Pelvic Pain Symptoms and Inflammation Among Adolescents and Adults with and Without Endometriosis
Abstract
1. Introduction
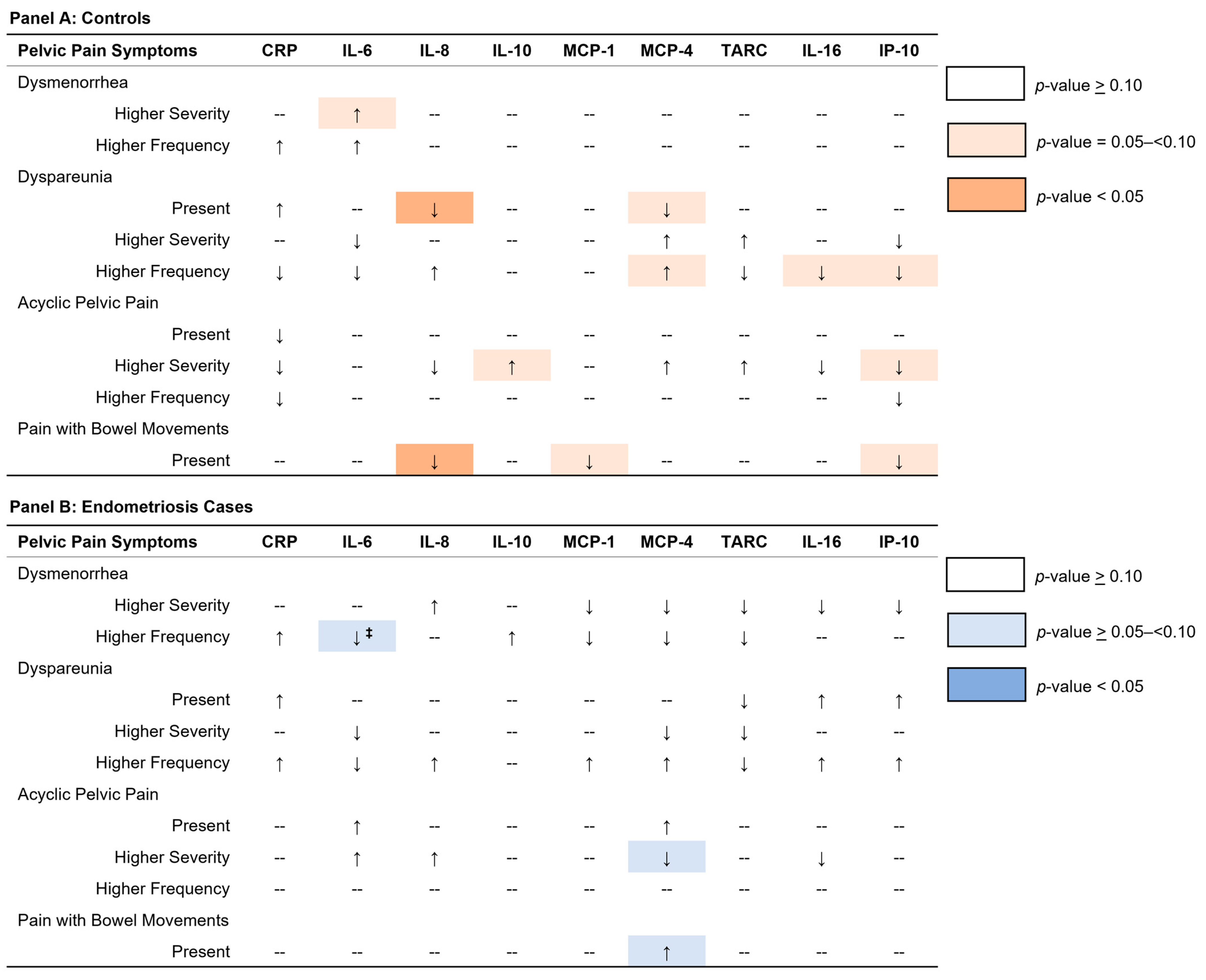
2. Results
