Moderate to high-risk germline pathogenic variants contribute to BC and EC risk independently. In many countries, variant screening of susceptibility genes (BRCA1, BRCA2, PALB2, TP53, STK11, CDH1, PTEN, ATM, BARD1, RAD51C, RAD51D and CHEK2 for BC and the DNA mismatch-repair genes, MLH1, MSH2, MSH6, and PMS2, in Lynch syndrome for EC) are offered in clinical practice of high-risk women [8,9,10]. Furthermore, genome-wide association studies (GWASs) have identified susceptibility loci that individually confer a low increase in risk for both cancers [11,12].
Von Wachenfeldt et al., 2007 demonstrated an over-representation of EC among Swedish families with a history of BC, which was further supported by a tumor spectrum study of non-BRCA BC families and an epidemiological registry-based study on familial cancer from the Swedish Multi-Generation registry [13,14,15]. A large American cohort study also revealed an overrepresentation of EC in individuals with prior breast cancer diagnosis, even after adjusting for shared environmental risk factors, suggesting a shared genetic risk could be involved [16]. Thus, besides environmental risk factors, EC and BC may share genetic susceptibility loci. Despite known variants in PTEN, which are rare (1:200,000), much remains unclear regarding the shared heritable factors between EC and BC, highlighting the need for new approaches to address this question [17].
Previously, we demonstrated the feasibility of using haplotype analysis alongside SNP analysis to identify novel risk loci for colorectal cancer, EC and BC independently within Swedish study populations [18,19,20]. By applying the same method and a comparable sample size, we conducted a haplotype GWAS to identify chromosomal regions that may harbor potential shared susceptibility loci for EC and BC. This analysis included Swedish cases of EC (N = 1116) and BC (N = 3200), along with controls (N = 5021). These samples were previously used together with various populations in large independent SNP GWASs in collaboration with the Endometrial Cancer Association Consortium (ECAC) and the Breast Cancer Association Consortium (BCAC) [11,12]. To confirm a combined effect of EC and BC, we compared the results with previous individual analyses of the same samples [18,19].
2. Results
In the combined analysis of EC and BC, we identified three candidate risk loci: 8p21.2, 16q24.3 and 17q11.2. In stage one, we identified six significant susceptibility loci for EC and BC on chromosome 8, 10, 11, 16 (two loci) and 17 (Table 1, Tables S1–S23).

Table 1.
Statistically significant risk loci in a combined endometrial and breast cancer GWAS.
In stage two, we compared the results of identical haplotypes from the present combined EC–BC analysis with the previous individual analysis of the same EC and BC samples and controls to examine a potential combined effect [18,19]. Three of the loci—8p21.2, 16q24.3, and 17q11.2—demonstrated lower p-values in the combined analysis compared to the previous individual EC and BC analyses, suggesting a shared genetic risk within these loci (Table 2). Notably, the latter loci did not show significant associations in previous independent analyses. In contrast, three well-known BC risk loci—10q26.13, 11q13.3, and 16q12.1—exhibited higher p-values in the combined analysis compared to previous individual BC analyses, indicating that the inclusion of EC samples diluted the association in these three regions. Moreover, one of the loci with combined EC–BC effect spanned multiple genes, and two loci spanned a single gene (Table 1, Figure 1).

Table 2.
Results from combined and individual endometrial and breast cancer GWASs.
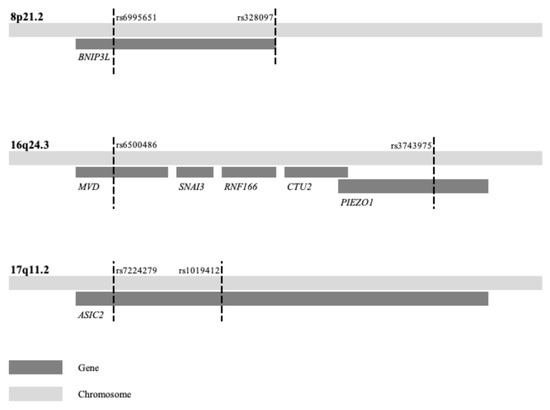
Figure 1.
Three loci associated with combined endometrial and breast cancer risk. The first and last SNP of the haplotypes listed in Table 1 are here indicated by dashed lines and variant rs-name along with gene(s) within the locus.
3. Discussion
We identified three candidate risk loci associated with EC and BC at 8p21.2, 16q24.3 and 17q11.2. The odds ratios (ORs) for these novel loci were similar in both the combined and individual analyses. The larger combined analysis likely allowed these regions to reach the significant threshold, which was not achievable in the individual analyses due to the rarity of the haplotypes, particularly at 8p21.2 and 16q24.3 (Table 1). The locus at 8p21.2 has previously been identified in an individual BC GWAS, using the same BC samples [18]. The other three significant loci (the known BC loci 10q26.13, 11q13.3, and 16q12.1) exhibited higher p-values in the combined analysis compared to the previous individual analyses of EC and BC, suggesting no shared EC–BC effect in these loci (Table 2) [11,19]. This was consistent with two targeted EC GWAS investigating a total of 16 known BC susceptibility loci, which reported no significant or inverse association between EC and BC at these loci [21,22]. Replication of the three novel candidate EC–BC loci, along with complementary analyses, such as sequencing and functional studies, is warranted for future research.
