Pharmacological HIF-PH Inhibition Suppresses Myoblast Differentiation Through Continued HIF-1α Stabilization †
Abstract
1. Introduction
2. Results
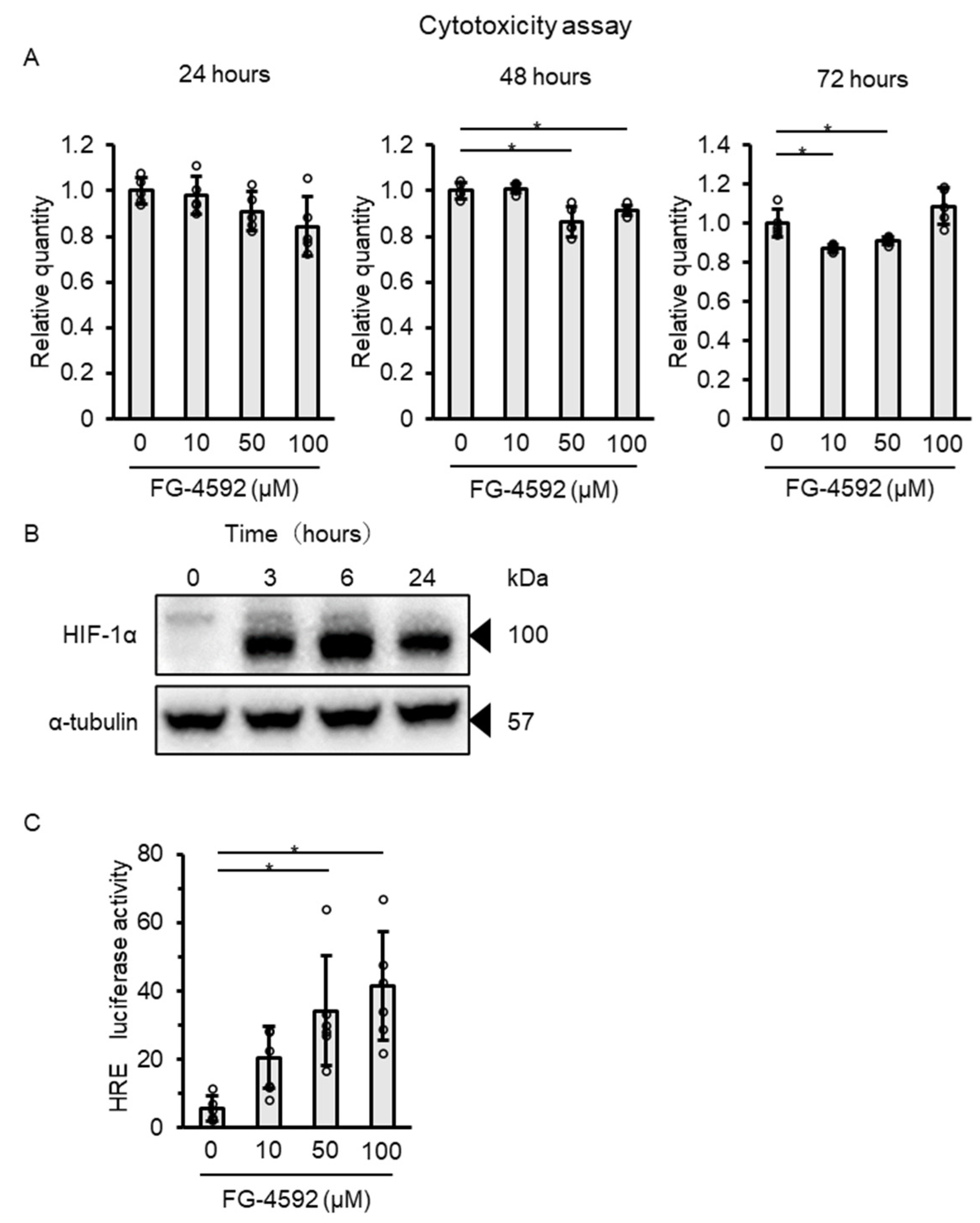
2.1. FG-4592 Stabilizes HIF-1α Protein Expression and Enhances Hypoxia-Response Element Activity in C2C12 Myoblasts
2.2. FG-4592 Suppresses the Differentiation of C2C12 Myoblasts into Myotubes
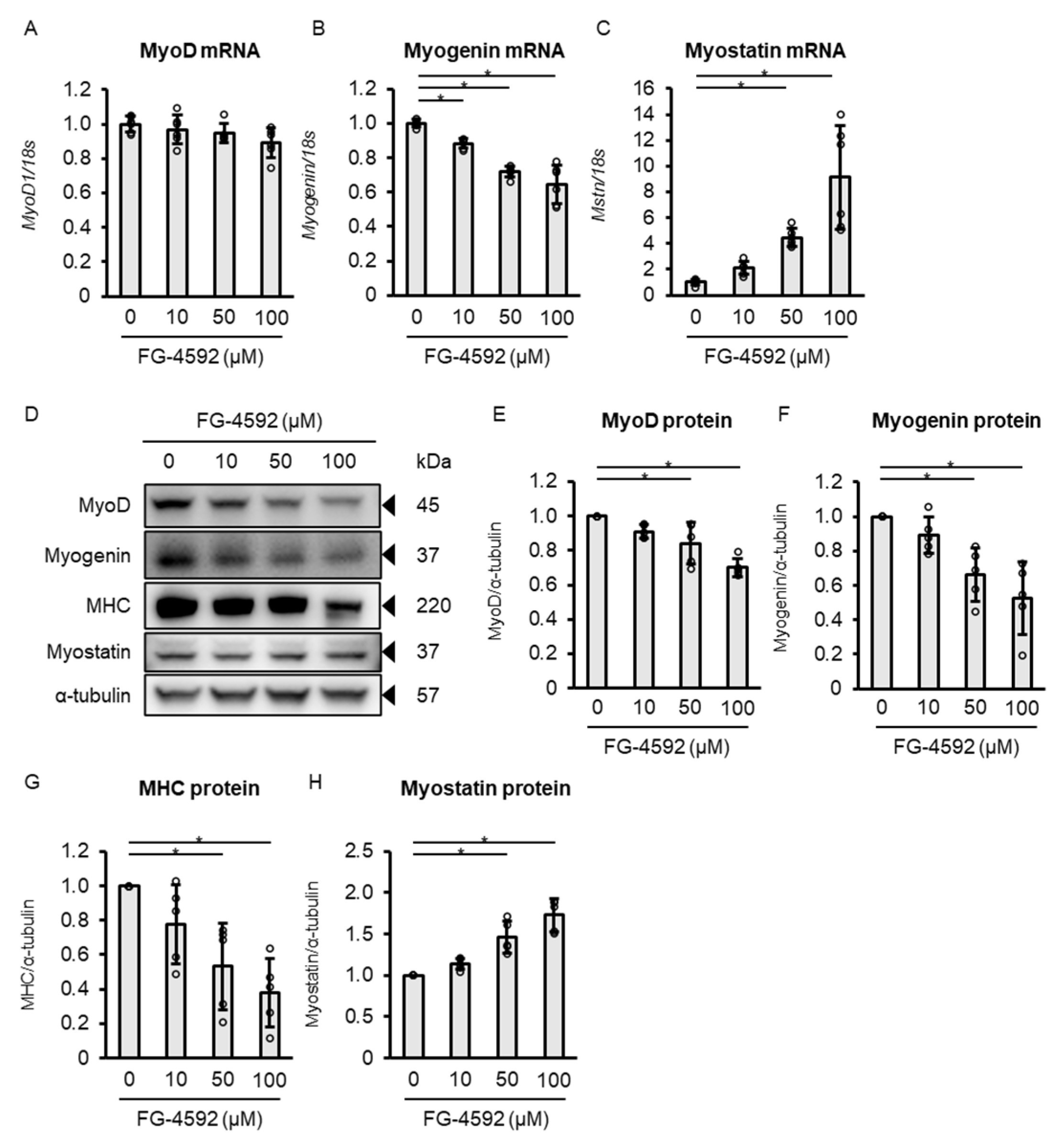
2.3. Concentration-Dependent Effects of FG-4592 on Muscle Differentiation-Related Markers
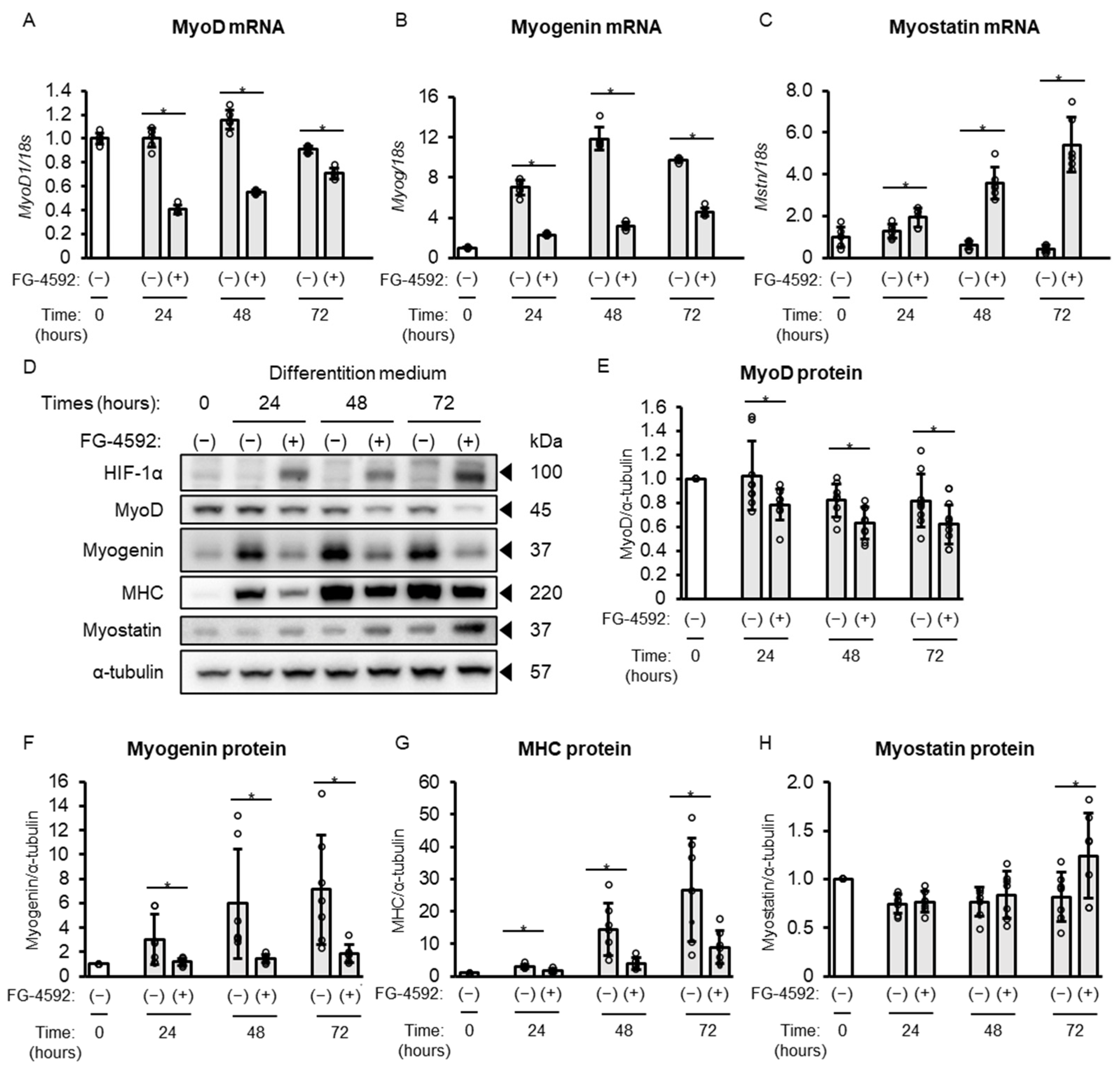
2.4. Time-Course Analysis of Effects of FG-4592 on Markers Related to Muscle Differentiation
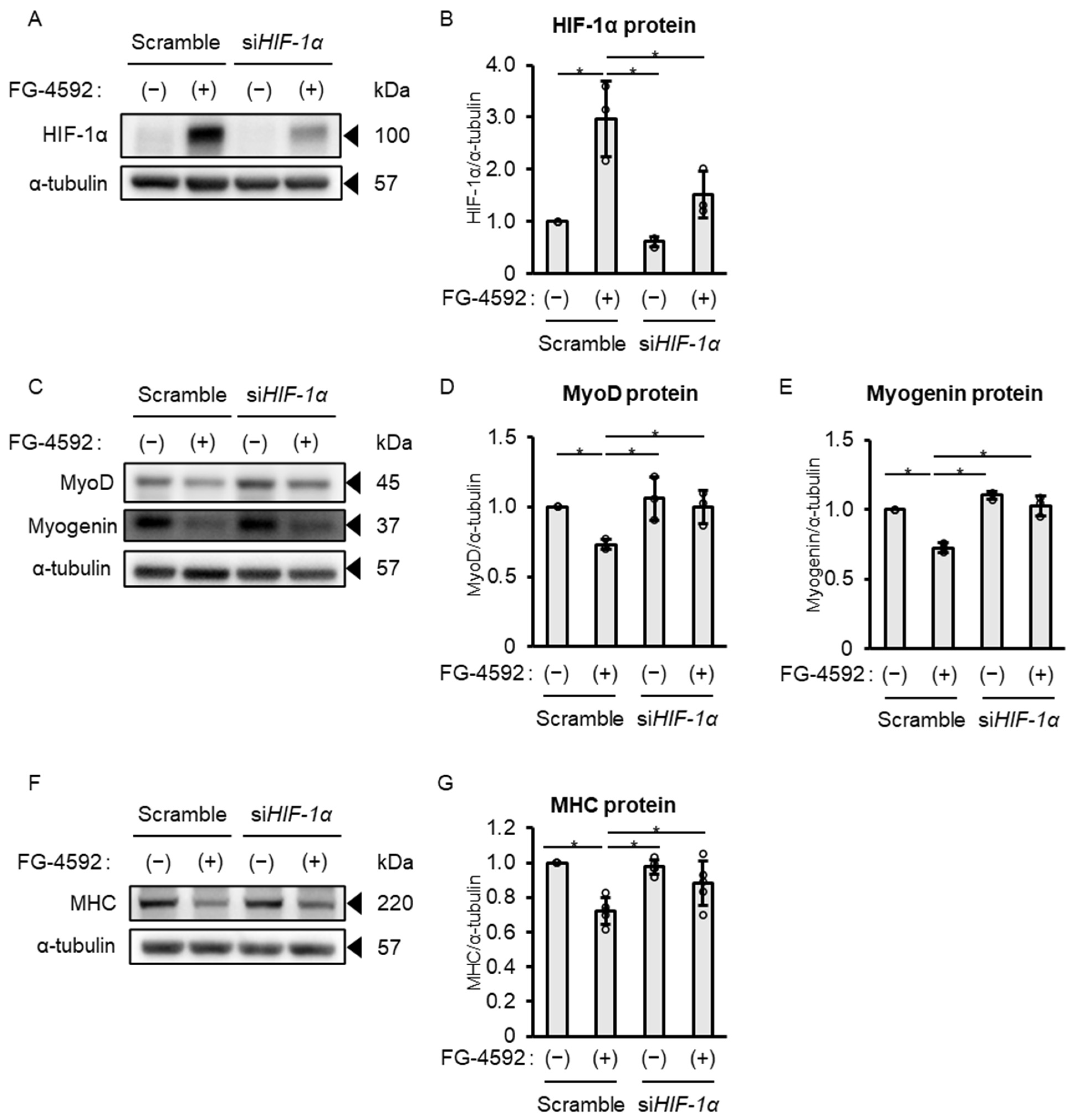
2.5. FG-4592 Suppresses Differentiation of C2C12 Myoblasts into Myotubes via HIF-1α Stabilization
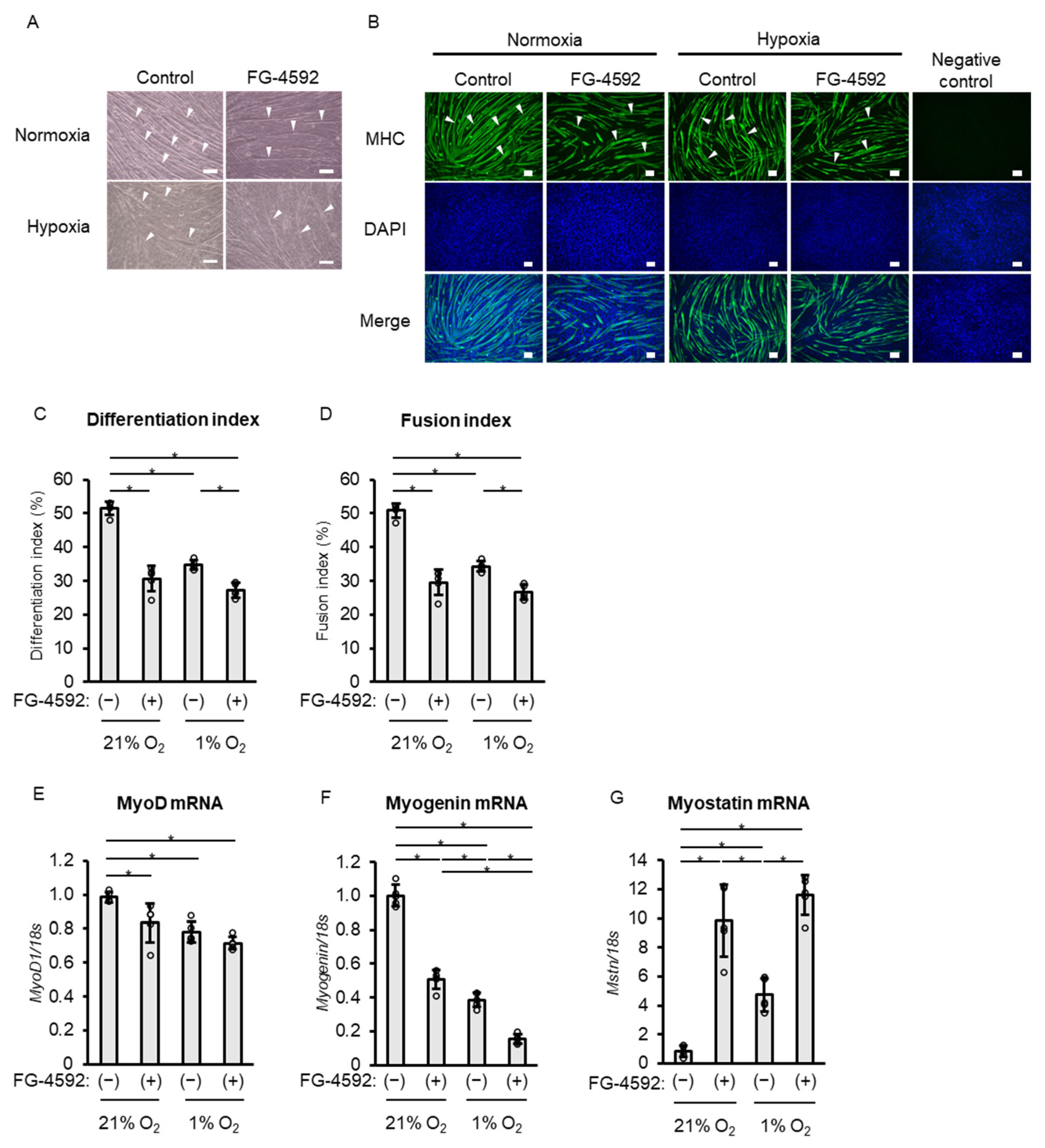
2.6. Effects of FG-4592 on Differentiation of C2C12 Myoblasts Similar to Those of Hypoxic Treatment
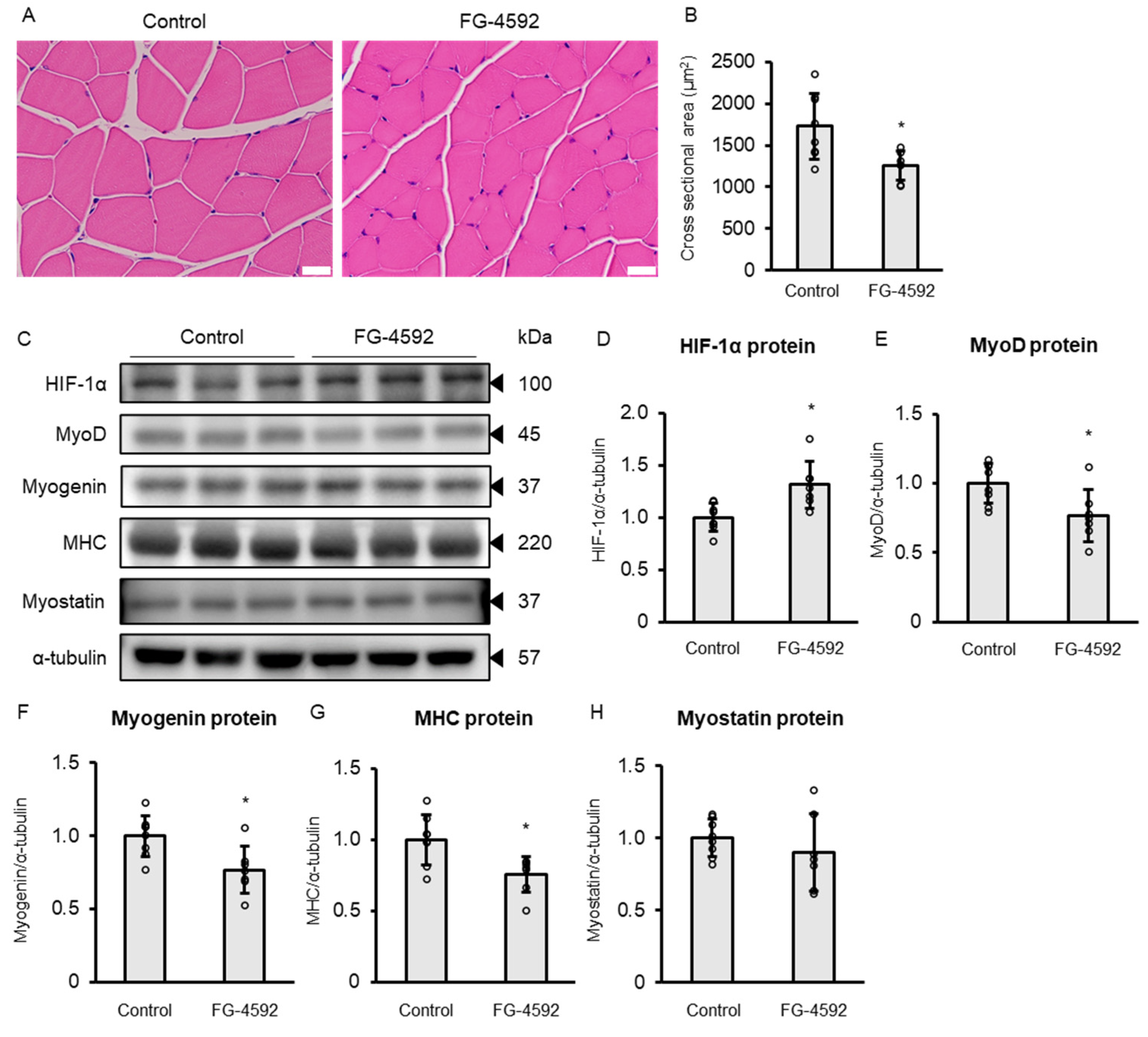
2.7. FG-4592 Suppresses Skeletal Muscle Differentiation In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Differentiation
4.2. Cytotoxicity Assay
4.3. Transfection and Dual Luciferase Reporter Assay
4.4. Western Blot Analysis
4.5. Real-Time Quantitative PCR
