Myopathic Ehlers-Danlos Syndrome (mEDS) Related to COL12A1: Two Novel Families and Literature Review
Abstract
1. Introduction
2. Results
2.1. Clinical and Laboratory Findings
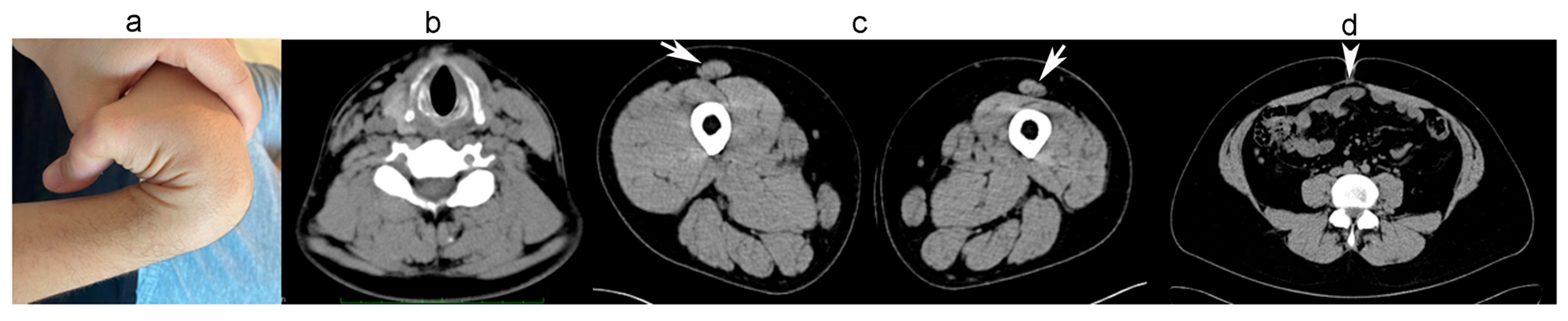
2.1.1. Family A
2.1.2. Family B
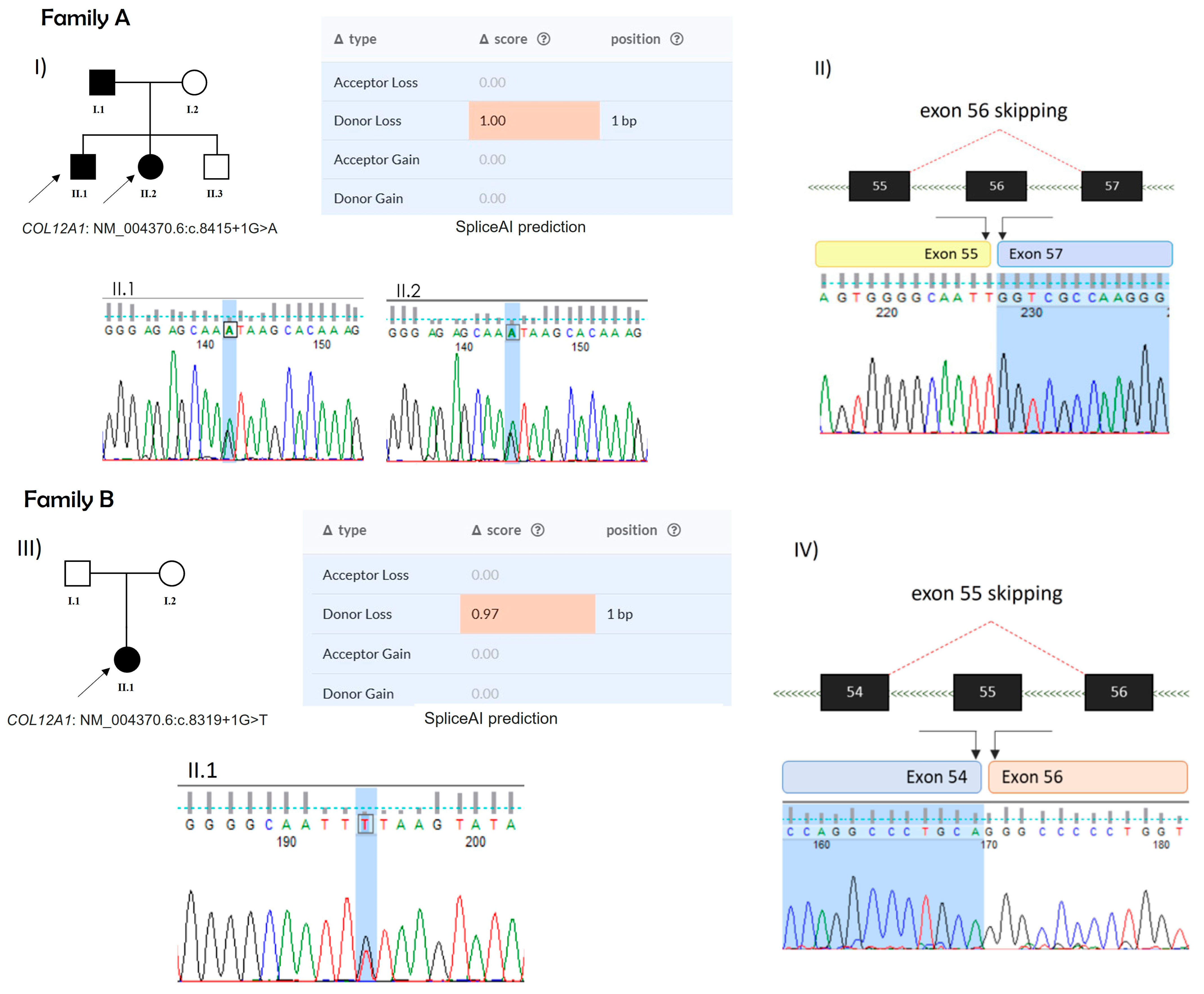
2.2. Mutation Analysis
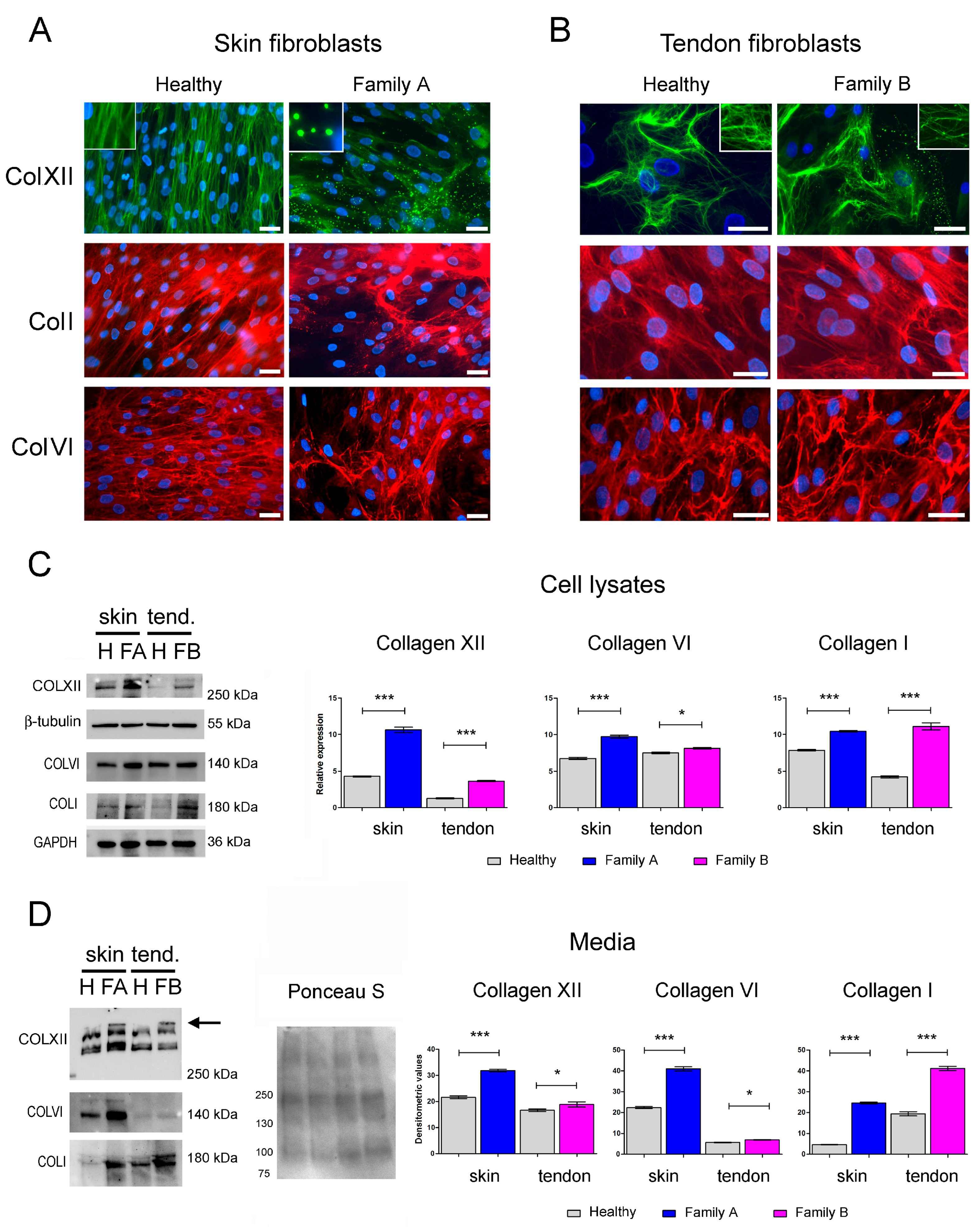
2.3. Immunofluorescence and Western Blot Analysis of Cell Cultures
3. Discussion
4. Materials and Methods
4.1. Patients and Methods
4.2. Genetic Analysis
4.3. Immunofluorescence of Dermal and Tendon Fibroblast Culture
4.4. Western Blotting
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiquet, M.; Birk, D.E.; Bonnemann, C.G.; Koch, M. Collagen XII: Protecting bone and muscle integrity by organizing collagen fibrils. Int. J. Biochem. Cell Biol. 2014, 53, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.K.; Gerecke, D.R.; Olsen, B.R. Type XII collagen: Distinct extracellular matrix component discovered by cDNA cloning. Proc. Natl. Acad. Sci. USA 1987, 84, 6040–6044. [Google Scholar] [CrossRef] [PubMed]
- Dublet, B.; Oh, S.; Sugrue, S.P.; Gordon, M.K.; Gerecke, D.R.; Olsen, B.R.; van der Rest, M. The structure of avian type XII collagen: α1(XII) chains contain 190-kDa non-triple helical amino-terminal domains and form homotrimeric molecules. J. Biol. Chem. 1989, 264, 13150–13156. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.P.; Taylor, R.W.; Gerecke, D.R.; Rochelle, J.M.; Seldin, M.F.; Olsen, B.R. The mouse α1(XII) and human α1(XII)-like collagen genes are localized on mouse chromosome 9 and human chromosome 6. Genomics 1992, 14, 225–231. [Google Scholar] [CrossRef]
- Yamagata, M.; Yamada, K.M.; Yamada, S.S.; Shinomura, T.; Tanaka, H.; Nishida, Y.; Obara, M.; Kimata, K. The complete primary structure of type XII collagen shows a chimeric molecule with reiterated fibronectin type III motifs, von Willebrand factor A motifs, a domain homologous to a noncollagenous region of type IX collagen, and short collagenous domains with an Arg-Gly-Asp site. J. Cell Biol. 1991, 115, 209–221. [Google Scholar] [CrossRef]
- Trueb, J.; Trueb, B. The two splice variants of collagen XII share a common 5′ end. Biochim. Biophys. Acta 1992, 1171, 97–98. [Google Scholar] [CrossRef]
- Keene, D.R.; Lunstrum, G.P.; Morris, N.P.; Stoddard, D.W.; Burgeson, R.E. Two type XII-like collagens localize to the surface of banded collagen fibrils. J. Cell Biol. 1991, 113, 971–978. [Google Scholar] [CrossRef]
- Font, B.; Eichenberger, D.; Rosenberg, L.M.; van der Rest, M. Characterization of the interactions of type XII collagen with two small proteoglycans from fetal bovine tendon, decorin and fibromodulin. Matrix Biol. 1996, 15, 341–348. [Google Scholar] [CrossRef]
