Decursin Suppresses Esophageal Squamous Cell Carcinoma Progression via Orchestrated Cell Cycle Deceleration, Apoptotic Activation, and Oncoprotein Degradation
Abstract
1. Introduction
2. Results
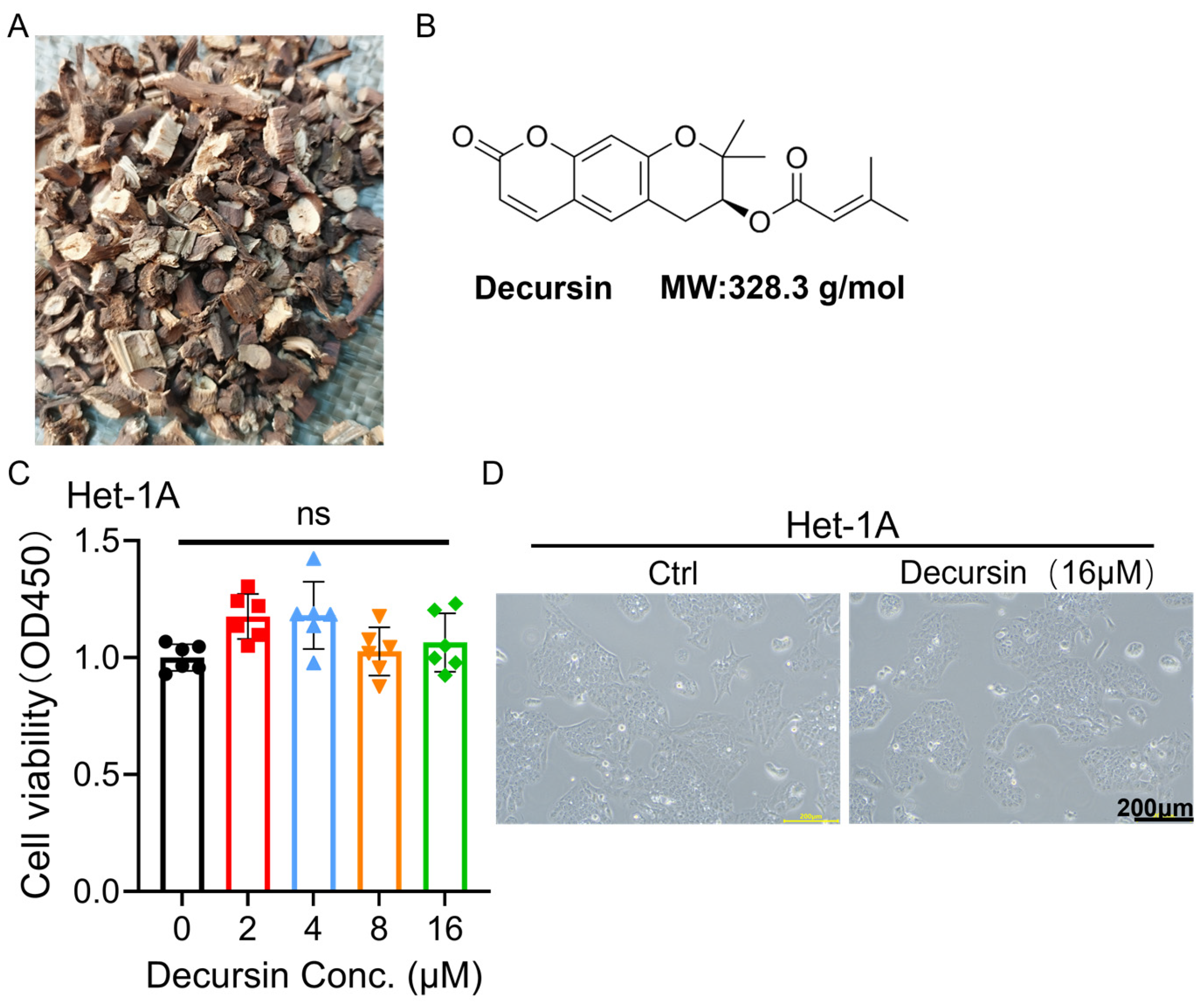
2.1. Decursin Spares Normal Esophageal Epithelium While Suppressing ESCC Viability
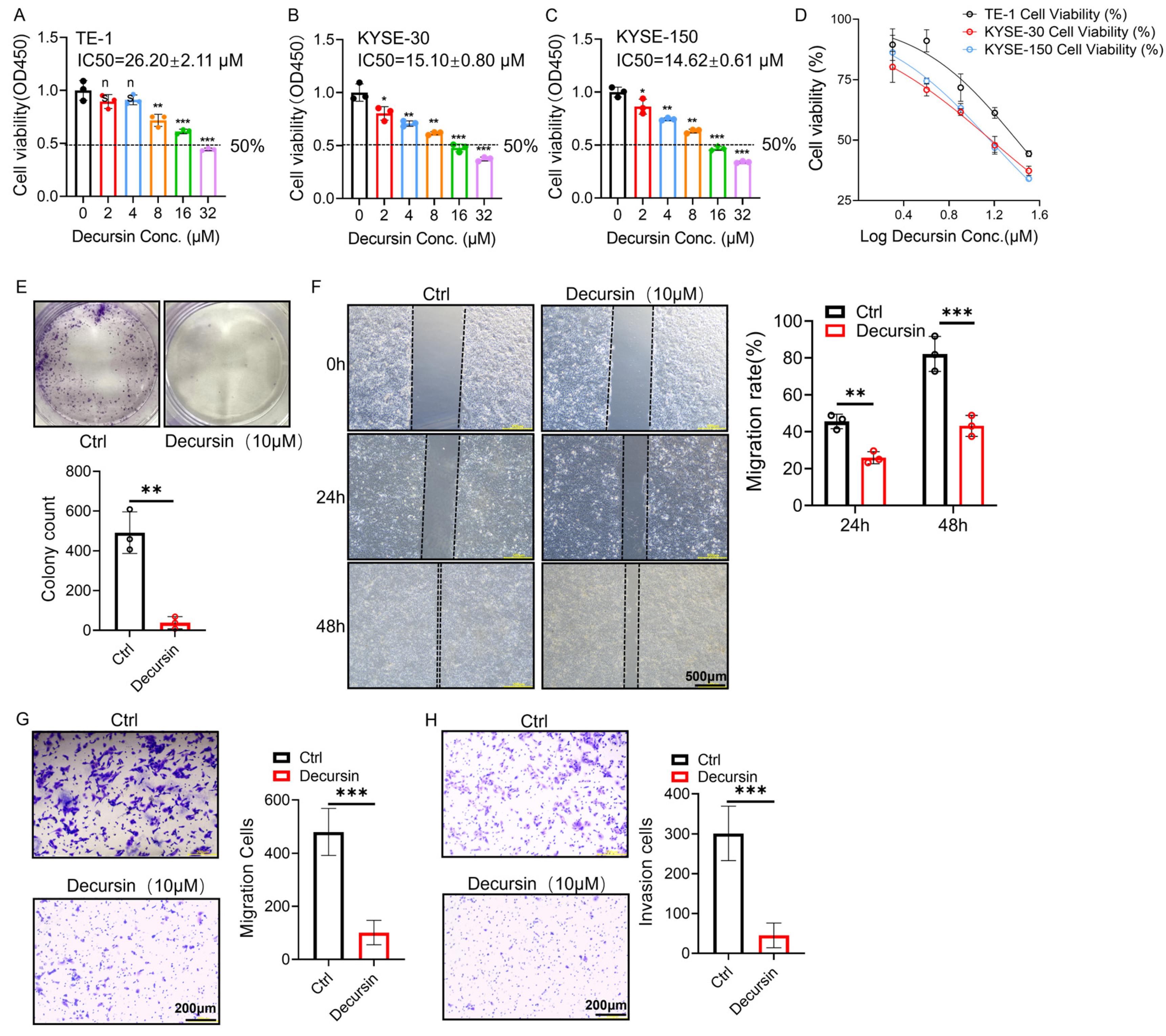
2.2. Decursin Suppresses Proliferation, Migration, and Invasion in ESCC Cells
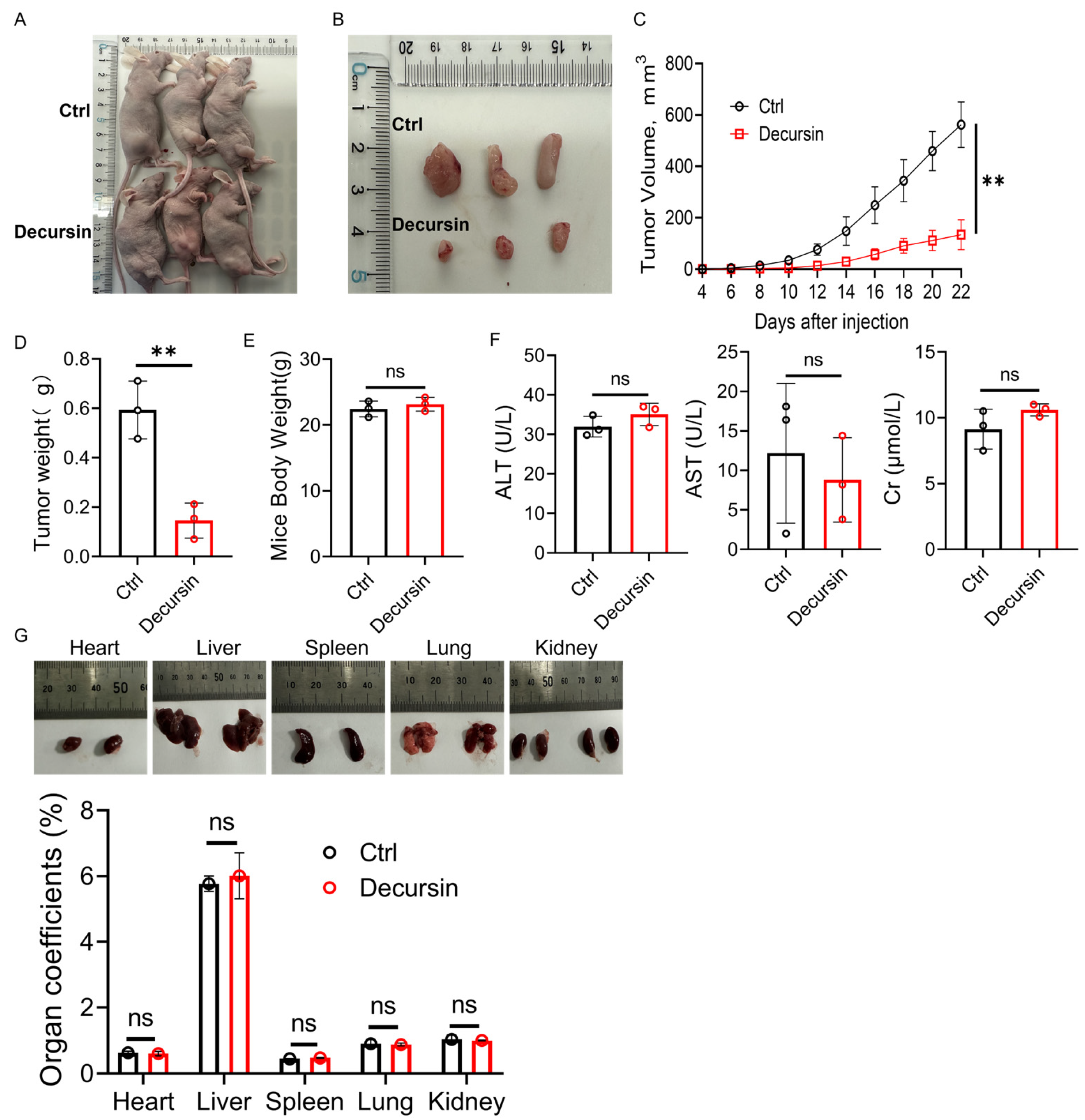
2.3. Decursin Attenuates ESCC Progression In Vivo
2.4. Decursin Suppresses ESCC via Dual Mechanisms: Cell Cycle Deceleration and Apoptosis Induction
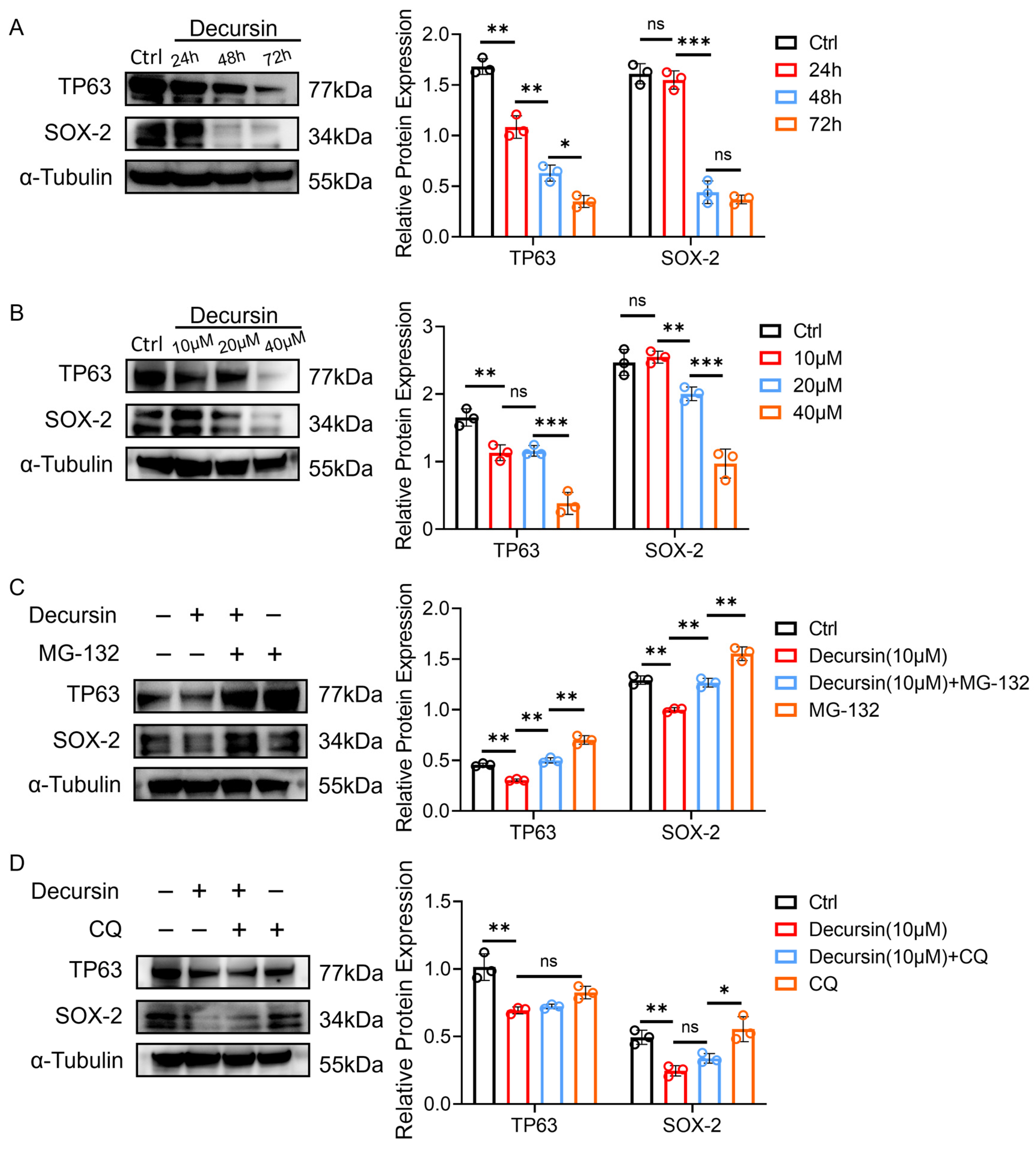
2.5. Decursin Promotes Oncoprotein Degradation Through Dose–Time Dependency and Ubiquitin–Proteasome Activation
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Animal Experiment
4.3. CCK-8 Assay
4.4. Colony Formation
4.5. Wound Healing
4.6. Transwell Assay
4.7. Cell Apoptosis Profiling
4.8. Cell Cycle Profiling
4.9. Western Blot
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ESCC | Esophageal squamous cell carcinoma |
| EAC | Esophageal adenocarcinoma |
| HIF-1α | Hypoxia-inducible factor 1α |
| VEGF | Vascular endothelial growth factor |
