Influence of Prosulfocarb and Polymer Supplementation on Soil Bacterial Diversity in Triticum aestivum L. Cultivation
Abstract
1. Introduction
2. Results
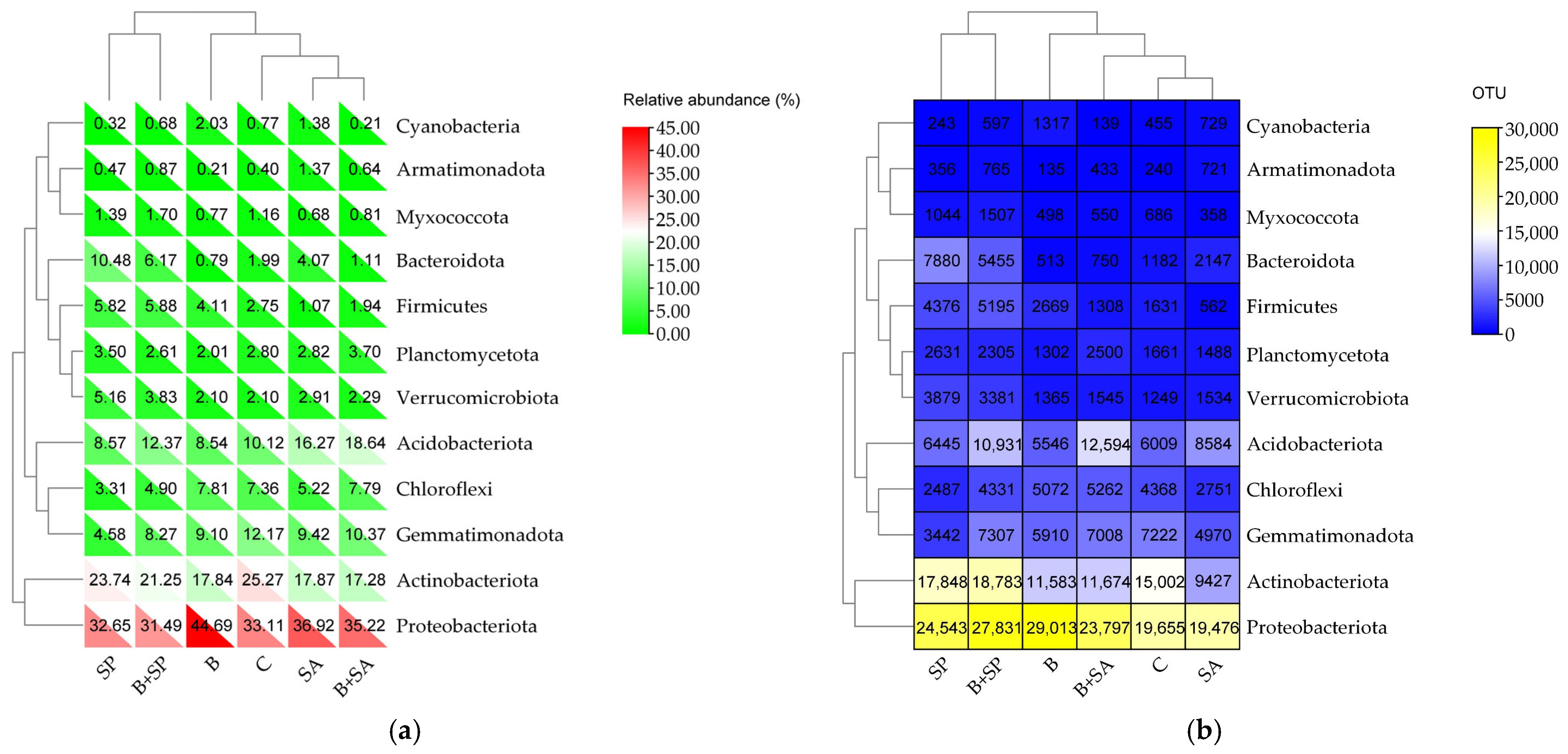
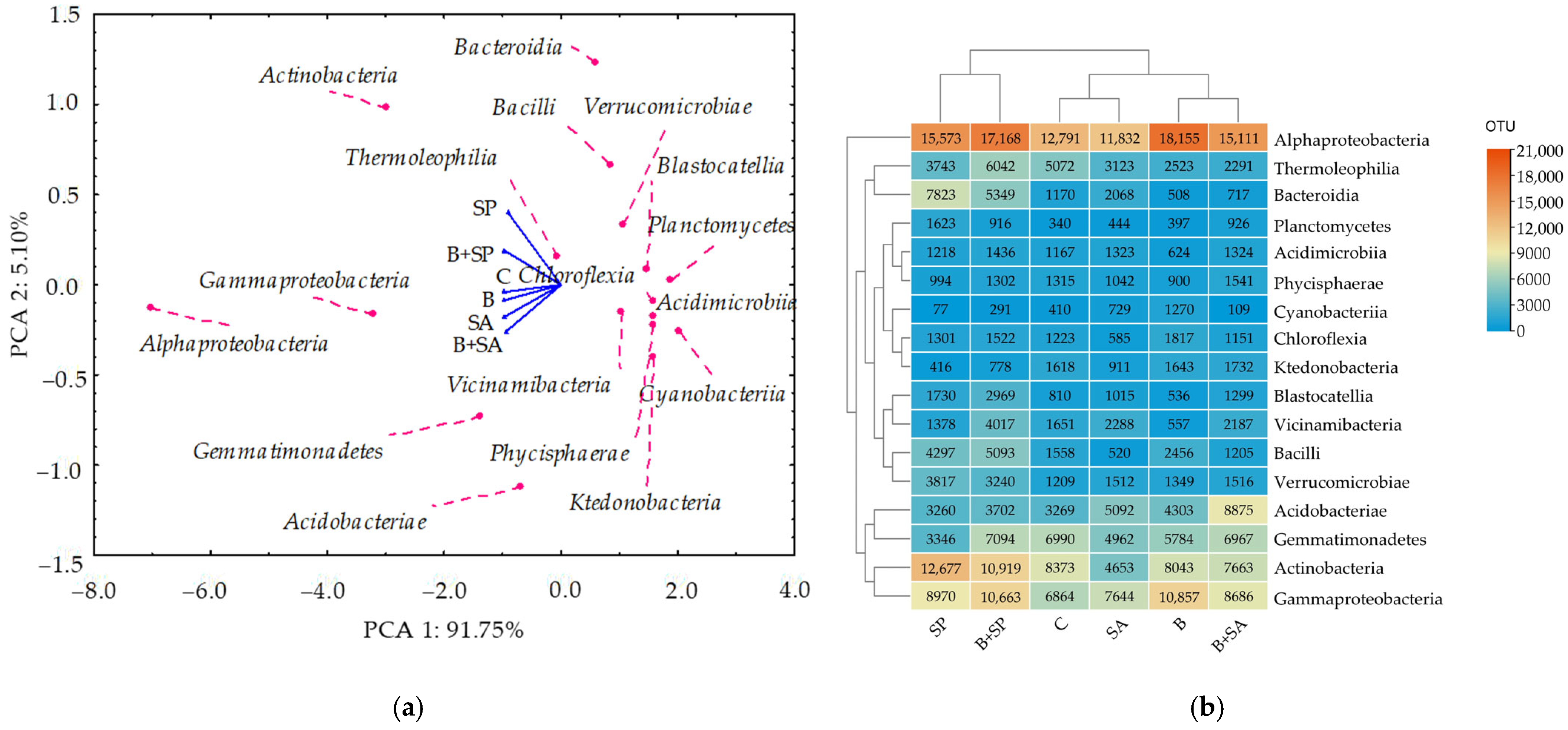
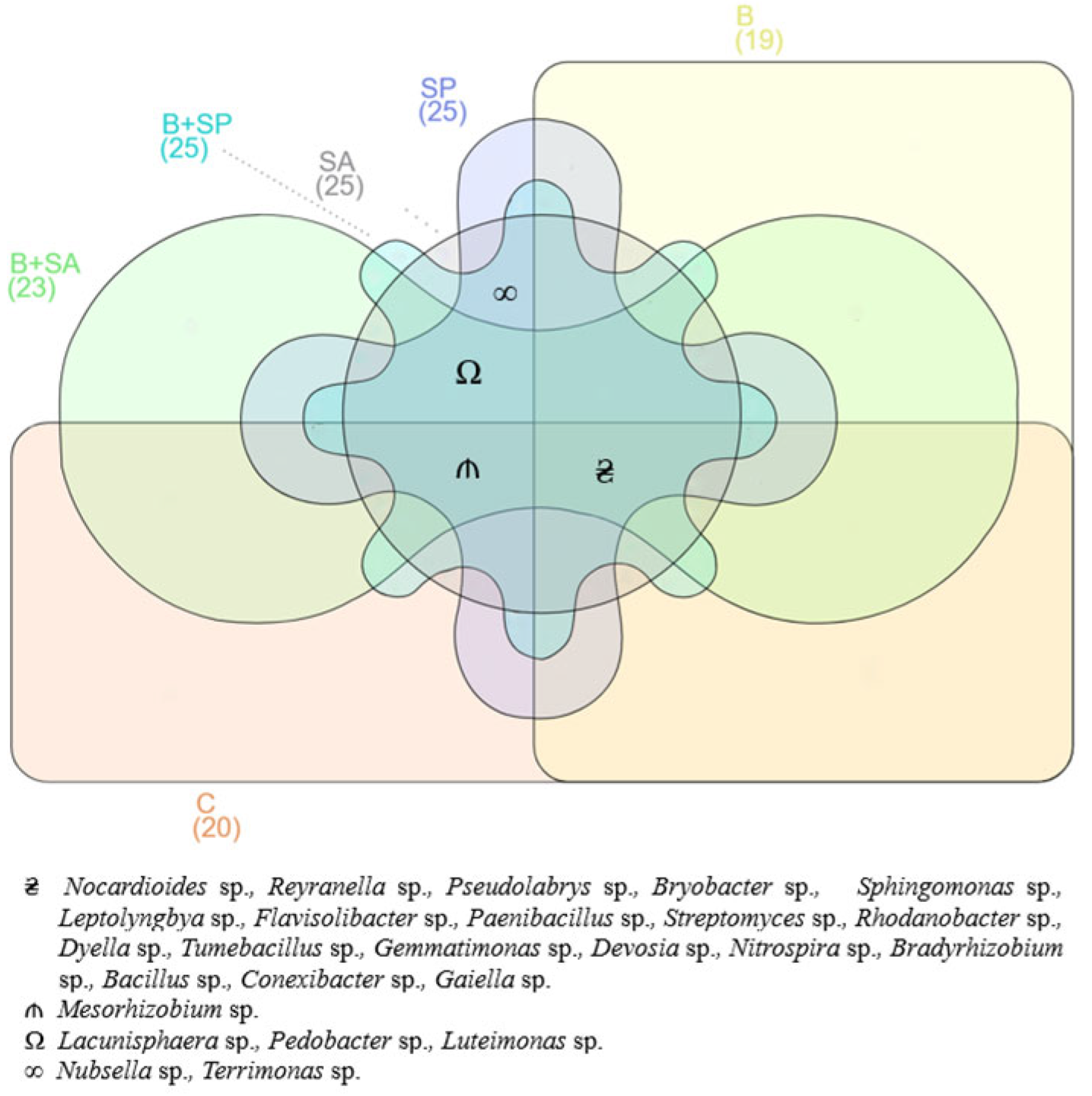
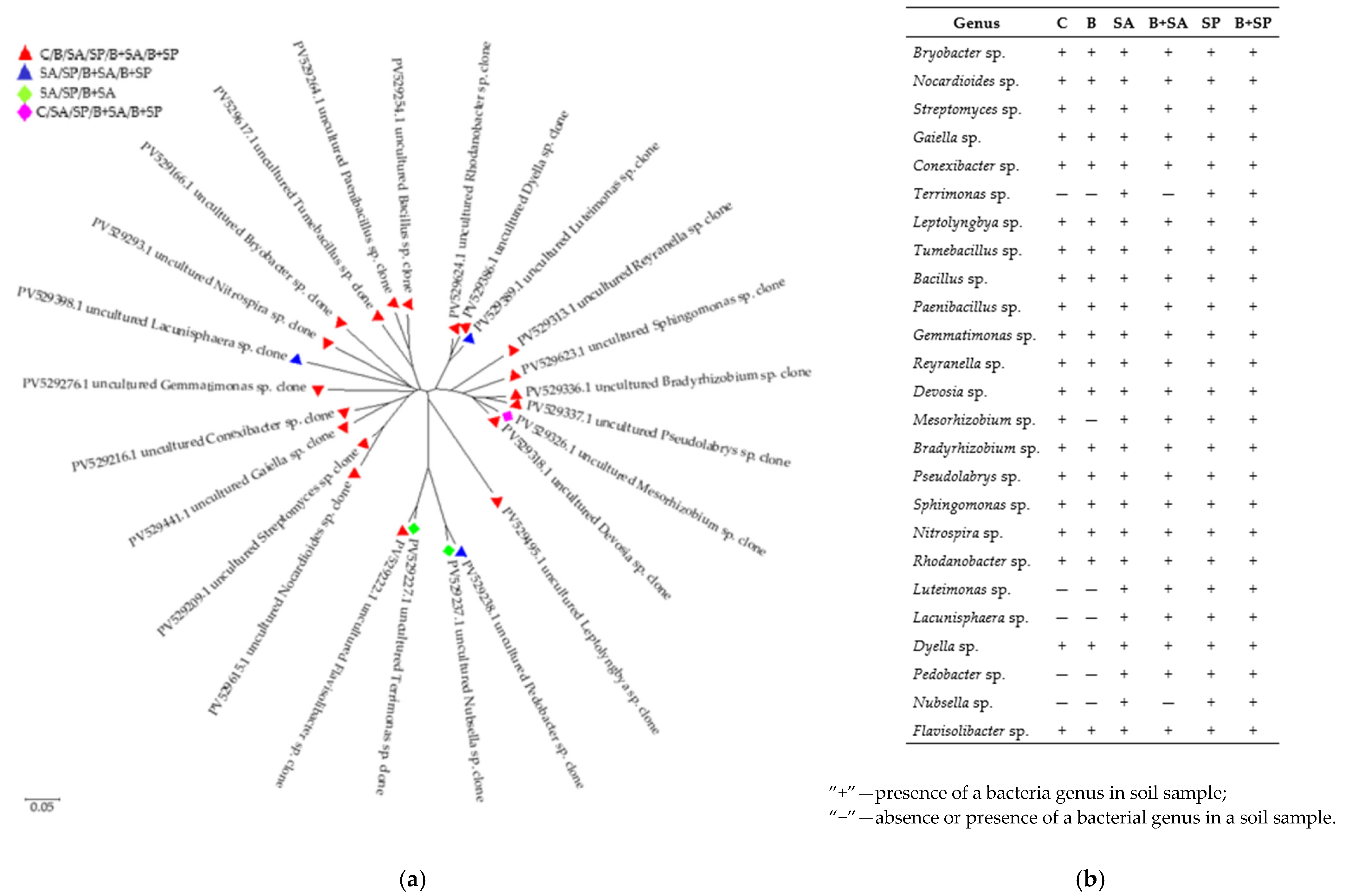
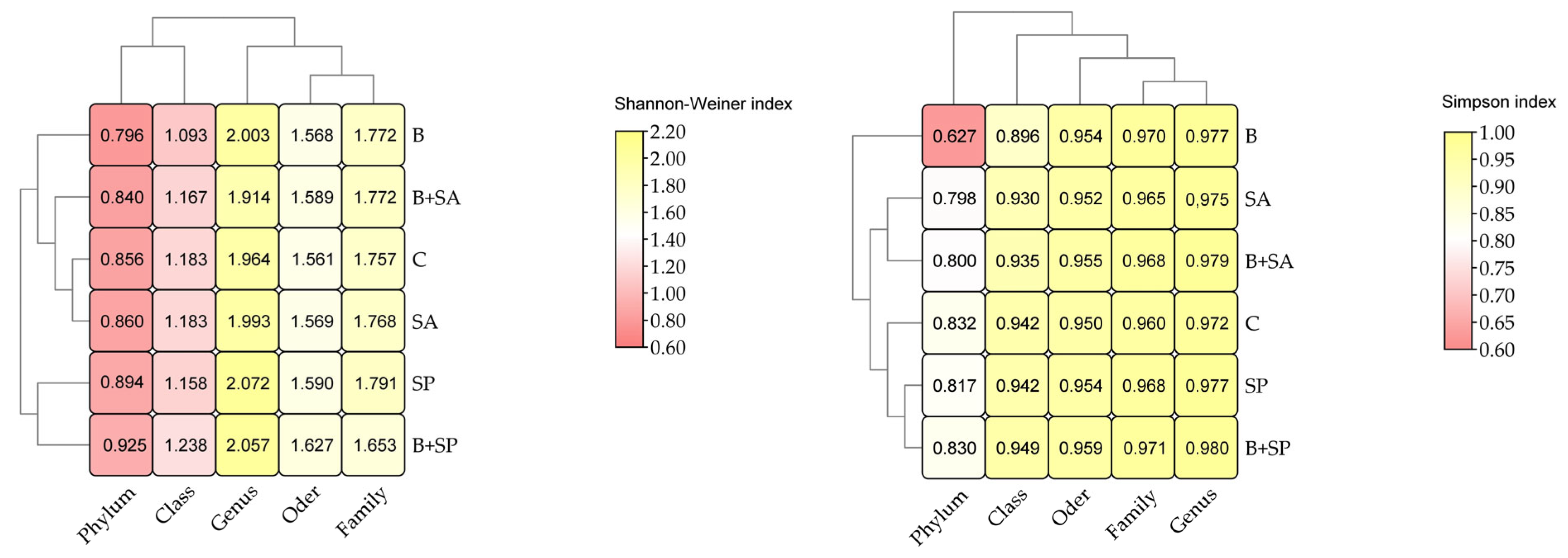
2.1. Soil Microbiota
2.2. Growth and Development of Spring Wheat
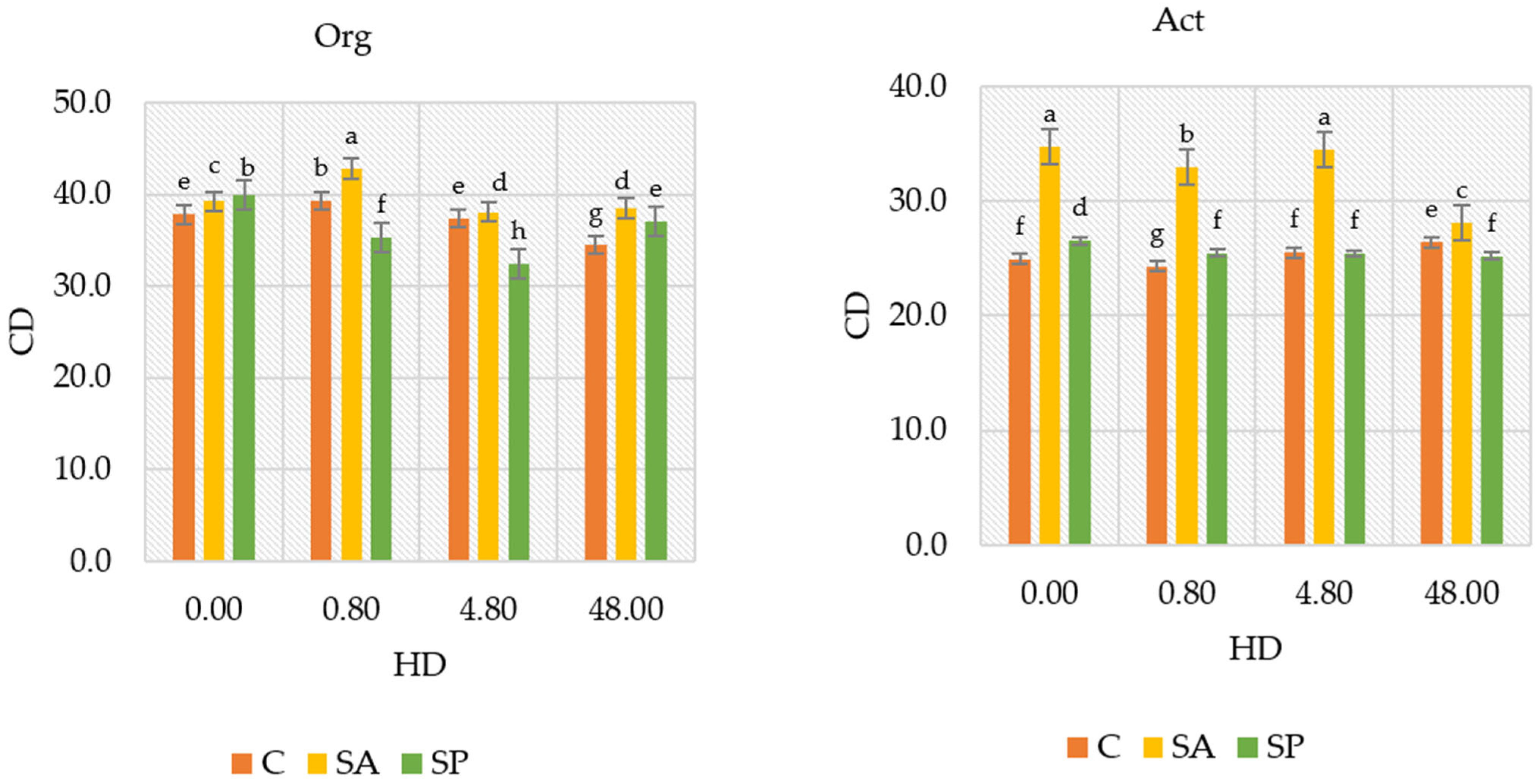
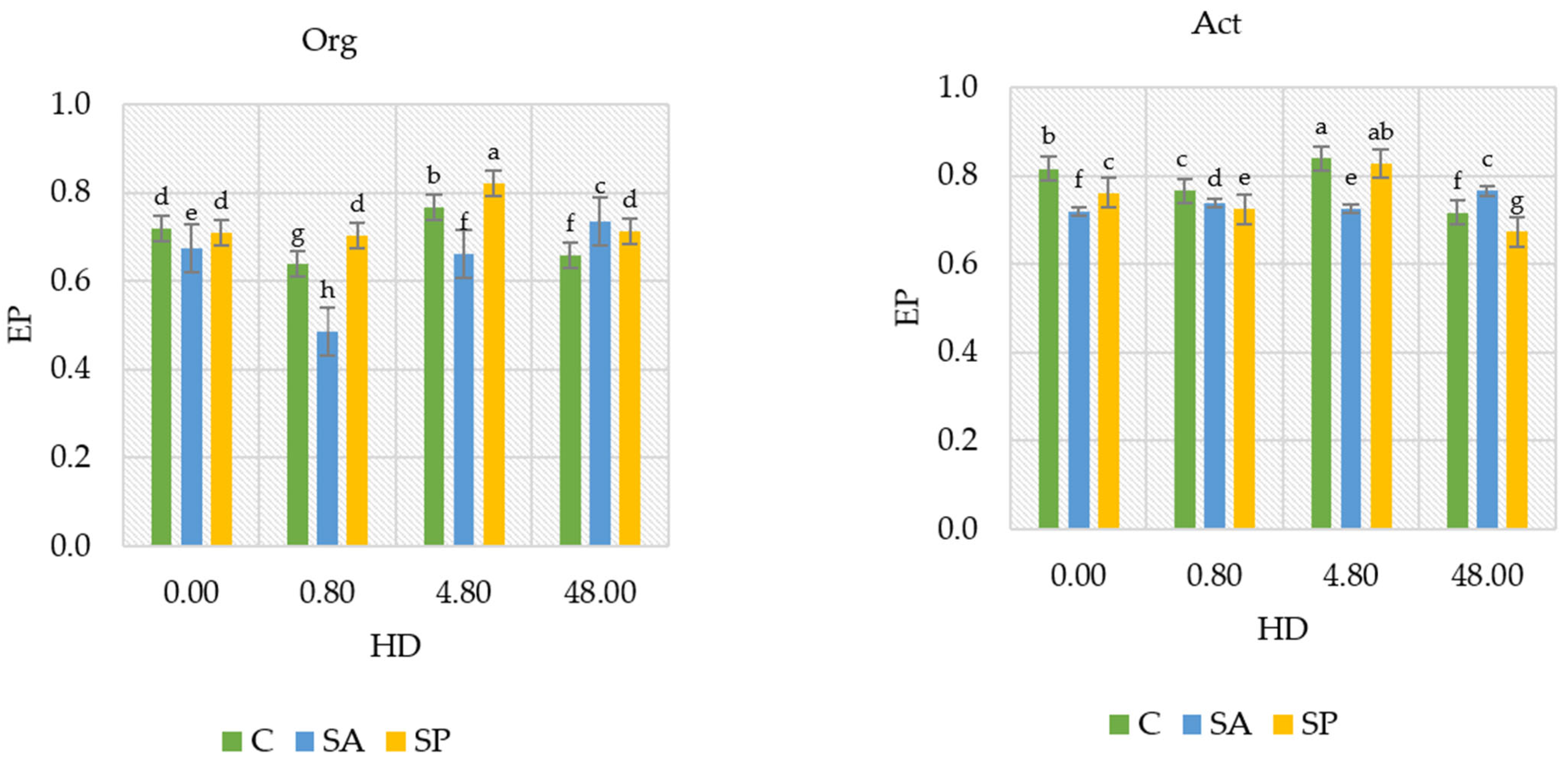
2.3. Physicochemical Properties of the Soil
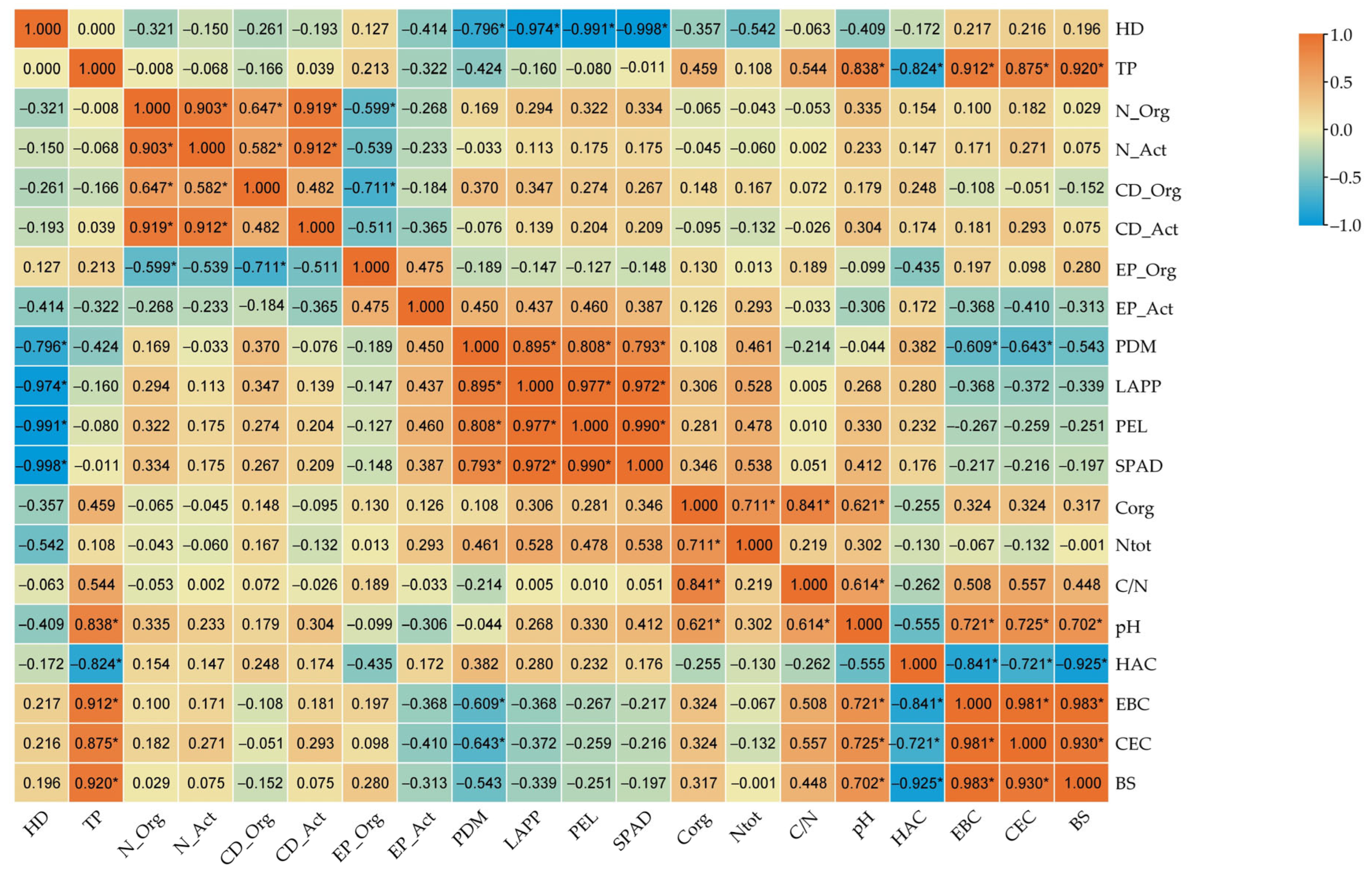
2.4. Correlations Between Soil Microbial Activity, Soil Physicochemical Properties, and Spring Wheat Growth and Development
3. Discussion
3.1. Soil Microbiota
3.2. Growth and Development of Spring Wheat
3.3. Physicochemical Properties of Soil
4. Materials and Methods
4.1. Soil Material
4.2. Herbicide Applications
- ▪
- sensitive weeds: Stellaria media (L.) Vill., Lamium purpureum L., Chenopodium album L., Apera spica-venti, Veronica hederifolia, Veronica persica, Galium aparine L.;
- ▪
- moderately sensitive weeds: Viola arvensis Murr., Papaver rhoeas L., Fallopia convolvulus (L.) Á. Löve, Matricaria chamomilla L., Poa annua L.;
- ▪
- resistant weeds: Panicum crus galli L.
4.3. Characteristics of Polymers
- ▪
- Sodium alginate (SA)—a solid substance with the molecular formula (NaC6H7O6) that occurs as a white powder with a pH of 5.5—8.0 and is produced by Agnex (Białystok, Poland). Its molecular weight ranges between 300 and 350 g mol−1. It is obtained from brown algae washed up on the Atlantic shore. It swells easily and binds water effectively. It has gelling properties at lower temperatures and reacts with calcium chloride to form a harder and stronger gel structure.
- ▪
- Sodium polyacrylate (SP)—an organic chemical compound with the molecular formula (C3H3NaO2)n that occurs as white granules characterized by a very high capacity to bind significant amounts of water. It stores water in the form of crystals and slowly releases it into the environment. It is produced by Biomus (Lublin, Poland) with the following characteristics: density: 700–800 kg m−3; water absorption capacity for distilled water: up to 20 g of water per 1 g of gel.
4.4. Characteristics of the Cultivated Plant
4.5. Establishment of and Procedure for Conducting the Experiment
- A quantity of 3.4 kg of soil, previously sieved through a mesh with a diameter of 5 mm, was weighed into plastic pots (capacity 3.5 dm3).
- Fertilizer, the herbicide Boxer 800 EC, sodium alginate, and sodium polyacrylate were then applied to the soil in the appropriate amounts.
- The soil was fertilized according to the nutrient requirements of Triticum aestivum L., in terms of pure components in mg kg−1 of soil dry matter: N—130 mg in the form of CO(NH2)2; P—60 mg in the form of KH2PO4, K—90 mg in the form of KH2PO4 + KCl; Mg—25 mg in the form of MgSO4 × 7H2O.
- The contents of the pots were thoroughly mixed, and the soil was sown with spring wheat, with 20 grains per pot. The whole mixture was brought to a moisture content of 50% of the capillary water capacity using deionized water.
- After the plants had germinated (7 days after sowing the spring wheat), 12 plants were left in each pot.
- Throughout the experiment, soil moisture was replenished 3 times per day with deionized water.
- Before harvesting the plants, the SPAD index (SPAD) was measured, while the length of the above-ground parts and ears of wheat was measured on the final day of the experiment (on the 50th day). After performing the plant biometric measurements, plant material was collected from each pot, and the fresh weight was determined.
- The plant roots were extracted from the soil, the entire soil was thoroughly homogenized, and 700 g of fresh soil was taken from each combination for microbiological analysis, while 800 g was taken for physicochemical analysis.
4.6. Physicochemical Analyses of Soil
4.7. Determination of Microbial Numbers