- Koch, M.; Bohrmann, B.; Matthison, M.; Hagios, C.; Trueb, B.; Chiquet, M. Large and small splice variants of collagen XII: Differential expression and ligand binding. J. Cell Biol. 1995, 130, 1005–1014. [Google Scholar] [CrossRef]
- Nishiyama, T.; McDonough, A.M.; Bruns, R.R.; Burgeson, R.E. Type XII and XIV collagens mediate interactions between banded collagen fibers in vitro and may modulate extracellular matrix deformability. J. Biol. Chem. 1994, 269, 28193–28199. [Google Scholar] [CrossRef]
- Veit, G.; Hansen, U.; Keene, D.R.; Bruckner, P.; Chiquet-Ehrismann, R.; Chiquet, M.; Koch, M. Collagen XII interacts with avian tenascin-X through its NC3 domain. J. Biol. Chem. 2006, 281, 27461–27470. [Google Scholar] [CrossRef] [PubMed]
- Massoudi, D.; Malecaze, F.; Soler, V.; Butterworth, J.; Erraud, A.; Fournie, P.; Koch, M.; Galiacy, S.D. NC1 long and NC3 short splice variants of type XII collagen are overexpressed during corneal scarring. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7246–7256. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Ma, J.; Lomeli, S. The coordinated activities of collagen VI and XII in maintenance of tissue structure, function and repair: Evidence for a physical interaction. Front. Mol. Biosci. 2024, 11, 1376091. [Google Scholar] [CrossRef] [PubMed]
- Izu, Y.; Adams, S.M.; Connizzo, B.K.; Beason, D.P.; Soslowsky, L.J.; Koch, M.; Birk, D.E. Collagen XII mediated cellular and extracellular mechanisms regulate establishment of tendon structure and function. Matrix Biol. 2021, 95, 52–67. [Google Scholar] [CrossRef]
- Nair, A.; Ambekar, Y.S.; Zevallos-Delgado, C.; Mekonnen, T.; Sun, M.; Zvietcovich, F.; Singh, M.; Aglyamov, S.; Koch, M.; Scarcelli, G.; et al. Multiple Optical Elastography Techniques Reveal the Regulation of Corneal Stiffness by Collagen XII. Investig. Ophthalmol. Vis. Sci. 2022, 63, 24. [Google Scholar] [CrossRef]
- Turko, A.J.; Kultz, D.; Fudge, D.; Croll, R.P.; Smith, F.M.; Stoyek, M.R.; Wright, P.A. Skeletal stiffening in an amphibious fish out of water is a response to increased body weight. J. Exp. Biol. 2017, 220, 3621–3631. [Google Scholar] [CrossRef]
- Zou, Y.; Zwolanek, D.; Izu, Y.; Gandhy, S.; Schreiber, G.; Brockmann, K.; Devoto, M.; Tian, Z.; Hu, Y.; Veit, G.; et al. Recessive and dominant mutations in COL12A1 cause a novel EDS/myopathy overlap syndrome in humans and mice. Hum. Mol. Genet. 2014, 23, 2339–2352. [Google Scholar] [CrossRef]
