Flaxseed Extracts Impact the Cellular Structure of a Keratinocyte Model for Oral Lichen Planus—A Preliminary Study
Abstract
1. Introduction
2. Results
2.1. Concentration of Polyphenols in Szafir and Jantarol Extracts
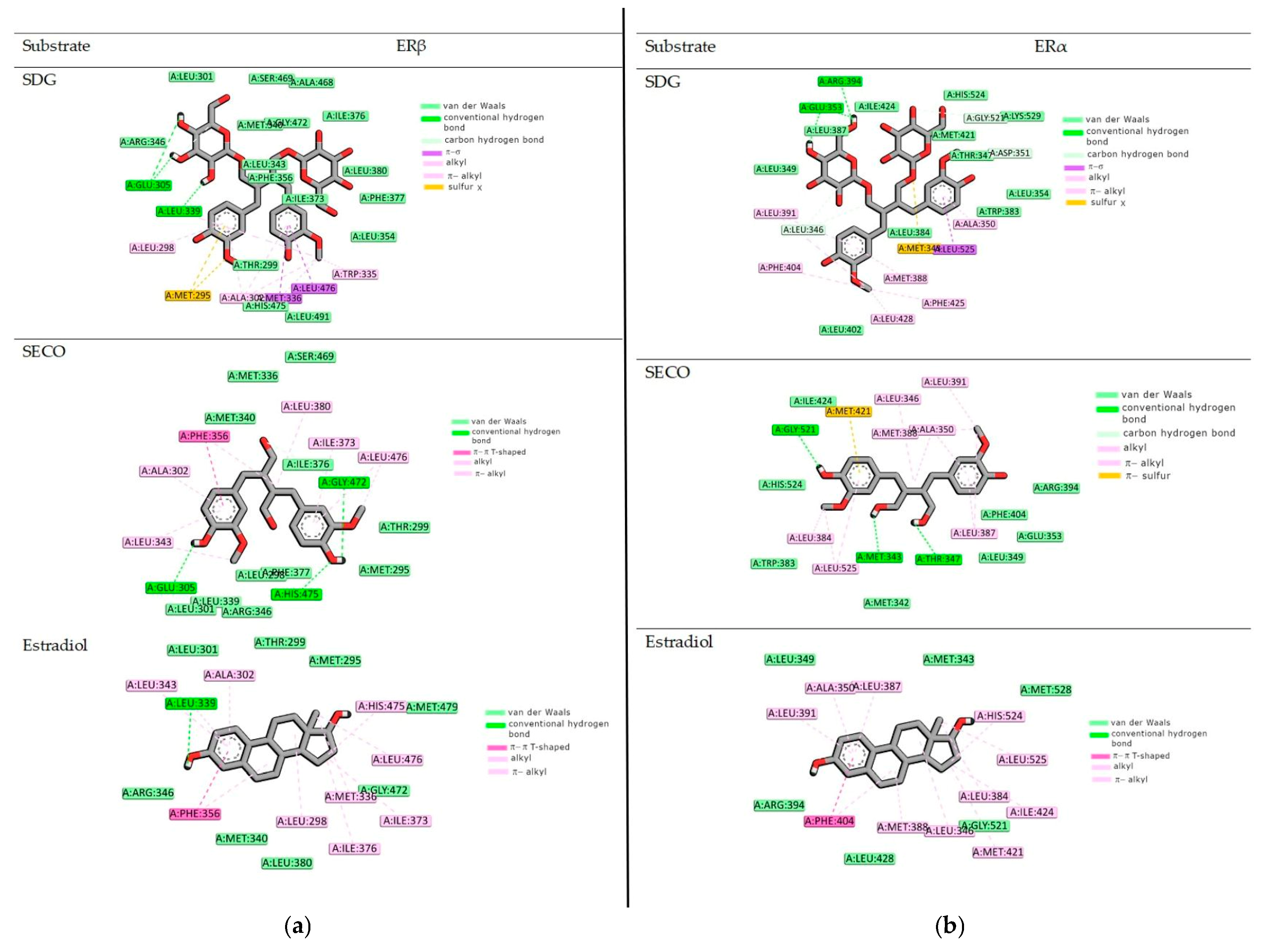
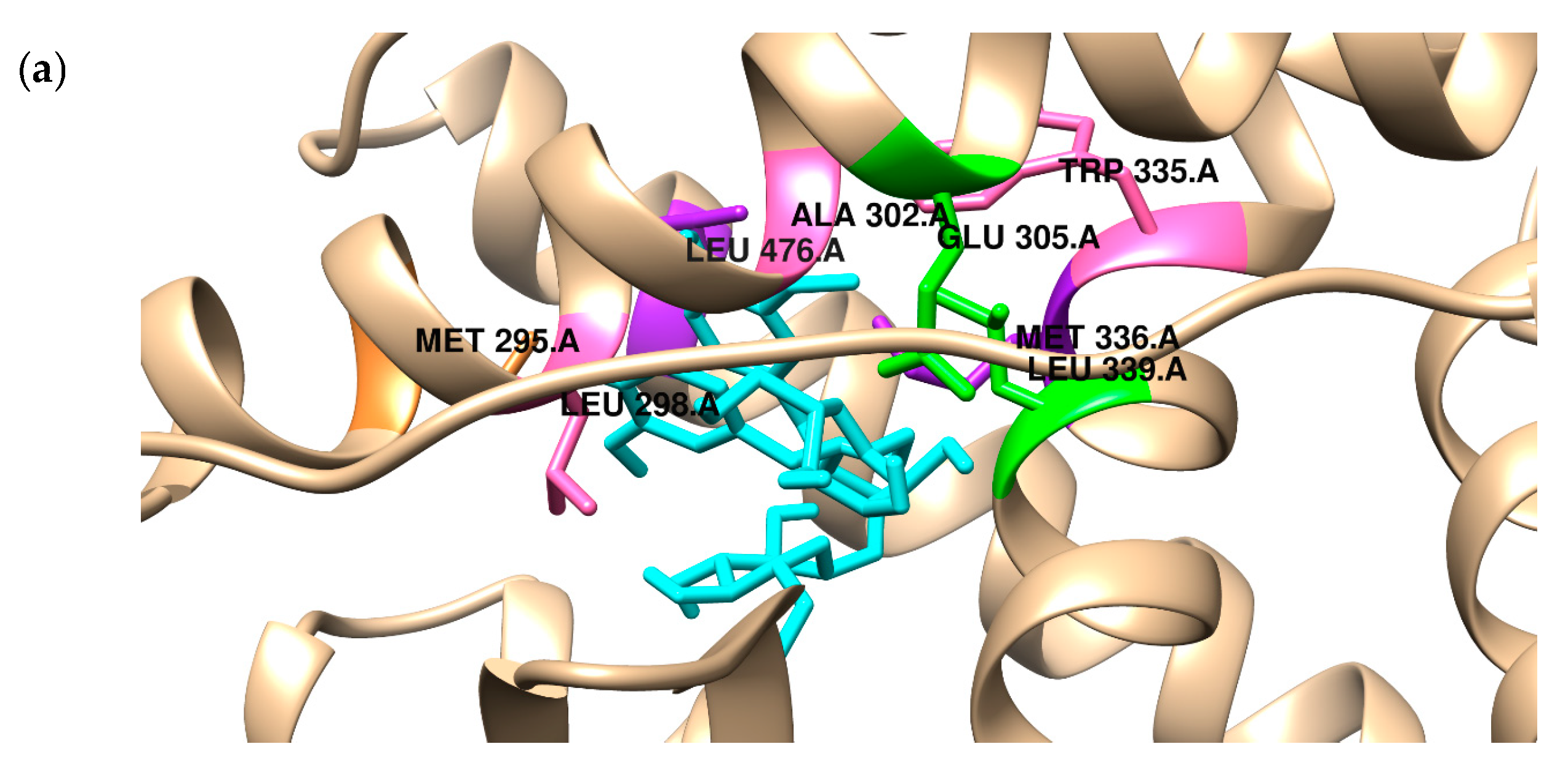
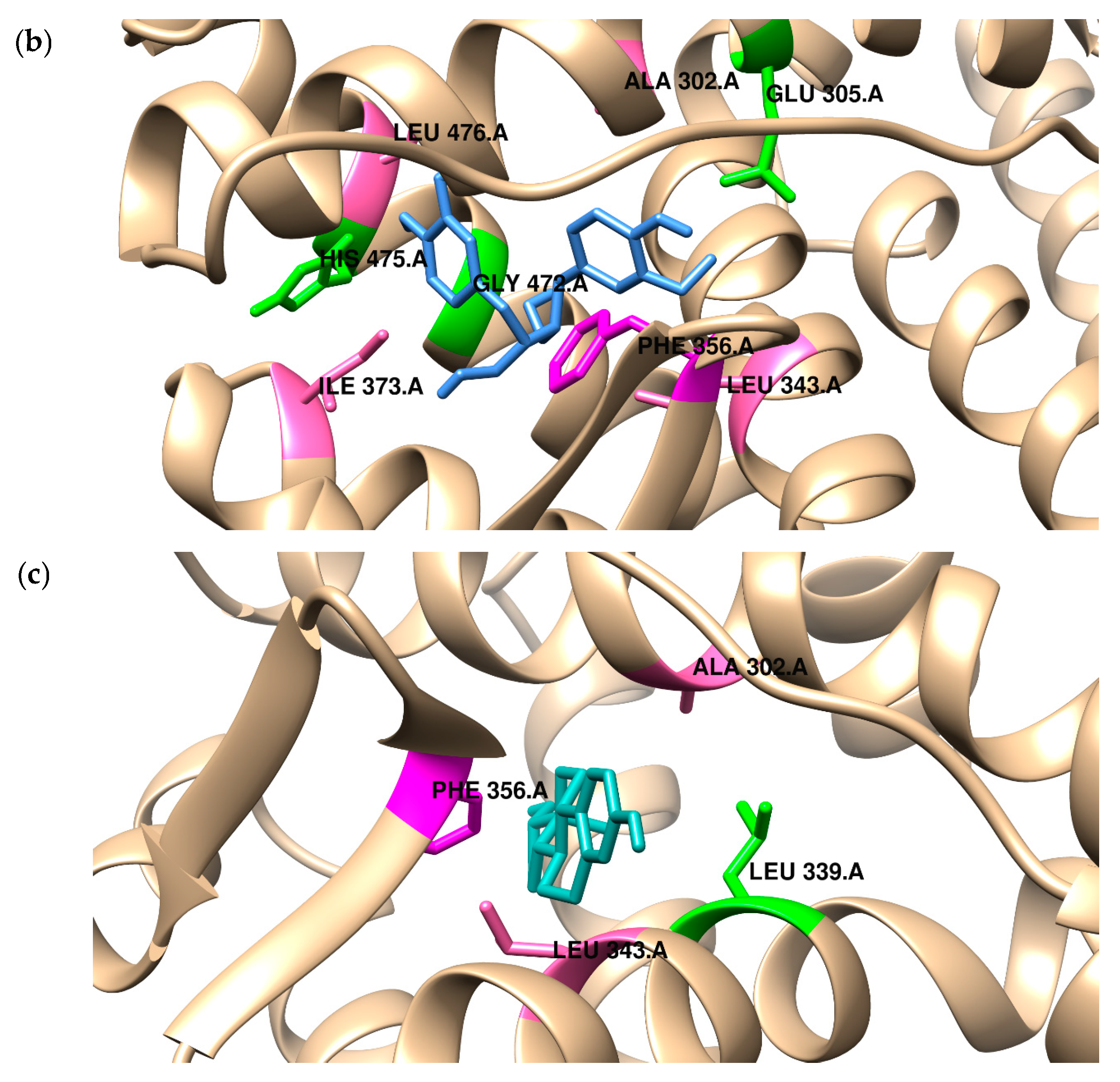
2.2. Binding to the Alpha and Beta Estrogen Receptor—Molecular Docking Study
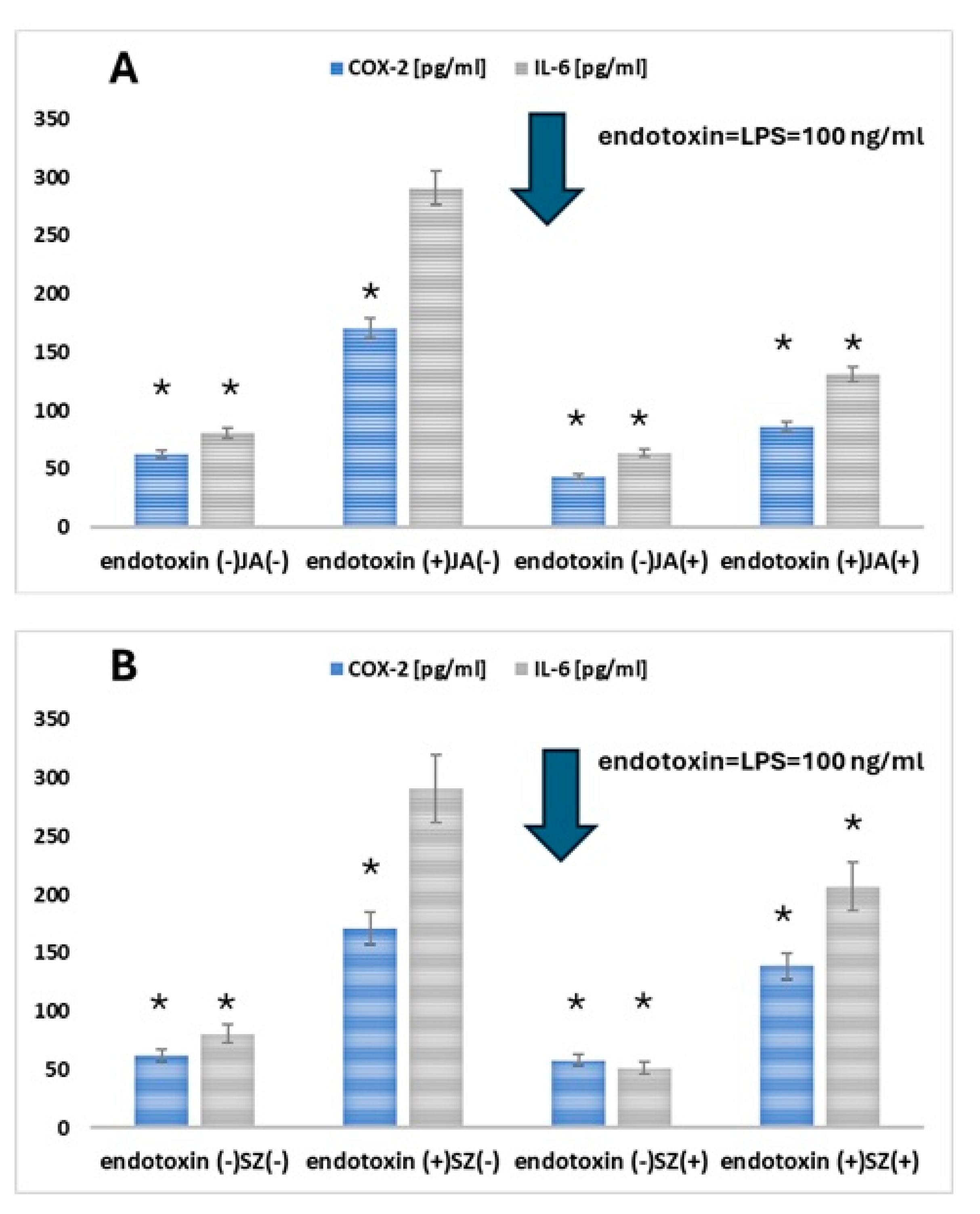