4.8. Metagenomic Analysis of the Soil
4.9. Determination of Plant Growth and Development
4.10. Statistical Analysis of the Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fusar Poli, E.; Fontefrancesco, M. Trends in the implementation of biopesticides in the Euro-Mediterranean region: A narrative literary review. Sustain. Earth Rev. 2024, 7, 14. [Google Scholar] [CrossRef]
- Nath, C.P.; Singh, R.G.; Choudhary, V.K.; Datta, D.; Nandan, R.; Singh, S.S. Challenges and Alternatives of Herbicide-Based Weed Management. Agronomy 2024, 14, 126. [Google Scholar] [CrossRef]
- Flor, R.J.; Maat, H.; Stuart, A.; Then, R.; Choun, S.; Chhun, S.; Hadi, B.A.R. Introducing an ecologically-based pest management approach in Cambodia through adaptive learning networks. Outlook Agric. 2023, 52, 200–211. [Google Scholar] [CrossRef]
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed management in 2050: Perspectives on the future of weed science. Weed Sci. 2018, 66, 275–285. [Google Scholar] [CrossRef]
- Parven, A.; Islam, M.; Kadiyala, V.; Mallavarapu, M. Herbicides in modern sustainable agriculture: Environmental fate, ecological implications, and human health concerns. Int. J. Environ. Sci. Technol. 2024, 22, 1181–1202. [Google Scholar] [CrossRef]
- Qu, R.Y.; He, B.; Yang, J.F.; Lin, H.Y.; Yang, W.C.; Wu, Q.Y.; Li, Q.; Yang, G.F. Where are the new herbicides? Pest Manag. Sci. 2021, 77, 2620–2625. [Google Scholar] [CrossRef]
- Tataridas, A.; Kanatas, P.; Chatzigeorgiou, A.; Zannopoulos, S.; Travlos, I. Sustainable crop and weed management in the era of the EU green deal: A survival guide. Agronomy 2022, 12, 589. [Google Scholar] [CrossRef]
- Davis, A.S.; Frisvold, G.B. Are herbicides a once in a century method of weed control? Pest Manag. Sci. 2017, 73, 2209–2220. [Google Scholar] [CrossRef]
- Li, Y.; Tang, J.; Liu, W.; Yan, W.; Sun, Y.; Che, J.; Tian, C.; Zhang, H.; Yu, L. The genetic architecture of grain yield in spring wheat based on genome-wide association study. Front. Genet. 2021, 12, 728472. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhang, C.Y.; Yuan, Y.; Hua, Y.F.; Asami, T.; Qin, Y.; Xiong, X.H.; Zhu, J.L.; Lu, Y.C. Molecular responses and degradation mechanisms of the herbicide diuron in rice crops. J. Agric. Food Chem. 2022, 70, 14352–14366. [Google Scholar] [CrossRef]
- Ruuskanen, S.; Fuchs, B.; Nissinen, R.; Puigbò, P.; Rainio, M.; Saikkonen, K.; Helander, M. Ecosystem consequences of herbicides: The role of microbiome. Trends Ecol. Evol. 2023, 38, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of agrochemicals on soil microbiota and management: A review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Bedano, J.C.; Lavelle, P.; Zou, X. Soil biodiversity for the sustainability of agroecosystems. Acta Oecol. 2021, 110, 103705. [Google Scholar] [CrossRef]
- Jeyaseelan, A.; Murugesan, K.; Thayanithi, S.; Palanisamy, S.B. A review of the impact of herbicides and insecticides on the microbial communities. Environ. Res. 2024, 245, 118020. [Google Scholar] [CrossRef]
- Khmelevtsova, L.; Azhogina, T.; Karchava, S.; Klimova, M.; Polienko, E.; Litsevich, A.; Chernyshenko, E.; Khammami, M.; Sazykin, I.; Sazykina, M. Effect of mineral fertilizers and pesticides application on bacterial community and antibiotic-resistance genes distribution in agricultural soils. Agronomy 2024, 14, 1021. [Google Scholar] [CrossRef]
- Andreasen, C.; Høgh, K.L.; Jensen, S.M. The effect of foliar and soil application of flufenacet and prosulfocarb on Italian ryegrass (Lolium multiflorum L.) control. Agriculture 2020, 10, 552. [Google Scholar] [CrossRef]
- Barba, V.; Marín-Benito, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Transport of 14C-prosulfocarb through soil columns under different amendment, herbicide incubation and irrigation regimes. Sci. Total Environ. 2020, 701, 134542. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Duan, G.; Sun, T.; Shen, G.; Tian, Z. Safe application of prosulfocarb in faba-bean fields in China. Chil. J. Agric. Res. 2024, 84, 718–728. [Google Scholar] [CrossRef]
