Changes in Gene Expression Patterns in Young and Senescent Fibroblasts in Glycated Three-Dimensional Collagen Matrices
Abstract
1. Introduction
2. Results
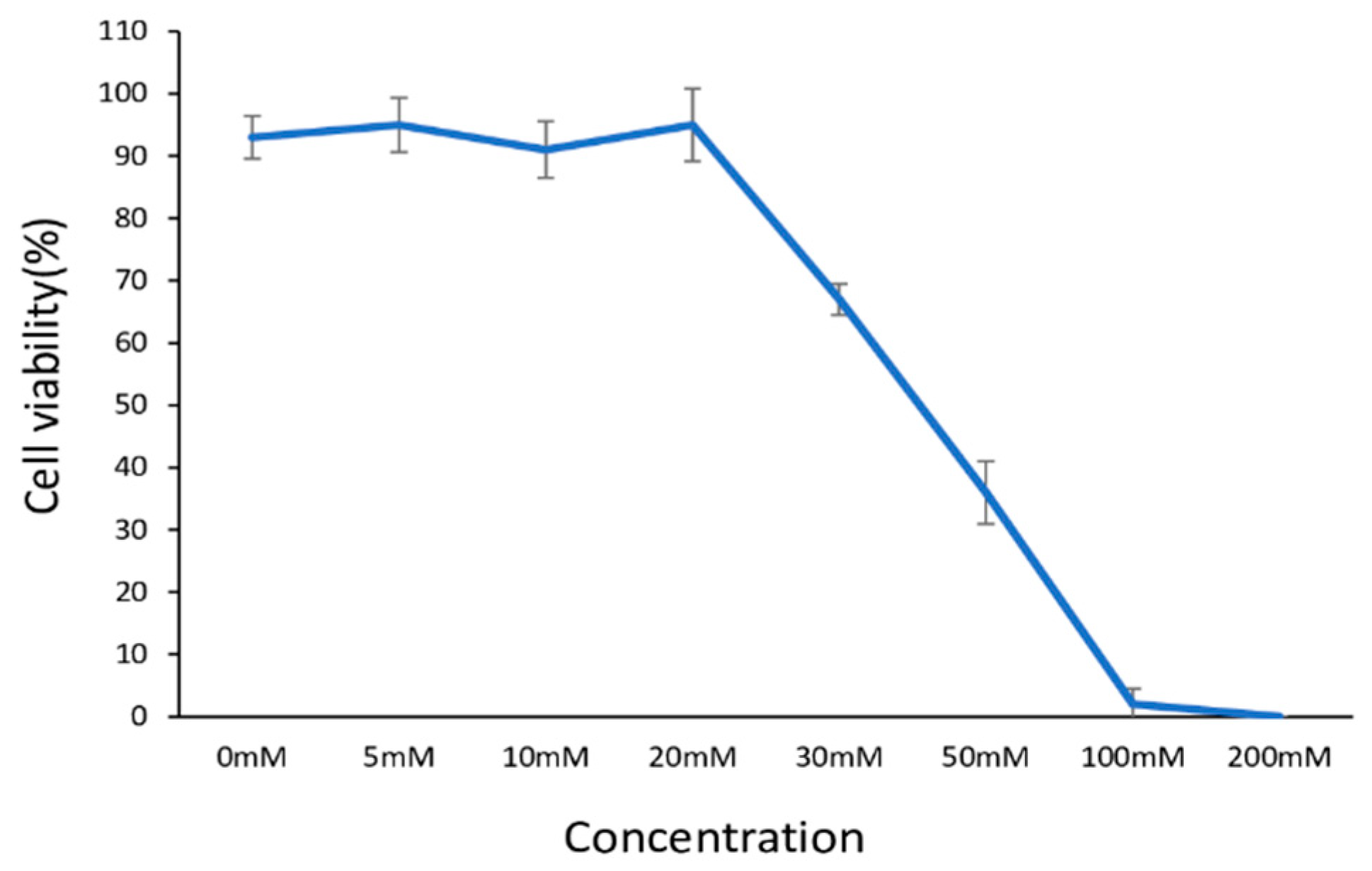
2.1. The Influence of D-Ribose Concentration on the Viability of Human Dermal Fibroblasts
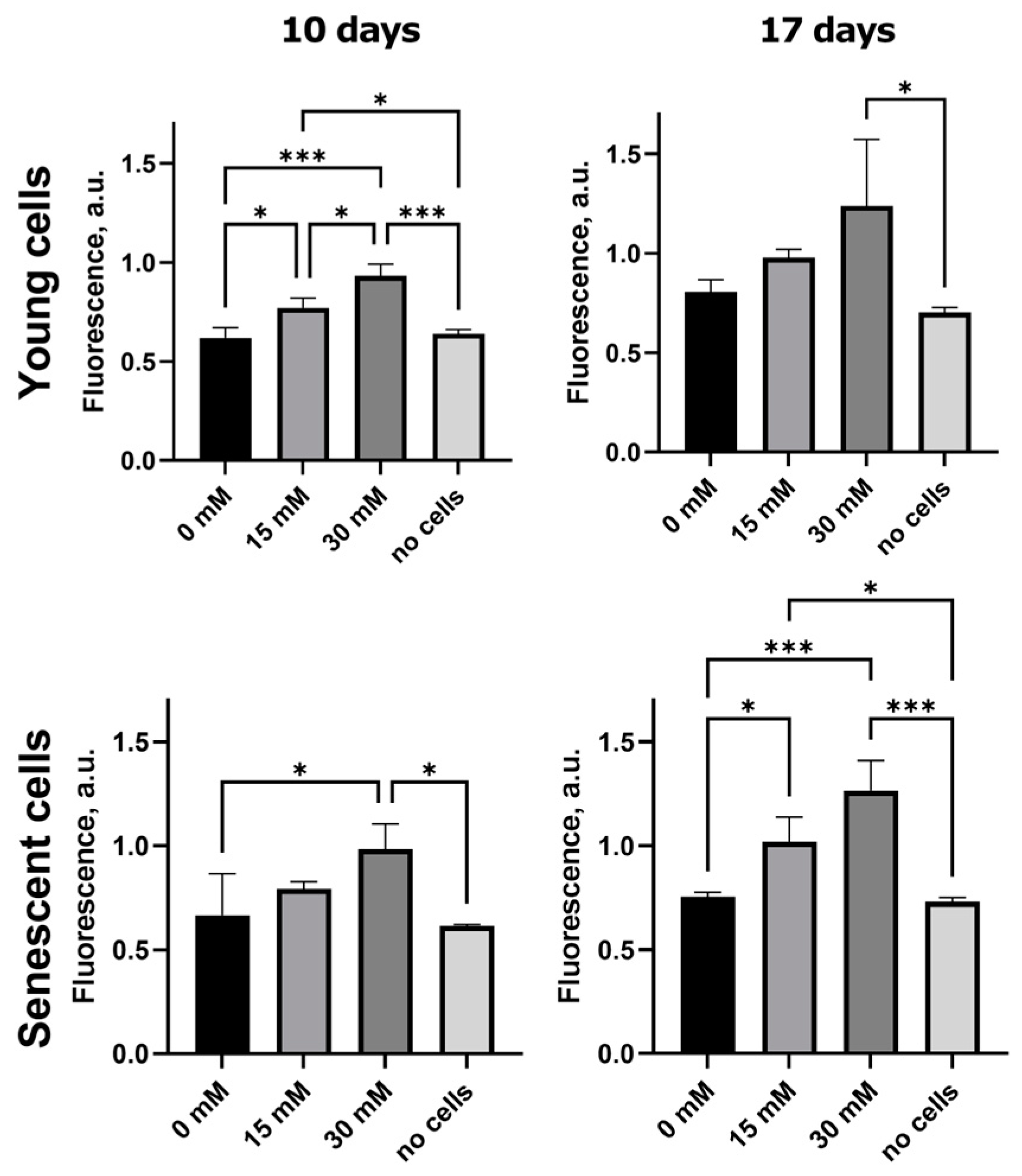
2.2. Formation of Fluorescent AGEs in Post-Glycated Collagen Matrices
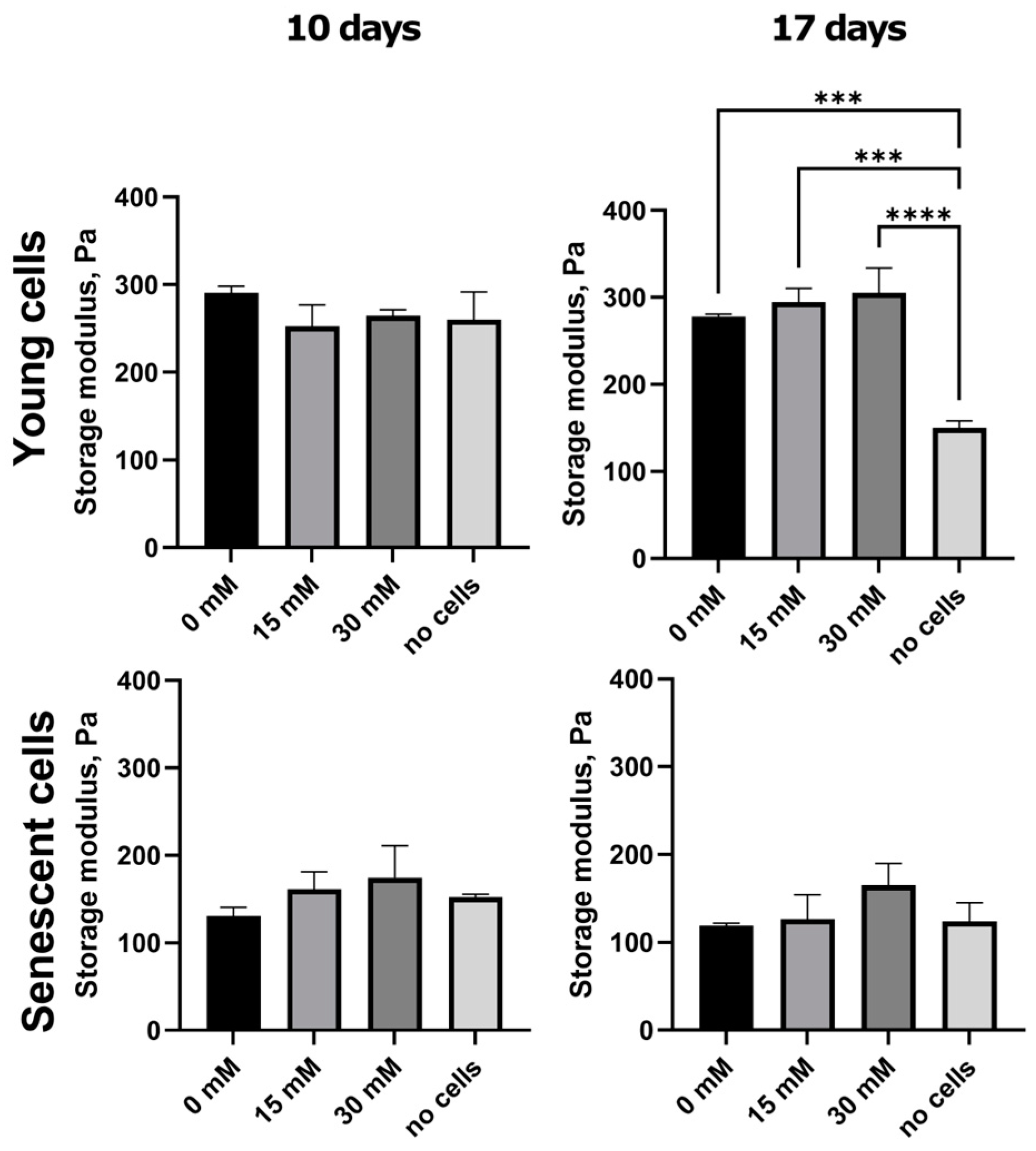
2.3. Ribosylation Alters the Mechanical Characteristics of the Collagen Matrices
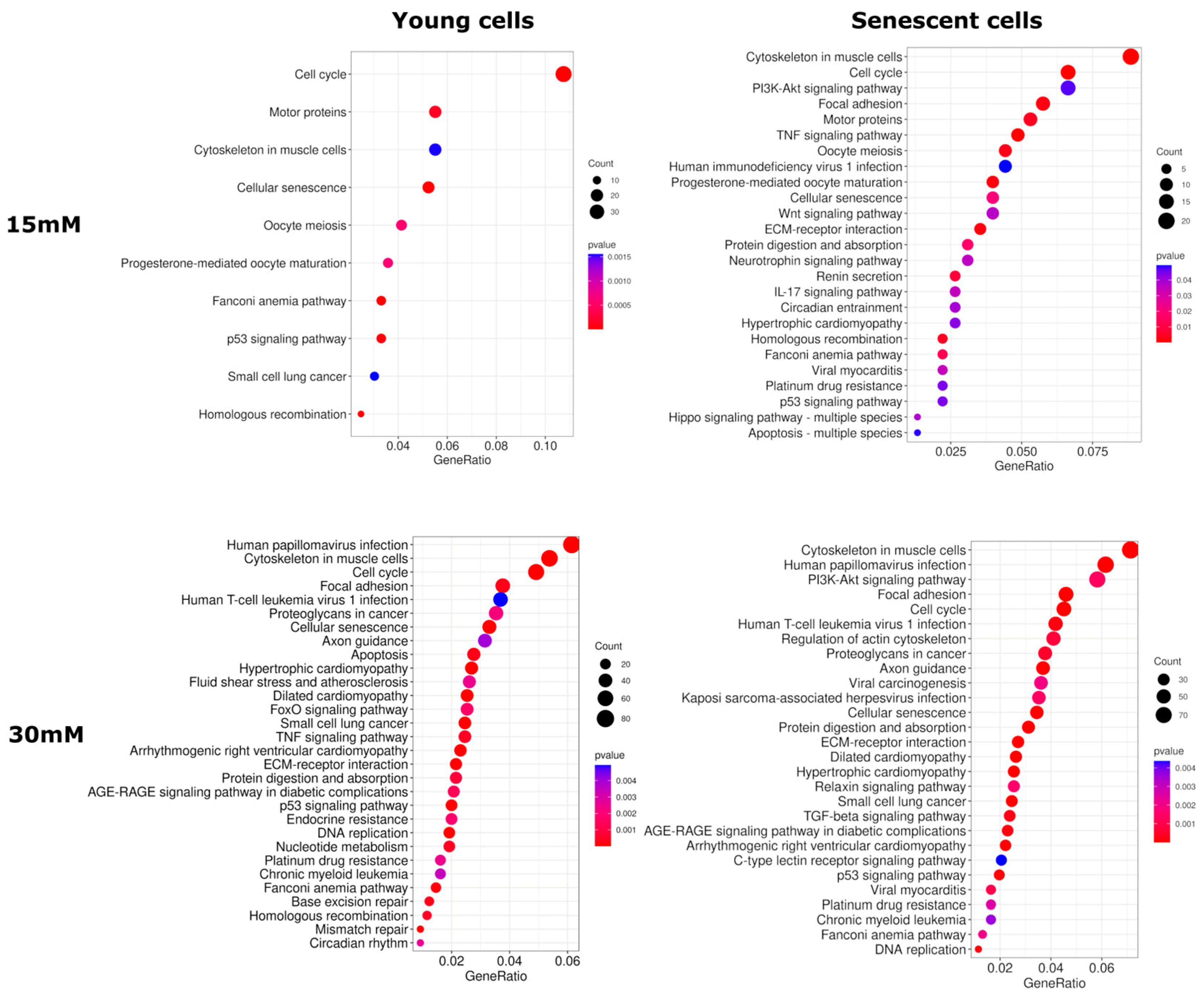
2.4. Transcriptome Analysis
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Formation of Collagen Gels and Glycation Process Using Ribose (Ribosylation)
4.3. Viability Assay
4.4. Autofluorescence Analysis
4.5. Rheology
4.6. Preparation of cDNA Libraries and Sequencing
4.7. Bioinformatics Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddy, V.P.; Aryal, P.; Darkwah, E.K. Advanced Glycation End Products in Health and Disease. Microorganisms 2022, 10, 1848. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.; Baynes, J.W. Glycation. In Encyclopedia of Biological Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 405–411. [Google Scholar]
- Fedintsev, A.; Moskalev, A. Stochastic Non-Enzymatic Modification of Long-Lived Macromolecules—A Missing Hallmark of Aging. Ageing Res. Rev. 2020, 62, 101097. [Google Scholar] [CrossRef] [PubMed]
- Selig, M.; Lauer, J.C.; Hart, M.L.; Rolauffs, B. Mechanotransduction and Stiffness-Sensing: Mechanisms and Opportunities to Control Multiple Molecular Aspects of Cell Phenotype as a Design Cornerstone of Cell-Instructive Biomaterials for Articular Cartilage Repair. Int. J. Mol. Sci. 2020, 21, 5399. [Google Scholar] [CrossRef]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- He, C.