The Impact of Zirconium Oxide Nanoparticles on the Mechanical and Physical Properties of Glass Ionomer Dental Materials
Abstract
1. Introduction
2. Results
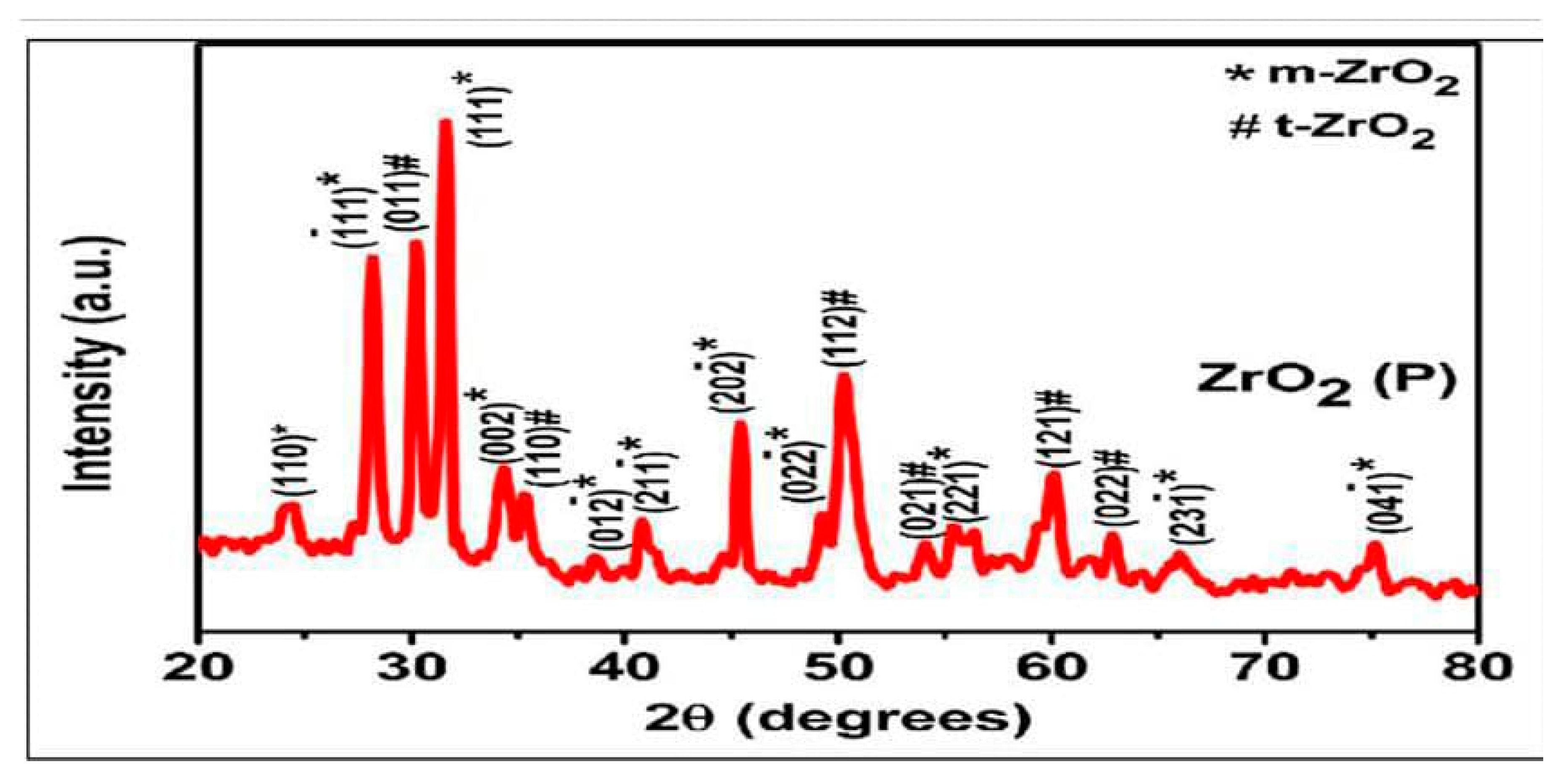
2.1. Characterization of ZrO2 Nanoparticles
X-Ray Diffraction (XRD)
3. Discussion
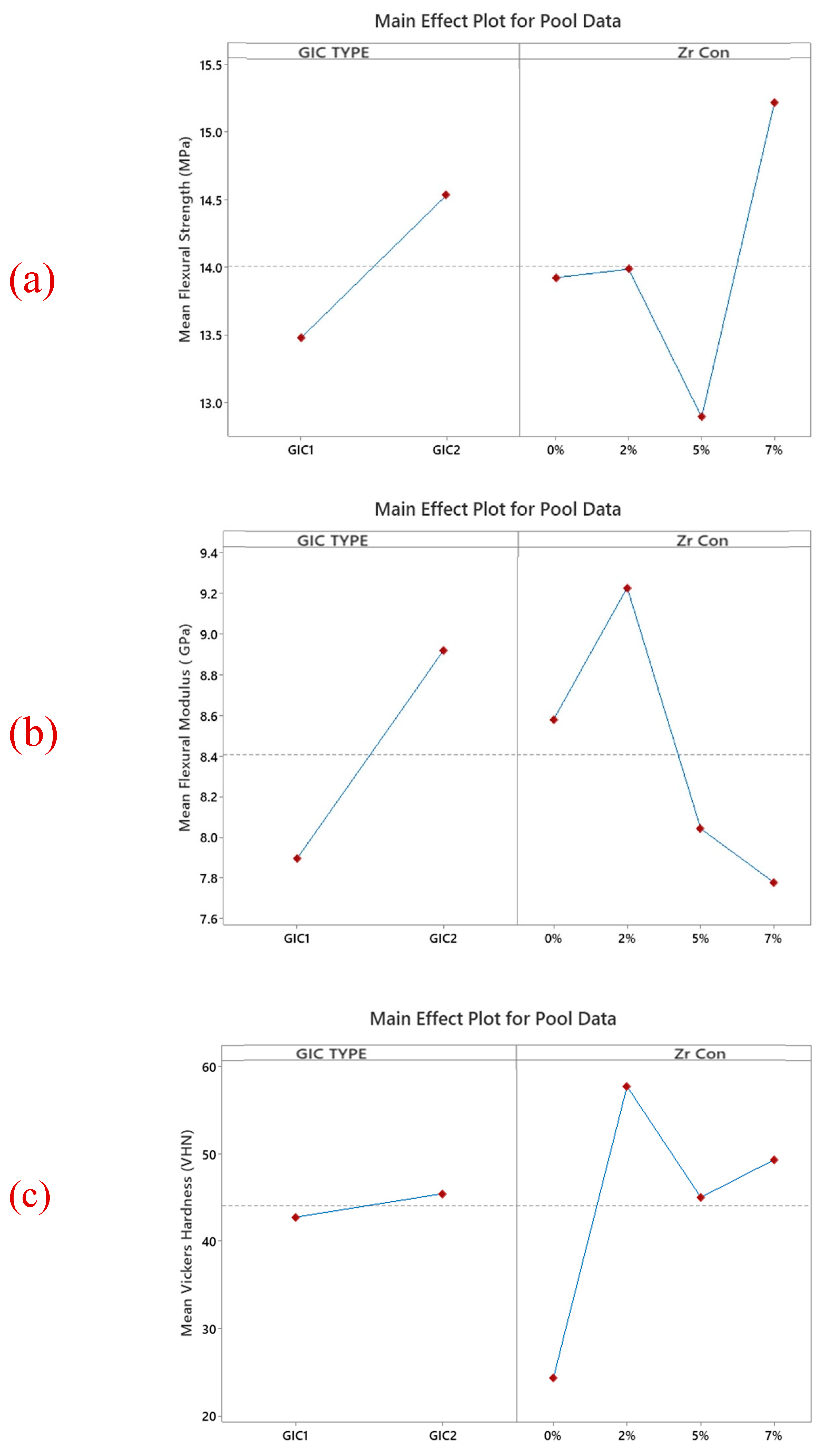
3.1. Flexural Strength
3.2. Flexural Modulus
3.3. Vickers Microhardness
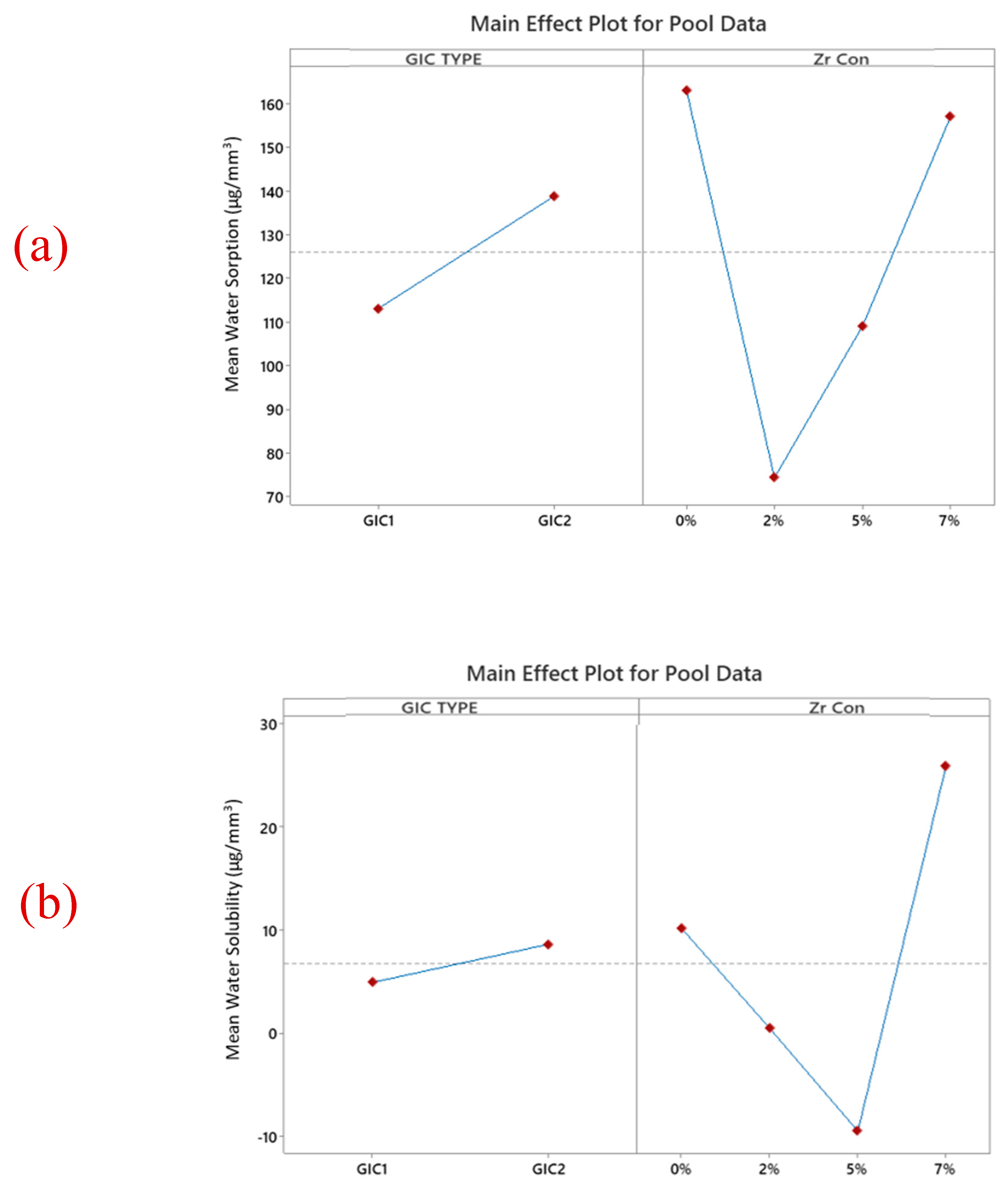
3.4. Water Sorption and Solubility
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Nano-ZrO2 Particles Preparation
4.2.2. Characterization of Nano-ZrO2 Particles
4.2.3. Incorporation of Nano-ZrO2 Particles into Glass Ionomer Cements
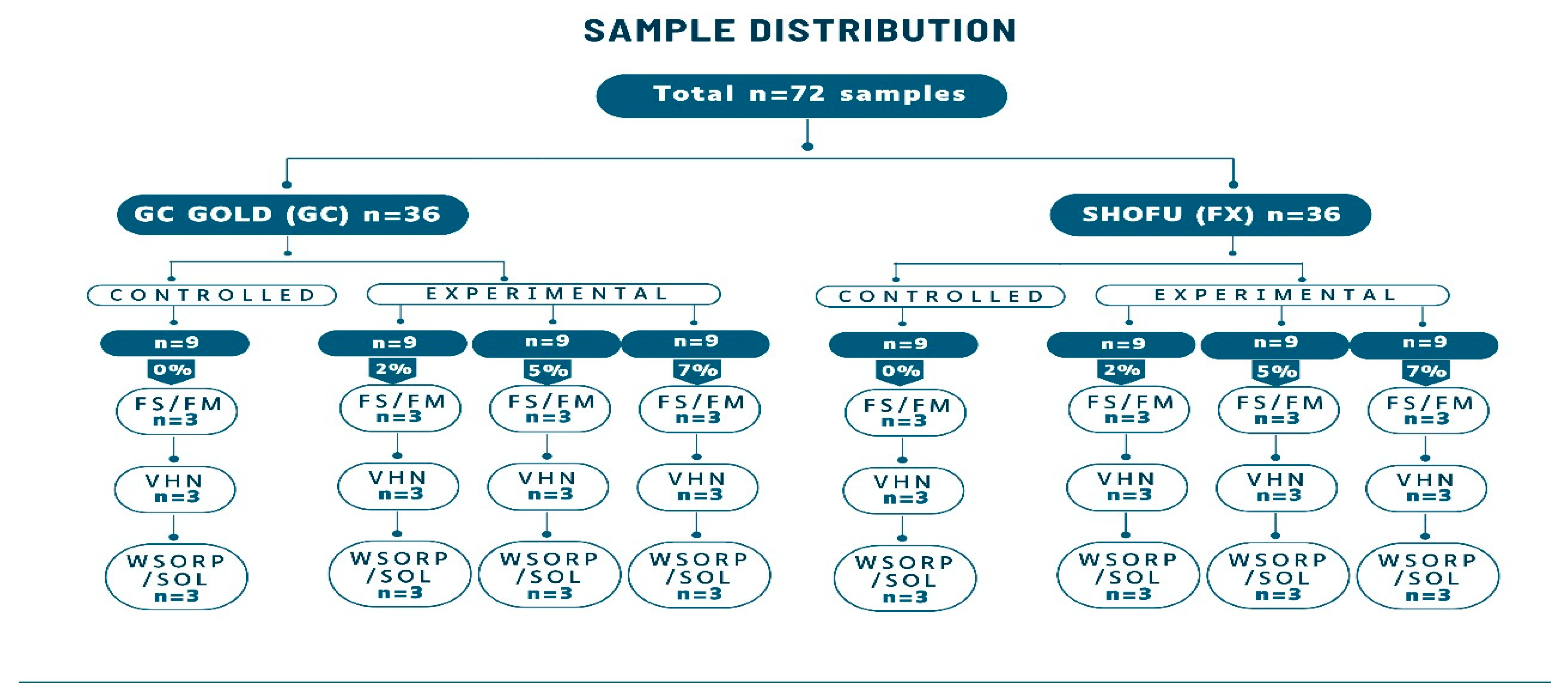
4.2.4. Specimens’ Preparation for Physio-Mechanical Properties
4.2.5. Flexural Strength Evaluation (FS)
4.2.6. Flexural Modulus Evaluation (FM)
4.2.7. Vickers Hardness Evaluation (VHN)
4.2.8. Water Sorption and Solubility Evaluation
4.2.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FS | Flexural strength. |
| FM | Flexural modulus. |
| VHN | Vickers microhardness. |
| NPs | Nanoparticles. |
References
- Heng, C. Tooth Decay Is the Most Prevalent Disease. Fed. Pract. 2016, 33, 31–33. [Google Scholar] [PubMed]
- Croll, T.P.; Nicholson, J.W. Glass ionomer cements in pediatric dentistry: Review of the literature. Pediatr. Dent. 2002, 24, 423–429. [Google Scholar] [PubMed]
- Berg, J.H.; Croll, T.P. Glass ionomer restorative cement systems: An update. Pediatr. Dent. 2015, 37, 116–124. [Google Scholar]
- Alobiedy, A.N.; Al-Helli, A.H.; Al-Hamaoy, A.R. Effect of adding micro and nano-carbon particles on conventional glass ionomer cement mechanical properties. Ain. Shams Eng. J. 2019, 10, 785–789. [Google Scholar] [CrossRef]