Initially, known hereditary cancer syndromes and their associated pathogenic variants were thought to be related to a single tumor type, such as BRCA1/2 in BC and APC or the DNA mismatch repair genes in colorectal cancer [23,24,25,26]. However, it has become increasingly clear that these variants are linked to an elevated risk across multiple tumor types [27,28,29]. To our knowledge, this GWAS is the only study employing a genome-wide syndrome approach that combines EC and BC to search for shared genetic risk. However, a meta-analysis of GWASs across various tumor types with a targeted approach, including EC and BC, identified associations between EC and BC with three SNPs on chromosome 2 and inverse association with one SNP on the same chromosome [30]. Notably, none of these four SNPs were analyzed in this study. Furthermore, EC is the most frequently observed secondary malignancy among BC survivors, which may partly reflect the adjuvant use of tamoxifen, known for its pro-estrogenic effect on the endometrium [31,32,33]. However, Sung et al. demonstrated a similar increase in risk following hormone receptor-negative BC, which is not treated with tamoxifen, suggesting that this increase may represent a shared genetic risk [34].
Several genes located in the three novel EC–BC loci have previously been discussed in the context of cancer, although many have not been reported to be involved in EC and BC. The gene BNIP3L at 8p21.2 has been suggested to have a tumor suppressive effect in various cancer types [35]. The locus 8p21.2 was early detected with loss of heterozygosity (LOH) in BC; prostate and ovarian cancer and somatic deletions in this region have been identified in EC and ovarian cancer [36,37,38,39]. Previous individual BC GWASs identified one significant haplotype at 8p21, while the present combined EC–BC GWAS revealed eight with a shared central haplotype and similar ORs but with various lengths, representing the same genetic loci. The mitophagy receptor BNIP3 is highly expressed in various cancers, including BC and EC, and functions as a tumor suppressor gene [40,41]. The gene BNIP3L (BNIP3-like) shares similar properties with BNIP3 [40,42]. One study using HCT116 cells demonstrated that BNIP3 is activated by the estrogen receptor beta (ERβ), promoting autophagy [43]. Furthermore, ERβ-mediated degradation of CyclinD1 was suggested to inhibit colon cancer cell growth via autophagy [43].
The locus at 16q24.3 is a known region of LOH associated with BC and prostate cancer [44,45]. This region contains five genes (Table 1, Figure 1). Among them, MVD, a member of the mevalonate pathway, interacts with RAC3 in HER2-positive breast cancer [46]. When hypomethylated, RAC3 can promote cell proliferation and invasion by increasing FASN expression in EC, potentially serving as a link between these two cancer types. [47]. The genes SNAI3 and PIEZO1, which are known to promote epithelial–mesenchymal transition (EMT), are noteworthy. EMT is a process that enables epithelial cells to acquire migratory and invasive properties, playing a crucial role in cancer progression, and it is considered a potential target for cancer therapy [48]. A genome-wide association may represent either a direct gene effect or an indirect effect through modifying genes influenced by environmental risk factors. Most EC and BC are estrogen-dependent tumors, and potential modifying gene effects could arise from variations in the estrogenic pathway. None of the genes in the novel EC–BC loci have been previously reported as hormonal modifiers, but the G protein-coupled estrogen receptor signaling has been suggested to suppress PIEZO1, which may influence proliferation [49]. Interestingly, PIEZO1 and WNT3 are often concurrently upregulated, and activation of WNT3 has been implicated in both BC and EC [50,51,52]. These relationships suggest that estrogen may modulate gene regulation and activate pathways, such as WNT3, contributing to the progression of both cancers. Furthermore, the nearby CTU2 gene has been implicated in hepatocellular carcinoma development [53]. Although the majority of EC and BC are hormone-dependent, there are triple negative BC and non-hormonal dependent HER2positive BC as well as type 2 EC which are not [54,55]. This study is based on unselected EC and BC cases; further studies on selected hormone-dependent EC and BC cases could be a future approach.
The locus at 17q11.2 has been highlighted in previous LOH studies related to BC [36]. Additionally, a study of Swedish high-risk BC families (≥3 first or second degree relatives of BC) identified a shared haplotype in this region [56], which was further supported by a sequence variant in the exome of the gene ASIC2 (previously known as ACCN1) [56]. The ASIC2 gene has been suggested to be involved in metastasis in triple-negative breast cancer and shown to promote metastasis in colorectal cancer through activation of the calcineurin/NFAT1 axis [57,58]. Another study highlighted a novel mechanism regulating IL-11 expression in endometrial adenocarcinoma cells via a prostaglandin receptor and calcineurin-1 [59]. Given that estrogen upregulates calcium-related proteins, this pathway could represent a potential link between BC and EC. The locus at 17q11.2 has previously been reported to be associated with EC and BC separately but at a distance from the current locus. An EC GWAS identified a susceptibility locus located 2 Mb away [12]. It is unlikely, but not excluded, that these represent the same genetic risk locus. Functional studies, such as mouse models, with gene knockout via CRISPR-Cas9 or modulating RNA expression with RNA interference, could be valuable methods for investigating the roles of these specific genes in cancer progression. Additionally, RNA expression analysis of tumor samples could also provide useful insights.