-P.; Chen, C.; Jiang, X.-C.; Li, H.; Zhu, L.-X.; Wang, P.-X.; Xiao, T. The Role of AGEs in Pathogenesis of Cartilage Destruction in Osteoarthritis. Bone Jt. Res. 2022, 11, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Vatner, S.F.; Zhang, J.; Vyzas, C.; Mishra, K.; Graham, R.M.; Vatner, D.E. Vascular Stiffness in Aging and Disease. Front. Physiol. 2021, 12, 762437. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and Extracellular Matrix Homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef]
- Flier, J.S.; Underhill, L.H.; Brownlee, M.; Cerami, A.; Vlassara, H. Advanced Glycosylation End Products in Tissue and the Biochemical Basis of Diabetic Complications. N. Engl. J. Med. 1988, 318, 1315–1321. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Hong, T.; Wang, H.; Gao, Z.; Wan, M. Advanced Glycosylation End Products Inhibit the Proliferation of Bone-Marrow Stromal Cells through Activating MAPK Pathway. Eur. J. Med. Res. 2021, 26, 94. [Google Scholar] [CrossRef]
- Omolaoye, T.S.; du Plessis, S.S. Male Infertility: A Proximate Look at the Advanced Glycation End Products. Reprod. Toxicol. 2020, 93, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Guvatova, Z.G.; Borisov, P.V.; Alekseev, A.A.; Moskalev, A.A. Age-Related Changes in Extracellular Matrix. Biochemistry 2022, 87, 1535–1551. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, L.; Wang, Y.; Wei, Y.; Xu, Y.; He, T.; He, R. D-Ribose Contributes to the Glycation of Serum Protein. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 2285–2292. [Google Scholar] [CrossRef]
- Wu, J.T.; Tu, M.C.; Zhung, P. Advanced Glycation End Product (AGE): Characterization of the Products from the Reaction between D-Glucose and Serum Albumin. J. Clin. Lab. Anal. 1996, 10, 21–34. [Google Scholar] [CrossRef]