4.6. Immunofluorescence Staining
4.7. siHIF-1α Transfection
4.8. Animal Experiments
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| ESA | Erythropoiesis-stimulating agent |
| HIF-PH | Hypoxia-inducible factor prolyl hydroxylase |
| HIF-1α | Hypoxia-inducible factor-1α |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal bovine serum |
| HRE | Hypoxia-response element |
| MHC | Myosin heavy chain |
| SD | Standard deviation |
References
- Moon, S.J.; Kim, T.H.; Yoon, S.Y.; Chung, J.H.; Hwang, H.J. Relationship between Stage of Chronic Kidney Disease and Sarcopenia in Korean Aged 40 Years and Older Using the Korea National Health and Nutrition Examination Surveys (KNHANES IV-2, 3, and V-1, 2), 2008–2011. PLoS ONE 2015, 10, e0130740. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Nishiguchi, S.; Fukutani, N.; Tanigawa, T.; Yukutake, T.; Kayama, H.; Aoyama, T.; Arai, H. Prevalence of sarcopenia in community-dwelling Japanese older adults. J. Am. Med. Dir. Assoc. 2013, 14, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Metter, E.J.; Talbot, L.A.; Schrager, M.; Conwit, R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B359–B365. [Google Scholar] [CrossRef]
- Astor, B.C.; Muntner, P.; Levin, A.; Eustace, J.A.; Coresh, J. Association of kidney function with anemia: The Third National Health and Nutrition Examination Survey (1988–1994). Arch. Intern. Med. 2002, 162, 1401–1408. [Google Scholar] [CrossRef]
- Szczech, L.A.; Barnhart, H.X.; Inrig, J.K.; Reddan, D.N.; Sapp, S.; Califf, R.M.; Patel, U.D.; Singh, A.K. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008, 74, 791–798. [Google Scholar] [CrossRef]
- Hsieh, M.M.; Linde, N.S.; Wynter, A.; Metzger, M.; Wong, C.; Langsetmo, I.; Lin, A.; Smith, R.; Rodgers, G.P.; Donahue, R.E.; et al. HIF prolyl hydroxylase inhibition results in endogenous erythropoietin induction, erythrocytosis, and modest fetal hemoglobin expression in rhesus macaques. Blood 2007, 110, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Takarada, Y.; Takazawa, H.; Sato, Y.; Takebayashi, S.; Tanaka, Y.; Ishii, N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J. Appl. Physiol. 2000, 88, 2097–2106. [Google Scholar] [CrossRef]
- Nishimura, A.; Sugita, M.; Kato, K.; Fukuda, A.; Sudo, A.; Uchida, A. Hypoxia increases muscle hypertrophy induced by resistance training. Int. J. Sports Physiol. Perform. 2010, 5, 497–508. [Google Scholar] [CrossRef]