- Gupta, A.A.; Mulay, S.; Mahajan, P.; Raj, A.T. Assessing the effect of ceramic additives on the physical, rheological and mechanical properties of conventional glass ionomer luting cement—An in-vitro study. Heliyon 2019, 5, 02094. [Google Scholar] [CrossRef]
- Simmons, J.J. The miracle mixture. Glass ionomer and alloy powder. Tex. Dent. J. 1983, 100, 6–12. [Google Scholar]
- Cho, E.; Kopel, H.; White, S.N. Moisture susceptibility of resin-modified glass ionomer materials. Quintessence Int. 1995, 26, 351–358. [Google Scholar]
- Yap, A.; Cheang, P.; Chay, P. Mechanical properties of two restorative reinforced glass-ionomer cements. J. Oral Rehabil. 2002, 29, 682–688. [Google Scholar] [CrossRef]
- Lucas, M.E.; Arita, K.; Nishino, M. Toughness, bonding and fluoride-release properties of hydroxyapatite-added glass ionomer cement. Biomaterials 2003, 24, 3787–3794. [Google Scholar] [CrossRef]
- Tian, K.V.; Yang, B.; Yue, Y.; Bowron, D.T.; Mayers, J.; Donnan, R.S.; Dobó-Nagy, C.; Nicholson, J.W.; Fang, D.C.; Greer, A.L.; et al. Atomic and vibrational origins of mechanical toughness in bioactive cement during setting. Nat. Commun. 2015, 6, 8631. [Google Scholar] [CrossRef]
- Yakop, F.; Abd Ghafar, S.A.; Yong, Y.K.; Saiful Yazan, L.; Mohamad Hanafiah, R.; Lim, V.; Eshak, Z. Silver NPs Clinacanthus Nutans leaves extract induced apoptosis towards oral squamous cell carcinoma cell lines. Artif. Cells Nanomed. Biotechnol. 2018, 46, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, G.R.; Swamy, M.K. Potential applications of engineering NPs in medicine and biology: An update. J. Biol. Inorg. Chem. 2018, 23, 1185–1204. [Google Scholar] [CrossRef] [PubMed]
- Semyari, H.; Sattari, M.; Atai, M.; Pournasir, M. The effect of nanozirconia mixed with glass-ionomer on proliferation of epithelial cells and adhesive molecules. J. Periodontol. Implant. Dent. 2011, 3, 63–68. [Google Scholar]