- de Magalhães, J.P.; Abidi, Z.; dos Santos, G.A.; Avelar, R.A.; Barardo, D.; Chatsirisupachai, K.; Clark, P.; De-Souza, E.A.; Johnson, E.J.; Lopes, I.; et al. Human Ageing Genomic Resources: Updates on Key Databases in Ageing Research. Nucleic Acids Res. 2024, 52, D900–D908. [Google Scholar] [CrossRef]
- Verzijl, N.; DeGroot, J.; Oldehinkel, E.; Bank, R.A.; Thorpe, S.R.; Baynes, J.W.; Bayliss, M.T.; Bijlsma, J.W.; Lafeber, F.P.; Tekoppele, J.M. Age-Related Accumulation of Maillard Reaction Products in Human Articular Cartilage Collagen. Biochem. J. 2000, 350 Pt 2, 381–387. [Google Scholar] [CrossRef]
- Wagner, N.; Rapp, A.E.; Braun, S.; Ehnert, M.; Imhof, T.; Koch, M.; Jenei-Lanzl, Z.; Zaucke, F.; Meurer, A. Generation of Matrix Degradation Products Using an In Vitro MMP Cleavage Assay. Int. J. Mol. Sci. 2022, 23, 6245. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Bourne, J.W.; Lippell, J.M.; Torzilli, P.A. Glycation Cross-Linking Induced Mechanical–Enzymatic Cleavage of Microscale Tendon Fibers. Matrix Biol. 2014, 34, 179–184. [Google Scholar] [CrossRef][Green Version]
- Martins, B.; Fernandes, R. Disturbed Matrix Metalloproteinases Activity in Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2023, 1415, 21–26. [Google Scholar]
- Chen, J.-J.; Huang, J.-F.; Du, W.-X.; Tong, P.-J. Expression and Significance of MMP3 in Synovium of Knee Joint at Different Stage in Osteoarthritis Patients. Asian Pac. J. Trop. Med. 2014, 7, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ao, P.; Li, J.; Wu, T.; Xu, L.; Deng, Z.; Chen, W.; Yin, C.; Cheng, X. Autophagy Protects Advanced Glycation End Product-Induced Apoptosis and Expression of MMP-3 and MMP-13 in Rat Chondrocytes. Biomed. Res. Int. 2017, 2017, 6341919. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.K.; Waters, J.G.; Kevorkian, L.; Darrah, C.; Cooper, A.; Donell, S.T.; Clark, I.M. Expression Profiling of Metalloproteinases and Their Inhibitors in Synovium and Cartilage. Arthritis Res. Ther. 2006, 8, R124. [Google Scholar] [CrossRef]