- Edwards, L.M.; Murray, A.J.; Tyler, D.J.; Kemp, G.J.; Holloway, C.J.; Robbins, P.A.; Neubauer, S.; Levett, D.; Montgomery, H.E.; Grocott, M.P.; et al. The effect of high-altitude on human skeletal muscle energetics: P-MRS results from the Caudwell Xtreme Everest expedition. PLoS ONE 2010, 5, e10681. [Google Scholar] [CrossRef]
- Mancini, D.M.; Walter, G.; Reichek, N.; Lenkinski, R.; McCully, K.K.; Mullen, J.L.; Wilson, J.R. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation 1992, 85, 1364–1373. [Google Scholar] [CrossRef]
- Plant, P.J.; Brooks, D.; Faughnan, M.; Bayley, T.; Bain, J.; Singer, L.; Correa, J.; Pearce, D.; Binnie, M.; Batt, J. Cellular markers of muscle atrophy in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2010, 42, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mao, J.; Han, X.; Zhang, W.; Li, Y.; Liu, Y.; Li, Q. Downregulated hypoxia-inducible factor 1α improves myoblast differentiation under hypoxic condition in mouse genioglossus. Mol. Cell. Biochem. 2021, 476, 1351–1364. [Google Scholar] [CrossRef]
- Di Carlo, A.; De Mori, R.; Martelli, F.; Pompilio, G.; Capogrossi, M.C.; Germani, A. Hypoxia inhibits myogenic differentiation through accelerated MyoD degradation. J. Biol. Chem. 2004, 279, 16332–16338. [Google Scholar] [CrossRef]
