The Profile of Retinal Ganglion Cell Death and Cellular Senescence in Mice with Aging
Abstract
1. Introduction
2. Results
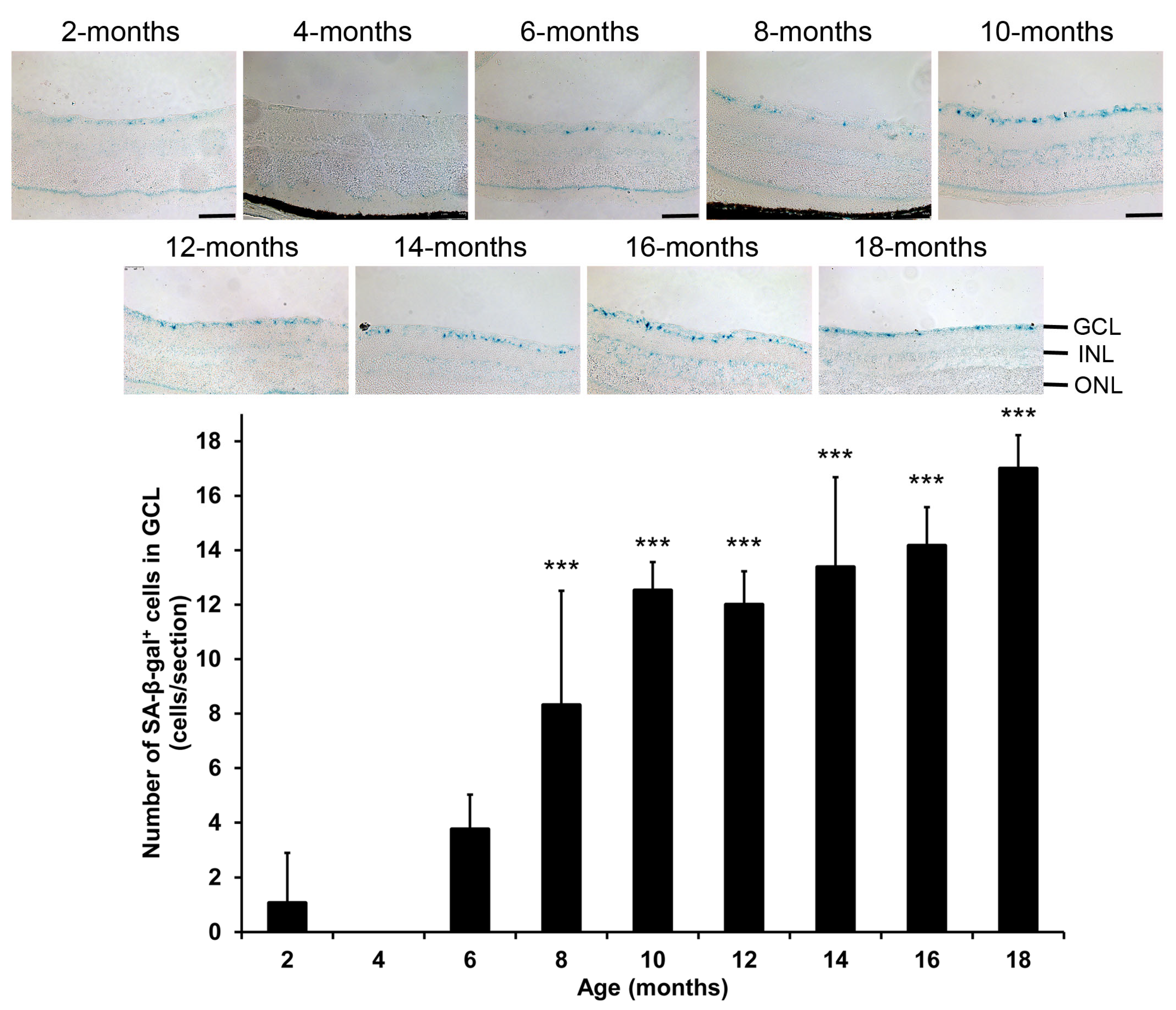
2.1. Changes of Senescence-Associated β-Galactosidase Expression in Mice During Aging
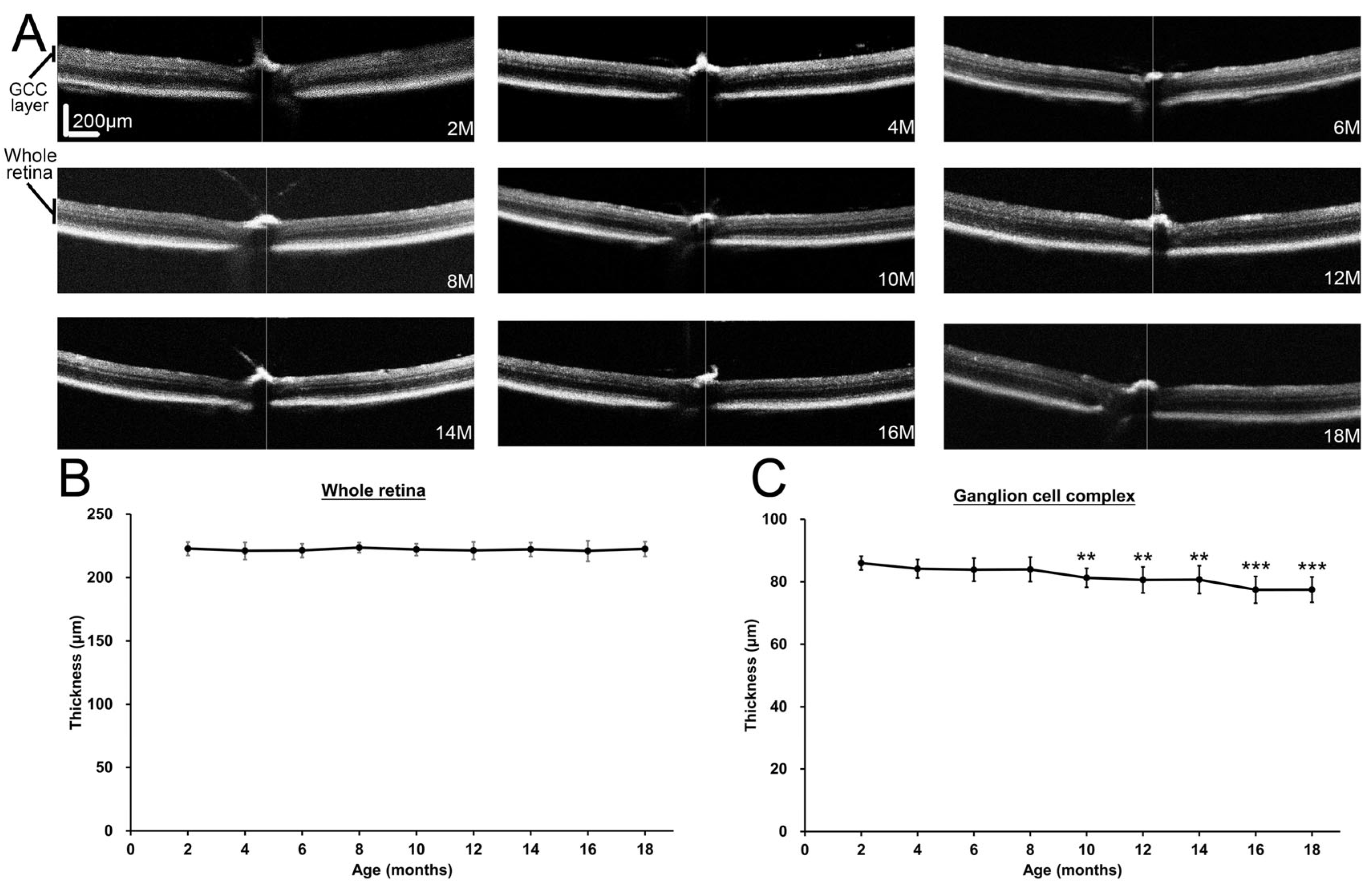
2.2. Changes of Ganglion Cell Complex Layer Thickness in Mice During Aging
2.3. Changes of Retinal Ganglion Cell Density in Mice During Aging
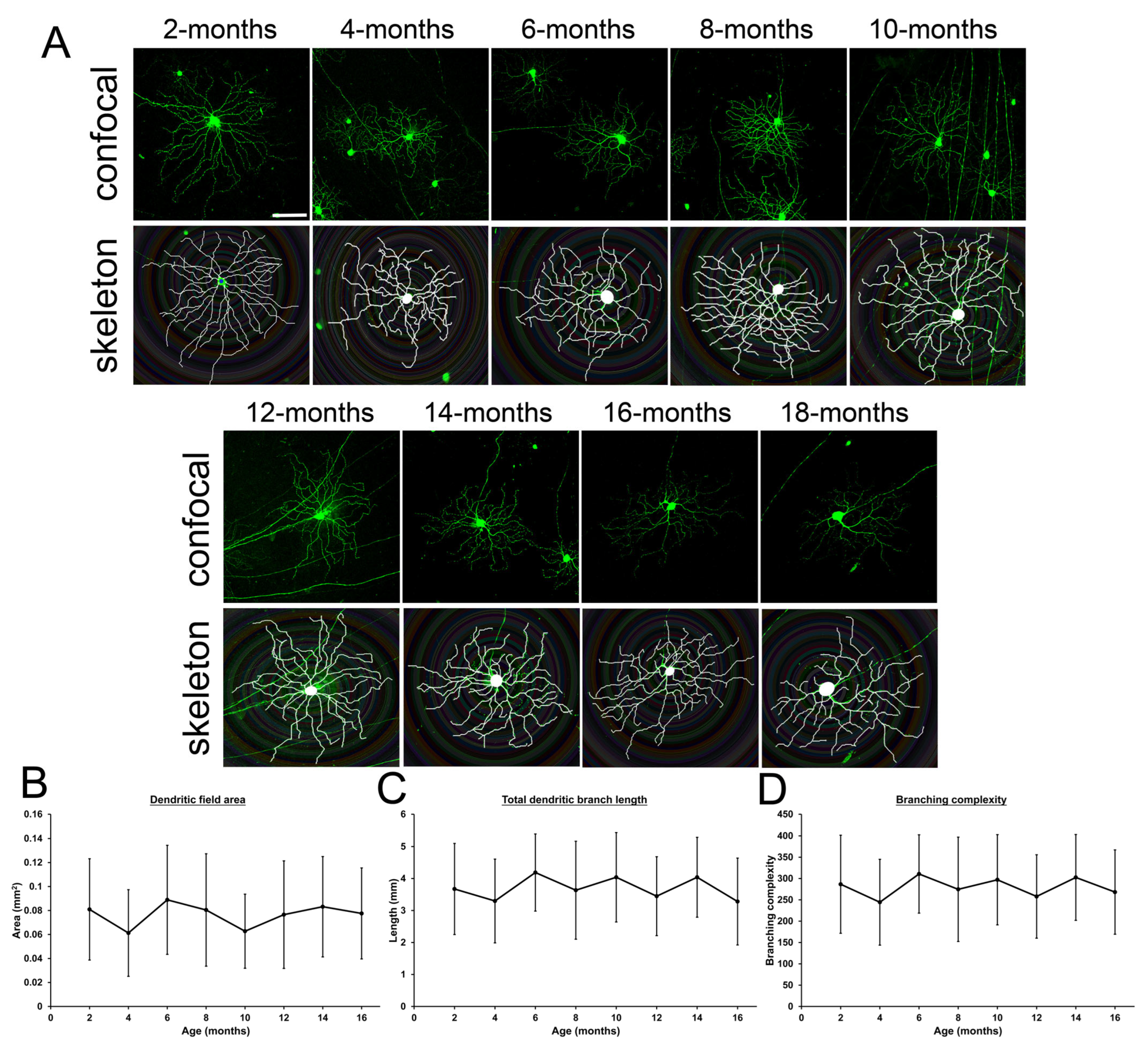
2.4. Changes of Retinal Ganglion Cell Dendrites in Mice During Aging
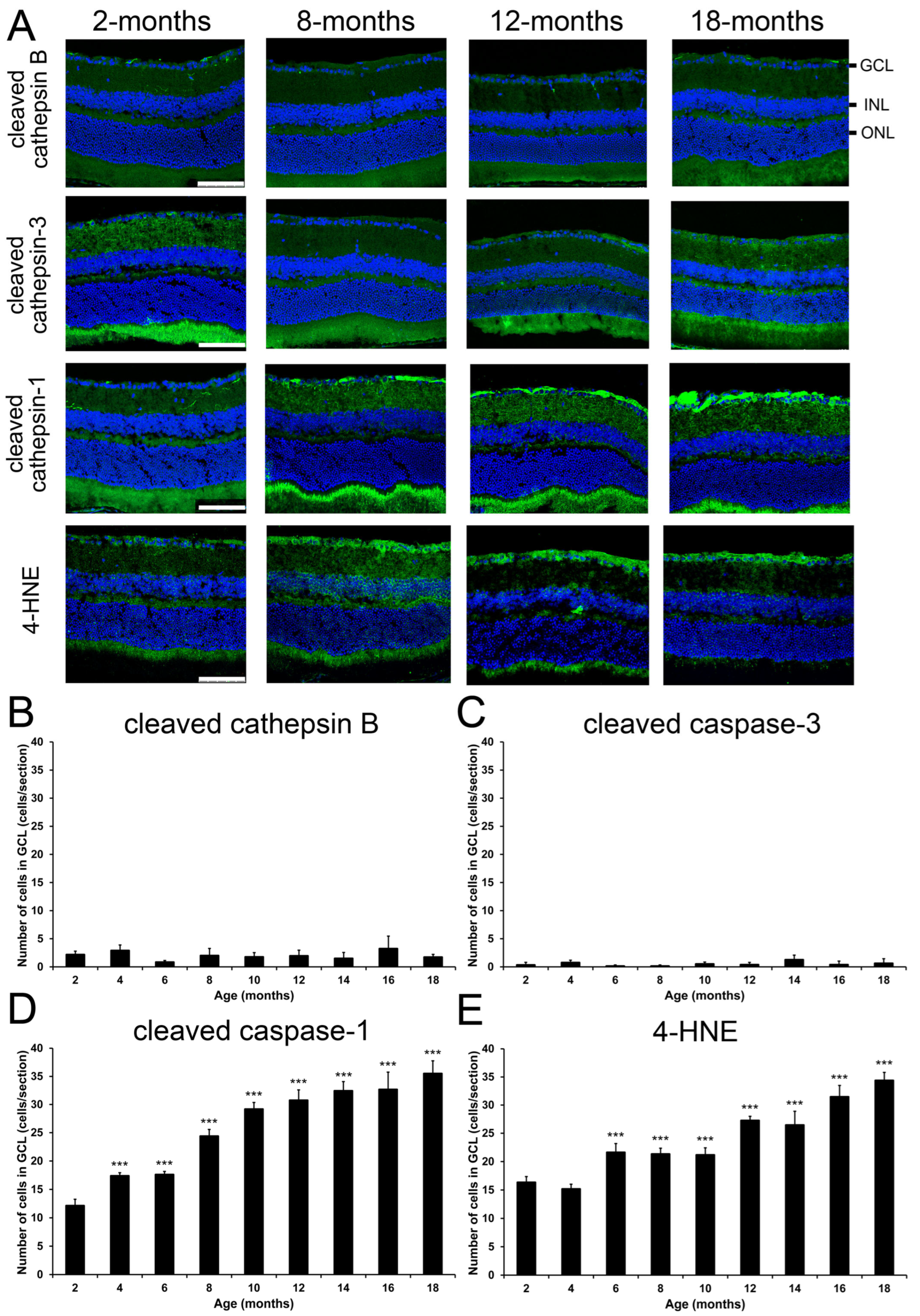
2.5. Changes of Cell Death Marker Expression in Mice During Aging
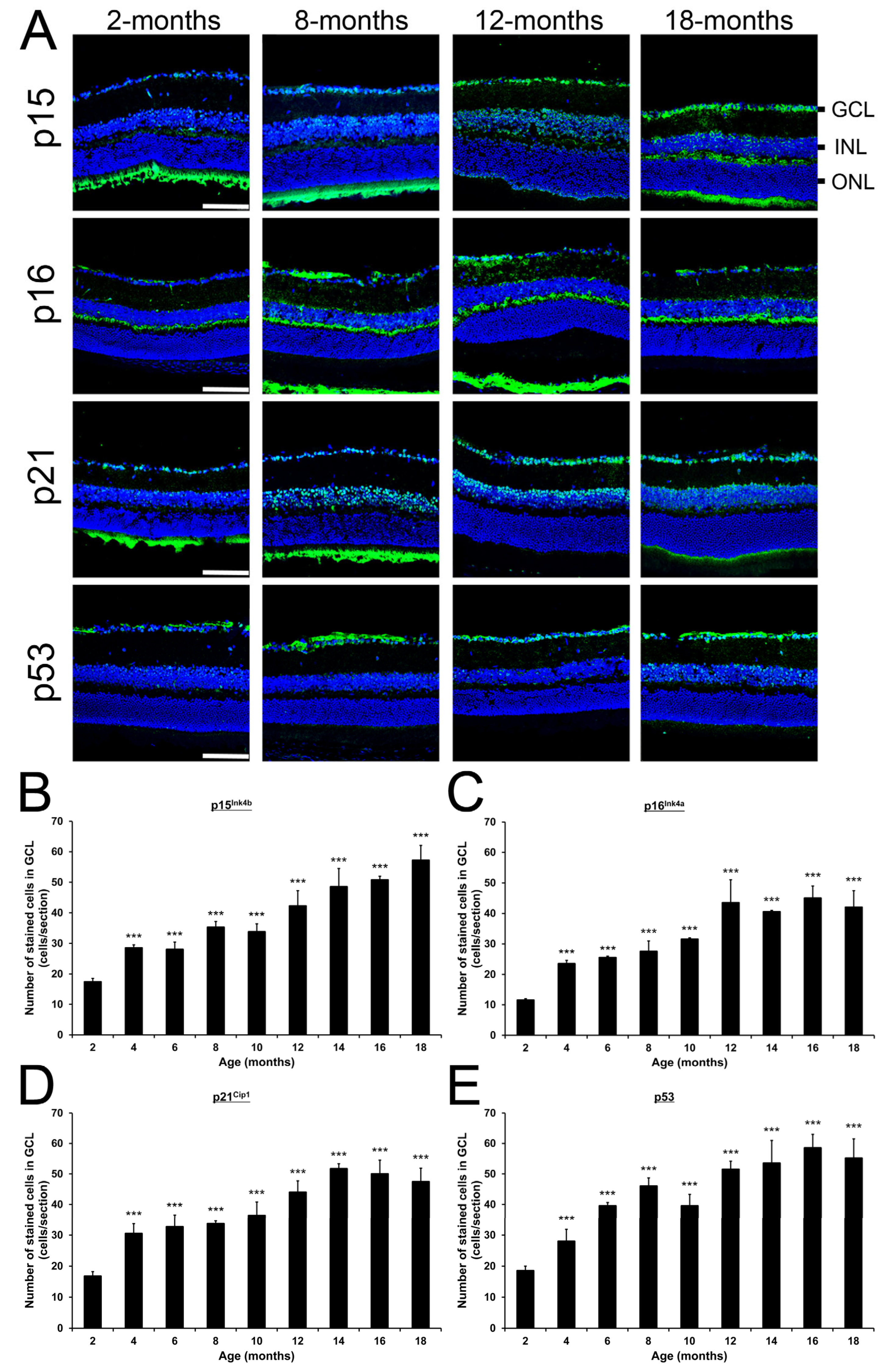
2.6. Changes of Cellular Senescence-Related Protein Expression in Mice During Aging
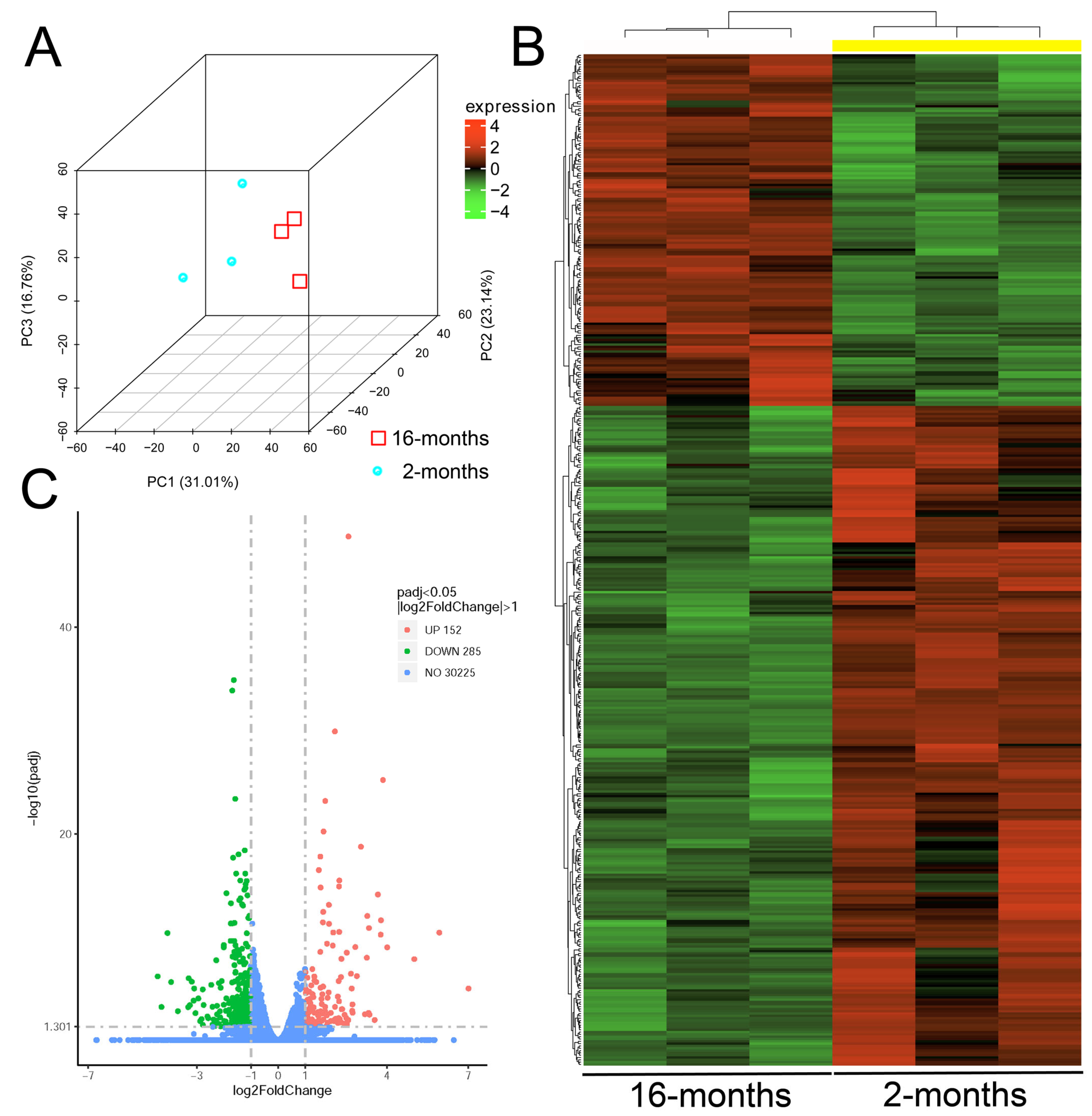
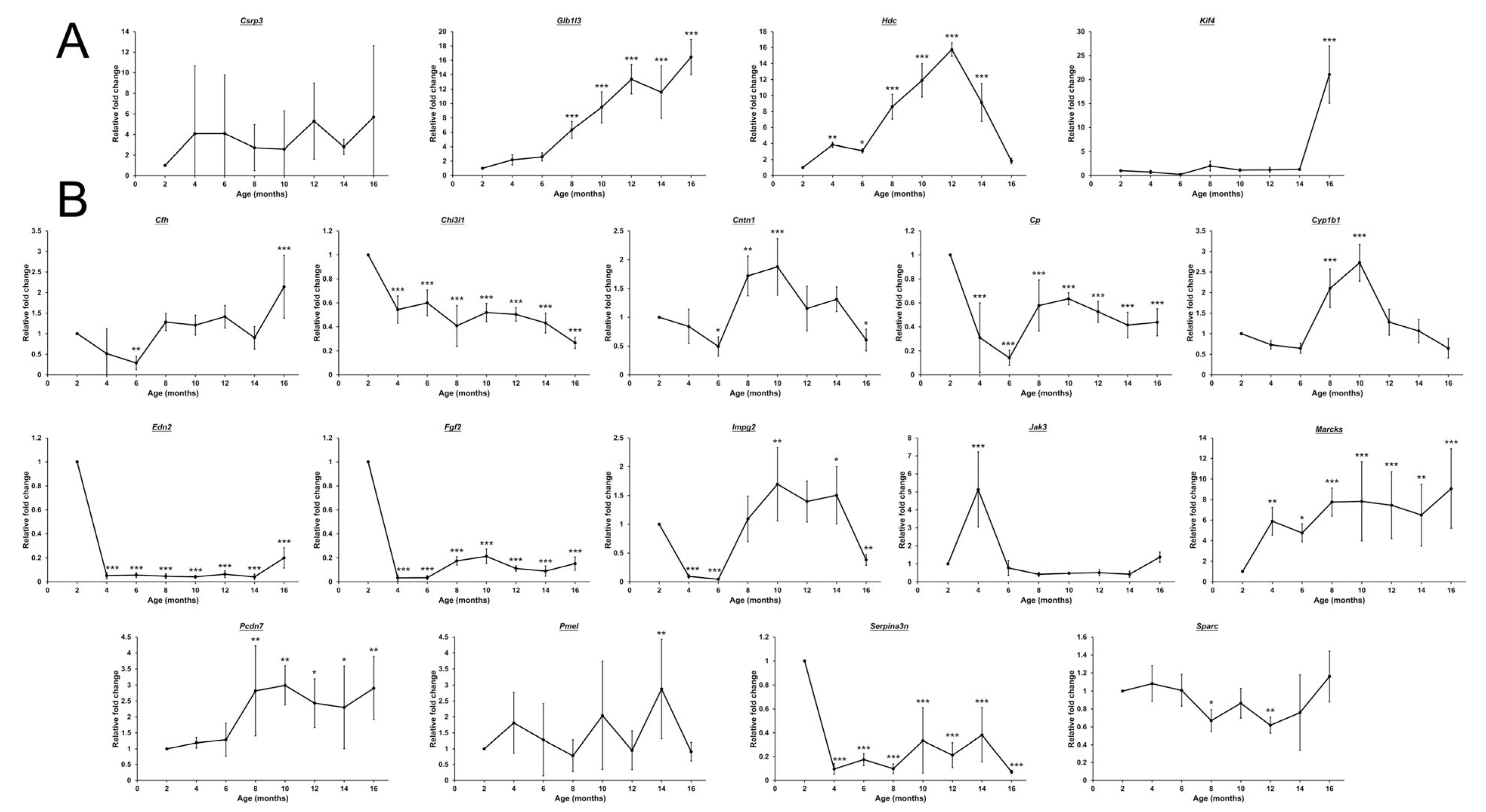
2.7. Retinal Transcriptomic Analysis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Senescence-Associated Β-Galactosidase Staining
4.3. Ganglion Cell Complex Layer Analysis
4.4. Retinal Ganglion Cell Survival Analysis
4.5. Retinal Ganglion Cell Dendrite Analysis
4.6. Immunofluorescence Analysis