- Dowling, A.H.; Schmitt, W.S.; Fleming, G.J.P. Modification of titanium dioxide particles to reinforce glass-ionomer restoratives. Dent. Mater. 2014, 30, 159–160. [Google Scholar] [CrossRef]
- Khademolhosseini, M.R.; Barounian, M.H.; Eskandari, A.; Aminzare, M.; Zahedi, A.M.; Ghahremani, D. Development of new Al2O3/TiO2 reinforced glass-ionomer cements (GICs) nanocomposites. J. Basic. Appl. Sci. Res. 2012, 2, 7526–7529. [Google Scholar]
- Cibim, D.D.; Saito, M.T.; Giovani, P.A.; Borges, A.F.S.; Pecorari, V.G.A.; Gomes, O.P.; Lisboa-Filho, P.N.; Niciti-Junior, F.H.; Puppin-Rontani, R.M.; Kantovitz, K.R. Novel nanotechnology of TiO2 improves physical-chemical and biological properties of glass ionomer cement. Int. J. Biomater. 2017, 2017, 7123919. [Google Scholar] [CrossRef]
- Gjorgievska, E.; Nicholson, J.W.; Grabić, D.; Guclu, Z.A.; Melitić, I.; Coleman, N.J. Assessment of the impact of the addition of NPs on the properties of glass-ionomer cements. Materials 2020, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Shinde, H.M.; Bhosale, T.T.; Gavade, N.L.; Babar, S.B.; Kamble, R.J.; Shirke, B.S.; Garadkar, K.M. Biosynthesis of ZrO2 NPs from Ficus benghalensis leaf extract for photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 14055–14064. [Google Scholar] [CrossRef]
- Jalill, R.D.A.; Jawad, M.M.H.M.; Abd, A.N. Plants extracts as green synthesis of nano-ZrO2 particles. J. Genet. Environ. Res. Conserv. 2017, 5, 623. [Google Scholar]
- Balaji, S.; Mandal, B.K.; Ranjan, S.; Dasgupta, N.; Chidambaram, R. Nano-zirconia–evaluation of its antioxidant and anticancer activity. J. Photochem. Photobiol. B 2017, 170, 125–133. [Google Scholar] [CrossRef]
- Shiekh, R.A.; Ab, R.I.; Masudi, S.A.; Luddin, N. Modification of glass ionomer cement by incorporating hydroxyapatite-silica nano-powder composite: Sol–gel synthesis and characterization. Ceram. Int. 2014, 40, 3165–3170. [Google Scholar] [CrossRef]