- Wagatsuma, A.; Arakawa, M.; Matsumoto, H.; Matsuda, R.; Hoshino, T.; Mabuchi, K. Cobalt chloride, a chemical hypoxia-mimicking agent, suppresses myoblast differentiation by downregulating myogenin expression. Mol. Cell. Biochem. 2020, 470, 199–214. [Google Scholar] [CrossRef]
- Wang, B.W.; Chang, H.; Kuan, P.; Shyu, K.G. Angiotensin II activates myostatin expression in cultured rat neonatal cardiomyocytes via p38 MAP kinase and myocyte enhance factor 2 pathway. J. Endocrinol. 2008, 197, 85–93. [Google Scholar] [CrossRef]
- Ríos, R.; Carneiro, I.; Arce, V.M.; Devesa, J. Myostatin is an inhibitor of myogenic differentiation. Am. J. Physiol. Cell Physiol. 2002, 282, C993–C999. [Google Scholar] [CrossRef] [PubMed]
- Langley, B.; Thomas, M.; Bishop, A.; Sharma, M.; Gilmour, S.; Kambadur, R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J. Biol. Chem. 2002, 277, 49831–49840. [Google Scholar] [CrossRef]
- Ichii, M.; Mori, K.; Miyaoka, D.; Sonoda, M.; Tsujimoto, Y.; Nakatani, S.; Shoji, T.; Emoto, M. Suppression of thyrotropin secretion during roxadustat treatment for renal anemia in a patient undergoing hemodialysis. BMC Nephrol. 2021, 22, 104. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Mehrotra, R.; Rhee, C.M.; Molnar, M.Z.; Lukowsky, L.R.; Patel, S.S.; Nissenson, A.R.; Kopple, J.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in peritoneal dialysis patients. Nephrol. Dial. Transpl. 2013, 28, 2146–2155. [Google Scholar] [CrossRef]
- Miki, Y.; Morioka, T.; Shioi, A.; Fujimoto, K.; Sakura, T.; Uedono, H.; Kakutani, Y.; Ochi, A.; Mori, K.; Shoji, T.; et al. Oncostatin M induces C2C12 myotube atrophy by modulating muscle differentiation and degradation. Biochem. Biophys. Res. Commun. 2019, 516, 951–956. [Google Scholar] [CrossRef]
- Markworth, J.F.; Cameron-Smith, D. Arachidonic acid supplementation enhances in vitro skeletal muscle cell growth via a COX-2-dependent pathway. Am. J. Physiol. Cell Physiol. 2013, 304, C56–C67. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, Y.T.; Setiawan, T.; Sari, I.N.; Park, K.; Lee, C.H.; Cho, K.W.; Lee, Y.K.; Lim, J.Y.; Yoon, J.K.; Lee, S.H.; et al. Ginsenoside Rd ameliorates muscle wasting by suppressing the signal transducer and activator of transcription 3 pathway. J. Cachexia Sarcopenia Muscle 2022, 13, 3149–3162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.K.; Guan, D.; Xu, J.; Li, X.; Zhang, N.; Yao, S.; Zhang, G.; Zhang, B.T. Long