- Quan, T.; Little, E.; Quan, H.; Qin, Z.; Voorhees, J.J.; Fisher, G.J. Elevated Matrix Metalloproteinases and Collagen Fragmentation in Photodamaged Human Skin: Impact of Altered Extracellular Matrix Microenvironment on Dermal Fibroblast Function. J. Investig. Dermatol. 2013, 133, 1362–1366. [Google Scholar] [CrossRef]
- Qin, Z.; Balimunkwe, R.M.; Quan, T. Age-related Reduction of Dermal Fibroblast Size Upregulates Multiple Matrix Metalloproteinases as Observed in Aged Human Skin in Vivo. Br. J. Dermatol. 2017, 177, 1337–1348. [Google Scholar] [CrossRef]
- van Goor, H.; Melenhorst, W.B.; Turner, A.J.; Holgate, S.T. Adamalysins in Biology and Disease. J. Pathol. 2009, 219, 277–286. [Google Scholar] [CrossRef]
- Conus, S.; Simon, H. Cathepsins and Their Involvement in Immune Responses. Swiss Med. Wkly. 2010, 140, w13042. [Google Scholar] [CrossRef]
- Janssen, B.J.C.; Robinson, R.A.; Pérez-Brangulí, F.; Bell, C.H.; Mitchell, K.J.; Siebold, C.; Jones, E.Y. Structural Basis of Semaphorin–Plexin Signalling. Nature 2010, 467, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Casazza, A.; Fazzari, P.; Tamagnone, L. Semaphorin Signals in Cell Adhesion and Cell Migration: Functional Role and Molecular Mechanisms. Adv. Exp. Med. Biol. 2007, 600, 90–108. [Google Scholar]
- Quan, T.; Fisher, G.J. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Liu, B.; Lv, L.; Wang, W.; Gao, D.; Zhang, Q.; Jiang, J.; Chai, M.; Yun, Z.; et al. Time-Resolved Extracellular Matrix Atlas of the Developing Human Skin Dermis. Front. Cell Dev. Biol. 2021, 9, 783456. [Google Scholar] [CrossRef] [PubMed]