- Braun, K.E.; Luks, A.-K.; Schmidt, B. Fate of the 14C-labeled herbicide prosulfocarb in a soil and in a sediment-water system. J. Environ. Sci. Health B 2017, 52, 122–130. [Google Scholar] [CrossRef]
- Bo, X.; Sun, J.; Mei, Q.; Wei, B.; An, Z.; Han, D.; Li, Z.; Xie, J.; Zhan, J.; He, M. Degradation of prosulfocarb by hydroxyl radicals in gas and aqueous phase: Mechanisms, kinetics and toxicity. Ecotoxicol. Environ. Saf. 2020, 191, 110175. [Google Scholar] [CrossRef]
- Muñoz, A.; Borras, E.; Rodenas, M.; Vera, T.; Pedersen, H.A. Atmospheric oxidation of a thiocarbamate herbicide used in winter cereals. Environ. Sci. Technol. 2018, 52, 9136–9144. [Google Scholar] [CrossRef] [PubMed]
- Palanivelu, S.D.; Armir, N.A.Z.; Zulkifli, A.; Hair, A.H.A.; Salleh, K.M.; Lindsey, K.; Che-Othman, M.H.; Zakaria, S. Hydrogel application in urban farming: Potentials and limitations—A review. Polymers 2022, 14, 2590. [Google Scholar] [CrossRef]
- Ali, K.; Asad, Z.; Agbna, G.H.D.; Saud, A.; Khan, A.; Zaidi, S.J. Progress and innovations in hydrogels for sustainable agriculture. Agronomy 2024, 14, 2815. [Google Scholar] [CrossRef]
- Grabowska-Polanowska, B.; Garbowski, T.; Bar-Michalczyk, D.; Kowalczyk, A. The benefits of synthetic or natural hydrogels application in agriculture: An overview article. J. Water Land Dev. 2021, 51, 208–224. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Z.; Jiang, H. Edible hydrogels with shrinkage tolerance in acids and stomach-friendly mechanical moduli. Appl. Mater. Today 2023, 32, 101786. [Google Scholar] [CrossRef]
- Choi, S.; Kim, H.R.; Kim, H.S. Fabrication of superabsorbent nanofibers based on sodium polyacrylate/polyvinyl alcohol and their water absorption characteristics. Polym. Int. 2019, 68, 764–771. [Google Scholar] [CrossRef]
- Özbakan, N.; Evirgen, B. The effect of sodium polyacrylate gel on the properties of liquefiable sandy soil under seismic condition. J. Eng. Res. 2023, 11, 100006. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Aslam, M.A.; Abdullah, M.F.B.; Al-Arjan, W.S.; Stojanovic, G.M.; Hasan, A. Hydrogels: Classifications, fundamental properties, applications, and scopes in recent advances in tissue engineering and regenerative medicine–A comprehensive review. Arab. J. Chem. 2024, 17, 105968. [Google Scholar] [CrossRef]
- Mikhailidi, A.; Ungureanu, E.; Tofanica, B.-M.; Ungureanu, O.C.; Fortună, M.E.; Belosinschi, D.; Volf, I. Agriculture 4.0: Polymer hydrogels as delivery agents of active ingredients. Gels 2024, 10, 368. [Google Scholar] [CrossRef]
- Patra, S.K.; Poddar, R.; Brestic, M.; Acharjee, P.U.; Bhattacharya, P.; Sengupta, S.; Pal, P.; Bam, N.; Biswas, B.; Barek, V.; et al. Prospects of hydrogels in agriculture for enhancing crop and water productivity under water deficit condition. Int. J. Polym. Sci. 2022, 2022, 4914836. [Google Scholar] [CrossRef]
- Garg, V.K.; Avashthi, H.; Tiwari, A.; Jain, P.A.; Ramkete, P.W.; Kayastha, A.M.; Singh, V.K. MFPPI–multi FASTA ProtParam interface. Bioinformation 2016, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Küçük, G.K. In silico characterization of esophageal cancer predominant genes. Sak. Univ. J. Sci. 2023, 27, 1255–1264. [Google Scholar] [CrossRef]
- Barba, V.; Marín-Benito, J.M.; García-Delgado, C.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Assessment of 14C-prosulfocarb dissipation mechanism in soil after amendment and its impact on the microbial community. Ecotoxicol. Environ. Saf. 2019, 182, 109395. [Google Scholar] [CrossRef]
- Carpio, M.J.; García-Delgado, C.; Marín-Benito, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Soil microbial community changes in a field treatment with chlorotoluron, flufenacet and diflufenican and two organic amendments. Agronomy 2020, 10, 1166. [Google Scholar] [CrossRef]
- Wang, Y.; Du, L.; Liu, H.; Long, D.; Huang, M.; Wang, Y.; Huang, S.; Jin, D. Halosulfuron methyl did not have a significant effect on diversity and community of sugarcane rhizosphere microflora. J. Hazard. Mater. 2020, 399, 123040. [Google Scholar] [CrossRef] [PubMed]
