Is COVID-19 Coagulopathy a Thrombotic Microangiopathy? A Prospective, Observational Study
Abstract
1. Introduction
2. Results
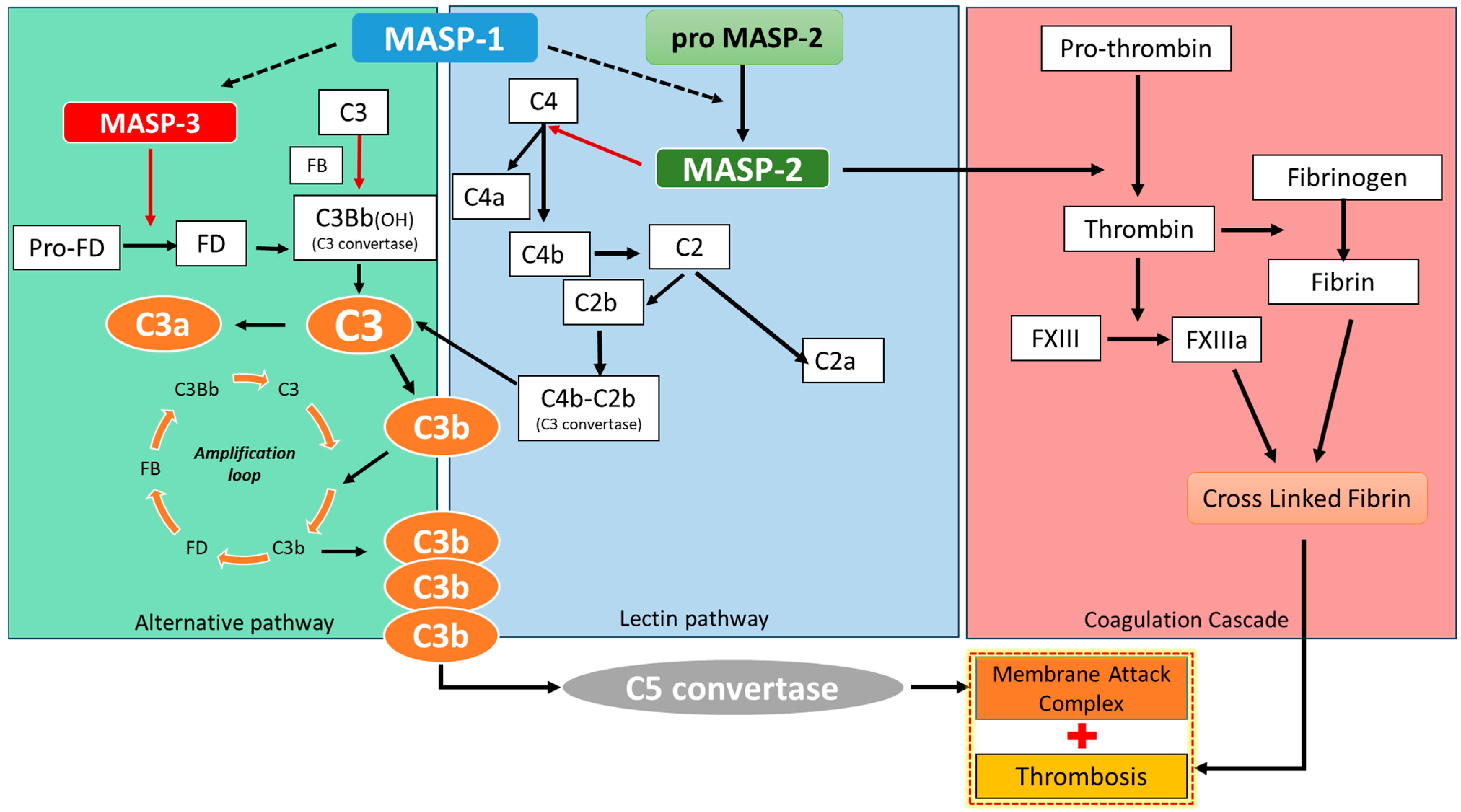
3. Discussion
4. Material and Methods
4.1. Study Design and Population
4.2. Laboratory Features
4.2.1. Blood Assays
4.2.2. Coagulation
4.2.3. Complement Proteins
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rostami, M.; Mansouritorghabeh, H.; Parsa-Kondelaji, M. High levels of Von Willebrand factor markers in COVID-19: A systematic review and meta-analysis. Clin. Exp. Med. 2022, 22, 347–357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, X.; Feng, Y.; Jia, Y.; Zhang, X.; Li, L.; Bai, X.; Jiao, L. Prognostic value of von Willebrand factor and ADAMTS13 in patients with COVID-19: A systematic review and meta-analysis. Thromb. Res. 2022, 218, 83–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conway, E.M.; Mackman, N.; Warren, R.Q.; Wolberg, A.S.; Mosnier, L.O.; Campbell, R.A.; Gralinski, L.E.; Rondina, M.T.; van de Veerdonk, F.L.; Hoffmeister, K.M.; et al. Understanding COVID-19-associated coagulopathy. Nat. Rev. Immunol. 2022, 22, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.; Guillén, E.; Quintana, L.F.; Garcia-Herrera, A.; Piñeiro, G.; Poch, E.; Carreras, E.; Campistol, J.M.; Diaz-Ricart, M.; Palomo, M. Thrombotic microangiopathies assessment: Mind the complement. Clin. Kidney J. 2020, 14, 1055–1066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malgaj Vrecko, M.; Veceric-Haler, Z. Coronavirus Disease 2019-Associated Thrombotic Microangiopathy. J. Hematol. 2022, 11, 148–153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, T.; Zhu, L.; Liu, H.; Zhang, X.; Wang, T.; Fu, Y.; Li, H.; Dong, Q.; Hu, Y.; Zhang, Z.; et al. Highly pathogenic coronavirus N protein aggravates inflammation by MASP-2-mediated lectin complement pathway overactivation. Signal Transduct. Target. Ther. 2022, 7, 318. [Google Scholar] [CrossRef]
- Jenny, L.; Dobó, J.; Gál, P.; Pál, G.; Lam, W.A.; Schroeder, V. MASP-1 of the complement system enhances clot formation in a microvascular whole blood flow model. PLoS ONE 2018, 13, e0191292. [Google Scholar] [CrossRef]
- Mangin, P.H.; Neeves, K.B.; Lam, W.A.; Cosemans, J.M.E.M.; Korin, N.; Kerrigan, S.W.; Panteleev, M.A.; Biorheology, S.O. The ADAMTS13–von Willebrand factor axis in COVID-19 patients. J. Thromb. Haemost. 2021, 19, 513–521. [Google Scholar] [CrossRef]