4.7. Retinal Transcriptomic Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Yao, Y.; Yang, Q.; Yuan, X.L.; Cen, L.P.; Ng, T.K. Diversified Treatment Options of Adult Stem Cells for Optic Neuropathies. Cell Transplant. 2022, 31, 9636897221123512. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- McMonnies, C.W. Glaucoma history and risk factors. J. Optom. 2017, 10, 71–78. [Google Scholar] [CrossRef]
- Harwerth, R.S.; Wheat, J.L.; Rangaswamy, N.V. Age-related losses of retinal ganglion cells and axons. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4437–4443. [Google Scholar] [CrossRef]
- Danias, J.; Lee, K.C.; Zamora, M.F.; Chen, B.; Shen, F.; Filippopoulos, T.; Su, Y.; Goldblum, D.; Podos, S.M.; Mittag, T. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/2NNia glaucomatous mice: Comparison with RGC loss in aging C57/BL6 mice. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5151–5162. [Google Scholar] [CrossRef]
- Ruzafa, N.; Pereiro, X.; Aspichueta, P.; Araiz, J.; Vecino, E. The Retina of Osteopontin deficient Mice in Aging. Mol. Neurobiol. 2018, 55, 213–221. [Google Scholar] [CrossRef]
- Kovács-Valasek, A.; Pöstyéni, E.; Dénes, V.; Mester, A.; Sétáló, G., Jr.; Gábriel, R. Age-Related Alterations of Proteins in Albino Wistar Rat Retina. Cells Tissues Organs 2021, 210, 135–150. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Ito, S.; Maeda, S.; Kato, G. A Ser75-to-Asp phospho-mimicking mutation in Src accelerates ageing-related loss of retinal ganglion cells in mice. Sci. Rep. 2017, 7, 16779. [Google Scholar] [CrossRef]