- Mhete, M.; Eze, P.N.; Rahube, T.O.; Akinyemi, F.O. Soil properties influence bacterial abundance and diversity under different land-use regimes in semi-arid environments. Sci. Afr. 2020, 7, e00246. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, J. Preparation of composites based on herbicide-degrading bacteria and their effects on soil bacterial communities. Microbe 2025, 6, 100234. [Google Scholar] [CrossRef]
- Du, M.; Zhang, J.; Wang, G.; Liu, C.; Wang, Z. Response of bacterial community composition and co-occurrence network to straw and straw biochar incorporation. Front. Microbiol. 2022, 13, 999399. [Google Scholar] [CrossRef]
- Onyango, L.; Ngonga, F.; Karanja, E.; Kuja, J.; Boga, H.; Cowan, D.; Mwangi, K.; Maghenda, M.; Lebre, P.; Kambura, A. The soil microbiomes of forest ecosystems in Kenya: Their diversity and environmental drivers. Sci. Rep. 2023, 13, 7156. [Google Scholar] [CrossRef]
- Petrić, I.; Karpouzas, D.G.; Bru, D.; Udiković-Kolić, N.; Kandeler, E.; Djuric, S.; Martin-Laurent, F. Nicosulfuron application in agricultural soils drives the selection towards NS-tolerant microorganisms harboring various levels of sensitivity to nicosulfuron. Environ. Sci. Pollut. Res. 2016, 23, 4320–4333. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Tian, L.; Li, X.; Tian, C. Microbial communities in peatlands along a chronosequence on the Sanjiang Plain, China. Sci. Rep. 2017, 7, 9567. [Google Scholar] [CrossRef] [PubMed]
- Tsoy, O.; Ravcheev, D.; Chuklina, J.; Gelfand, M. Nitrogen fixation and molecular oxygen: Comparative genomic reconstruction of transcription regulation in Alphaproteobacteria. Front. Microbiol. 2016, 7, 1343. [Google Scholar] [CrossRef]
- Shivlata, L.; Satyanarayana, T. Thermophilic and alkaliphilic Actinobacteria: Biology and potential applications. Front. Microbiol. 2015, 6, 1014. [Google Scholar] [CrossRef]
- Doolotkeldieva, T.; Konurbaeva, M.; Bobusheva, S. Microbial communities in pesticide-contaminated soils in Kyrgyzstan and bioremediation possibilities. Environ. Sci. Pollut. Res. 2018, 25, 31848–31862. [Google Scholar] [CrossRef]
- Wang, F.; Wei, Y.; Yan, T.; Wang, C.; Chao, Y.; Jia, M.; An, L.; Sheng, H. Sphingomonas sp. Hbc-6 alters physiological metabolism and recruits beneficial rhizosphere bacteria to improve plant growth and drought tolerance. Front. Plant Sci. 2022, 13, 1002772. [Google Scholar] [CrossRef] [PubMed]
- Mazoyon, C.; Hirel, B.; Pecourt, A.; Catterou, M.; Gutierrez, L.; Sarazin, V.; Dubois, F.; Duclercq, J. Sphingomonas sediminicola is an endosymbiotic bacterium able to induce the formation of root nodules in pea (Pisum sativum L.) and to enhance plant biomass production. Microorganisms 2023, 11, 199. [Google Scholar] [CrossRef]
- Kaur, A.; Pati, P.K.; Pati, A.M.; Nagpal, A.K. Physico-chemical characterization and topological analysis of pathogenesis-related proteins from Arabidopsis thaliana and Oryza sativa using in-silico approaches. PLoS ONE 2020, 15, e0239836. [Google Scholar] [CrossRef]
- Sun, C.; Liu, Y.; Bei, K.; Zheng, W.; Wang, Q.; Wang, Q. Impact of biochar on the degradation rates of three pesticides by vegetables and its effects on soil bacterial communities under greenhouse conditions. Sci. Rep. 2024, 14, 19986. [Google Scholar] [CrossRef]
- Strachel, R.; Wyszkowska, J.; Baćmaga, M. An evaluation of the effectiveness of sorbents in the remediation of soil contaminated with zinc. Water Air Soil Pollut. 2018, 229, 235. [Google Scholar] [CrossRef]
- Bajestani, M.S.; Kiani, F.; Ebrahimi, S.; Malekzadeh, E.; Tatari, A. Effects of bentonite/sodium alginate/nanocellulose composites on soil properties and their biodegradability over time. Sci. Rep. 2025, 15, 10596. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. The role of sodium alginate hydrogel in maintaining soil homeostasis exposed to sulcotrione. Agriculture 2024, 14, 2081. [Google Scholar] [CrossRef]