- Matsuoka, A.; Koami, H.; Shinada, K.; Sakamoto, Y. Investigation of differences in coagulation characteristics between hospitalized patients with SARS-CoV-2 Alpha, Delta, and Omicron variant infection using rotational thromboelastometry (ROTEM): A single-center, retrospective, observational study. J. Clin. Lab. Anal. 2022, 36, e24796. [Google Scholar] [CrossRef]
- Noone, D.G.; Riedl, M.; Pluthero, F.G.; Bowman, M.L.; Liszewski, M.K.; Lu, L.; Quan, Y.; Balgobin, S.; Schneppenheim, R.; Schneppenheim, S.; et al. Von Willebrand factor regulates complement on endothelial cells. Kidney Int. 2016, 90, 123–134. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Henry, B.M.; Lippi, G. Increased VWF and Decreased ADAMTS-13 in COVID-19: Creating a Milieu for (Micro)Thrombosis. Semin Thromb. Hemost. 2021, 47, 400–418. [Google Scholar] [CrossRef] [PubMed]
- Philippe, A.; Gendron, N.; Bory, O.; Beauvais, A.; Mirault, T.; Planquette, B.; Sanchez, O.; Diehl, J.L.; Chocron, R.; Smadja, D.M. Von Willebrand factor collagen-binding capacity predicts in-hospital mortality in COVID-19 patients: Insight from VWF/ADAMTS13 ratio imbalance. Angiogenesis 2021, 24, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Tiscia, G.; Favuzzi, G.; De Laurenzo, A.; Cappucci, F.; Fischetti, L.; Colaizzo, D.; Chinni, E.; Florio, L.; Miscio, G.; Piscitelli, A.P.; et al. The Prognostic Value of ADAMTS-13 and von Willebrand Factor in COVID-19 Patients: Prospective Evaluation by Care Setting. Diagnostics 2021, 11, 1648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dolgushina, N.; Gorodnova, E.; Beznoshenco, O.; Romanov, A.; Menzhinskaya, I.; Krechetova, L.; Sukhikh, G. Von Willebrand Factor and ADAMTS-13 Are Associated with the Severity of COVID-19 Disease. J. Clin. Med. 2022, 11, 4006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Q.; Bignotti, A.; Yada, N.; Ye, Z.; Liu, S.; Han, Z.; Zheng, X.L. Dynamic Assessment of Plasma von Willebrand Factor and ADAMTS13 Predicts Mortality in Hospitalized Patients with SARS-CoV-2 Infection. J. Clin. Med. 2023, 12, 7174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grandone, E.; Vimercati, A.; Sorrentino, F.; Colaizzo, D.; Ostuni, A.; Ceci, O.; Capozza, M.; Tiscia, G.; De Laurenzo, A.; Mastroianno, M.; et al. Obstetric outcomes in pregnant COVID-19 women: The imbalance of von Willebrand