- Hicks, D.; Farsani, G.T.; Laval, S.; Collins, J.; Sarkozy, A.; Martoni, E.; Shah, A.; Zou, Y.; Koch, M.; Bonnemann, C.G.; et al. Mutations in the collagen XII gene define a new form of extracellular matrix-related myopathy. Hum. Mol. Genet. 2014, 23, 2353–2363. [Google Scholar] [CrossRef]
- Punetha, J.; Kesari, A.; Hoffman, E.P.; Gos, M.; Kaminska, A.; Kostera-Pruszczyk, A.; Hausmanowa-Petrusewicz, I.; Hu, Y.; Zou, Y.; Bonnemann, C.G.; et al. Novel Col12A1 variant expands the clinical picture of congenital myopathies with extracellular matrix defects. Muscle Nerve 2017, 55, 277–281. [Google Scholar] [CrossRef]
- Witting, N.; Krag, T.; Werlauff, U.; Duno, M.; Oestergaard, S.T.; Dahlqvist, J.R.; Vissing, J. Collagen XII myopathy with rectus femoris atrophy and collagen XII retention in fibroblasts. Muscle Nerve 2018, 57, 1026–1030. [Google Scholar] [CrossRef]
- Jezela-Stanek, A.; Walczak, A.; Lazniewski, M.; Kosinska, J.; Stawinski, P.; Murcia Pienkowski, V.; Biernacka, A.; Rydzanicz, M.; Kostrzewa, G.; Krajewski, P.; et al. Novel COL12A1 variant as a cause of mild familial extracellular matrix-related myopathy. Clin. Genet. 2019, 95, 736–738. [Google Scholar] [CrossRef]
- Mohassel, P.; Liewluck, T.; Hu, Y.; Ezzo, D.; Ogata, T.; Saade, D.; Neuhaus, S.; Bolduc, V.; Zou, Y.; Donkervoort, S.; et al. Dominant collagen XII mutations cause a distal myopathy. Ann. Clin. Transl. Neurol. 2019, 6, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Delbaere, S.; Dhooge, T.; Syx, D.; Petit, F.; Goemans, N.; Destree, A.; Vanakker, O.; De Rycke, R.; Symoens, S.; Malfait, F. Novel defects in collagen XII and VI expand the mixed myopathy/Ehlers-Danlos syndrome spectrum and lead to variant-specific alterations in the extracellular matrix. Genet. Med. 2020, 22, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Araujo, D.; Antunes, H. A novel mutation in the COL12A1 gene. Gene 2021, 768, 145266. [Google Scholar] [CrossRef]
- Coppens, S.; Desmyter, L.; Koch, M.; Ozcelik, S.; O’Heir, E.; Van Bogaert, P.; Vilain, C.; Christiaens, F. Ehlers-Danlos/myopathy overlap syndrome caused by a large de novo deletion in COL12A1. Am. J. Med. Genet. A 2022, 188, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Deng, L.; Zhang, Y.; Wang, Y.; Yan, D.; Lin, X.; Wang, Y.; Wang, Y.; Hu, X. Novel variant of COL12A1 gene causing neonatal hypotonia and respiratory failure. Neurol. Sci. 2025, 46, 2847–2854. [Google Scholar] [CrossRef] [PubMed]
- Furuhata-Yoshimura, M.; Yamaguchi, T.; Izu, Y.; Kosho, T. Homozygous splice site variant affecting the first von Willebrand factor A domain of COL12A1 in a patient with myopathic Ehlers-Danlos syndrome. Am. J. Med. Genet. A 2023, 191, 2631–2639. [Google Scholar] [CrossRef]