- Ahn, S.; Ryou, J.-E.; Ahn, K.; Lee, C.; Lee, J.-D.; Jung, J. Evaluation of dynamic properties of sodium-alginate-reinforced soil using a resonant-column test. Materials 2021, 14, 2743. [Google Scholar] [CrossRef] [PubMed]
- Dingley, C.; Cass, P.; Adhikari, B.; Daver, F. Application of superabsorbent natural polymers in agriculture. Polym. Renew. Resour. 2024, 15, 210–255. [Google Scholar] [CrossRef]
- Sethi, D.; Mohanty, S.; Mohapatra, K.K.; Dash, P.K.; Sahoo, S.K.; Padhan, K.; Kusumavathi, K.; Majhi, R.; Panda, N.; Pattanayak, S.K. Exploring the influence of polymers on soil ecosystems: Prospective from agricultural contexts. Front. Chem. Eng. 2024, 6, 1485534. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Q.; Yu, M.; Yin, M.; Chen, F. Effect of sodium alginate–gelatin–polyvinyl pyrrolidone microspheres on cucumber plants, soil, and microbial communities under lead stress. Int. J. Biol. Macromol. 2023, 247, 125688. [Google Scholar] [CrossRef]
- Quezada, G.R.; Retamal, F.; Jeldres, M.; Jeldres, R.I. Understanding the behavior of sodium polyacrylate in suspensions of silica and monovalent salts. Polymers 2023, 15, 3861. [Google Scholar] [CrossRef]
- Yakimenko, O.; Pozdnyakov, L.; Kadulin, M.; Gruzdenko, D.; Panova, I.; Yaroslavov, A. Effects of binary polymer-humic soil amendments on soil carbon cycle and detoxication ability of heavy metal pollution. Chem. Biol. Technol. Agric. 2024, 11, 174. [Google Scholar] [CrossRef]
- Wang, H.; Bai, W.; Peng, X.; Han, W.; He, J.; Song, J.; Lv, G. Effect of super absorbent polymer sodium polyacrylate on the bacterial community and associated chemistry of loessial soil. Arch. Agron. Soil Sci. 2019, 66, 70–82. [Google Scholar] [CrossRef]
- Adjuik, T.A.; Nokes, S.E.; Montross, M.D.; Wendroth, O. The impacts of bio-based and synthetic hydrogels on soil hydraulic properties: A review. Polymers 2022, 14, 4721. [Google Scholar] [CrossRef]
- Zumstein, M.T.; Schintlmeister, A.; Nelson, T.F.; Baumgartner, R.; Woebken, D.; Wagner, M.; Kohler, H.-P.; McNeil, K.; Sander, M. Biodegradation of synthetic polymers in soils: Tracking carbon into CO2 and microbial biomass. Sci. Adv. 2018, 4, eaas9024. [Google Scholar] [CrossRef]
- Tang, S.; Li, J.; Huang, G.; Yan, L. Predicting protein surface property with its surface hydrophobicity. Protein Pept. Lett. 2021, 28, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Ferenczy, G.G.; Kellermayer, M. Contribution of hydrophobic interactions to protein mechanical stability. Comput. Struct. Biotechnol. J. 2022, 20, 1946–1956. [Google Scholar] [CrossRef] [PubMed]
- Gitsopoulos, T.; Georgoulas, I.; Botsoglou, D.; Vazanelli, E. Response of wheat to pre-emergence and early post-emergence herbicides. Agronomy 2024, 14, 1875. [Google Scholar] [CrossRef]
- Zhang, B.; Lv, F.; Yang, J. Pesticides toxicity, removal and detoxification in plants: A review. Agronomy 2024, 14, 1260. [Google Scholar] [CrossRef]
- Liu, N.; Li, J.; Lv, J.; Yu, J.; Xie, J.; Wu, Y.; Tang, Z. Melatonin alleviates imidacloprid phytotoxicity to cucumber (Cucumis sativus L.) through modulating redox homeostasis in plants and promoting its metabolism by enhancing glutathione dependent detoxification. Ecotoxicol. Environ. Saf. 2021, 217, 112248. [Google Scholar] [CrossRef]
- Panfili, I.; Bartucca, M.L.; Marrollo, G.; Povero, G.; Del Buono, D. Application of a plant biostimulant to improve maize (Zea mays) tolerance to metolachlor. J. Agric. Food Chem. 2019, 67, 12164–12171. [Google Scholar] [CrossRef]
- Mendoza-Meneses, C.J.; García-Trejo, J.F.; Macías-Bobadilla, G.; Aguirre-Becerra, H.; Soto-Zarazúa, G.M.; Feregrino-Pérez, A.A. Review and Perspectives of the Use of Alginate as a Polymer Matrix for Microorganisms Applied in Agro-Industry. Molecules 2022, 27, 4248. [Google Scholar] [CrossRef]