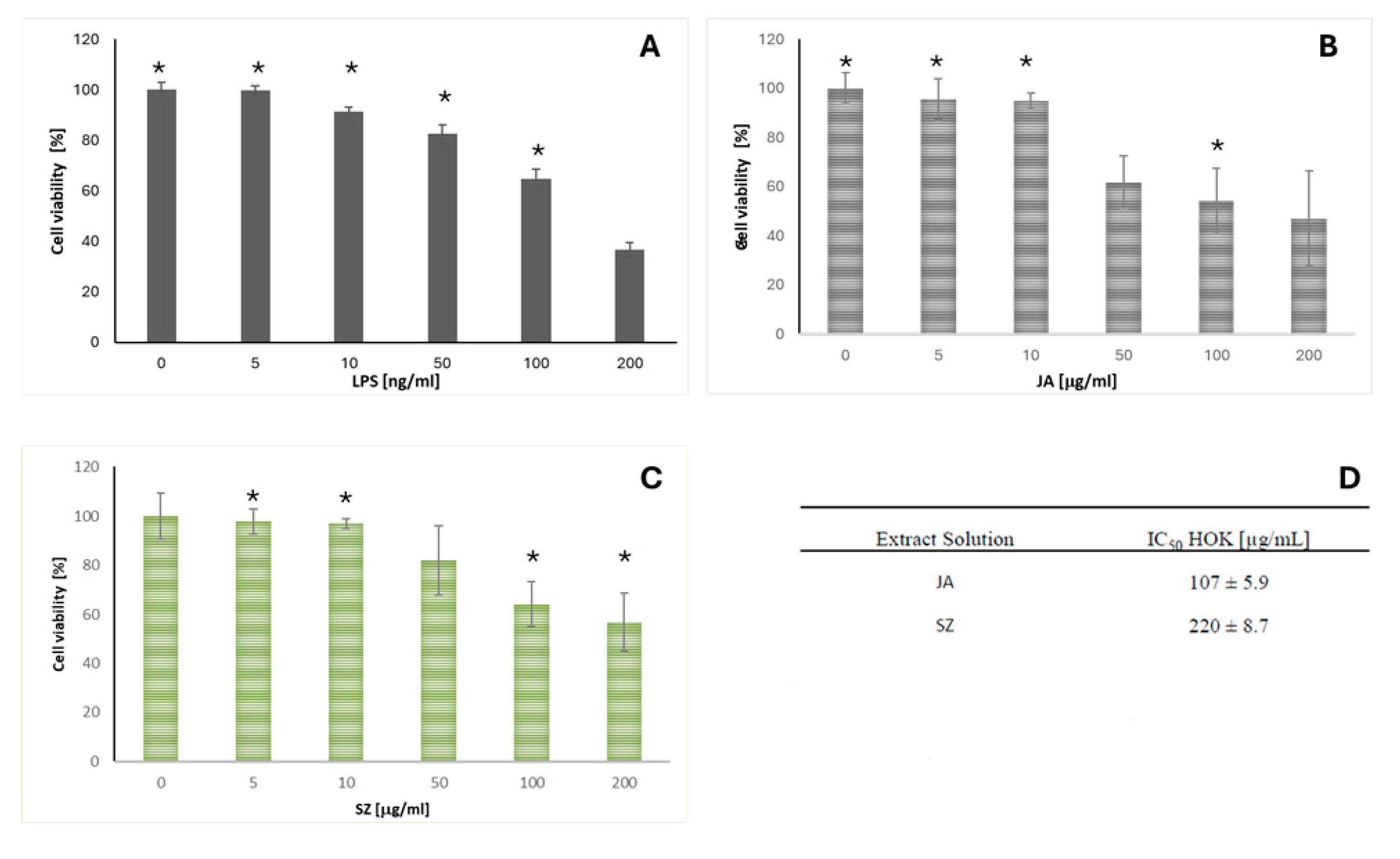
2.3. Analysis of the Effect of Flaxseed Extracts on the Keratinocytes
3. Discussion
4. Materials and Methods
- Jantarol (breeding name of the cultivar BOH 505)—germination capacity (germ.): 72%, thousand-grain weight (MTZ): 7.4 g, 2020 harvest, seed color: yellow
- Szafir (breeding name of the cultivar BOH 191)—germ.: 80%, MTZ: 8.2 g, 2020 harvest, seed color: brown.
4.1. In Silico Docking for the ERα and ERβ Affinity Methodology
4.2. Preparation of the Extracts from Szafir and Jantarol Cultivars
4.3. Determination of SDG and Other Phenolic Compounds Content in Methanol Extracts of Phenolic Acids via Ultra-Performance Liquid Chromatography (UPLC)
4.4. Cell Culture Cell Preparation
4.5. Cell Viability Assay
4.6. Quantification of Interleukin-6 (IL-6) and Cyclooxygenase 2 (COX-2) Using Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Data Analysis
5. Conclusions
- The extracts from the JA and SZ flax cultivars showed the presence of other phenolic compounds characterized by anti-inflammatory properties.
- The use of Jantarol and Szafir flaxseed extracts showed anti-inflammatory action on oral keratinocyte cell lines. Further clinical studies have to be performed to confirm its action in vivo.
- There was a high in silico affinity of SDG to ERs.