- Moheet, I.A.; Luddin, N.; Ab, R.I.; Masudi, S.A.; Kannan, T.P.; Abd Ghani, N.R. Evaluation of mechanical properties and bond strength of nano-hydroxyapatite-silica added glass ionomer cement. Ceram. Int. 2018, 44, 9899–9906. [Google Scholar] [CrossRef]
- Barandehfard, F.; Rad, M.K.; Hosseinnia, A.; Khoshroo, K.; Tahriri, M.; Jazayeri, H.E.; Moharamzadeh, K.; Tayebi, L. The addition of synthesized hydroxyapatite and fluorapatite nanoparticles to a glass-ionomer cement for dental restoration and its effects on mechanical properties. Ceram. Int. 2016, 42, 17866–17875. [Google Scholar] [CrossRef]
- ISO 9917-2:2017; Dentistry—Water-Based Cements—Part 2: Resin-Modified Cements. 3rd ed. International Organization for Standardization: Geneva, Switzerland, 2017.
- Bresciani, E.; Barata, T.D.J.E.; Fagundes, T.C.; Adachi, A.; Terrin, M.M.; Navarro, M.F.D.L. Compressive and diametral tensile strength of glass ionomer cements. J. Appl. Oral Sci. 2004, 12, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M. Materials Selection in Mechanical Design; Butterworth-Heinemann: Oxford, UK, 2011; p. 40. [Google Scholar]
- Kutuk, Z.B.; Vural, U.K.; Cakir, F.Y.; Miletic, I.; Gurgan, S. Mechanical properties and water sorption of two experimental glass ionomer cements with hydroxyapatite or calcium fluorapatite formulation. Dent. Mater. J. 2019, 38, 471–479. [Google Scholar] [CrossRef]
- Sajjad, A.; Bakar, W.Z.W.; Mohamad, D.; Kannan, T.P. Characterization and enhancement of physicomechanical properties of glass ionomer cement by incorporating a novel nano zirconia silica hydroxyapatite composite synthesized via sol-gel. AIMS Mater. Sci. 2019, 6, 730–747. [Google Scholar] [CrossRef]
- Bariker, R.H.; Mandroli, S.P. An in-vitro evaluation of antibacterial effect of Amalgomer CR and Fuji VII against bacteria causing severe early childhood caries. J. Indian. Soc. Pedod. Prev. Dent. 2016, 34, 23–29. [Google Scholar]