- Qin, C.; Wang, H.; Zhao, Y.; Qi, Y.; Wu, N.; Zhang, S.; Xu, W. Recent advances of hydrogel in agriculture: Synthesis, mechanism, properties and applications. Eur. Polym. J. 2024, 2019, 113376. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, W.; Wang, Y.; Guo, Y.; Duan, S.; Zhao, K.; Li, S. Metal ions increase mechanical strength and barrier properties of collagen-sodium polyacrylate composite films. Inter. J. Biol. Macromol. 2018, 119, 15–22. [Google Scholar] [CrossRef]
- Shankarappa, S.K.; Muniyandi, S.J.; Chandrashekar, A.B.; Singh, A.K.; Nagabhushanaradhya, P.; Shivashankar, B.; El-Ansary, D.O.; Wani, S.H.; Elansary, H.O. Standardizing the hydrogel application rates and foliar nutrition for enhancing yield of lentil (Lens culinaris). Processes 2020, 8, 420. [Google Scholar] [CrossRef]
- Ma, L.; Chai, C.; Wu, W.; Qi, P.; Liu, X.; Hao, J. Hydrogels as the plant culture substrates: A review. Carbohydr. Polym. 2023, 305, 120544. [Google Scholar] [CrossRef] [PubMed]
- Ataikiru, T.L.; Okpokwasili, G.S.C.; Okerentugba, P.O. Impact of pesticides on microbial diversity and enzymes in soil. S. Asian J. Res. Microbiol. 2019, 4, 1–16. [Google Scholar] [CrossRef]
- Omar, G.; Tasi’u, B. Effect of herbicides application on soil physico-chemical properties and performance of maize in Sudan Savanna zone of Nigeria. Int. J. Plant Soil Sci. 2020, 32, 47–58. [Google Scholar] [CrossRef]
- Tudararo-Aherobo, L.E.; Ataikiru, T.L. Effects of chronic use of herbicides on soil physicochemical and microbiological characteristics. Microbiol. Res. J. Int. 2020, 30, 9–19. [Google Scholar] [CrossRef]
- Fasinmirin, J. Modelling cation exchange capacity and water holding capacity from basic soil properties. Eurasian J. Soil Sci. 2016, 5, 266–274. [Google Scholar]
- Estrada, R.; Porras, T.; Romero, Y.; Pérez, W.E.; Vilcara, E.A.; Cruz, J.; Arbizu, C.I. Soil depth and physicochemical properties influence microbial dynamics in the rhizosphere of two Peruvian superfood trees, cherimoya and lucuma, as shown by PacBio-HiFi sequencing. Sci. Rep. 2024, 14, 19508. [Google Scholar] [CrossRef] [PubMed]
- Rauthan, K.; Joshi, S.; Kumar, L.; Goel, D.; Kumar, S. Functional annotation of uncharacterized proteins from Fusobacterium nucleatum: Identification of virulence factors. Genom. Inform. 2023, 21, e21. [Google Scholar] [CrossRef]
- Tasneem, M.; Gupta, S.D.; Momin, M.B.; Hossain, K.M.; Osman, T.B.; Rabbi, M.F. In silico annotation of a hypothetical protein from Listeria monocytogenes EGD-e unfolds a toxin protein of the type II secretion system. Genom. Inform. 2023, 21, e7. [Google Scholar] [CrossRef]
- Piccoli, I.; Camarotto, C.; Squartini, A.; Longo, M.; Gross, S.; Maggini, M.; Cabrera, M.L.; Morari, F. Hydrogels for agronomical application: From soil characteristics to crop growth: A review. Agron. Sustain. Dev. 2024, 44, 22. [Google Scholar] [CrossRef]
- Hou, X.; Li, R.; He, W.; Dai, X.; Ma, K.; Liang, Y. Superabsorbent polymers influence soil physical properties and increase potato tuber yield in a dry-farming region. J. Soils Sediments 2018, 18, 816–826. [Google Scholar] [CrossRef]
- Erci, V.; Seker, C.; Basaran, M.; Erpul, G. Determining the effectiveness of some soil stabilizers in wind erosion prevention using wind tunnel experiments. L. Degrad. Dev. 2021, 32, 2962–2977. [Google Scholar] [CrossRef]
- Abrisham, E.S.; Jafari, M.; Tavili, A.; Rabii, A.; Chahoki, M.A.Z.; Zare, S.; Egan, T.; Yazdanshenas, H.; Ghasemian, D.; Tahmoures, M. Effects of a super absorbent polymer on soil properties and plant growth for use in land reclamation. Arid. L. Res. Manag. 2018, 32, 407–420. [Google Scholar] [CrossRef]
- El-Saied, H.; El-Hady, O.A.; Basta, A.H.; El-Dewiny, C.T.; Abo-Sedera, S.A. Bio-chemical properties of sandy calcareous soil treated with rice straw-based hydrogels. J. Saudi Soc. Agric. Sci. 2016, 15, 188–194. [Google Scholar] [CrossRef]
- IUSS Working Group World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Reference Base for Soil Resources, Update 2015; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014. [Google Scholar]
- PPDB (Pesticide Properties DataBase). Available online: http://sitem.herts.ac.uk/aeru/ppdb/ (accessed on 15 February 2025).