- Naghipoor, K.; Khosravi, T.; Oladnabi, M. Whole exome sequencing identifies a novel variant in the COL12A1 gene in a family with Ullrich congenital muscular dystrophy 2. Mol. Biol. Rep. 2023, 50, 7427–7435. [Google Scholar] [CrossRef]
- Padmanabha, H.; Arunachal, G.; Kishore, P.; Sharma, P.P.; Mailankody, P.; Mahale, R.R.; Nashi, S.; Mathuranath, P.S.; Chandra, S.R. Collagen XII-Related Myopathy: An Emerging Spectrum of Extracellular Matrix-Related Myopathy. Neurol. India 2023, 71, 1257–1259. [Google Scholar] [CrossRef]
- Ipek, R.; Cavdartepe, B.E.; Bozdogan, S.T.; Yis, U. COL12A1 Gene Variant and a Review of the Literature: A Case Report of Ullrich Congenital Muscular Dystrophy. Mol. Syndromol. 2024, 15, 311–316. [Google Scholar] [CrossRef]
- El Sherif, R.; Saito, Y.; Hussein, R.S.; Izu, Y.; Koch, M.; Noguchi, S.; Nishino, I. A novel homozygous nonsense variant in COL12A1 causes myopathic Ehlers-Danlos syndrome: A case report and literature review. Neuropathol. Appl. Neurobiol. 2024, 50, e13004. [Google Scholar] [CrossRef] [PubMed]
- McCarty, R.M.; Saade, D.; Munot, P.; Laverty, C.G.; Pinz, H.; Zou, Y.; McAnally, M.; Yun, P.; Tian, C.; Hu, Y.; et al. Clinical characterization of Collagen XII-related disease caused by biallelic COL12A1 variants. Ann. Clin. Transl. Neurol. 2025, 12, 602–614. [Google Scholar] [CrossRef] [PubMed]
- Allamand, V.; Merlini, L.; Bushby, K.; Consortium for Collagen VI-Related Myopathies. 166th ENMC International Workshop on Collagen type VI-related Myopathies, 22–24 May 2009, Naarden, The Netherlands. Neuromuscul. Disord. NMD 2010, 20, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Schessl, J.; Goemans, N.M.; Magold, A.I.; Zou, Y.; Hu, Y.; Kirschner, J.; Sciot, R.; Bonnemann, C.G. Predominant fiber atrophy and fiber type disproportion in early ullrich disease. Muscle Nerve 2008, 38, 1184–1191. [Google Scholar] [CrossRef]
- Yonekawa, T.; Nishino, I. Ullrich congenital muscular dystrophy: Clinicopathological features, natural history and pathomechanism(s). J. Neurol. Neurosurg. Psychiatry 2015, 86, 280–287. [Google Scholar] [CrossRef]
- Panades-de Oliveira, L.; Rodriguez-Lopez, C.; Cantero Montenegro, D.; Marcos Toledano, M.D.M.; Fernandez-Marmiesse, A.; Esteban Perez, J.; Hernandez Lain, A.; Dominguez-Gonzalez, C. Bethlem myopathy: A series of 16 patients and description of seven new associated mutations. J. Neurol. 2019, 266, 934–941. [Google Scholar] [CrossRef]
- Yonekawa, T.; Komaki, H.; Okada, M.; Hayashi, Y.K.; Nonaka, I.; Sugai, K.; Sasaki, M.; Nishino, I. Rapidly progressive scoliosis and respiratory deterioration in Ullrich congenital muscular dystrophy. J. Neurol. Neurosurg. Psychiatry 2013, 84, 982–988. [Google Scholar] [CrossRef]