- Garcia-Contreras, R.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Sakagami, H.; Morales-Luckie, R.A.; Nakajima, H. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J. Appl. Oral Sci. 2015, 23, 321–328. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Ansari, S.; Moshaverinia, M.; Roohpour, N.; Darr, J.A.; Rehman, I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 2008, 4, 432–440. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Ansari, S.; Movasaghi, Z.; Billington, R.W.; Darr, J.A.; Rehman, I. Modification of conventional glass-ionomer cements with N-vinylpyrrolidone containing polyacids, nano-hydroxy and fluoroapatite to improve mechanical properties. Dent. Mater. 2008, 24, 1381–1390. [Google Scholar] [CrossRef]
- Kumar, N. Exploring the Variability in Mechanical Property Testing of Dental Resin Composites. Doctoral’s Dissertation, University of Birmingham, Birmingham, UK, 2011. [Google Scholar]
- Elsaka, S.E.; Hamouda, I.M.; Swain, M.V. Titanium dioxide NPs addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. J. Dent. 2011, 39, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Prentice, L.H.; Tyas, M.J.; Burrow, M.F. The effect of ytterbium fluoride and barium sulphate NPs on the reactivity and strength of a glass-ionomer cement. Dent. Mater. J. 2006, 22, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Elshenawy, E.A.; El-Ebiary, M.A.; Kenawy, E.R.; El-Olimy, G.A. Modification of glass-ionomer cement properties by quaternized chitosan-coated nanoparticles. Odontology 2023, 111, 328–341. [Google Scholar] [CrossRef] [PubMed]
- ISO 9917-1:2007; Dentistry—Water-Based Cements—Part 1: Powder/Liquid Acid-Base Cements. 2nd ed. International Organization for Standardization: Geneva, Switzerland, 2007.