- AK, M. Skin Aging and Modern Age Anti-Aging Strategies. Int. J. Clin. Dermatol. Res. 2019, 6, 209–240. [Google Scholar] [CrossRef]
- Li, N.; Gao, W.; Zhang, Y.-F.; Ho, M. Glypicans as Cancer Therapeutic Targets. Trends Cancer 2018, 4, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.-C.; Wang, J.-W.; Yuan, G.; Wang, Y.-P.; Xu, K.-Q.; Zhang, L.; Xu, X.-F.; Mao, W.-J.; Liu, Y. AGEs Promote Calcification of HASMCs by Mediating Pi3k/AKT-GSK3β Signaling. Front. Biosci. 2021, 26, 125–134. [Google Scholar] [CrossRef]
- Tominaga, K.; Suzuki, H.I. TGF-β Signaling in Cellular Senescence and Aging-Related Pathology. Int. J. Mol. Sci. 2019, 20, 5002. [Google Scholar] [CrossRef]
- Meng, X.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The Master Regulator of Fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Giroud, J.; Bouriez, I.; Paulus, H.; Pourtier, A.; Debacq-Chainiaux, F.; Pluquet, O. Exploring the Communication of the SASP: Dynamic, Interactive, and Adaptive Effects on the Microenvironment. Int. J. Mol. Sci. 2023, 24, 10788. [Google Scholar] [CrossRef]
- Bauwens, E.; Parée, T.; Meurant, S.; Bouriez, I.; Hannart, C.; Wéra, A.-C.; Khelfi, A.; Fattaccioli, A.; Burteau, S.; Demazy, C.; et al. Senescence Induced by UVB in Keratinocytes Impairs Amino Acids Balance. J. Investig. Dermatol. 2023, 143, 554–565.e9. [Google Scholar] [CrossRef]
- Kim, Y.; Byun, H.; Jee, B.A.; Cho, H.; Seo, Y.; Kim, Y.; Park, M.H.; Chung, H.; Woo, H.G.; Yoon, G. Implications of Time-series Gene Expression Profiles of Replicative Senescence. Aging Cell 2013, 12, 622–634. [Google Scholar] [CrossRef]
- Godwin, A.R.F.; Singh, M.; Lockhart-Cairns, M.P.; Alanazi, Y.F.; Cain, S.A.; Baldock, C. The Role of Fibrillin and Microfibril Binding Proteins in Elastin and Elastic Fibre Assembly. Matrix Biol. 2019, 84, 17–30. [Google Scholar] [CrossRef]