- Harman, A.M.; MacDonald, A.; Meyer, P.; Ahmat, A. Numbers of neurons in the retinal ganglion cell layer of the rat do not change throughout life. Gerontology 2003, 49, 350–355. [Google Scholar] [CrossRef] [PubMed]
- García-Ayuso, D.; Salinas-Navarro, M.; Agudo, M.; Cuenca, N.; Pinilla, I.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Retinal ganglion cell numbers and delayed retinal ganglion cell death in the P23H rat retina. Exp. Eye Res. 2010, 91, 800–810. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, Y.; Liang, J.J.; Zhuang, X.; Ng, T.K. Longitudinal and simultaneous profiling of 11 modes of cell death in mouse retina post-optic nerve injury. Exp. Eye Res. 2022, 222, 109159. [Google Scholar] [CrossRef]
- Yao, Y.; Bin, X.; Xu, Y.; Chen, S.; Chen, S.; Yuan, X.L.; Cao, Y.; Ng, T.K. Cellular senescence mediates retinal ganglion cell survival regulation post-optic nerve crush injury. Cell Prolif. 2024, 57, e13719. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Haverkamp, S.; Reinhard, K.; Peichl, L.; Mietsch, M. No evidence for age-related alterations in the marmoset retina. Front. Neuroanat. 2022, 16, 945295. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Leggio, G.M.; Maltese, A.; Castorina, A.; D’Agata, V.; Drago, F. Dopamine-3 receptor modulates intraocular pressure: Implications for glaucoma. Biochem. Pharmacol. 2012, 83, 680–686. [Google Scholar] [CrossRef]