- Alshali, R.Z.; Salim, N.A.; Satterthwaite, J.D.; Silikas, N. Long-term sorption and solubility of bulk-fill and conventional resin-composites in water and artificial saliva. J. Dent. 2015, 43, 1511–1518. [Google Scholar] [CrossRef]
- Lima, R.B.; Faris, J.F.; Andrade, A.M.; Silva, F.D.; Duarte, R.M. Water sorption and solubility of glass ionomer cements indicated for atraumatic restorative treatment considering the time and the pH of the storage solution. RGO-Rev. Gaúcha De Odontol. 2018, 66, 29–34. [Google Scholar] [CrossRef]
- Mustafa, R.; Alshali, R.Z.; Silikas, N. The effect of desiccation on water sorption, solubility and hygroscopic volumetric expansion of dentine replacement materials. Dent. Mater. J. 2018, 34, 205–213. [Google Scholar] [CrossRef]
- Sunbul, H.A.; Silikas, N.; Watts, D.C. Resin-based composites show similar kinetic profiles for dimensional change and recovery with solvent storage. Dent. Mater. 2015, 31, 201–217. [Google Scholar] [CrossRef]
- Alrahlah, A.; Silikas, N.; Watts, D.C. Hygroscopic expansion kinetics of dental resin-composites. Dent. Mater. 2014, 30, 143–148. [Google Scholar] [CrossRef]
- Ferracane, J.L. Elution of leachable components from composites. J. Oral Rehabil. 1994, 21, 441–452. [Google Scholar] [CrossRef]
- Toledano, M.B.; Osorio, R.; Osorio, E.; Aguilera, F.S.; Romeo, A.; De La Higuera, B.; García-Godoy, F. Sorption and solubility testing of orthodontic bonding cements in different solutions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 76, 251–256. [Google Scholar] [CrossRef]
- Keyf, F.; Tuna, S.H.; ¸Sen, M.; Safrany, A. Water sorption and solubility of different luting and restorative dental cements. Turk. J. Med. Sci. 2007, 37, 47–55. [Google Scholar]
- Sinthawornkul, S.; Thunyakitpisal, P.; Thunyakitpisal, N.; Jiemsirilers, S. Comparison of Shear Bond Strength, Water Sorption and Solubility of 3 Glass Ionomer Cements for Direct Bonding of Orthodontic Brackets in vitro. J. Dent. Assoc. Thail. 2017, 67, 225–235. [Google Scholar]