- Latorre, M.; Humphrey, J.D. Numerical Knockouts–In Silico Assessment of Factors Predisposing to Thoracic Aortic Aneurysms. PLoS Comput. Biol. 2020, 16, e1008273. [Google Scholar] [CrossRef]
- Kaneko-Kawano, T.; Suzuki, K. Mechanical Stress Regulates Gene Expression via Rho/Rho-Kinase Signaling Pathway. J. Phys. Fit. Sports Med. 2015, 4, 53–61. [Google Scholar] [CrossRef]
- Johnson, L.A.; Rodansky, E.S.; Haak, A.J.; Larsen, S.D.; Neubig, R.R.; Higgins, P.D.R. Novel Rho/MRTF/SRF Inhibitors Block Matrix-Stiffness and TGF-β–Induced Fibrogenesis in Human Colonic Myofibroblasts. Inflamm. Bowel Dis. 2014, 20, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.N.I.; Mitsuhashi, S.; Sigetomi, K.; Ubukata, M. Quercetin Inhibits Advanced Glycation End Product Formation via Chelating Metal Ions, Trapping Methylglyoxal, and Trapping Reactive Oxygen Species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Oh, J.-G.; Kim, J.S.; Lee, K.-W. Effects of Chebulic Acid on Advanced Glycation Endproducts-Induced Collagen Cross-Links. Biol. Pharm. Bull. 2014, 37, 1162–1167. [Google Scholar] [CrossRef]
- de Freitas, L.; Valli, M.; Dametto, A.C.; Pennacchi, P.C.; Andricopulo, A.D.; Maria-Engler, S.S.; Bolzani, V.S. Advanced Glycation End Product Inhibition by Alkaloids from Ocotea paranapiacabensis for the Prevention of Skin Aging. J. Nat. Prod. 2020, 83, 649–656. [Google Scholar] [CrossRef]
- André, A.; Touré, A.K.; Stien, D.; Eparvier, V. 2,5-diketopiperazines Mitigate the Amount of Advanced Glycation End Products Accumulated with Age in Human Dermal Fibroblasts. Int. J. Cosmet. Sci. 2020, 42, 596–604. [Google Scholar] [CrossRef]