In our previous haplotype GWASs, we identified novel susceptibility loci with higher ORs, ranging from 1.27 to 3.6, than those generally reported in GWAS of single variants, a finding that is also supported by this study [18,20,60,61]. The higher ORs may be attributed to the multiallelic haplotype strategy, which enables the capture of the associated region of interest—specifically, an SNP with designated neighboring variants (a haplotype). Since haplotype analyses require homogeneous populations, this is not always feasible; however, it has been achieved with these Swedish samples. The significance level can be debated, given that multiple tests were conducted in each window. In this study, no corrections for multiple testing were performed, as the haplotypes of various lengths within a region represent the same genetic loci. This can be exemplified by the locus 8p21.2, where eight significant (along with several insignificant) haplotypes with similar odds ratios share a common central haplotype. A similar phenomenon is observed at all three loci, although the number of significant haplotypes varies. We propose that the haplotype with the lowest p-value most accurately delineates the area of interest (Table 1). The underlying biological mechanism is likely associated with the cycles of homologous recombination that occur, which subsequently “condense” the genetic risk. Therefore, conventional genome-wide significance, with a p < 5 × 10−8, was applied.
The strength of this study is the relatively homogenous and large Swedish EC and BC cohorts which enabled comprehensive haplotype analysis. In addition, all SNPs and haplotypes were examined without preconceived assumptions, highlighting potential areas that previously targeted GWASs or limited analyses of known BC regions may have missed. However, a limitation of this study is that it included individuals affected by either EC or BC, without considering other cancers or family history of cancer among the cases. Ideally, an equal number of EC and BC cases should have been selected for this syndrome approach GWAS. The lower number of EC cases (n = 1133) compared to BC cases (n = 3215) in this study may have introduced bias and led to an underestimation of loci associated with a higher risk for EC than for BC. Furthermore, a concern regarding the reproducibility of these findings in other populations is the known global diversity in genetic backgrounds [62]. This study is based on Swedish cases and controls. Therefore, future haplotype GWASs in diverse populations are warranted.
4. Materials and Methods
4.1. Study Population
In this study, we included 3215 invasive BC cases, 1133 invasive EC cases, and 5032 controls (Figure 2). The BC cases were obtained from three Swedish cohorts: KARMA (n = 2712), KARBAC1 (n = 394) and KARBAC2 (n = 109) [14,63,64]. The KARMA cohort is a Swedish population-based cohort that underwent a screening or clinical mammogram in Sweden between October 2010 and March 2013 [63]. KARBAC1 (N = 394) is a Swedish hospital-based cohort of consecutive breast patients recruited from October 1998 to May 2000 [64]. KARBAC2 (N = 109) is a cohort with germline BRCA1/BRCA2 negative Swedish BC cases recruited from a clinical genetic counselling department between February 2000 and January 2012 [14]. The EC cases were obtained from two Swedish cohorts—RENDOCAS (n = 555) and CAHRES (n = 578) [65,66]. RENDOCAS is a hospital-based cohort of Swedish consecutive EC cases who underwent surgery between 2008 and 2011 [65]. CAHRES is a Swedish population-based nationwide cohort recruited between January 1994 and December 1995 [66]. The controls were sourced from the KARMA cohort, consisting of healthy Swedish women aged 40–74 years who underwent mammographic screening. All five cohorts have been previously analyzed in single nucleotide GWAS for EC or BC separately as part of the global collaborations of BCAC and ECAC [11,12]. Based on our previous Swedish haplotype GWAS with comparable sample sizes of endometrial, breast, and colorectal cancers, we were confident that the study was adequately powered.
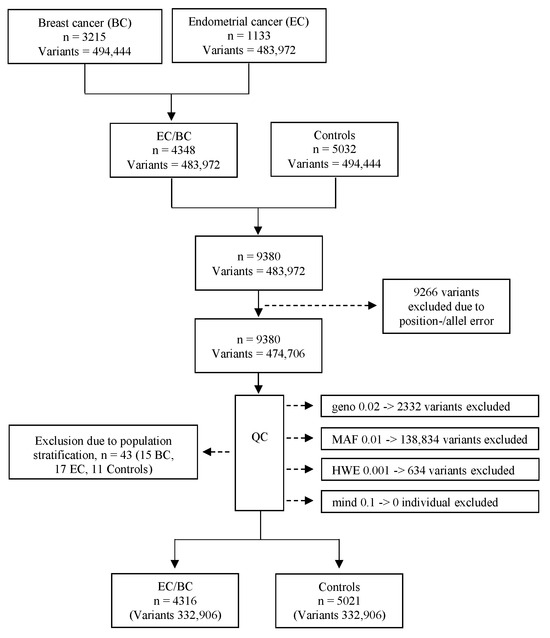
Figure 2.
Flowchart of included individuals and variants. n = individuals; Geno 0.02 = excluded variants with a genotyping rate < 2%; MAF 0.01 = excluded variants with a minor allele frequency < 0.01; HWE 0.001 = excluded variants that failed the Hardy–Weinberg equilibrium test at p < 0.001; mind 0.1 = excluded individuals with missing genotypes > 10%.