factor and ADAMTS13 axis. BMC Pregnancy Childbirth 2022, 22, 142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hadique, S.; Badami, V.; Sangani, R.; Forte, M.; Alexander, T.; Goswami, A.; Garrison, A.; Wen, S. Coagulation Studies Are Not Predictive of Hematological Complications of COVID-19 Infection. TH Open 2022, 6, e1–e9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gauchel, N.; Rieder, M.; Krauel, K.; Goller, I.; Jeserich, M.; Salzer, U.; Venhoff, A.C.; Baldus, N.; Pollmeier, L.; Wirth, L.; et al. Complement system component dysregulation is a distinctive feature of COVID-19 disease: A prospective and comparative analysis of patients admitted to the emergency department for suspected COVID-19 disease. J. Thromb. Thrombolysis 2022, 53, 788–797. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sinkovits, G.; Mező, B.; Réti, M.; Müller, V.; Iványi, Z.; Gál, J.; Gopcsa, L.; Reményi, P.; Szathmáry, B.; Lakatos, B.; et al. Complement Overactivation and Consumption Predicts In-Hospital Mortality in SARS-CoV-2 Infection. Front. Immunol. 2021, 12, 663187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tantillo, S.; Cilloni, N.; Guarnera, M.; Talarico, F.; Citino, M.; Silingardi, M.; Catalano, L.; Imbriani, M. Does COVID-19 vaccination protect against pulmonary embolism? J. Anesth. Analg. Crit. Care 2023, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J. The Role of the von Willebrand Factor Collagen-Binding Assay (VWF:CB) in the Diagnosis and Treatment of von Willebrand Disease (VWD) and Way Beyond: A Comprehensive 36-Year History. Semin Thromb. Hemost. 2023, 50, 43–80. [Google Scholar] [CrossRef] [PubMed]
- Philippe, A.; Chocron, R.; Gendron, N.; Bory, O.; Beauvais, A.; Peron, N.; Khider, L.; Guerin, C.L.; Goudot, G.; Levasseur, F.; et al. Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis 2021, 24, 505–517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, J.; Yuan, X.; Chen, H.; Chaturvedi, S.; Braunstein, E.M.; Brodsky, R.A. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood 2020, 136, 2080–2089. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, K.; Pfefferle, S.; Bertram, S.; Glowacka, I.; Drosten, C.; Pöhlmann, S.; Simmons, G. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J. Virol. 2010, 84, 8753–8764. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Nooijer, A.H.; Grondman, I.; Janssen, N.A.F.; Netea, M.G.; Willems, L.; van de Veerdonk, F.L.; Giamarellos-Bourboulis, E.J.; Toonen, E.J.M.; Joosten, L.A.B. RCI-COVID-19 study group. Complement Activation in the Disease Course of Coronavirus Disease 2019 and Its Effects on Clinical Outcomes. J. Infect. Dis. 2021, 223, 214–224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noris, M.; Benigni, A.; Remuzzi, G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020, 98, 314–322. [Google Scholar] [CrossRef]
- Bilgin, E.; Ertenli, A.İ. Proposal of a new nomenclature for the underlying pathogenetic mechanism of severe Coronavirus Disease-19: “Inflammatory Thrombosis with Immune Endotheliitis-ITIE”. Rheumatol. Int. 2021, 41, 679–680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anderson, P.; Kremser, L.; Newby, C. The role of inflammatory cytokines and cytokine storm in COVID-19-induced coagulopathy. Thromb. Haemost. 2021, 121, 1302–1312. [Google Scholar] [CrossRef]
- Jing, H.; Wu, X.; Xiang, M.; Liu, L.; Novakovic, V.A.; Shi, J. Pathophysiological mechanisms of thrombosis in acute and long COVID-19. Front Immunol. 2022, 13, 992384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ambrosino, P.; Calcaterra, I.L.; Mosella, M.; Formisano, R.; D’Anna, S.E.; Bachetti, T.; Marcuccio, G.; Galloway, B.; Mancini, F.P.; Papa, A.; et al. Endothelial Dysfunction in COVID-19: A Unifying Mechanism and a Potential Therapeutic Target. Biomedicines 2022, 10, 812. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hess, K.; Ajjan, R.; Phoenix, F.; Dobó, J.; Gál, P.; Schroeder, V. Effects of MASP-1 of the complement system on activation of coagulation factors and plasma clot formation. PLoS ONE 2012, 7, e35690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Mild (n = 28) | Severe (n = 15) | p | |
|---|---|---|---|
| Male sex, % | 71.40% | 73.30% | 0.594 |
| Age (years), median (IQR) | 62.00 (49.25–69.50) | 61.00 (53.00–68.75) | 0.894 |
| BMI (kg/m2), median (IQR) | 26.75 (25.00–30.98) | 29.40 (36.50–36.00) | 0.157 |
| Diabetes, n (%) | 6 (30%) | 2 (18.20%) | 0.394 |
| Hypertension, n (%) | 9 (45%) | 7 (63.60%) | 0.269 |
| Mild (n = 28) | Severe (n = 15) | p * | |
|---|---|---|---|
| WBC (109/L) | 8.4750 (6.395–10.665) | 8.690 (5.740–12.230) | 0.819 |
| Hb (gr/L) | 12.9 (11.63–13.70) | 12.1 (11.60–12.80) | 0.296 |
| PLT count (109/L) | 292.50 (235.75–382.00) | 248 (197–285.00) | 0.048 |