- Fathi, U.A.; Maha, A.A.; Ahmad, Z.A. The Effect of the Incorporation of Titanium Dioxide NPs on the Mechanical and Physical Properties of Glass Ionomer Cement. JRMDS 2022, 10, 88–91. [Google Scholar]
- Dehis, W.; Eissa, S.; Elawady, A.; Elhotaby, M. Impact of nano-TiO2 particles on water sorption and solubility in different denture base materials. J. Arab. Soc. Med. Res. 2018, 13, 99–105. [Google Scholar] [CrossRef]
- Foo, Y.T.; Abdullah, A.Z.; Horri, B.A.; Salamatinia, B. Optimised Co-Precipitation synthesis condition for oxalate-derived zirconia NPs. Ceram. Int. 2019, 45, 22930–22939. [Google Scholar] [CrossRef]
- ISO 4049:2009; Dentistry—Polymer-Based Restorative Materials. 4th ed. International Organization for Standardization: Geneva, Switzerland, 2009.
- Curtis, A.R. The Influence of ‘Nanocluster’ Reinforcement on the Mechanical Properties of a Resin-Based Composite Material. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2009. [Google Scholar]
- Wang, Y.; Zhu, M.; Zhu, X.X. Functional fillers for dental resin composites. Acta Biomater. 2021, 122, 50–65. [Google Scholar] [CrossRef]
- Yilmaz, M.N.; Gul, P.; Kiziltunc, A. Water sorption and solubility of a high-viscous glass-ionomer cement after the application of different surface-coating agents. Eur. J. Gen. Dent. 2020, 9, 118–121. [Google Scholar] [CrossRef]
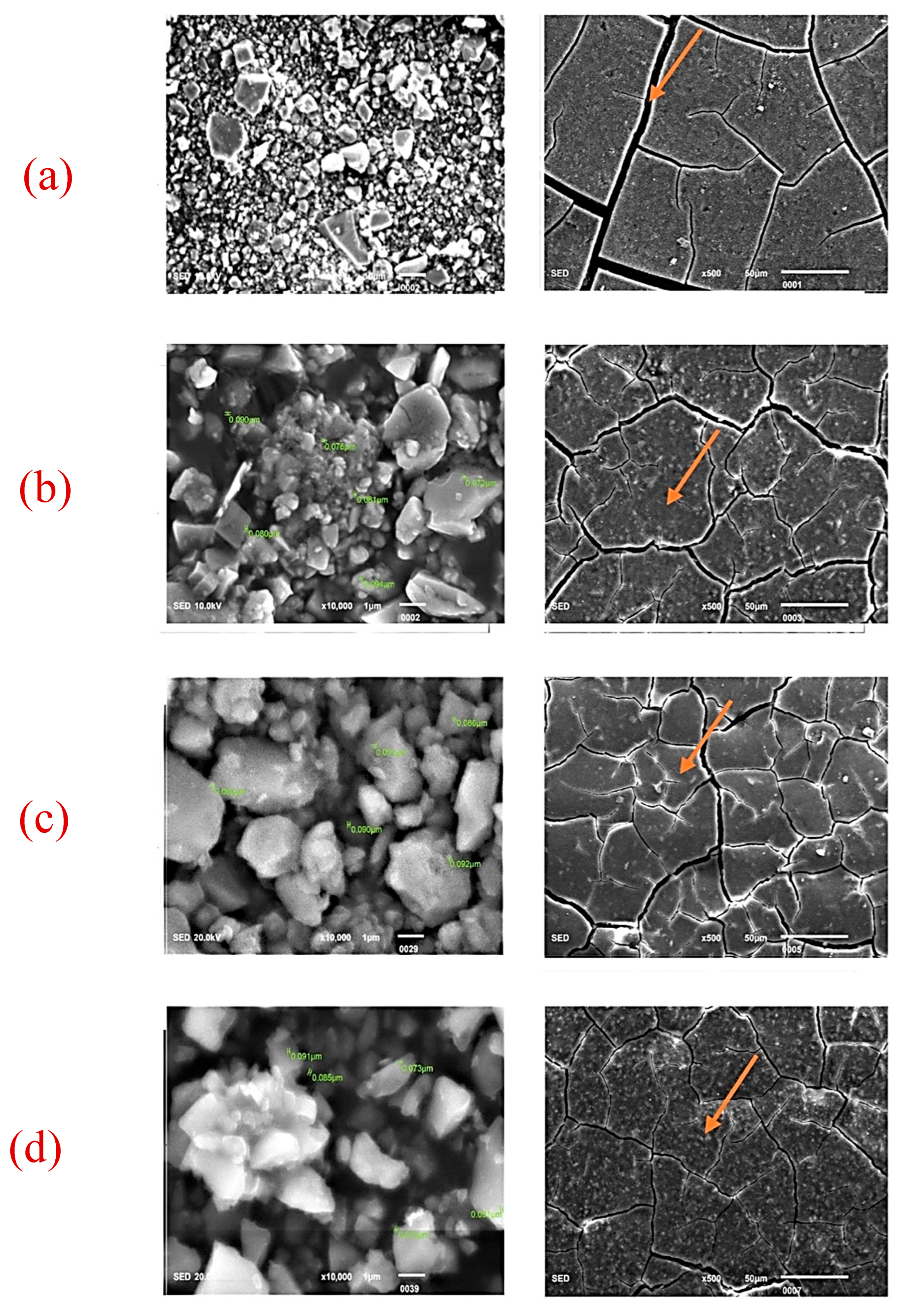
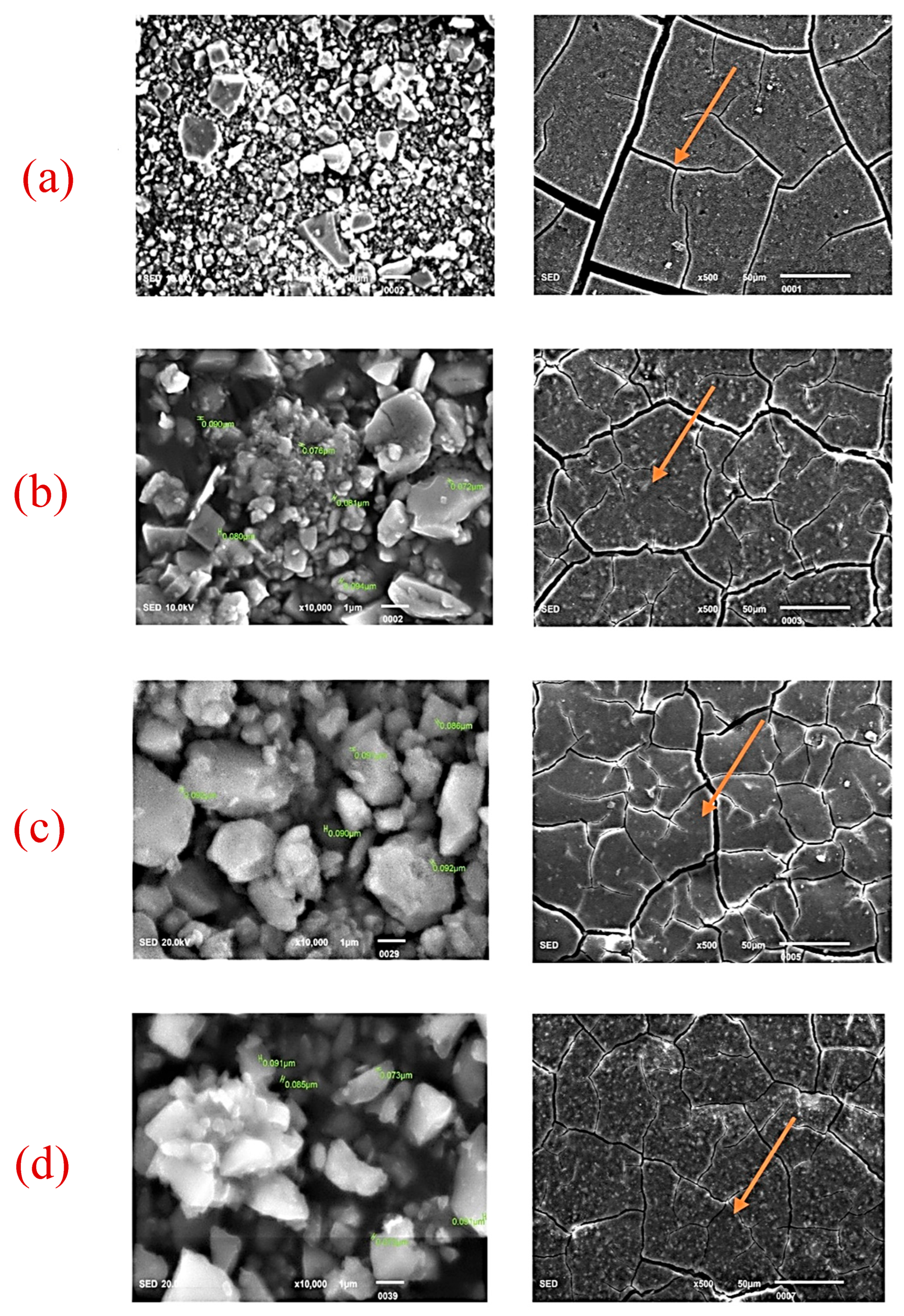
| Nano-ZrO2 Particles Present in GIC | 0 wt% | 2 wt% | 5 wt% | 7 wt% |
|---|---|---|---|---|
| Mean (SD) Flexural Strength (MPa) | ||||
| GIC 1 | 6.00 (0.4) c | 8.00 (0.21) bc | 10.38 (1.2) b | 14.00 (1.8) a |
| GIC 2 | 9.3 (1.3) a | 5.00 (0.2) c | 7.00 (0.6) bc | 8.2 (0.9) ab |
| Mean (SD) Flexural Modulus (GPa) | ||||
| GIC 1 | 8.41 (3.2) a | 8.00 (2.0) a | 9.41 (5.0) a | 12.00 (5.1) a |
| GIC 2 | 9.0 (4.0) a | 7.0 (3) a | 3.0 (2.0) a | 10.00 (2.0) a |
| Mean (SD) Surface Hardness (VHN) | ||||
| GIC 1 | 17.00 (0.3) c | 53.0 (0.3) a | 55.24 (1.6) a | 46.00 (0.2) b |
| GIC 2 | 32.00 (5.36) c | 62.41 (2.39) a | 35.00 (1.3) c | 53.00 (0.2) b |
| Nano-ZrO2 Particles Present in GIC | 0 wt% | 2 wt% | 5 wt% | 7 wt% |
|---|---|---|---|---|
| Mean (SD) Water Sorption (μg/mm3) | ||||