All studies were approved by the local ethical boards, and all individuals gave written informed consent. Specifically, the approvals were as follows: KARMA—Regional Ethics Committee, Stockholm, Dnr 2010/958-31/1; KARBAC1—Ethical Committee of Karolinska Institutet, Dnr 98-232; KARBAC2—Regional Ethical Committee, Stockholm, Dnr 2010/1156-31/2 Dnr 2012/1453-32 and 2011/1686-32; RENDOCAS—Regional Ethical Committee, Stockholm, Dnr 2010/1536-31/2; CAHRES—Ethical Committee of Karolinska Institutet, Dnr 98-036.
4.2. Genotyping and Quality Control
Individuals were genotyped using the Illumina Infinium OncoArray-500K B Bead-Chip. The five cohorts shared a total of 483,972 SNPs, and the datasets were merged using PLINK v1.9. During quality control (QC), 2332 variants were excluded due to a genotype call rate of less than 98% (Geno 0.02), 138,834 variants were removed for having a minor allele frequency of less than 0.01 (MAF 0.01), and 634 variants were excluded for deviating from the Hardy–Weinberg equilibrium at p < 0.001 (HWE 0.001). No individual was excluded due to missing genotype data (Figure 2).
To identify ethnic outliers, a multidimensional scaling (MDS) was performed on the remaining individuals and variants across four dimensions using predefined MDS coordinates 1 (C1), C2, C3 or C4. Samples with MDS values above +0.04 or below −0.04 were classified as ethnic outliers and subsequently excluded from the analysis (15 BC cases, 17 EC cases, and 11 controls). The final dataset consisted of 4316 cases (1116 EC and 3200 BC), 5021 controls, and a total of 332,906 variants (Figure 2). The reference genome panel GRCh37 was utilized.
4.3. Statistical Methods
To examine the associations between haplotypes of various lengths (exposure) and the outcomes of EC and BC, we conducted a sliding window haplotype GWAS with window sizes ranging from 1 to 25 SNPs in PLINK v1.07 [67]. The sliding window strategy was chosen to define the candidate region of interest from the first to the last SNP, with window sizes ranging from 1 to 25 SNPs. This range was chosen based on previous studies, which showed that the sizes of the candidate regions varied, making this range appropriate [18,19,20]. We applied the default setting of minor haplotype frequency threshold of 0.01 in PLINK v.1.07, meaning that each haplotype or SNP with a frequency above 1% was tested individually against all other SNPs/haplotypes in this window exceeding this threshold [67,68].
To adjust for population stratification, we used a logistic regression model, where the MDS coordinates C1–C4 (refer to “Genotyping and quality control”) were included as covariates. ORs and p-values were calculated using the default settings for haplotype analysis in PLINK v1.07 [67]. The 95% confidence intervals for the haplotypes reported in Table 1 were calculated manually from PLINK output based on OR and “STAT”. The aim of this study was to identify candidate loci associated with EC and BC risk, so only loci with OR > 1 were reported. A genome-wide significance level of p < 5 × 10−8 was considered statistically significant [69]. The definition of a shared EC–BC candidate risk locus is statistically significant in the present combined analysis, with a lower p-value compared to previous individual analyses of EC and BC. No further correction for multiple testing beyond the genome-wide significance level was performed, as we assumed that all haplotypes of various lengths within each sublocus represented the same genetic risk locus.
5. Conclusions
In conclusion, this combined haplotype GWAS of EC and BC samples identified three loci associated with a shared genetic risk, two of which are novel. Further studies are warranted to replicate these findings, identify the variants, and integrate them into a polygenic risk score for use in clinical practice.
Supplementary Materials
The following supporting information can be downloaded at: https://doi.org/10.5281/zenodo.15239435, accessed on 17 April 2025.
Author Contributions
Conceptualization, E.B., A.v.W., S.M. and A.L.; methodology, E.B., W.L., M.A.F., S.M. and A.L.; software, W.L.; DNA samples and phenotypic data, P.H., E.T., M.M., S.M. and C.W.; formal analysis, E.B., M.A.F., S.M. and A.L.; writing—original draft preparation, E.B.; writing—review and editing, E.B., W.L., M.A.F., A.v.W., C.W., E.T., M.M., T.A.O., P.H., S.M. and A.L.; visualization, E.B.; supervision, S.M. and A.L.; project administration, E.B. and A.L.; funding acquisition, A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Swedish Cancer Society, grant number 18-0700 (A.L). Swedish Research Council, grant number 2019-01441 (A.L.); The Cancer Research Funds of Radiumhemmet, grant number 191203 (A.L.); and Stockholm County Council (ALFproject), grant number 500395 (A.L.). The KARMA study was funded by Märit and Hans Rausing’s Initiative Against Breast Cancer (P.H.), and the European Community’s Seventh Framework Programme under grant agreement 223175 (HEALTH-F2-2009-223175) (P.H.). OncoArray endometrial cancer analysis was supported by NHMRC project grants (ID#1031333 and ID#1109286). OncoArray genotyping of ECAC cases was performed with the generous assistance of the Ovarian Cancer Association Consortium (OCAC). The OCAC OncoArray genotyping project was funded by grants from the US National Institutes of Health (CA1X01HG007491-01, U19-CA148112, R01-CA149429, and R01-CA058598; Canadian Institutes of Health Research, MOP-86727; and the Ovarian Cancer Research Fund. CIDR genotyping for the OncoArray was conducted under contract, 268201200008I. OncoArray genotyping of the BCAC controls was funded by the Genome Canada Grant GPH-129344, NIH Grant U19 CA148065, and Cancer UK Grant C1287/A16563. T.A.O’M. was supported by an Australian National Health and Medical Research Council Fellowship (ID1111246 and ID1173170). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The approvals from the ethics committees are as follows: KARMA—Regional Ethics Committee, Stockholm, Dnr 2010/958-31/1; KARBAC1—Ethical Committee of Karolinska Institutet, Dnr 98-232; KARBAC2—Regional Ethical Committee, Stockholm, Dnr 2010/1156-31/2 Dnr 2012/1453-32 and 2011/1686-32; RENDOCAS—Regional Ethical Committee, Stockholm, Dnr 2010/1536-31/2; CAHRES—Ethical Committee of Karolinska Institutet, Dnr 98-036.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original data presented in the study are openly available in Zenodo at https://doi.org/10.5281/zenodo.15239435. Due to the Excel-sheet row limitation, the complete data are not included. The complete data of this study are available from the corresponding authors upon request.