- The research presented concludes the potential use of flaxseed in developing clinically available products.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosa, E.A.; Hurtado-Puerto, A.M.; Falcão, D.P.; Brietzke, A.P.; De Almeida Prado Franceschi, L.E.; Cavalcanti Neto, F.F.; Tiziane, V.; Carneiro, F.P.; Kogawa, E.M.; Moreno, H.; et al. Oral lichen planus and malignant transformation: The role of p16, Ki-67, Bub-3 and SOX4 in assessing precancerous potential. Exp. Ther. Med. 2018, 15, 4157–4166. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Sen, S.; Dutta, A.; Abhinandan, A.; Kumar, V.; Singh, A.K. Oral manifestation and its management in postmenopausal women: An integrated review. Prz. Menopauzalny 2020, 19, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Arrica, M.; Lucchese, A.; Valente, M.; Pannone, G.; Lajolo, C.; Ninivaggi, R.; Petruzzi, M. Oral lichen planus clinical characteristics in Italian patients: A retrospective analysis. Head Face Med. 2016, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, F.; Sakurai, J.; Uesugi, A.; Oikawa, Y.; Ohsako, T.; Mochizuki, Y.; Hirai, H.; Kayamori, K.; Harada, H. Malignant transformation of oral lichen planus: A retrospective study of 565 Japanese patients. BMC Oral Health 2021, 21, 298. [Google Scholar] [CrossRef]
- Gangeshetty, N. Oral lichen planus: Etiology, pathogenesis, diagnosis, and management. World J. Stomatol. 2015, 4, 12. [Google Scholar] [CrossRef]
- Lavanya, N.; Jayanthi, P.; Rao, U.; Ranganathan, K. Oral lichen planus: An update on pathogenesis and treatment. J. Oral Maxillofac. Pathol. 2011, 15, 127–132. [Google Scholar] [CrossRef]
- Daume, L.; Kreis, C.; Bohner, L.; Jung, S.; Kleinheinz, J. Clinical characteristics of oral lichen planus and its causal context with dental restorative materials and oral health-related quality of life. BMC Oral Health 2021, 21, 262. [Google Scholar] [CrossRef]
- Parlatescu, I.; Tovaru, M.; Nicolae, C.L.; Sfeatcu, R.; Didilescu, A.C. Oral health-related quality of life in different clinical forms of oral lichen planus. Clin. Oral Investig. 2020, 24, 301–308. [Google Scholar] [CrossRef]
- Wardrop, R.W.; Hailes, J.; Burger, H.; Reade, P.C. Oral discomfort at menopause. Oral Surgery, Oral Med. Oral Pathol. 1989, 67, 535–540. [Google Scholar] [CrossRef]
- Radwan-Oczko, M.; Rybińska, A.; Mierzwicka, A.; Duś-Ilnicka, I. Salivary Histamine Levels in Patients with Oral Lichen Planus Lesions. Medicina 2024, 60, 1038. [Google Scholar] [CrossRef]
- Mohan, R.P.S.; Gupta, A.; Kamarthi, N.; Malik, S.; Goel, S.; Gupta, S. Incidence of Oral Lichen Planus in Perimenopausal Women: A Cross-sectional Study in Western Uttar Pradesh Population. J. Mid-Life Health 2017, 8, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Pertyński, T.; Stachowiak, G. Menopauza—Fakty i kontrowersje. Endokrynol. Pol. 2006, 57, 525–534. [Google Scholar] [PubMed]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Ström, A.; Treuter, E.; Warner, M.; et al. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, W.; Haitel, A.; Holzer, G.; Huber, J.C.; Kolbus, A.; Tschugguel, W. Estrogen Receptor-β Is the Predominant Estrogen Receptor Subtype in Normal Human Synovia. J. Soc. Gynecol. Investig. 2006, 13, 512–517. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen Receptors Alpha and Beta Subtype—Selective Ligands and Clinical Potential. Steroids 2011, 18, 1492–1501. [Google Scholar] [CrossRef]
- Alshahrani, I.; Hameed, M.S.; Syed, S.; Amanullah, M.; Togoo, R.A.; Kaleem, S. Changes in essential salivary parameters in patients undergoing fixed orthodontic treatment: A longitudinal study. Niger. J. Clin. Pract. 2019, 22, 707–712. [Google Scholar] [CrossRef]
- Chi, A.C.; Neville, B.W.; Krayer, J.W.; Gonsalves, W.C. Oral manifestations of systemic disease. Am. Fam. Physician 2010, 82, 1381–1388. [Google Scholar] [CrossRef]
- Basch, E.; Bent, S.; Collins, J.; Dacey, C.; Hammerness, P.; Harrison, M.; Smith, M.; Szapary, P.; Ulbricht, C.; Vora, M.; et al. Flax and flaxseed oil (Linum usitatissimum): A review by the Natural Standard Research Collaboration. J. Soc. Integr. Oncol. 2007, 5, 92–105. [Google Scholar] [CrossRef]