| GIC 1 | 179.4 (56.7) a | 90.56 (11.88) ab | 122.5 (53.3) ab | 61.05 (2.60) b |
| GIC 2 | 318 (178) a | 119.9 (30.3) a | 157.4 (37.4) a | 130 (17.8) a |
| Mean (SD) Water solubility (μg/mm3) | ||||
| GIC 1 | −2.52 (14.47) a | 15.2 (22.8) a | 22.22 (6.38) a | 5.22 (5.34) a |
| GIC 2 | 27.3 (24.1) a | 15.91 (9.16) a | 5.66 (5.42) a | 35.1 (26.0) a |
| Glass Ionomer Cement | Powder | Liquid | PD/LIQ Ratio | Setting Time | Manufacturer |
|---|---|---|---|---|---|
| GIC 1 | Flouroaluminosilicate glass and pigments | Polyacrylic acid, Polybasic Carboxylic acid | 2.7 g:1 g | 2 min 20 s | GIC CO Tokoyo Japan GC GOLD LABEL 2 |
| GIC 2 | Flouroaluminosilicate glass and pigments | Acrylic acid Tricarboxylic Acid Copolymer solution and tartaric acid | 2.7 g:1 g | 2 min 30 s | SHOFU INC Kyoto Japan FX Ultra shofu |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, F.; Moin, S.F.; Kumar, N.; Asghar, M.A.; Mahmood, S.J.; Palma, P.J. The Impact of Zirconium Oxide Nanoparticles on the Mechanical and Physical Properties of Glass Ionomer Dental Materials. Int. J. Mol. Sci. 2025, 26, 5382. https://doi.org/10.3390/ijms26115382
Amin F, Moin SF, Kumar N, Asghar MA, Mahmood SJ, Palma PJ. The Impact of Zirconium Oxide Nanoparticles on the Mechanical and Physical Properties of Glass Ionomer Dental Materials. International Journal of Molecular Sciences. 2025; 26(11):5382. https://doi.org/10.3390/ijms26115382
Chicago/Turabian StyleAmin, Faiza, Syed Faraz Moin, Naresh Kumar, Muhammad Asif Asghar, Syed Junaid Mahmood, and Paulo J. Palma. 2025. "The Impact of Zirconium Oxide Nanoparticles on the Mechanical and Physical Properties of Glass Ionomer Dental Materials" International Journal of Molecular Sciences 26, no. 11: 5382. https://doi.org/10.3390/ijms26115382
APA StyleAmin, F., Moin, S. F., Kumar, N., Asghar, M. A., Mahmood, S. J., & Palma, P. J. (2025). The Impact of Zirconium Oxide Nanoparticles on the Mechanical and Physical Properties of Glass Ionomer Dental Materials. International Journal of Molecular Sciences, 26(11), 5382. https://doi.org/10.3390/ijms26115382