Acknowledgments
The authors thank all individuals who took part in the KARMA, KARBAC, CAHRES and RENDOCAS studies as well as all clinicians, technicians, and administrative staff involved. BCAC, ECAC and Amanda Spurdle are acknowledged for enabling the genotyping to be carried out in the four cohorts.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BC | Breast cancer |
| BCAC | Breast Cancer Association Consortium |
| EC | Endometrial cancer |
| ECAC | Endometrial Cancer Association Consortium |
| GWAS | Genome-wide association study |
| LOH | Loss of heterozygosity |
| MDS | Multidimensional scaling |
| OR | Odds ratio |
| QC | Quality control |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef]
- Titus-Ernstoff, L.; Longnecker, M.P.; Newcomb, P.A.; Dain, B.; Greenberg, E.R.; Mittendorf, R.; Stampfer, M.; Willett, W. Menstrual factors in relation to breast cancer risk. Cancer Epidemiol. Biomark. Prev. 1998, 7, 783–789. [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: Collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet 2002, 360, 187–195. [Google Scholar] [CrossRef]
- Albrektsen, G.; Heuch, I.; Hansen, S.; Kvale, G. Breast cancer risk by age at birth, time since birth and time intervals between births: Exploring interaction effects. Br. J. Cancer 2005, 92, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J. Endogenous oestrogens and breast cancer risk in premenopausal and postmenopausal women. Steroids. 2011, 76, 812–815. [Google Scholar] [CrossRef]
- Endogenous Hormones and Breast Cancer Collaborative Group; Key, T.J.; Appleby, P.N.; Reeves, G.K.; Travis, R.C.; Alberg, A.J.; Barricarte, A.; Berrino, F.; Krogh, V.; Sieri, S.; et al. Sex hormones and risk of breast cancer in premenopausal women: A collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013, 14, 1009–1019. [Google Scholar]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Patel, M.M.; Adrada, B.E. Hereditary Breast Cancer: BRCA Mutations and Beyond. Radiol. Clin. N. Am. 2024, 62, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Graffeo, R.; Rana, H.Q.; Conforti, F.; Bonanni, B.; Cardoso, M.J.; Paluch-Shimon, S.; Pagani, O.; Goldhirsch, A.; Partridge, A.H.; Lambertini, M.; et al. Moderate penetrance genes complicate genetic testing for breast cancer diagnosis: ATM, CHEK2, BARD1 and RAD51D. Breast 2022, 65, 32–40. [Google Scholar] [CrossRef]
- Michailidou, K.; Lindstrom, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemacon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017, 551, 92–94. [Google Scholar] [CrossRef] [PubMed]
- O’Mara, T.A.; Glubb, D.M.; Amant, F.; Annibali, D.; Ashton, K.; Attia, J.; Auer, P.L.; Beckmann, M.W.; Black, A.; Bolla, M.K.; et al. Identification of nine new susceptibility loci for endometrial cancer. Nat. Commun. 2018, 9, 3166. [Google Scholar] [CrossRef]
- von Wachenfeldt, A.; Lindblom, A.; South Swedish Oncogenetic Study Group; Gronberg, H.; Einbeigi, Z.; Rosenquist, R.; Gardman, C.; Iselius, L. A hypothesis-generating search for new genetic breast cancer syndromes—A national study in 803 Swedish families. Hered. Cancer Clin. Pract. 2007, 5, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Wendt, C.; Lindblom, A.; Arver, B.; von Wachenfeldt, A.; Margolin, S. Tumour spectrum in non-BRCA hereditary breast cancer families in Sweden. Hered. Cancer Clin. Pract. 2015, 13, 15. [Google Scholar] [CrossRef]
- Zheng, G.; Yu, H.; Hemminki, A.; Forsti, A.; Sundquist, K.; Hemminki, K. Familial associations of female breast cancer with other cancers. Int. J. Cancer 2017, 141, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Kazerouni, N.; Schairer, C.; Friedman, H.B.; Lacey, J.V., Jr.; Greene, M.H. Family history of breast cancer as a determinant of the risk of developing endometrial cancer: A nationwide cohort study. J. Med. Genet. 2002, 39, 826–832. [Google Scholar] [CrossRef][Green Version]
- Yehia, L.; Keel, E.; Eng, C. The Clinical Spectrum of PTEN Mutations. Annu. Rev. Med. 2020, 71, 103–116. [Google Scholar] [CrossRef]