- Foley, A.R.; Quijano-Roy, S.; Collins, J.; Straub, V.; McCallum, M.; Deconinck, N.; Mercuri, E.; Pane, M.; D’Amico, A.; Bertini, E.; et al. Natural history of pulmonary function in collagen VI-related myopathies. Brain J. Neurol. 2013, 136, 3625–3633. [Google Scholar] [CrossRef]
- Pepe, G.; Bertini, E.; Bonaldo, P.; Bushby, K.; Giusti, B.; de Visser, M.; Guicheney, P.; Lattanzi, G.; Merlini, L.; Muntoni, F.; et al. Bethlem myopathy (BETHLEM) and Ullrich scleroatonic muscular dystrophy: 100th ENMC international workshop, 23–24 November 2001, Naarden, The Netherlands. Neuromuscul. Disord. NMD 2002, 12, 984–993. [Google Scholar] [CrossRef]
- Pepe, G.; de Visser, M.; Bertini, E.; Bushby, K.; Vanegas, O.C.; Chu, M.L.; Lattanzi, G.; Merlini, L.; Muntoni, F.; Urtizberea, A. Bethlem myopathy (BETHLEM) 86th ENMC international workshop, 10–11 November 2000, Naarden, The Netherlands. Neuromuscul. Disord. NMD 2002, 12, 296–305. [Google Scholar] [CrossRef]
- Bonnemann, C.G.; Brockmann, K.; Hanefeld, F. Muscle ultrasound in Bethlem myopathy. Neuropediatrics 2003, 34, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Lampe, A.; Allsop, J.; Knight, R.; Pane, M.; Kinali, M.; Bonnemann, C.; Flanigan, K.; Lapini, I.; Bushby, K.; et al. Muscle MRI in Ullrich congenital muscular dystrophy and Bethlem myopathy. Neuromuscul. Disord. NMD 2005, 15, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Sabatelli, P.; Merlini, L.; Di Martino, A.; Cenni, V.; Faldini, C. Early Morphological Changes of the Rectus Femoris Muscle and Deep Fascia in Ullrich Congenital Muscular Dystrophy. Int. J. Environ. Res. Public Health 2022, 19, 1252. [Google Scholar] [CrossRef]
- Birk, D.E.; Fitch, J.M.; Babiarz, J.P.; Doane, K.J.; Linsenmayer, T.F. Collagen fibrillogenesis in vitro: Interaction of types I and V collagen regulates fibril diameter. J. Cell Sci. 1990, 95 Pt 4, 649–657. [Google Scholar] [CrossRef]
- Cenni, V.; Sabatelli, P.; Di Martino, A.; Merlini, L.; Antoniel, M.; Squarzoni, S.; Neri, S.; Santi, S.; Metti, S.; Bonaldo, P.; et al. Collagen VI Deficiency Impairs Tendon Fibroblasts Mechanoresponse in Ullrich Congenital Muscular Dystrophy. Cells 2024, 13, 378. [Google Scholar] [CrossRef]
- Sardone, F.; Traina, F.; Bondi, A.; Merlini, L.; Santi, S.; Maraldi, N.M.; Faldini, C.; Sabatelli, P. Tendon Extracellular Matrix Alterations in Ullrich Congenital Muscular Dystrophy. Front. Aging Neurosci. 2016, 8, 131. [Google Scholar] [CrossRef][Green Version]
- Beenakker, E.A.; van der Hoeven, J.H.; Fock, J.M.; Maurits, N.M. Reference values of maximum isometric muscle force obtained in 270 children aged 4–16 years by hand-held dynamometry. Neuromuscul. Disord. NMD 2001, 11, 441–446. [Google Scholar] [CrossRef]