- Spanic, V.; Lalic, Z.; Berakovic, I.; Jukic, G.; Varnica, I. Morphological characterization of 1322 winter wheat (Triticum aestivum L.) varieties from EU referent collection. Agriculture 2024, 14, 551. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Cacak-Pietrzak, G.; Lenc, L.; Stalenga, J. Rating of spring wheat varieties (Triticum aestivum L.) according to their suitability for organic agriculture. Agronomy 2020, 10, 1900. [Google Scholar] [CrossRef]
- Lackmann, C.; Velki, M.; Bjedov, D.; Ečimović, S.; Seiler, T.-B.; Hollert, H. Commercial preparations of pesticides exert higher toxicity and cause changes at subcellular level in earthworm Eisenia andrei. Environ. Sci. Eur. 2021, 33, 12. [Google Scholar] [CrossRef]
- Devault, D.A.; Guillemin, J.P.; Millet, M.; Hulin, M.; Merlo, M. Prosulfocarb at center stage! Environ. Sci. Pollut. Res. 2022, 29, 61–67. [Google Scholar] [CrossRef]
- Adjuik, T.A.; Nokes, S.E.; Montross, M.D. Biodegradability of bio-based and synthetic hydrogels as sustainable soil amendments: A review. J. Appl. Polym. Sci. 2023, 140, e53655. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Mohawesh, O.; Gharaibeh, M.A.; Alghamdi, A.G.; Alajlouni, M.A.; Alqudah, A.M. Effect of hydrogel on corn growth, water use efficiency, and soil properties in a semi-arid region. J. Saudi Soc. Agric. Sci. 2022, 21, 518–524. [Google Scholar] [CrossRef]
- Sepehri, S.; Abdoli, S.; Asgari Lajayer, B.; Astatkie, T.; Price, G.W. Changes in phytochemical properties and water use efficiency of peppermint (Mentha piperita L.) using superabsorbent polymer under drought stress. Sci. Rep. 2023, 13, 21989. [Google Scholar] [CrossRef]
- Abdelghafar, R.; Abdelfattah, A.; Mostafa, H. Effect of super absorbent hydrogel on hydro-physical properties of soil under deficit irrigation. Sci. Rep. 2024, 14, 7655. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Boros-Lajszner, E.; Kucharski, J. The impact of soil contamination with lead on the biomass of maize intended for energy purposes, and the biochemical and physicochemical properties of the soil. Energies 2024, 17, 1156. [Google Scholar] [CrossRef]
- Komorek, D.; Wyszkowska, J.; Borowik, A.; Zaborowska, M. Microbial activity and diversity in soil sown with Zea mays and Triticosecale. Agriculture 2024, 14, 1070. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Kucharski, J. The role of grass compost and Zea mays in alleviating toxic effects of tetracycline on the soil bacteria community. Int. J. Environ. Res. Public Health 2022, 19, 7357. [Google Scholar] [CrossRef]
- Sarathchandra, S.U.; Burch, G.; Cox, N.R. Growth patterns of bacterial communites in the rhizoplane and rhizosphere of with clover (Trifolium repens L.) and perennial ryegrass (Lolium perenne L.) in long-term pasture. Appl. Soil Ecol. 1997, 6, 293–299. [Google Scholar] [CrossRef]
- De Leij, F.A.A.M.; Whipps, J.M.; Lynch, J.M. The use of colony development for the characterization of bacterial communities in soil and on roots. Microb. Ecol. 1994, 27, 81–97. [Google Scholar] [CrossRef]
- Lipińska, A.; Wyszkowska, J.; Kucharski, J. Microbiological and biochemical activity in soil contaminated with pyrene subjected to bioaugmentation. Water Air Soil Pollut. 2021, 232, 45. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. Response of soil microbiota, enzymes, and plants to the fungicide azoxystrobin. Int. J. Mol. Sci. 2024, 25, 8104. [Google Scholar] [CrossRef] [PubMed]
- TIBCO Software Inc. Statistica. Data Analysis Software System, Version 13. 2017. Available online: https://www.statistica.com (accessed on 10 February 2025).
- Zhang, W.; Xu, J.; Dong, F.; Liu, X.; Zhang, Y.; Wu, X.; Zheng, Y. Effect of tetraconazole application on the soil microbial community. Environ. Sci. Pollut. Res. 2014, 21, 8323–8332. [Google Scholar] [CrossRef]
- MEGA 7 Software. Available online: https://www.megasoftware.net/show_eua (accessed on 15 March 2025).
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant. 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A Web-Based Tool for the Analysis of Sets through Venn Diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- ProtParam Software. Available online: https://web.expasy.org/protparam (accessed on 3 March 2025).
- ISIS-Draw, MDL, Version 2.3. 2004. Available online: https://mdl-isis-draw.software.informer.com/2.3/ (accessed on 15 March 2025).