- Samuel, M.A.; Zhang, Y.; Meister, M.; Sanes, J.R. Age-related alterations in neurons of the mouse retina. J. Neurosci. 2011, 31, 16033–16044. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Zhao, D.; Wong, V.H.Y.; Chrysostomou, V.; Crowston, J.G.; Bui, B.V. The Effect of Aging on Retinal Function and Retinal Ganglion Cell Morphology Following Intraocular Pressure Elevation. Front. Aging Neurosci. 2022, 14, 859265. [Google Scholar] [CrossRef]
- Lehallier, B.; Gate, D.; Schaum, N.; Nanasi, T.; Lee, S.E.; Yousef, H.; Moran Losada, P.; Berdnik, D.; Keller, A.; Verghese, J.; et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 2019, 25, 1843–1850. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L. p15(INK4b) regulates cell cycle signaling in hippocampal astrocytes of aged rats. Aging Clin. Exp. Res. 2016, 28, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Suryadevara, V.; Hudgins, A.D.; Rajesh, A.; Pappalardo, A.; Karpova, A.; Dey, A.K.; Hertzel, A.; Agudelo, A.; Rocha, A.; Soygur, B.; et al. SenNet recommendations for detecting senescent cells in different tissues. Nat. Rev. Mol. Cell Biol. 2024, 25, 1001–1023. [Google Scholar] [CrossRef]