- van der Ploeg, R.J.; Fidler, V.; Oosterhuis, H.J. Hand-held myometry: Reference values. J. Neurol. Neurosurg. Psychiatry 1991, 54, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Merlini, L.; Mazzone, E.S.; Solari, A.; Morandi, L. Reliability of hand-held dynamometry in spinal muscular atrophy. Muscle Nerve 2002, 26, 64–70. [Google Scholar] [CrossRef]
- Musacchia, F.; Ciolfi, A.; Mutarelli, M.; Bruselles, A.; Castello, R.; Pinelli, M.; Basu, S.; Banfi, S.; Casari, G.; Tartaglia, M.; et al. VarGenius executes cohort-level DNA-seq variant calling and annotation and allows to manage the resulting data through a PostgreSQL database. BMC Bioinform. 2018, 19, 477. [Google Scholar] [CrossRef]
- Sabatelli, P.; Gualandi, F.; Gara, S.K.; Grumati, P.; Zamparelli, A.; Martoni, E.; Pellegrini, C.; Merlini, L.; Ferlini, A.; Bonaldo, P.; et al. Expression of collagen VI α5 and α6 chains in human muscle and in Duchenne muscular dystrophy-related muscle fibrosis. Matrix Biol. J. Int. Soc. Matrix Biol. 2012, 31, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Antoniel, M.; Traina, F.; Merlini, L.; Andrenacci, D.; Tigani, D.; Santi, S.; Cenni, V.; Sabatelli, P.; Faldini, C.; Squarzoni, S. Tendon Extracellular Matrix Remodeling and Defective Cell Polarization in the Presence of Collagen VI Mutations. Cells 2020, 9, 409. [Google Scholar] [CrossRef] [PubMed]

| Family/ Patient | Gender | Onset | Age (years) | Able to Walk | FVC% Predicted | NIV Age (Years) | Muscle Weakness (MRC) | CK (U/L) | Hypermobility (BS) | COL12A1 Variant # | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1/P1 | M | Birth | 3 | y | - | - | - | - | D | c.7001T>C, p.Ile2334Thr | [17] |
| F2/P2 | F | Childhood | 42 | y | Normal | - | Mild P and N | 93 | Generalized | c.8357G>A, p.Gly2786Asp | [18] |
| F2/P3 | F | Birth | 6 | y | Normal | - | Mild N, min P | 747 | Generalized | Same family variant | |
| F3/P4 | M | Childhood | 48 | y | Normal | - | P | 973 | - | c.5893Arg, p.Arg1965Cys | |
| F3/P5 | M | Adolescence | 22 | y | Normal | - | P | 1310 | n | Same family variant | |
| F3/P6 | M | Adolescence | 27 | y | 79% | - | P | 824 | n | Same family variant | |
| F4/P7 | F | Birth | 8 | y | - | - | Global | Normal | Marked | c.8329G>C, p.Gly2777Arg | [19] |
| F5/P8 | M | Birth | 15 | y | 58% | - | A-P and D (4+) | Normal | Generalized | c.8100+2T>C | [20] |
| F5/P9 | M | Birth | 12 | y | 91% | - | A and P (5-), D (4) | - | Generalized | Same family variant | |
| F5/P10 | F | Birth | 40 | y | 105% | - | A-P and D (4+) | - | Generalized | Same family variant | |