Noncoding RNA lncRNA-3 Recruits PRC2 for MyoD1 Silencing to Suppress Muscle Regeneration During Aging. Int. J. Mol. Sci. 2024, 25, 12478. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ’EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Miki, Y.; Ochi, A.; Uedono, H.; Kakutani, Y.; Nagata, Y.; Mori, K.; Imanishi, Y.; Shoji, T.; Morioka, T.; Emoto, M. Continued Activation of Hypoxia Inducible Factor 1α by Roxadustat and Inhibition of Differentiation in Skeletal Muscles. In Proceedings of the Kidney Week 2024, American Society of Nephrology, San Diego, CA, USA, 25 October 2024. [Google Scholar]

| Control (n = 8) | FG-4592 (n = 7) | p-Value | |
|---|---|---|---|
| Body weight (g) | 26.38 ± 1.22 | 25.99 ± 1.50 | 0.586 |
| Gastrocnemius muscle (mg) | 318.28 ± 28.59 | 297.69 ± 42.25 | 0.313 |
| Extensor digitorum longus muscle (mg) | 23.34 ± 0.93 | 22.54 ± 1.90 | 0.283 |
| Soleus muscle (mg) | 19.33 ± 2.68 | 19.54 ± 1.91 | 0.861 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miki, Y.; Ochi, A.; Uedono, H.; Kakutani, Y.; Ichii, M.; Nagata, Y.; Mori, K.; Imanishi, Y.; Shoji, T.; Morioka, T.; et al. Pharmacological HIF-PH Inhibition Suppresses Myoblast Differentiation Through Continued HIF-1α Stabilization. Int. J. Mol. Sci. 2025, 26, 5410. https://doi.org/10.3390/ijms26115410