| CA9 | Carbonic anhydrase IX |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| Cr | Creatinine |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Primers 2017, 3, 17048. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhao, D.; Zhi, Y.; Wu, Q.; Ma, J.; Xu, J.; Liu, T.; Zhang, J.; Wang, P.; Hu, Y.; et al. RTN4IP1 Contributes to ESCC via Regulation of Amino Acid Transporters. Adv. Sci. 2025, 12, e2406220. [Google Scholar] [CrossRef]
- Sohda, M.; Kuwano, H. Current Status and Future Prospects for Esophageal Cancer Treatment. Ann. Thorac. Cardiovasc. Surg. 2017, 23, 1–11. [Google Scholar] [CrossRef]
- Yang, Y.M.; Hong, P.; Xu, W.W.; He, Q.Y.; Li, B. Advances in targeted therapy for esophageal cancer. Signal Transduct. Target. Ther. 2020, 5, 229. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Li, M.; Guo, T.; Lin, J.; Huang, X.; Ke, Q.; Wu, Y.; Fang, C.; Hu, C. Corrigendum to “Curcumin inhibits the invasion and metastasis of triple negative breast cancer via Hedgehog/Gli1 signaling pathway” [J. Ethnopharmacol. 283 (2022) 114689]. J. Ethnopharmacol 2023, 312, 116524. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, X.; Wang, L.; Li, X.; Xie, T.; Zhao, J.; Yan, J.; Wang, L.; Ye, H.; Jiao, S.; et al. Herb-sourced emodin inhibits angiogenesis of breast cancer by targeting VEGFA transcription. Theranostics 2020, 10, 6839–6853. [Google Scholar] [CrossRef]
- Shen, Z.; Zhao, L.; Yoo, S.A.; Lin, Z.; Zhang, Y.; Yang, W.; Piao, J. Emodin induces ferroptosis in colorectal cancer through NCOA4-mediated ferritinophagy and NF-κb pathway inactivation. Apoptosis 2024, 29, 1810–1823. [Google Scholar] [CrossRef]