- Ramasamy, R.; Shekhtman, A.; Schmidt, A.M. The Multiple Faces of RAGE–Opportunities for Therapeutic Intervention in Aging and Chronic Disease. Expert. Opin. Ther. Targets 2016, 20, 431–446. [Google Scholar] [CrossRef]
- Sell, D.R.; Biemel, K.M.; Reihl, O.; Lederer, M.O.; Strauch, C.M.; Monnier, V.M. Glucosepane Is a Major Protein Cross-Link of the Senescent Human Extracellular Matrix. J. Biol. Chem. 2005, 280, 12310–12315. [Google Scholar] [CrossRef]
- Piperi, C. Dietary Advanced Glycation End-Products: Molecular Mechanisms and Preventive Tools. Curr. Nutr. Rep. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Roy, R.; Boskey, A.; Bonassar, L.J. Processing of Type I Collagen Gels Using Nonenzymatic Glycation. J. Biomed. Mater. Res. A 2010, 93A, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Girton, T.S.; Oegema, T.R.; Tranquillo, R.T. Exploiting Glycation to Stiffen and Strengthen Tissue Equivalents for Tissue Engineering. J. Biomed. Mater. Res. 1999, 46, 87–92. [Google Scholar] [CrossRef]
- Sroga, G.E.; Siddula, A.; Vashishth, D. Glycation of Human Cortical and Cancellous Bone Captures Differences in the Formation of Maillard Reaction Products between Glucose and Ribose. PLoS ONE 2015, 10, e0117240. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. ClusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]

| Gene Symbol | Young Cells | Senescent Cells | ||
|---|---|---|---|---|
| LogFC, 15 mMR | LogFC, 30 mMR | LogFC, 15 mMR | LogFC, 30 mMR | |
| AP005018.2 | −1.17 | −2.5 | −1.31 | −2.66 |
| COL11A1 | −1.72 | −2.93 | −1.65 | −2.81 |
| WFDC1 | −1.8 | −3.69 | −1.59 | −5.35 |
| TMEM158 | 1.08 | 3.03 | 1.44 | 4.16 |
| GRIK2 | −1.23 | −2.83 | −1.12 | −2.96 |
| GTSE1 | −1.38 | −4.26 | −1.1 | −2.24 |
| CDCA3 | −1.03 | −3.65 | −1.07 | −2.97 |
| PHACTR3 | −1.12 | −3.19 | −1.01 | −3.87 |
| EPGN | 1.65 | 2.86 | 1.06 | 1.95 |