- Li, Y.; Schlamp, C.L.; Poulsen, G.L.; Jackson, M.W.; Griep, A.E.; Nickells, R.W. p53 regulates apoptotic retinal ganglion cell death induced by N-methyl-D-aspartate. Mol. Vis. 2002, 8, 341–350. [Google Scholar]
- Tawarayama, H.; Feng, Q.; Murayama, N.; Suzuki, N.; Nakazawa, T. Cyclin-Dependent Kinase Inhibitor 2b Mediates Excitotoxicity-Induced Death of Retinal Ganglion Cells. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4479–4488. [Google Scholar] [CrossRef] [PubMed]
- Li, L.U.; Zhao, Y.; Zhang, H. P16INK4a upregulation mediated by TBK1 induces retinal ganglion cell senescence in ischemic injury. Cell Death Dis. 2017, 8, e2752. [Google Scholar] [CrossRef] [PubMed]
- de Mera-Rodríguez, J.A.; Álvarez-Hernán, G.; Gañán, Y.; Martín-Partido, G.; Rodríguez-León, J.; Francisco-Morcillo, J. Senescence-associated β-galactosidase activity in the developing avian retina. Dev. Dyn. 2019, 248, 850–865. [Google Scholar] [CrossRef]
- Hari, P.; Millar, F.R.; Tarrats, N.; Birch, J.; Quintanilla, A.; Rink, C.J.; Fernández-Duran, I.; Muir, M.; Finch, A.J.; Brunton, V.G.; et al. The innate immune sensor Toll-like receptor 2 controls the senescence-associated secretory phenotype. Sci. Adv. 2019, 5, eaaw0254. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Y.; Bin, X.; Chan, K.P.; Chen, S.; Qian, Z.; Yao, Y.; Yuan, X.L.; Qiu, K.; Huang, Y.; et al. Combined treatment of human mesenchymal stem cells and green tea extract on retinal ganglion cell regeneration in rats after optic nerve injury. Exp. Eye Res. 2024, 239, 109787. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Zheng, K.; Xu, Y.; Chen, S.; Chen, S.; Liang, J.; Cao, Y.; Ng, T.K.; Qiu, K. Longitudinal in vivo evaluation of retinal ganglion cell complex layer and dendrites in mice with experimental autoimmune encephalomyelitis. Exp. Eye Res. 2023, 237, 109708. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Longair, M.H.; Baker, D.A.; Armstrong, J.D. Simple Neurite Tracer: Open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics 2011, 27, 2453–2454. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Blackman, A.V.; Oyrer, J.; Jayabal, S.; Chung, A.J.; Watt, A.J.; Sjöström, P.J.; van Meyel, D.J. Neuronal morphometry directly from bitmap images. Nat. Methods 2014, 11, 982–984. [Google Scholar] [CrossRef]
- Yuan, X.L.; Chen, S.L.; Xu, Y.; Yao, Y.; Liang, J.J.; Zhuang, X.; Hald, E.S.; Ng, T.K. Green tea extract enhances retinal ganglion cell survival and axonal regeneration in rats with optic nerve injury. Green tea extract enhances retinal ganglion cell survival and axonal regeneration in rats with optic nerve injury. J. Nutr. Biochem. 2023, 117, 109333. [Google Scholar] [CrossRef]