| Variables | N_Org | N_Act | CD_Org | CD_Act | EP_Org | EP_Act |
|---|---|---|---|---|---|---|
| HD | 17.014 | 1.653 | 41.347 | 10.436 | 10.592 | 25.558 |
| TP | 72.701 | 96.742 | 46.092 | 64.233 | 11.990 | 18.913 |
| HD×TP | 10.017 | 0.143 | 9.723 | 24.940 | 74.891 | 51.418 |
| error | 0.269 | 1.462 | 2.837 | 0.391 | 2.526 | 4.111 |
| HD | Org | Act | ||||
|---|---|---|---|---|---|---|
| C | SA | SP | C | SA | SP | |
| 0.00 | 3.536 ± 0.266 h | 18.006 ± 0.446 a | 4.232 ± 0.342 f | 2.536 ± 0.172 f | 9.866 ± 0.844 c | 2.411 ± 0.278 g |
| 0.80 | 5.480 ± 0.971 e | 16.383 ± 0.402 b | 3.849 ± 0.427 g | 3.731 ± 0.541 e | 11.431 ± 0.931 b | 2.255 ± 0.024 h |
| 4.80 | 3.054 ± 0.653 i | 11.785 ± 0.981 c | 2.949 ± 0.027 j | 2.467 ± 0.665 g | 12.537 ± 0.657 a | 1.934 ± 0.196 i |
| 48.0 | 1.417 ± 0.244 l | 8.200 ± 0.166 d | 2.049 ± 0.475 k | 1.700 ± 0.294 j | 9.205 ± 0.779 d | 1.097 ± 0.206 k |
| Genus | Aa | MW | pI | ExC | Iix | Aix | GRAVY |
|---|---|---|---|---|---|---|---|
| Bryobacter | 427 | 33.639 | 5.07 | 6250 | 40.01 | 23.19 | 0.745 |
| Nocardioides | 407 | 31.996 | 5.08 | 5250 | 42.23 | 23.83 | 0.672 |
| Streptomyces | 402 | 32.133 | 5.08 | 5500 | 39.98 | 23.23 | 0.678 |
| Gaiella | 426 | 33.425 | 5.07 | 6125 | 44.43 | 24.41 | 0.754 |
| Conexibacter | 427 | 33.639 | 5.07 | 6250 | 40.01 | 23.19 | 0.745 |
| Flavisolibacter | 422 | 33.968 | 5.09 | 5250 | 41.14 | 27.73 | 0.717 |
| Terrimonas | 422 | 33.881 | 5.08 | 4875 | 42.07 | 28.67 | 0.704 |
| Nubsella | 422 | 33.731 | 5.09 | 5125 | 39.66 | 27.49 | 0.708 |
| Pedobacter | 422 | 33.669 | 5.08 | 5500 | 42.99 | 27.01 | 0.736 |
| Leptolyngbya | 405 | 31.798 | 5.09 | 4750 | 41.46 | 27.41 | 0.694 |
| Tumebacillus | 428 | 33.778 | 5.07 | 6125 | 44.94 | 23.83 | 0.742 |
| Bacillus | 428 | 33.753 | 5.08 | 5625 | 38.81 | 25.93 | 0.723 |
| Paenibacillus | 426 | 33.603 | 5.08 | 5375 | 33.55 | 26.29 | 0.712 |
| Gemmatimonas | 419 | 32.918 | 5.07 | 6125 | 46.61 | 21.28 | 0.699 |
| Reyranella | 402 | 31.625 | 5.09 | 5000 | 41.42 | 25.87 | 0.695 |
| Devosia | 402 | 31.773 | 5.09 | 5125 | 40.48 | 26.12 | 0.706 |
| Mesorhizobium | 402 | 31.827 | 5.09 | 5000 | 39.38 | 26.37 | 0.695 |
| Bradyrhizobium | 402 | 31.779 | 5.09 | 5250 | 40.90 | 25.37 | 0.704 |
| Pseudolabrys | 402 | 31.703 | 5.08 | 5500 | 40.83 | 24.63 | 0.729 |
| Sphingomonas | 428 | 33.866 | 5.08 | 5875 | 42.43 | 25.47 | 0.740 |
| Dyella | 427 | 33.646 | 5.08 | 5375 | 40.69 | 26.00 | 0.706 |
| Rhodanobacter | 427 | 33.698 | 5.09 | 5250 | 36.61 | 26.23 | 0.686 |
| Luteimonas | 427 | 33.634 | 5.08 | 5500 | 37.38 | 25.76 | 0.706 |
| Nitrspira | 427 | 33.742 | 5.08 | 5750 | 45.37 | 26.70 | 0.756 |
| Lacunisphaera | 426 | 33.858 | 5.08 | 5625 | 46.33 | 27.46 | 0.765 |
| Variables | PDM | LAPP | PEL | SPAD |
|---|---|---|---|---|
| HD | 82.047 | 98.919 | 99.349 | 98.211 |
| TP | 7.495 | 0.396 | 0.118 | 0.009 |
| HD × TP | 7.495 | 0.396 | 0.118 | 0.009 |
| error | 2.964 | 0.289 | 0.414 | 1.770 |
| HD | PDM | LAPP | PEL | SPAD |
|---|---|---|---|---|
| Soil without the addition of polymers (SWAP) | ||||
| 0.00 | 25.750 a | 66.000 a | 8.000 b | 45.600 b |
| 0.80 | 25.970 a | 65.750 a | 8.125 a | 47.525 a |
| 4.80 | 21.633 b | 62.250 b | 8.250 a | 39.617 e |
| 48.0 | 0.000 g | 0.000 g | 0.000 f | 0.000 f |
| Soil with the addition of sodium alginate (SA) | ||||
| 0.00 | 18.165 c | 61.750 b | 7.750 c | 45.783 b |
| 0.80 | 16.815 d | 55.500 d | 8.000 b | 44.767 c |
| 4.80 | 6.508 f | 48.500 e | 7.750 c | 44.050 c |
| 48.0 | 0.000 g | 0.000 g | 0.000 f | 0.000 f |
| Soil with the addition of sodium polyacrylate (SP) | ||||
| 0.00 | 11.980 e | 59.250 c | 7.500 d | 44.758 c |
| 0.80 | 16.205 d | 54.000 d | 7.125 e | 45.433 b |
| 4.80 | 6.370 f | 40.500 f | 7.125 e | 40.475 d |
| 48.0 | 0.000 g | 0.000 g | 0.000 f | 0.000 f |
| Variables | Corg | Ntot | C/N | pH | HAC | EBC | CEC | BS |
|---|---|---|---|---|---|---|---|---|
| HD | 23.389 | 48.895 | 17.377 | 31.481 | 22.325 | 15.004 | 15.917 | 11.838 |
| TP | 13.465 | 16.642 | 31.543 | 43.519 | 46.225 | 73.354 | 63.586 | 73.746 |
| HD × TP | 62.324 | 32.173 | 49.130 | 23.222 | 29.244 | 7.932 | 17.572 | 11.275 |
| error | 0.822 | 2.289 | 1.951 | 1.778 | 2.206 | 3.710 | 2.924 | 3.140 |
| HD | Corg | Ntot | C/N | pH | HAC | EBC | CEC | BS |
|---|---|---|---|---|---|---|---|---|
| Soil without the addition of polymers (SWAP) | ||||||||
| 0.00 | 7.575 d | 1.285 a | 5.896 h | 4.725 e | 15.375 d | 29.000 h | 44.375 h | 65.220 g |
| 0.80 | 7.210 f | 1.120 e | 6.437 f | 4.775 d | 15.750 c | 29.500 g | 45.250 g | 65.206 g |
| 4.80 | 7.110 g | 1.060 f | 6.708 d | 4.725 e | 16.125 b | 30.000 f | 46.125 f | 65.044 g |
| 48.0 | 6.715 h | 1.050 f | 6.398 g | 4.700 f | 16.500 a | 29.500 g | 46.000 f | 64.136 h |
| Soil with the addition of sodium alginate (SA) | ||||||||
| 0.00 | 7.130 g | 1.095 f | 6.517 e | 4.825 b | 15.375 d | 32.500 e | 47.875 d | 67.888 f |
| 0.80 | 7.310 e | 1.130 d | 6.467 f | 4.825 b | 15.750 c | 33.000 d | 48.750 d | 67.703 f |
| 4.80 | 7.515 d | 1.130 d | 6.668 d | 4.825 b | 15.375 d | 34.500 c | 49.875 d | 69.172 e |
| 48.0 | 8.045 c | 1.120 e | 7.222 c | 4.775 d | 14.625 f | 35.500 b | 50.125 b | 70.822 c |
| Soil with the addition of sodium polyacrylate (SP) | ||||||||
| 0.00 | 9.545 a | 1.225 b | 7.797 a | 4.875 a | 15.000 e | 34.500 d | 49.500 e | 69.710 d |
| 0.80 | 8.825 b | 1.210 c | 7.318 b | 4.875 a | 14.663 f | 35.000 c | 49.663 e | 70.478 c |
| 4.80 | 7.375 e | 1.115 e | 6.619 e | 4.825 b | 14.288 g | 35.500 b | 49.788 c | 71.301 b |
| 48.0 | 6.560 i | 1.040 f | 6.324 g | 4.800 c | 13.913 h | 36.500 a | 50.413 a | 72.405 a |
| Prosulfocarb | Parameter | Formula/Value | |
|---|---|---|---|
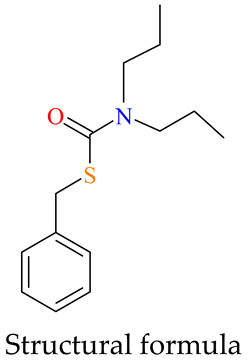 | Chemical formula | C14H21NOS | |
| Substance groups | thiocarbamate | ||
| Solubility in water at 20 °C (mg dm⁻3) | 13.2 | ||
| Solubility in organic solvents at 20 °C (mg dm⁻3) | 250,000 (acetone) | ||
| Vapor pressure at 20 °C (mPa) | 0.79 | ||
| Octanol–water partition coefficient at pH 7, 20 °C | P | 3.02 × 104 | |
| Log P | 4.48 | ||
| Soil degradation aerobic (days) | DT50 (typical) | 11.9 | |
| DT50 (lab at 20 °C) | 11.9 | ||
| DT50 (field) | 9.8 | ||
| Kf (cm3 g−1) | 23.1 | ||
| Kfoc (cm3 g−1) | 1693 | ||
| Parameter | C | B | SA | B + SA | SP | B + SP |
|---|---|---|---|---|---|---|
| DNA concentration (µg cm−3) | 15.1 | 8.96 | 11.10 | 6.74 | 11.9 | 14.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baćmaga, M.; Wyszkowska, J.; Kucharski, J. Influence of Prosulfocarb and Polymer Supplementation on Soil Bacterial Diversity in Triticum aestivum L. Cultivation. Int. J. Mol. Sci. 2025, 26, 5452. https://doi.org/10.3390/ijms26125452
Baćmaga M, Wyszkowska J, Kucharski J. Influence of Prosulfocarb and Polymer Supplementation on Soil Bacterial Diversity in Triticum aestivum L. Cultivation. International Journal of Molecular Sciences. 2025; 26(12):5452. https://doi.org/10.3390/ijms26125452
Chicago/Turabian StyleBaćmaga, Małgorzata, Jadwiga Wyszkowska, and Jan Kucharski. 2025. "Influence of Prosulfocarb and Polymer Supplementation on Soil Bacterial Diversity in Triticum aestivum L. Cultivation" International Journal of Molecular Sciences 26, no. 12: 5452. https://doi.org/10.3390/ijms26125452
APA StyleBaćmaga, M., Wyszkowska, J., & Kucharski, J. (2025). Influence of Prosulfocarb and Polymer Supplementation on Soil Bacterial Diversity in Triticum aestivum L. Cultivation. International Journal of Molecular Sciences, 26(12), 5452. https://doi.org/10.3390/ijms26125452