- Barnekow, E.; Liu, W.; Helgadottir, H.T.; Michailidou, K.; Dennis, J.; Bryant, P.; Thutkawkorapin, J.; Wendt, C.; Czene, K.; Hall, P.; et al. A Swedish Genome-Wide Haplotype Association Analysis Identifies a Novel Breast Cancer Susceptibility Locus in 8p21.2 and Characterizes Three Loci on Chromosomes 10, 11 and 16. Cancers 2022, 14, 1206. [Google Scholar] [CrossRef]
- Barnekow, E.; Liu, W.; Andersson, E.; Wang, X.; Helgadottir, H.T.; Thutkawkorapin, J.; Barilla, S.; Vermani, L.; Mints, M.; Tham, E.; et al. A Swedish genome-wide haplotype association analysis identifies novel candidate loci associated with endometrial cancer risk. PLoS ONE 2025, 20, e0316086. [Google Scholar] [CrossRef]
- Barot, S.; Vermani, L.; Blom, J.; Larsson, S.; Liljegren, A.; Lindblom, A. Candidate Genetic Loci Modifying the Colorectal Cancer Risk Caused by Lifestyle Risk Factors. Clin. Transl. Gastroenterol. 2025, 16, e00790. [Google Scholar] [CrossRef]
- McGrath, M.; Lee, I.M.; Buring, J.; Hunter, D.J.; De Vivo, I. Novel breast cancer risk alleles and endometrial cancer risk. Int. J. Cancer 2008, 123, 2961–2964. [Google Scholar] [CrossRef]
- Healey, C.S.; Ahmed, S.; ANECS; AOCS Management Group; O’Mara, T.A.; Ferguson, K.; Lambrechts, D.; Garcia-Dios, D.A.; Vergote, I.; Amant, F.; et al. Breast cancer susceptibility polymorphisms and endometrial cancer risk: A Collaborative Endometrial Cancer Study. Carcinogenesis 2011, 32, 1862–1866. [Google Scholar] [CrossRef]
- Miki, Y.; Swensen, J.; Shattuck-Eidens, D.; Futreal, P.A.; Harshman, K.; Tavtigian, S.; Liu, Q.; Cochran, C.; Bennett, L.M.; Ding, W.; et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994, 266, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Wooster, R.; Neuhausen, S.L.; Mangion, J.; Quirk, Y.; Ford, D.; Collins, N.; Nguyen, K.; Seal, S.; Tran, T.; Averill, D.; et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12–13. Science 1994, 265, 2088–2090. [Google Scholar] [CrossRef]
- Leach, F.S.; Nicolaides, N.C.; Papadopoulos, N.; Liu, B.; Jen, J.; Parsons, R.; Peltomaki, P.; Sistonen, P.; Aaltonen, L.A.; Nystrom-Lahti, M.; et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 1993, 75, 1215–1225. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Nicolaides, N.C.; Wei, Y.F.; Ruben, S.M.; Carter, K.C.; Rosen, C.A.; Haseltine, W.A.; Fleischmann, R.D.; Fraser, C.M.; Adams, M.D.; et al. Mutation of a mutL homolog in hereditary colon cancer. Science 1994, 263, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J. Natl. Cancer Inst. 1999, 91, 1310–1316. [Google Scholar] [CrossRef]
- Ford, D.; Easton, D.F.; Bishop, D.T.; Narod, S.A.; Goldgar, D.E. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet 1994, 343, 692–695. [Google Scholar] [CrossRef]
- Bonadona, V.; Bonaiti, B.; Olschwang, S.; Grandjouan, S.; Huiart, L.; Longy, M.; Guimbaud, R.; Buecher, B.; Bignon, Y.J.; Caron, O.; et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. Jama Requir. Manag. 2011, 305, 2304–2310. [Google Scholar]
- Lindstrom, S.; Wang, L.; Feng, H.; Majumdar, A.; Huo, S.; Macdonald, J.; Harrison, T.; Turman, C.; Chen, H.; Mancuso, N.; et al. Genome-wide analyses characterize shared heritability among cancers and identify novel cancer susceptibility regions. J. Natl. Cancer Inst. 2023, 115, 712–732. [Google Scholar] [CrossRef]
- Wijayabahu, A.T.; Egan, K.M.; Yaghjyan, L. Uterine cancer in breast cancer survivors: A systematic review. Breast Cancer Res. Treat. 2020, 180, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Chen, L. Second malignancies after breast cancer: The impact of adjuvant therapy. Mol. Clin. Oncol. 2014, 2, 331–336. [Google Scholar] [CrossRef]
- Swerdlow, A.J.; Jones, M.E.; British Tamoxifen Second Cancer Study Group. Tamoxifen treatment for breast cancer and risk of endometrial cancer: A case-control study. J. Natl. Cancer Inst. 2005, 97, 375–384. [Google Scholar] [CrossRef]
- Sung, H.; Freedman, R.A.; Siegel, R.L.; Hyun, N.; DeSantis, C.E.; Ruddy, K.J.; Jemal, A. Risks of subsequent primary cancers among breast cancer survivors according to hormone receptor status. Cancer 2021, 127, 3310–3324. [Google Scholar] [CrossRef]