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, P3. [Google Scholar] [CrossRef]

| Functional Annotation Clusters | Enrichment Score | Gene Count | % | p |
|---|---|---|---|---|
| Secreted | 5.59 | 53 | 14.21 | 1.61 × 10−7 |
| Glycoprotein | 4.87 | 95 | 25.47 | 5.80 × 10−6 |
| Pathways of neurodegeneration | 4.21 | 19 | 5.09 | 4.13 × 10−4 |
| Cell adhesion | 4.01 | 29 | 7.77 | 4.36 × 10−8 |
| Krueppel-associated box | 3.82 | 21 | 5.63 | 1.01 × 10−6 |
| Innate immunity | 2.89 | 18 | 4.83 | 5.49 × 10−5 |
| Mitochondrial ATP synthesis coupled proton transport | 2.76 | 8 | 2.14 | 2.81 × 10−5 |
| Plastocyanin-like | 2.63 | 3 | 0.80 | 5.38 × 10−4 |
| Extracellular matrix organization | 2.57 | 13 | 3.49 | 1.25 × 10−5 |
| Immunoglobulin subtype 2 | 1.83 | 11 | 2.95 | 0.002 |
| Cytoskeleton | 1.82 | 36 | 9.65 | 4.73 × 10−4 |
| Proton-transporting ATP synthase complex, coupling factor F(o) | 1.81 | 3 | 0.80 | 0.017 |
| PI3K-Akt signaling pathway | 1.71 | 16 | 4.29 | 5.13 × 10−4 |
| Developmental protein | 1.65 | 25 | 6.70 | 0.025 |
| Metal ion binding | 1.62 | 77 | 20.64 | 5.47 × 10−4 |
| Leukocyte cell-cell adhesion | 1.50 | 4 | 1.07 | 0.009 |
| Glycoside hydrolase superfamily | 1.49 | 4 | 1.07 | 0.044 |
| ECM-receptor interaction | 1.41 | 7 | 1.88 | 0.003 |
| Synapse disassembly | 1.40 | 4 | 1.07 | 9.18 × 10−5 |