- Morales-Bozo, I.; Ortega-Pinto, A.; Rojas Alcayaga, G.; Aitken Saavedra, J.P.; Salinas Flores, O.; Lefimil Puente, C.; Lozano Moraga, C.; Manríquez Urbina, J.M.; Urzúa Orellana, B. Evaluation of the effectiveness of a chamomile (Matricaria chamomilla) and linseed (Linum usitatissimum) saliva substitute in the relief of xerostomia in elders. Gerodontology 2017, 34, 42–48. [Google Scholar] [CrossRef]
- Andersson, G.; Johansson, G.; Attström, R.; Edwardsson, S.; Glantz, P.O.; Larsson, K. Comparison of the effect of the linseed extract Salinum and a methyl cellulose preparation on the symptoms of dry mouth. Gerodontology 1995, 12, 12–17. [Google Scholar] [CrossRef]
- Ameri, A.; Heydarirad, G.; Jafari, J.M.; Ghobadi, A.; Rezaeizadeh, H.; Choopani, R. Medicinal plants contain mucilage used in traditional Persian medicine (TPM). Pharm. Biol. 2015, 53, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Aitken-Saavedra, J.; Chaves Tarquinio, S.B.; De Oliveira Da Rosa, W.L.; Fernandes Da Silva, A.; Almeida MacHado, B.M.E.; Santos Castro, I.; Oliveira Wennesheimer, A.; Morales-Bozo, I.; Uchoa Vasconcelos, A.C.; Neutzling Gomes, A.P. Effect of a Homemade Salivary Substitute Prepared Using Chamomile Matricaria chamomilla L. Flower and Flax Linum usitatissimum L. Seed to Relieve Primary Burning Mouth Syndrome: A Preliminary Report. J. Altern. Complement. Med. 2020, 26, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Kręgielczak, A.; Łukaszewska-Kuska, M.; Mania-Końsko, A.; Dorocka-Bobkowska, B. Flaxseed (Linum usitatissimum), Chamomile (Matricariae flos) and Marshmallow (Althaea officinalis) Mouth Rinses in the Therapy of Oral Mucosa Diseases—A Review. J. Nat. Fibers 2023, 20, 2228487. [Google Scholar] [CrossRef]
- Dzuvor, C.K.O.; Taylor, J.T.; Acquah, C.; Pan, S.; Agyei, D. Bioprocessing of functional ingredients from flaxseed. Molecules 2018, 23, 2444. [Google Scholar] [CrossRef]
- Morris, D.H. Backgrounder on Lignans. In Flax: A Health Nutrition Primer; Flax Council of Canada: Winnipeg, MB, Canada, 2007; pp. 44–52. [Google Scholar]
- Toure, A.; Xu, X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Strandås, C.; Kamal-Eldin, A.; Andersson, R.; Åman, P. Composition and properties of flaxseed phenolic oligomers. Food Chem. 2008, 110, 106–112. [Google Scholar] [CrossRef]
- Lehraiki, A.; Attoumbre, J.; Matifat, F. Extraction of Lignans from Flaxseed and Evaluation of Their Biological Effects on Breast Cancer MCF-7 and MDA-MB-231 Cell Lines Abdelali. J. Med. Food 2010, 13, 834–841. [Google Scholar] [CrossRef]
- Sainvitu, P.; Nott, K.; Richard, G.; Blecker, C.; Jérôme, C.; Wathelet, J.P.; Paquot, M.; Deleu, M. Structure, properties and obtention routes of flaxseed lignan secoisolariciresinol: A review. Biotechnol. Agron. Soc. Environ. 2012, 16, 115–124. [Google Scholar]
- Powers, C.N.; Setzer, W.N. A molecular docking study of phytochemical estrogen mimics from dietary herbal supplements. Silico Pharmacol. 2015, 3, 4. [Google Scholar] [CrossRef]
- Martynowicz, H.; Jodkowska, A.; Poręba, R.; Mazur, G.; Więckiewicz, M. Demographic, clinical, laboratory, and genetic risk factors associated with COVID-19 severity in adults: A narrative review. Dent. Med. Probl. 2021, 58, 115–121. [Google Scholar] [CrossRef]
- Orzechowska-Wylęgała, B.E.; Wylęgała, A.A.; Zalejska-Fiolka, J.; Czuba, Z.; Toborek, M. Pro-inflammatory cytokines and antioxidative enzymes as salivary biomarkers of dentofacial infections in children. Dent. Med. Probl. 2024, 61. [Google Scholar] [CrossRef] [PubMed]
- Kerstjens, A.; De Winter, H. LEADD: Lamarckian evolutionary algorithm for de novo drug design. J. Cheminform. 2022, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Menche, G.; Power, T.D.; Sower, L.; Peterson, J.W.; Schein, C.H. Accounting for ligand-bound metal ions in docking small molecules on adenylyl cyclase toxins. Proteins 2007, 67, 593–605. [Google Scholar] [CrossRef]