- Poole, L.P.; Macleod, K.F. Mitophagy in tumorigenesis and metastasis. Cell. Mol. Life Sci. 2021, 78, 3817–3851. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Yu, J.C.; Lo, Y.L.; Kuo, C.H.; Yue, C.T.; Jou, Y.S.; Huang, C.S.; Lung, J.C.; Wu, C.W. Genome-wide search for loss of heterozygosity using laser capture microdissected tissue of breast carcinoma: An implication for mutator phenotype and breast cancer pathogenesis. Cancer Res. 2000, 60, 3884–3892. [Google Scholar] [PubMed]
- Lai, J.; Flanagan, J.; Phillips, W.A.; Chenevix-Trench, G.; Arnold, J. Analysis of the candidate 8p21 tumour suppressor, BNIP3L, in breast and ovarian cancer. Br. J. Cancer 2003, 88, 270–276. [Google Scholar] [CrossRef][Green Version]
- Kaveh, F.; Baumbusch, L.O.; Nebdal, D.; Borresen-Dale, A.L.; Lingjaerde, O.C.; Edvardsen, H.; Kristensen, V.N.; Solvang, H.K. A systematic comparison of copy number alterations in four types of female cancer. BMC Cancer 2016, 16, 913. [Google Scholar] [CrossRef]
- Zeegers, M.P.; Nekeman, D.; Khan, H.S.; van Dijk, B.A.; Goldbohm, R.A.; Schalken, J.; Shajahan, S.; Pearlman, A.; Oddoux, C.; van den Brandt, P.A.; et al. Prostate cancer susceptibility genes on 8p21–23 in a Dutch population. Prostate Cancer Prostatic Dis. 2013, 16, 248–253. [Google Scholar] [CrossRef]
- Chourasia, A.H.; Macleod, K.F. Tumor suppressor functions of BNIP3 and mitophagy. Autophagy 2015, 11, 1937–1938. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Koukourakis, M.I.; Gatter, K.C.; Harris, A.L.; Sivridis, E. BNIP3 expression in endometrial cancer relates to active hypoxia inducible factor 1alpha pathway and prognosis. J. Clin. Pathol. 2008, 61, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Bellot, G.; Garcia-Medina, R.; Gounon, P.; Chiche, J.; Roux, D.; Pouyssegur, J.; Mazure, N.M. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell Biol. 2009, 29, 2570–2581. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, C.; Wu, H.; Huang, J. Estrogen Receptor Beta (ERbeta) Mediated-CyclinD1 Degradation via Autophagy Plays an Anti-Proliferation Role in Colon Cells. Int. J. Biol. Sci. 2019, 15, 942–952. [Google Scholar] [CrossRef]
- Rakha, E.A.; Green, A.R.; Powe, D.G.; Roylance, R.; Ellis, I.O. Chromosome 16 tumor-suppressor genes in breast cancer. Genes Chromosome. Cancer 2006, 45, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Harkonen, P.; Kyllonen, A.P.; Nordling, S.; Vihko, P. Loss of heterozygosity in chromosomal region 16q24.3 associated with progression of prostate cancer. Prostate 2005, 62, 267–274. [Google Scholar] [CrossRef]
- Crocamo, S.; Binato, R.; Dos Santos, E.C.; de Paula, B.; Abdelhay, E. Translational Results of Zo-NAnTax: A Phase II Trial of Neoadjuvant Zoledronic Acid in HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2022, 23, 15515. [Google Scholar] [CrossRef]
- Meijuan, C.; Fang, L.; Min, F.; Qian, W. Hypomethylated gene RAC3 induces cell proliferation and invasion by increasing FASN expression in endometrial cancer. Int. J. Biochem. Cell Biol. 2022, 150, 106274. [Google Scholar] [CrossRef]
- Dudas, J.; Ladanyi, A.; Ingruber, J.; Steinbichler, T.B.; Riechelmann, H. Epithelial to Mesenchymal Transition: A Mechanism that Fuels Cancer Radio/Chemoresistance. Cells 2020, 9, 428. [Google Scholar] [CrossRef]
- Sun, Y.; Leng, P.; Guo, P.; Gao, H.; Liu, Y.; Li, C.; Li, Z.; Zhang, H. G protein coupled estrogen receptor attenuates mechanical stress-mediated apoptosis of chondrocyte in osteoarthritis via suppression of Piezo1. Mol. Med. 2021, 27, 96. [Google Scholar] [CrossRef]
- Wu, R.W.; Lian, W.S.; Chen, Y.S.; Ko, J.Y.; Wang, S.Y.; Jahr, H.; Wang, F.S. Piezoelectric Microvibration Mitigates Estrogen Loss-Induced Osteoporosis and Promotes Piezo1, MicroRNA-29a, and Wnt3a Signaling in Osteoblasts. Int. J. Mol. Sci. 2021, 22, 9476. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Liu, Q.; Lu, W.; Bu, G. Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: Implication for breast cancer tumorigenesis. Oncogene 2010, 29, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Du, X.M.; Si, W.; Zhao, X.H.; Zhou, Z.Q. Role of INPP4B in the proliferation, migration, invasion, and survival of human endometrial cancer cells. Histol. Histopathol. 2024, 39, 1197–1208. [Google Scholar]