| F5/P11 | F | - | 76 | y | 58% | - | P (5-), D (4-) | - | D | Same family variant | |
| F6/P12 | F | Birth | 4 | y | - | - | - | - | D | c.7196G>A, p.Gly2399Glu | [21] |
| F6/P13 | M | - | 34 | y | - | - | - | - | D | Same family variant | |
| F6/P14 | M | - | 34 | y | - | - | - | - | D | Same family variant | |
| F7/P15 | M | Birth | 5 | y | 95% | - | A (2/5), P and D (4) | 174 | D and P | c.7951-630_8100+991del1771ins10 | [22] |
| F7/P16 | F | Birth | 37 | y | 104% | - | D (4+) | 60 | D and P | Same family variant | |
| F7/P17 | M | Birth | 33 | y | 96% | - | D (4) | 101 | D and P | Same family variant | |
| F8/P18 | M | Childhood | 62 | y | 115% | - | D (4) | 106 | n | c.8276G>A, p.Gly2759Asp | |
| F9/P19 | M | Birth | 4 | y | 81% | - | Mild P and D | 103 | D and P | c.8453G>A, p.Gly2818Glu | |
| F10/P20 | M | Birth | 3 | y | - | - | A (2/5), mild P and D | 99 | D and P | c.8065G>A, p.Gly2689Arg | |
| F11/P21 | M | Birth | 4 | - | - | - | P | 198 | Generalized | c.8415+1_8415+10del | [23] |
| F12/P22 | F | Birth | 4 | y | - | - | - | 258 | Generalized (7/9) | c.8265+1G>A | |
| F13/P23 | F | Birth | 13 | y | - | - | - | 73 | Generalized (9/9) | c.8178+3A>C | |
| F13/P24 | F | Birth | 10 | y | - | - | - | - | Generalized (9/9) | Same family variant | |
| F13/P25 | M | Birth | 39 | y | - | - | - | 254 | Moderate | Same family variant | |
| F14/P26 | M | Birth | 7 | y | - | - | - | - | D | c.8100+3_8100+6delGAGT | |
| F15/P27 | M | Birth | 18 | y | - | - | - | 146 | - | c.5587C>T, p.Arg1863Cys | |
| F16/P28 | F | - | 14 | y | - | - | Upper limbs (4/5) | 42 | Generalized (7/9) | c.8336G>A, p.Arg2779His | [24] |
| F17/P29 | F | Birth | 8 | y | 73 | - | Shoulder, marked | - | - | In-frame deletion of exons 45–54 | [25] |
| F18/P30 | F | Birth | 0.1 | - | - | 0.1 | - | 380 | - | c.7622C>T, p.Ser2541Phe | [26] |
| F19/P31 | M | Birth | 29 | + | 90 | - | A and D (4/5) | Normal | D (5/9) | c.8415+1G>A | This report |
| F19/P32 | F | Adolescence | 14 | + | Normal | - | A and D (4/5) | Normal | Generalized (6/9) | Same family variant | |
| F20/P33 | F | Birth | 41 | + | 86 | - | N, hip, knee (4/5) | Normal | Generalized (6/9) | c.8319+1G>T |
| Family/ Patient | Gender | Onset | Age (Years) | Able to Walk | NIV Age (Years) | CK (U/L) | Muscle Weakness (MRC) | Hypermobility (BS) | COL12A1 Variant: | Status | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1/P1 | M | Birth | 9 | Never | <3 | Normal | Profound, mild facial | D | c.8006+1G>A | Hom | [17] |
| F1/P2 | M | - | - | Never | n | Normal | Profound | D | Same family variant | Hom | |
| F2/P3 | M | Birth | 47 | y | n | 93 | P and D | D | c.395-1G>A | Hom | [27] |