| KIF20A | −1.27 | −6.07 | −1.01 | −5.65 |
| HAPLN1 | −1.68 | −4.32 | −1.66 | −3.33 |
| LINC00578 | −1.36 | −2.43 | −1.41 | −4.7 |
| MMP3 | 1.15 | 3.89 | 1.37 | 5.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guvatova, Z.G.; Kudasheva, E.R.; Efremov, Y.M.; Timashev, P.S.; Fedorova, M.S.; Pudova, E.A.; Snezhkina, A.V.; Kudryavtseva, A.V.; Kobelyatskaya, A.A.; Moskalev, A.A. Changes in Gene Expression Patterns in Young and Senescent Fibroblasts in Glycated Three-Dimensional Collagen Matrices. Int. J. Mol. Sci. 2025, 26, 4769. https://doi.org/10.3390/ijms26104769
Guvatova ZG, Kudasheva ER, Efremov YM, Timashev PS, Fedorova MS, Pudova EA, Snezhkina AV, Kudryavtseva AV, Kobelyatskaya AA, Moskalev AA. Changes in Gene Expression Patterns in Young and Senescent Fibroblasts in Glycated Three-Dimensional Collagen Matrices. International Journal of Molecular Sciences. 2025; 26(10):4769. https://doi.org/10.3390/ijms26104769
Chicago/Turabian StyleGuvatova, Zulfiya G., Evelina R. Kudasheva, Yuri M. Efremov, Peter S. Timashev, Maria S. Fedorova, Elena A. Pudova, Anastasiya V. Snezhkina, Anna V. Kudryavtseva, Anastasiya A. Kobelyatskaya, and Alexey A. Moskalev. 2025. "Changes in Gene Expression Patterns in Young and Senescent Fibroblasts in Glycated Three-Dimensional Collagen Matrices" International Journal of Molecular Sciences 26, no. 10: 4769. https://doi.org/10.3390/ijms26104769
APA StyleGuvatova, Z. G., Kudasheva, E. R., Efremov, Y. M., Timashev, P. S., Fedorova, M. S., Pudova, E. A., Snezhkina, A. V., Kudryavtseva, A. V., Kobelyatskaya, A. A., & Moskalev, A. A. (2025). Changes in Gene Expression Patterns in Young and Senescent Fibroblasts in Glycated Three-Dimensional Collagen Matrices. International Journal of Molecular Sciences, 26(10), 4769. https://doi.org/10.3390/ijms26104769