- Xue, C.; Wei, Z.; Zhang, Y.; Liu, Y.; Zhang, S.; Li, Q.; Feng, K.; Yang, X.; Liu, G.; Chen, Y.; et al. Activation of CTU2 expression by LXR promotes the development of hepatocellular carcinoma. Cell Biol. Toxicol. 2024, 40, 23. [Google Scholar] [CrossRef]
- Miki, Y. New Insights into Breast and Endometrial Cancers. Cancers 2020, 12, 2595. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef] [PubMed]
- Maguire, P.; Holmberg, K.; Kost-Alimova, M.; Imreh, S.; Skoog, L.; Lindblom, A. CGH analysis of familial non-BRCA1/BRCA2 breast tumors and mutation screening of a candidate locus on chromosome 17q11.2–12. Int. J. Mol. Med. 2005, 16, 135–141. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, N.; Zhang, B.; Chen, Y. Identification of an immune cell infiltration-related gene signature for prognosis prediction in triple-negative breast cancer. Cell. Mol. Biol. 2024, 70, 91–98. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Song, J.W.; Li, W.; Liu, X.; Cao, L.; Wan, L.M.; Tan, Y.X.; Ji, S.P.; Liang, Y.M.; Gong, F. The acid-sensing ion channel, ASIC2, promotes invasion and metastasis of colorectal cancer under acidosis by activating the calcineurin/NFAT1 axis. J. Exp. Clin. Cancer Res. 2017, 36, 130. [Google Scholar] [CrossRef] [PubMed]
- Sales, K.J.; Grant, V.; Cook, I.H.; Maldonado-Perez, D.; Anderson, R.A.; Williams, A.R.; Jabbour, H.N. Interleukin-11 in endometrial adenocarcinoma is regulated by prostaglandin F2alpha-F-prostanoid receptor interaction via the calcium-calcineurin-nuclear factor of activated T cells pathway and negatively regulated by the regulator of calcineurin-1. Am. J. Pathol. 2010, 176, 435–445. [Google Scholar] [CrossRef]
- Vermani, L.; Barnekow, E.; Liu, W.; Wendt, C.; Hall, P.; Margolin, S.; Lindblom, A. Swedish Genome-Wide Haplotype Association Analysis Suggests Breast Cancer Loci with Varying Risk-Modifying Effects. Genes 2024, 15, 1616. [Google Scholar] [CrossRef]
- Barnekow, E.; Hasslow, J.; Liu, W.; Bryant, P.; Thutkawkorapin, J.; Wendt, C.; Czene, K.; Hall, P.; Margolin, S.; Lindblom, A. A Swedish Familial Genome-Wide Haplotype Analysis Identified Five Novel Breast Cancer Susceptibility Loci on 9p24.3, 11q22.3, 15q11.2, 16q24.1 and Xq21.31. Int. J. Mol. Sci. 2023, 24, 4468. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Gignoux, C.R.; Walters, R.K.; Wojcik, G.L.; Neale, B.M.; Gravel, S.; Daly, M.J.; Bustamante, C.D.; Kenny, E.E. Human Demographic History Impacts Genetic Risk Prediction across Diverse Populations. Am. J. Hum. Genet. 2020, 107, 788–789. [Google Scholar] [CrossRef] [PubMed]
- Gabrielson, M.; Eriksson, M.; Hammarstrom, M.; Borgquist, S.; Leifland, K.; Czene, K.; Hall, P. Cohort Profile: The Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA). Int. J. Epidemiol. 2017, 46, 1740–1741g. [Google Scholar] [CrossRef] [PubMed]
- Margolin, S.; Werelius, B.; Fornander, T.; Lindblom, A. BRCA1 mutations in a population-based study of breast cancer in Stockholm County. Genet. Test. 2004, 8, 127–132. [Google Scholar] [CrossRef]
- Weiderpass, E.; Baron, J.A.; Adami, H.O.; Magnusson, C.; Lindgren, A.; Bergstrom, R.; Correia, N.; Persson, I. Low-potency oestrogen and risk of endometrial cancer: A case-control study. Lancet 1999, 353, 1824–1828. [Google Scholar] [CrossRef]
- Tzortzatos, G.; Wersall, O.; Danielsson, K.G.; Lindblom, A.; Tham, E.; Mints, M. Familial cancer among consecutive uterine cancer patients in Sweden. Hered. Cancer Clin. Pract. 2014, 12, 14. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Purcell, S. Haplotype-Based Association Tests with GLMs. Available online: https://zzz.bwh.harvard.edu/plink/haplo.shtml#hap3 (accessed on 5 April 2025).
- Dudbridge, F.; Gusnanto, A. Estimation of significance thresholds for genomewide association scans. Genet. Epidemiol. 2008, 32, 227–234. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).