| Haptoglobin (mg/dL) | 274.50 (196.25–354.75) | 289.50 (173.50–371.25) | 0.915 |
| LDH (UI/L) | 340.00 (258.00–403.00) | 357.00 (327.00–499.00) | 0.860 |
| D-dimer (mg/L FEU) | 0.70 (0.41–1.63) | 1.87 (0.76–3.57) | 0.041 |
| Fibrinogen (mg/dL) | 458.00 (314.00–554.00) | 467.00 (370.00–605.00) | 0.397 |
| AT (%) | 103.50 (95.2–117.00) | 108.00 (91.00–119.00) | 0.855 |
| Ferritin (ng/mL) | 648.00 (241.00–963.00) | 944.00 (330.00–1854.00) | 0.317 |
| CRP (mg/dL) | 2.58 (1.25–9.79) | 4.01 (0.99–14.72) | 0.449 |
| IL-6 (pg/mL) | 15.30 (6.65–155.18) | 31.05 (11.70–82.88) | 0.510 |
| PCT (ng/mL) | 0.05 (0.05–0.20) | 0.05 (0.05–0.53) | 0.375 |
| Serum creatinine (mg/dL) | 0.76 (0.64–0.92) | 0.80 (0.67–1.45) | 0.629 |
| Mild (n = 28) | Severe (n = 15) | p * | |
|---|---|---|---|
| vWF:CBA (UI/mL) | 3.16 (2.24–3.64) | 3.44 (3.13–4.90) | 0.023 |
| ADAMTS 13 (UI/mL) | 0.89 (0.82–1.14) | 0.80 (0.70–1.07) | 0.093 |
| vWF:RICOF (UI/mL) | 2.40 (1.79–3.57) | 3.65 (2.30–3.82) | 0.169 |
| vW:AG (UI/mL) | 2.88 (1.94–3.78) | 3.65 (2.30–4.11) | 0.090 |
| Factor VIII (UI/mL) | 3.15 (2.50–3.51) | 3.14 (2.58–3.82) | 0.646 |
| Mild (n = 28) | Severe (n = 15) | p * | |
|---|---|---|---|
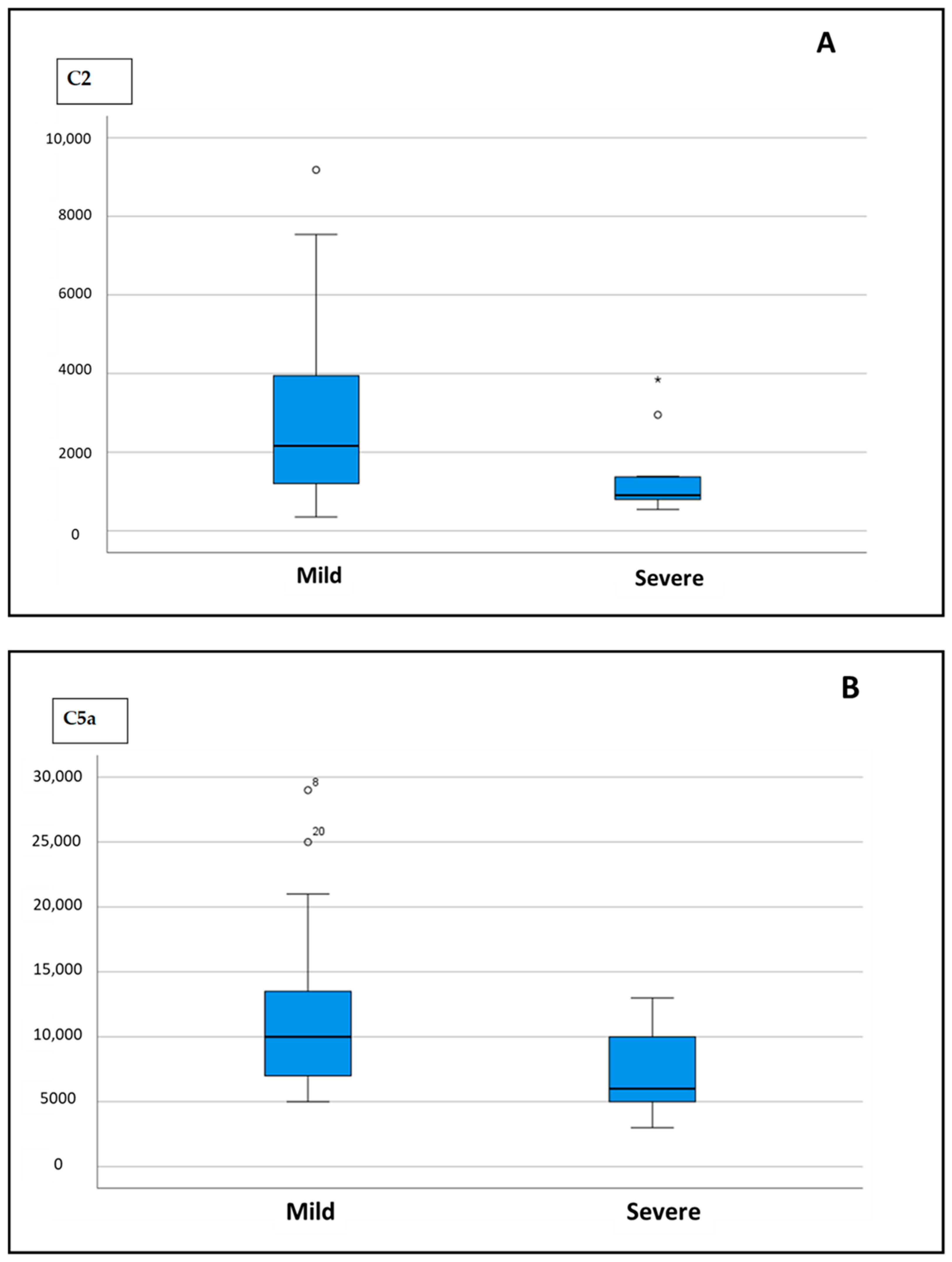
| C2 | 2161.11 (185.700–3947.700) | 904.70 (789.800–1370.400) | 0.006 |
| C4b | 9930.50 (5545.550–12,996.850) | 8794.49 (7043.900–23,535.800) | 0.999 |
| C5 | 16,904.30 (13,078.550–24,302.050) | 16,164.80 (12,696.900–35,980.000) | 0.962 |
| C5a | 10,000 (7000–13,750) | 6000 (4925–10,500) | 0.015 |
| CFI | 43,946.30 (29,112.000–55,291.700) | 32,804.20 (25,427.700–60,340.800) | 0.445 |
| MBL | 2428.60 (2004.050–4868.900) | 3811.00 (2693.600–5010.800) | 0.176 |
| C1q | 22,840.00 (19,800.00–25,740.00) | 28,880.00 (23,080.00–31,440.00) | 0.860 |
| C3 | 22,360 (14,150.00–32,450.00) | 25,560.00 (12,680.00–34,100.00) | 0.703 |
| C3b/iC3b | 166,660.00 (164,710.000–163,830.00) | 164,840.000 (161,060.00–165,380.00) | 0.037 |
| C4 | 537,400.00 (345,250.00–746,300.00) | 759,720.00 (415,460.00–861,680.00) | 0.192 |
| Factor B | 232,840.00 (164,910.00–276,460.00) | 247,880.00 (197,840.00–294,389.00) | 0.611 |
| Factor H | 253,000.00 (212,510.00–314,790.00) | 304,480.00 (257,300.00–353,460.00) | 0.086 |
| Hematology | Biochemistry | Coagulation Tests | Complement |
|---|---|---|---|
| White blood cells (WBC) | Lactate dehydrogenase (LDH) | von Willebrand factor collagen-binding activity (vWF:CBA) | Complement C2 (C2) |