- Duś-Ilnicka, I. Neglected area of oral cancer: A word about the International Agency for Research on Cancer (IARC) “Handbook of Oral Cancer Prevention”. Dent. Med. Probl. 2024, 61, 479–480. [Google Scholar] [CrossRef]
- Soria, A.; Agbo-Godeau, S.; Taïeb, A.; Francès, C. Treatment of refractory oral erosive lichen planus with topical rapamycin: 7 Cases. Dermatology 2008, 218, 22–25. [Google Scholar] [CrossRef]
- Maloth, K.N.; Sunitha, K.; Boyapati, R.; Shravan Kumar, D.R. Bullous lichen planus treated with oral minipulse therapy: A rare case report. J. Clin. Diagn. Res. 2014, 8, ZD17–ZD19. [Google Scholar] [CrossRef]
- Kazanowska-Dygdała, M.; Duś, I.; Radwan-Oczko, M. The presence of Helicobacter pylori in oral cavities of patients with leukoplakia and oral lichen planus. J. Appl. Oral Sci. 2016, 24, 18–23. [Google Scholar] [CrossRef]
- Agha-Hosseini, F.; Mirzaii-Dizgah, I.; Mikaili, S.; Abdollahi, M. Increased salivary lipid peroxidation in human subjects with oral lichen planus. Int. J. Dent. Hyg. 2009, 7, 246–250. [Google Scholar] [CrossRef]
- Eliasson, L.; Carlén, A.; Laine, M.; Birkhed, D. Minor gland and whole saliva in postmenopausal women using a low potency oestrogen (oestriol). Arch. Oral Biol. 2003, 48, 511–517. [Google Scholar] [CrossRef]
- Leimola-Virtanen, R.; Salo, T.; Toikkanen, S.; Pulkkinen, J.; Syrjänen, S. Expression of estrogen receptor (ER) in oral mucosa and salivary glands. Maturitas 2000, 36, 131–137. [Google Scholar] [CrossRef]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef] [PubMed]
- EMA. European Union Herbal Monograph on Linum usitatissimum L., Semen; European Medicines Agency: Amsterdam, The Netherlands, 2005; Volume 44, pp. 1–8. [Google Scholar]
- Barbary, O.M.; El-Sohaimy, S.A.; El-Saadani, M.A.; Zeitoun, A.M. Antioxidant, Antimicrobial and Anti-HCV Activities of Lignan Extracted from Flaxseed. Res. J. Agric. Biol. Sci. 2010, 6, 247–256. [Google Scholar]
- Chen, M.N.; Lin, C.C.; Liu, C.F. Efficacy of phytoestrogens for menopausal symptoms: A meta-analysis and systematic review. Climacteric 2015, 18, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.Å. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Yaşar, P.; Ayaz, G.; User, S.D.; Güpür, G.; Muyan, M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod. Med. Biol. 2017, 16, 4–20. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Souza, P.C.T.; Textor, L.C.; Melo, D.C.; Nascimento, A.S.; Skaf, M.S.; Polikarpov, I. An alternative conformation of ERβ bound to estradiol reveals H12 in a stable antagonist position. Sci. Rep. 2017, 7, 3509. [Google Scholar] [CrossRef]
- Sharma, P.; Joshi, T.; Joshi, T.; Chandra, S.; Tamta, S. In silico screening of potential antidiabetic phytochemicals from Phyllanthus emblica against therapeutic targets of type 2 diabetes. J. Ethnopharmacol. 2020, 248, 112268. [Google Scholar] [CrossRef]
- Sayed, A.A.; Elfiky, A.A. In silico estrogen-like activity and in vivo osteoclastogenesis inhibitory effect of Cicer arietinum extract. Cell. Mol. Biol. 2018, 64, 29–39. [Google Scholar] [CrossRef]
- Zeng, W.; Takashima, K.; Tang, Q.; Zou, X.; Ojiro, R.; Ozawa, S.; Jin, M.; Ando, Y.; Yoshida, T.; Shibutani, M. Natural antioxidant formula ameliorates lipopolysaccharide-induced impairment of hippocampal neurogenesis and contextual fear memory through suppression of neuroinflammation in rats. J. Chem. Neuroanat. 2023, 131, 102285. [Google Scholar] [CrossRef]
- Baby, T.K.; Bindhu, P.R.; Pillai, R.K.; Jayanthi, P. Immunohistochemical expression of cyclooxygenase-2 in oral lichen planus and normal oral mucosa. Indian J. Pathol. Microbiol. 2022, 65, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Beristain-Colorado, M.D.P.; Castro-Gutiérrez, M.E.M.; Torres-Rosas, R.; Vargas-Treviño, M.; Moreno-Rodríguez, A.; Fuentes-Mascorro, G.; Argueta-Figueroa, L. Application of neural networks for the detection of oral cancer: A systematic review. Dent. Med. Probl. 2024, 61, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Tanenbaum, D.M.; Wang, Y.; Williams, S.P.; Sigler, P.B. Crystallographic comparison of the estrogen and progesterone receptor’s ligand binding domains. Proc. Natl. Acad. Sci. USA 1998, 95, 5998–6003. [Google Scholar] [CrossRef]