Miki Y, Ochi A, Uedono H, Kakutani Y, Ichii M, Nagata Y, Mori K, Imanishi Y, Shoji T, Morioka T, et al. Pharmacological HIF-PH Inhibition Suppresses Myoblast Differentiation Through Continued HIF-1α Stabilization. International Journal of Molecular Sciences. 2025; 26(11):5410. https://doi.org/10.3390/ijms26115410
Chicago/Turabian StyleMiki, Yuya, Akinobu Ochi, Hideki Uedono, Yoshinori Kakutani, Mitsuru Ichii, Yuki Nagata, Katsuhito Mori, Yasuo Imanishi, Tetsuo Shoji, Tomoaki Morioka, and et al. 2025. "Pharmacological HIF-PH Inhibition Suppresses Myoblast Differentiation Through Continued HIF-1α Stabilization" International Journal of Molecular Sciences 26, no. 11: 5410. https://doi.org/10.3390/ijms26115410
APA StyleMiki, Y., Ochi, A., Uedono, H., Kakutani, Y., Ichii, M., Nagata, Y., Mori, K., Imanishi, Y., Shoji, T., Morioka, T., & Emoto, M. (2025). Pharmacological HIF-PH Inhibition Suppresses Myoblast Differentiation Through Continued HIF-1α Stabilization. International Journal of Molecular Sciences, 26(11), 5410. https://doi.org/10.3390/ijms26115410