| Hemoglobin (Hb) | Creatinine (Cr) | ADAM metallopeptidase with thrombospondin type 1 motif 13 (ADAMTS 13) | Complement C4b (C4b) |
| Platelets (PLT) | C-reactive protein (CRP) | von Willebrand factor ristocetin cofactor activity (vWF:RICOF) | Complement C5 (C5) |
| Procalcitonin (PCT) | von Willebrand factor antigen (vW:AG) | Complement C5a (C5a) | |
| Interleukin 6 (Il-6) | Anticardiolipin IgM and IgG (IgM ACA, IgG ACA) | Adipsin mannose-binding lectin (MBL) | |
| Aptoglobin | Anti-β2-Glycoprotein IgM (Anti-β2-GPI IgM) | Complement factor I (Factor I) | |
| Ferritin | Anti-β2-Glycoprotein IgG (Anti-β2-GPI IgG) | Complement C1q (C1q) | |
| Fibrinogen | Complement C3 (C3) | ||
| Complement C3b/iC3b (C3b/iC3b) | |||
| Complement C4 (C4) | |||
| Complement factor B (Factor B) | |||
| Complement factor H (Factor H) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silingardi, M.; Zappulo, F.; Dormi, A.; Pizzini, A.M.; Donadei, C.; Cappuccilli, M.; Fantoni, C.; Zaccaroni, S.; Pizzuti, V.; Cilloni, N.; et al. Is COVID-19 Coagulopathy a Thrombotic Microangiopathy? A Prospective, Observational Study. Int. J. Mol. Sci. 2025, 26, 5395. https://doi.org/10.3390/ijms26115395
Silingardi M, Zappulo F, Dormi A, Pizzini AM, Donadei C, Cappuccilli M, Fantoni C, Zaccaroni S, Pizzuti V, Cilloni N, et al. Is COVID-19 Coagulopathy a Thrombotic Microangiopathy? A Prospective, Observational Study. International Journal of Molecular Sciences. 2025; 26(11):5395. https://doi.org/10.3390/ijms26115395
Chicago/Turabian StyleSilingardi, Mauro, Fulvia Zappulo, Ada Dormi, Attilia Maria Pizzini, Chiara Donadei, Maria Cappuccilli, Chiara Fantoni, Stefania Zaccaroni, Valeria Pizzuti, Nicola Cilloni, and et al. 2025. "Is COVID-19 Coagulopathy a Thrombotic Microangiopathy? A Prospective, Observational Study" International Journal of Molecular Sciences 26, no. 11: 5395. https://doi.org/10.3390/ijms26115395
APA StyleSilingardi, M., Zappulo, F., Dormi, A., Pizzini, A. M., Donadei, C., Cappuccilli, M., Fantoni, C., Zaccaroni, S., Pizzuti, V., Cilloni, N., Tantillo, S., Guidi, A., Mancini, R., La Manna, G., & Comai, G. (2025). Is COVID-19 Coagulopathy a Thrombotic Microangiopathy? A Prospective, Observational Study. International Journal of Molecular Sciences, 26(11), 5395. https://doi.org/10.3390/ijms26115395