- Ji, X.; Chen, Z.; Lin, W.; Wu, Q.; Wu, Y.; Hong, Y.; Tong, H.; Wang, C.; Zhang, Y. Esculin induces endoplasmic reticulum stress and drives apoptosis and ferroptosis in colorectal cancer via PERK regulating eIF2α/CHOP and Nrf2/HO-1 cascades. J. Ethnopharmacol. 2024, 328, 118139. [Google Scholar] [CrossRef]
- Chen, P.; Zhong, X.; Song, Y.; Zhong, W.; Wang, S.; Wang, J.; Huang, P.; Niu, Y.; Yang, W.; Ding, Z.; et al. Triptolide induces apoptosis and cytoprotective autophagy by ROS accumulation via directly targeting peroxiredoxin 2 in gastric cancer cells. Cancer Lett. 2024, 587, 216622. [Google Scholar] [CrossRef] [PubMed]
- Muralikrishnan, A.; Sekar, M.; Kumarasamy, V.; Gan, S.H.; Ravi, S.; Subramaniyan, V.; Wong, L.S.; Wu, Y.S.; Khattulanuar, F.S.; Mat Rani, N.N.I. Chemistry, Pharmacology and Therapeutic Potential of Decursin: A Promising Natural Lead for New Drug Discovery and Development. Drug Des. Devel Ther. 2024, 18, 3741–3763. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Parveen, S.; Qureshi, M.; Subhan, F.; Lee, Y.S. Decursin and decursinol angelate: Molecular mechanism and therapeutic potential in inflammatory diseases. Inflamm. Res. 2018, 67, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Yoon, S.H.; Jang, H.; Jeong, J.H.; Lee, Y.M. Decursin promotes HIF-1α proteasomal degradation and immune responses in hypoxic tumour microenvironment. Phytomedicine 2020, 78, 153318. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.E.; Kim, N.; Joo, M.; Lee, M.W.; Jeon, H.J.; Ryu, H.; Song, I.C.; Song, G.Y.; Lee, H.J. Decursin inhibits tumor growth, migration, and invasion in gastric cancer by down-regulating CXCR7 expression. Am. J. Cancer Res. 2019, 9, 2007–2018. [Google Scholar]
- Jiang, Y.Y.; Jiang, Y.; Li, C.Q.; Zhang, Y.; Dakle, P.; Kaur, H.; Deng, J.W.; Lin, R.Y.; Han, L.; Xie, J.J.; et al. TP63, SOX2, and KLF5 Establish a Core Regulatory Circuitry That Controls Epigenetic and Transcription Patterns in Esophageal Squamous Cell Carcinoma Cell Lines. Gastroenterology 2020, 159, 1311–1327.e1319. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, Z.; Beasley, A.; D’Amico, T.; Chen, X.L. Personalized and targeted therapy of esophageal squamous cell carcinoma: An update. Ann. N. Y. Acad. Sci. 2016, 1381, 66–73. [Google Scholar] [CrossRef]
- Abnet, C.C.; Arnold, M.; Wei, W.Q. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018, 154, 360–373. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, M.; Huang, H.; Jin, W.L. Drug repurposing for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Du, H.; Geng, G.; Zhou, H.; Xu, M.; Cao, H.; Zhang, B.; Song, G.; Hu, T. Matrine inhibits proliferation and induces apoptosis via BID-mediated mitochondrial pathway in esophageal cancer cells. Mol. Biol. Rep. 2014, 41, 3009–3020. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; He, L.; Zhang, Y.; Zhong, Z.; Tan, W. Immune-inflammatory modulation by natural products derived from edible and medicinal herbs used in Chinese classical prescriptions. Phytomedicine 2024, 130, 155684. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.I.; Kim, N.; Joo, M.; Lee, K.H.; Lee, M.W.; Jeon, H.J.; Ryu, H.; Kim, J.M.; Sul, J.Y.; et al. Decursin inhibits cell growth and autophagic flux in gastric cancer via suppression of cathepsin C. Am. J. Cancer Res. 2021, 11, 1304–1320. [Google Scholar]
- Yang, Y.; Hu, Y.E.; Zhao, M.Y.; Jiang, Y.F.; Fu, X.; You, F.M. Decursin affects proliferation, apoptosis, and migration of colorectal cancer cells through PI3K/Akt signaling pathway. Zhongguo Zhong Yao Za Zhi 2023, 48, 2334–2342. [Google Scholar] [CrossRef]
- Kim, D.; Go, S.H.; Song, Y.; Lee, D.K.; Park, J.R. Decursin Induces G1 Cell Cycle Arrest and Apoptosis through Reactive Oxygen Species-Mediated Endoplasmic Reticulum Stress in Human Colorectal Cancer Cells in In Vitro and Xenograft Models. Int. J. Mol. Sci. 2024, 25, 9939. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Huang, L.; Yu, X.; Wang, T.; Zhang, N.; Yang, M. The TDP-43/TP63 Positive Feedback Circuit Promotes Esophageal Squamous Cell Carcinoma Progression. Adv. Sci. 2024, 11, e2402913. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, S.; Liu, T.; Zhao, X.; Xiang, T.; Hu, X.; Wu, C.; Lin, D. Aberrant epithelial cell interaction promotes esophageal squamous-cell carcinoma development and progression. Signal Transduct. Target. Ther. 2023, 8, 453. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, J.; Zhang, X.; Zhang, Z.; Xie, Y.; Liu, J.B.; Ho, Z.V.; Panda, A.; Qiu, X.; Cejas, P.; et al. Reprogramming of the esophageal squamous carcinoma epigenome by SOX2 promotes ADAR1 dependence. Nat. Genet. 2021, 53, 881–894. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614. [Google Scholar] [CrossRef]
- Talib, W.H.; Awajan, D.; Hamed, R.A.; Azzam, A.O.; Mahmod, A.I.; Al-Yasari, I.H. Combination Anticancer Therapies Using Selected Phytochemicals. Molecules 2022, 27, 5452. [Google Scholar] [CrossRef]
- Castillo-Pichardo, L.; Dharmawardhane, S.F. Grape polyphenols inhibit Akt/mammalian target of rapamycin signaling and potentiate the effects of gefitinib in breast cancer. Nutr. Cancer 2012, 64, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- La, X.; Zhang, L.; Li, Z.; Li, H.; Yang, Y. (−)-Epigallocatechin Gallate (EGCG) Enhances the Sensitivity of Colorectal Cancer Cells to 5-FU by Inhibiting GRP78/NF-κB/miR-155-5p/MDR1 Pathway. J. Agric. Food Chem. 2019, 67, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xie, S.; Chen, X.; Pan, S.; Qian, H.; Zhu, X. Effects of Quercetin on the Efficacy of Various Chemotherapeutic Drugs in Cervical Cancer Cells. Drug Des. Devel Ther. 2021, 15, 577–588. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, C.; Wu, L.; Yang, X.; Xie, K.; Zhang, P.; Feng, Y.; Ma, H.; Tong, X. Decursin Suppresses Esophageal Squamous Cell Carcinoma Progression via Orchestrated Cell Cycle Deceleration, Apoptotic Activation, and Oncoprotein Degradation. Int. J. Mol. Sci. 2025, 26, 5391. https://doi.org/10.3390/ijms26115391
Fang C, Wu L, Yang X, Xie K, Zhang P, Feng Y, Ma H, Tong X. Decursin Suppresses Esophageal Squamous Cell Carcinoma Progression via Orchestrated Cell Cycle Deceleration, Apoptotic Activation, and Oncoprotein Degradation. International Journal of Molecular Sciences. 2025; 26(11):5391. https://doi.org/10.3390/ijms26115391
Chicago/Turabian StyleFang, Chen, Lin Wu, Xiangzhe Yang, Kai Xie, Peng Zhang, Yu Feng, Haitao Ma, and Xing Tong. 2025. "Decursin Suppresses Esophageal Squamous Cell Carcinoma Progression via Orchestrated Cell Cycle Deceleration, Apoptotic Activation, and Oncoprotein Degradation" International Journal of Molecular Sciences 26, no. 11: 5391. https://doi.org/10.3390/ijms26115391
APA StyleFang, C., Wu, L., Yang, X., Xie, K., Zhang, P., Feng, Y., Ma, H., & Tong, X. (2025). Decursin Suppresses Esophageal Squamous Cell Carcinoma Progression via Orchestrated Cell Cycle Deceleration, Apoptotic Activation, and Oncoprotein Degradation. International Journal of Molecular Sciences, 26(11), 5391. https://doi.org/10.3390/ijms26115391