| F3/P4 | M | Birth | 6 | Never | - | - | Profound | - | c.8828C>T, p.(Pro2943Leu) | Hom | [28] |
| F4/P5 | F | - | 4 | Few steps | - | 90 | P and D | D | c.7541A>G, c.8369C>T | CompHet | [29] |
| F5/P6 | F | - | 3 | y | n | Normal | Four Limbs (4/5) | D | c.8903C>T, p.Pro2968Leu | Hom | [30] |
| F6/P7 | F | Birth | 1.9 | Never | <2 | 51 | - | D | c.4240C>T, p.R1414X | Hom | [31] |
| F7/P8 | F | Congenital | 1.25 | n | n | - | ULs (3/5), LLs (2/5) | D and P | c.57904+2T>A; c.5269C>T | CompHet | [32] |
| F8/P9 | F | Congenital | 18 | n | y | - | Four Limbs (4/5), N (2/5) | D and P | c.8464C>T | Hom | |
| F9/P10 | M | Congenital | 4 | - | y | - | Generalized | D | c.6340+1G>TinsA | Hom | |
| F10/P11 | M | Congenital | 13 | y, run | y | - | Mild P and A | D | c.946_947insA | Hom | |
| F11/P12 | F | Congenital | 3 | y | n | - | Four Limbs (4/5),N(2/5) | D and P | c.3329C>A, c.4177del | CompHet | |
| F11/P13 | M | Congenital | 1.25 | y | n | - | Four Limbs (3/5) | D and P | Same family variant | CompHet | |
| F12/P14 | M | Congenital | 3 | n | y | - | ULs and LLs (3/5) | Hands and feet | c.5664+1G>A; c.6127delG | CompHet | |
| F13/P15 | M | Congenital | 16 | Never | y | - | D > P, LLs > ULs | D and P | c.5230+1G>A | Hom |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merlini, L.; Sabatelli, P.; Cenni, V.; Zanobio, M.; Di Martino, A.; Traina, F.; Faldini, C.; Nigro, V.; Torella, A. Myopathic Ehlers-Danlos Syndrome (mEDS) Related to COL12A1: Two Novel Families and Literature Review. Int. J. Mol. Sci. 2025, 26, 5387. https://doi.org/10.3390/ijms26115387
Merlini L, Sabatelli P, Cenni V, Zanobio M, Di Martino A, Traina F, Faldini C, Nigro V, Torella A. Myopathic Ehlers-Danlos Syndrome (mEDS) Related to COL12A1: Two Novel Families and Literature Review. International Journal of Molecular Sciences. 2025; 26(11):5387. https://doi.org/10.3390/ijms26115387
Chicago/Turabian StyleMerlini, Luciano, Patrizia Sabatelli, Vittoria Cenni, Mariateresa Zanobio, Alberto Di Martino, Francesco Traina, Cesare Faldini, Vincenzo Nigro, and Annalaura Torella. 2025. "Myopathic Ehlers-Danlos Syndrome (mEDS) Related to COL12A1: Two Novel Families and Literature Review" International Journal of Molecular Sciences 26, no. 11: 5387. https://doi.org/10.3390/ijms26115387
APA StyleMerlini, L., Sabatelli, P., Cenni, V., Zanobio, M., Di Martino, A., Traina, F., Faldini, C., Nigro, V., & Torella, A. (2025). Myopathic Ehlers-Danlos Syndrome (mEDS) Related to COL12A1: Two Novel Families and Literature Review. International Journal of Molecular Sciences, 26(11), 5387. https://doi.org/10.3390/ijms26115387