| Positive regulation of tumor necrosis factor production | 1.37 | 7 | 1.88 | 0.016 |
| Retina development | 1.31 | 5 | 1.34 | 0.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-Y.; Bin, X.; Xu, Y.; Chen, S.; Zhou, S.; Chen, S.; Cao, Y.; Qiu, K.; Ng, T.K. The Profile of Retinal Ganglion Cell Death and Cellular Senescence in Mice with Aging. Int. J. Mol. Sci. 2025, 26, 5436. https://doi.org/10.3390/ijms26125436
Wang W-Y, Bin X, Xu Y, Chen S, Zhou S, Chen S, Cao Y, Qiu K, Ng TK. The Profile of Retinal Ganglion Cell Death and Cellular Senescence in Mice with Aging. International Journal of Molecular Sciences. 2025; 26(12):5436. https://doi.org/10.3390/ijms26125436
Chicago/Turabian StyleWang, Wen-Ying, Xin Bin, Yanxuan Xu, Si Chen, Shuyi Zhou, Shaowan Chen, Yingjie Cao, Kunliang Qiu, and Tsz Kin Ng. 2025. "The Profile of Retinal Ganglion Cell Death and Cellular Senescence in Mice with Aging" International Journal of Molecular Sciences 26, no. 12: 5436. https://doi.org/10.3390/ijms26125436
APA StyleWang, W.-Y., Bin, X., Xu, Y., Chen, S., Zhou, S., Chen, S., Cao, Y., Qiu, K., & Ng, T. K. (2025). The Profile of Retinal Ganglion Cell Death and Cellular Senescence in Mice with Aging. International Journal of Molecular Sciences, 26(12), 5436. https://doi.org/10.3390/ijms26125436