- Möcklinghoff, S.; Rose, R.; Carraz, M.; Visser, A.; Ottmann, C.; Brunsveld, L. Synthesis and crystal structure of a phosphorylated estrogen receptor ligand binding domain. Chembiochem 2010, 11, 2251–2254. [Google Scholar] [CrossRef]
- Velázquez-Libera, J.L.; Durán-Verdugo, F.; Valdés-Jiménez, A.; Núñez-Vivanco, G.; Caballero, J. LigRMSD: A web server for automatic structure matching and RMSD calculations among identical and similar compounds in protein-ligand docking. Bioinformatics 2020, 36, 2912–2914. [Google Scholar] [CrossRef]
- Szczęśniak-Sięga, B.M.; Wiatrak, B.; Czyżnikowska, Ż.; Janczak, J.; Wiglusz, R.J.; Maniewska, J. Synthesis and biological evaluation as well as in silico studies of arylpiperazine-1,2-benzothiazine derivatives as novel anti-inflammatory agents. Bioorg. Chem. 2021, 106, 104476. [Google Scholar] [CrossRef]
- Janek, T.; Czyżnikowska, Ż.; Łukaszewicz, M.; Gałęzowska, J. The effect of Pseudomonas fluorescens biosurfactant pseudofactin II on the conformational changes of bovine serum albumin: Pharmaceutical and biomedical applications. J. Mol. Liq. 2019, 288, 111001. [Google Scholar] [CrossRef]
- Zeitoun, A.M.; Preisner, M.; Kulma, A.; Dymińska, L.; Hanuza, J.; Starzycki, M.; Szopa, J. Does biopolymers composition in seeds contribute to the flax resistance against the Fusarium infection? Biotechnol. Prog. 2014, 30, 992–1004. [Google Scholar] [CrossRef]

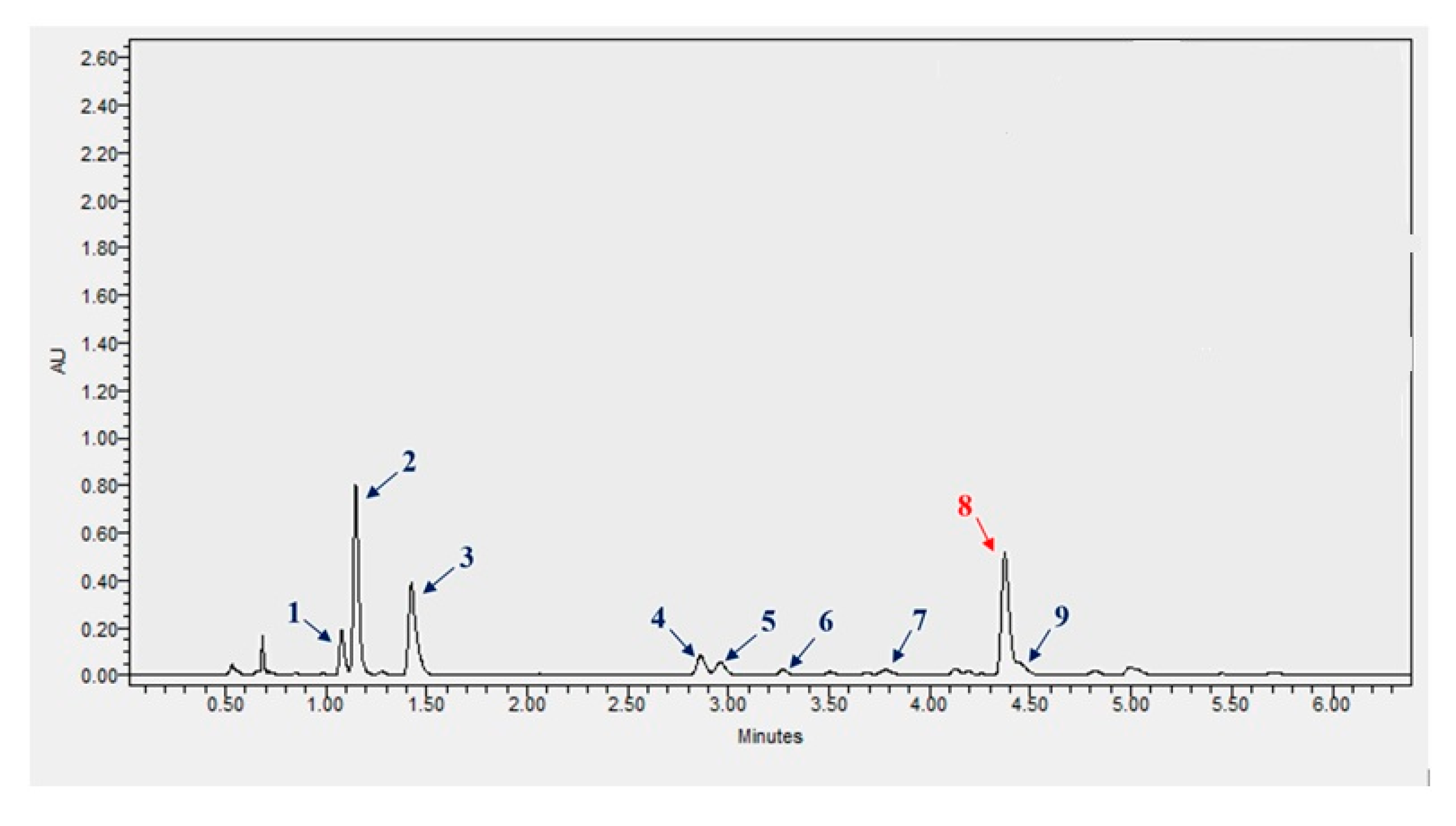
| Phenolic Compounds | Contents [mg/100 mL of Defatted Seeds] | |
|---|---|---|
| Szafir (93.14 g of Dry Weight) | Jantarol (92.15 g of Dry Weight) | |
| caffeic acid glucoside | 142.10 | 56.06 |
| coumaric acid glucoside | 468.62 | 133.87 |
| ferulic acid glucoside | 566.59 | 186.85 |
| p-coumaric–ferulic glucoside | 43.33 | 29.26 |
| caffeic-p-–coumaric glucoside | 33.87 | 23.00 |
| coumaric acid | 28.11 | 9.73 |
| ferulic acid | 179.96 | 31.89 |
| Total phenolic acids | 1462.58 | 470.66 |
| SDG | 1436.59 | 1467.13 |
| Total phenolic compounds | 2899.17 | 1937.79 |
| a | ΔGbind | ΔEint | ΔE1 | ΔE2 |
| SDG | −12.3 | −16.1 | −16.1 | 0.0 |
| SECO | −13.3 | −20.8 | −20.8 | 0.0 |
| Estradiol | −11.2 | −11.2 | −11.0 | 0.0 |
| b | ΔGbind | ΔEint | ΔE1 | ΔE2 |
| SDG | −10.95 | −14.5 | −14.5 | 0.0 |
| SECO | −11.5 | −15.5 | −15.5 | 0.0 |
| Estradiol | −11.9 | −12.5 | −12.5 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duś-Ilnicka, I.; Prescha, A.; Mordal, A.; Środa-Pomianek, K.; Sobieszczańska, B.; Bielecka, M.; Czyżnikowska, Ż.; Szperlik, J.; Matkowski, A.; Radwan-Oczko, M. Flaxseed Extracts Impact the Cellular Structure of a Keratinocyte Model for Oral Lichen Planus—A Preliminary Study. Int. J. Mol. Sci. 2025, 26, 5462. https://doi.org/10.3390/ijms26125462
Duś-Ilnicka I, Prescha A, Mordal A, Środa-Pomianek K, Sobieszczańska B, Bielecka M, Czyżnikowska Ż, Szperlik J, Matkowski A, Radwan-Oczko M. Flaxseed Extracts Impact the Cellular Structure of a Keratinocyte Model for Oral Lichen Planus—A Preliminary Study. International Journal of Molecular Sciences. 2025; 26(12):5462. https://doi.org/10.3390/ijms26125462
Chicago/Turabian StyleDuś-Ilnicka, Irena, Anna Prescha, Amanda Mordal, Kamila Środa-Pomianek, Beata Sobieszczańska, Monika Bielecka, Żaneta Czyżnikowska, Jakub Szperlik, Adam Matkowski, and Małgorzata Radwan-Oczko. 2025. "Flaxseed Extracts Impact the Cellular Structure of a Keratinocyte Model for Oral Lichen Planus—A Preliminary Study" International Journal of Molecular Sciences 26, no. 12: 5462. https://doi.org/10.3390/ijms26125462
APA StyleDuś-Ilnicka, I., Prescha, A., Mordal, A., Środa-Pomianek, K., Sobieszczańska, B., Bielecka, M., Czyżnikowska, Ż., Szperlik, J., Matkowski, A., & Radwan-Oczko, M. (2025). Flaxseed Extracts Impact the Cellular Structure of a Keratinocyte Model for Oral Lichen Planus—A Preliminary Study. International Journal of Molecular Sciences, 26(12), 5462. https://doi.org/10.3390/ijms26125462