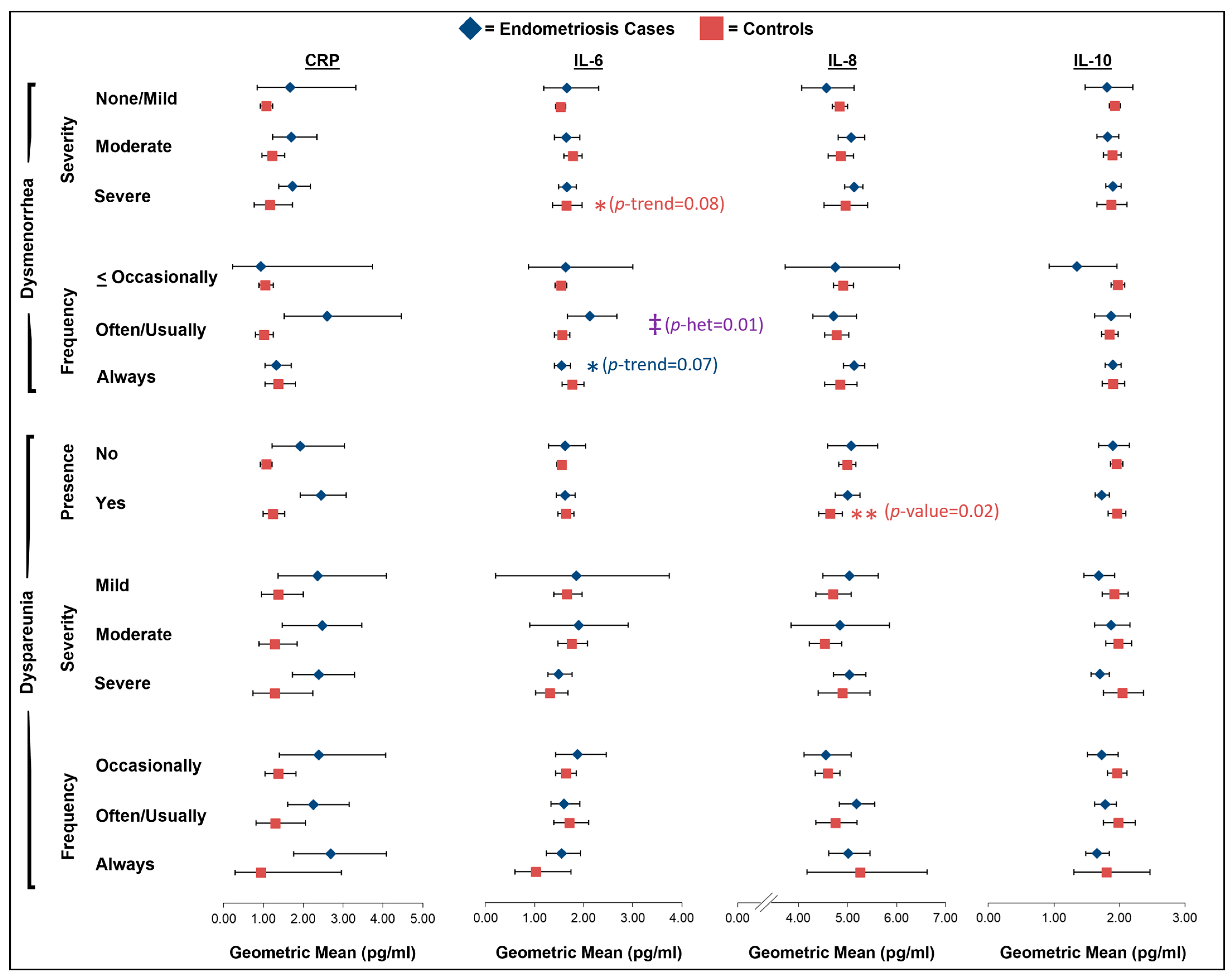
2.1. Dysmenorrhea
2.2. Dyspareunia
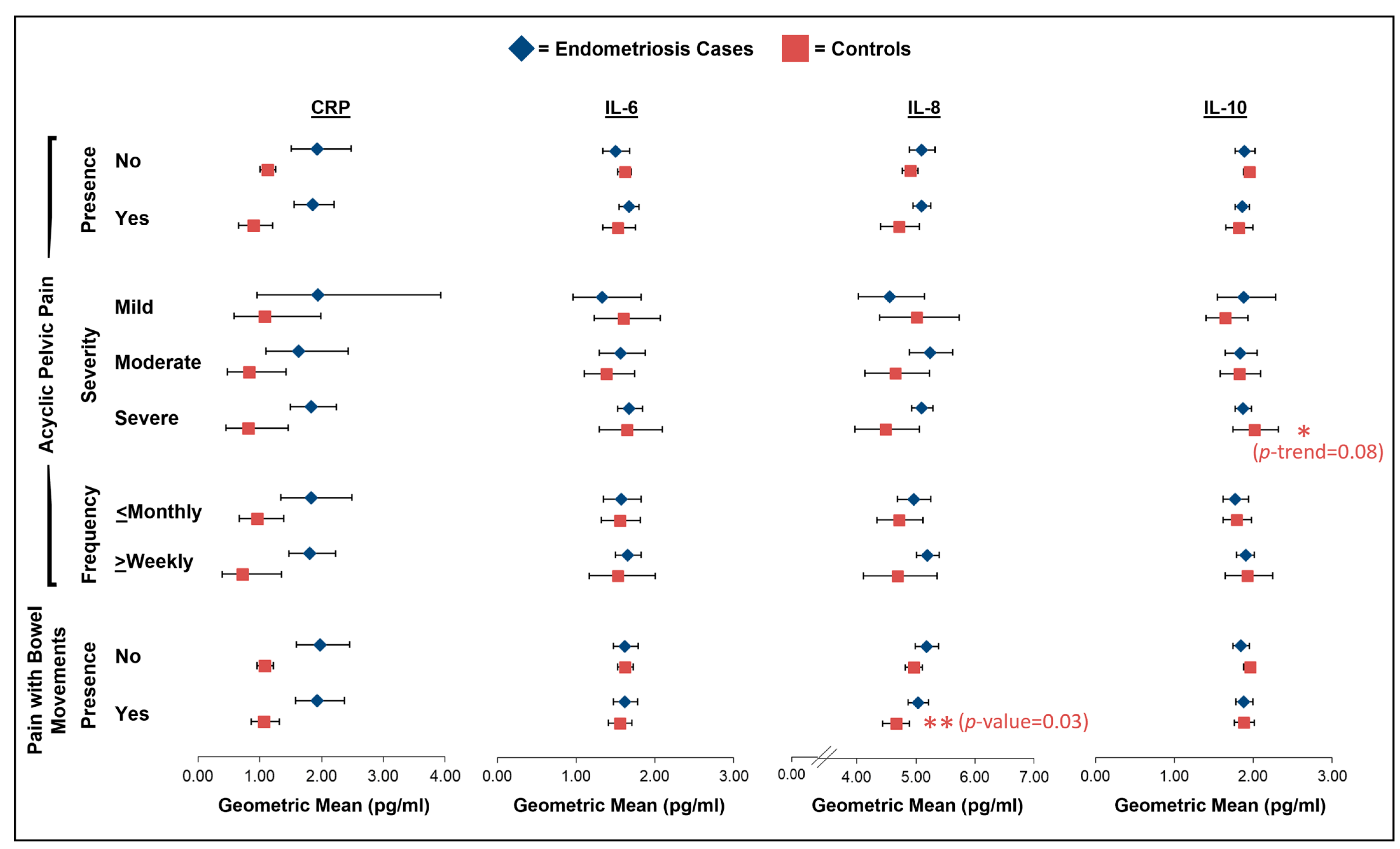
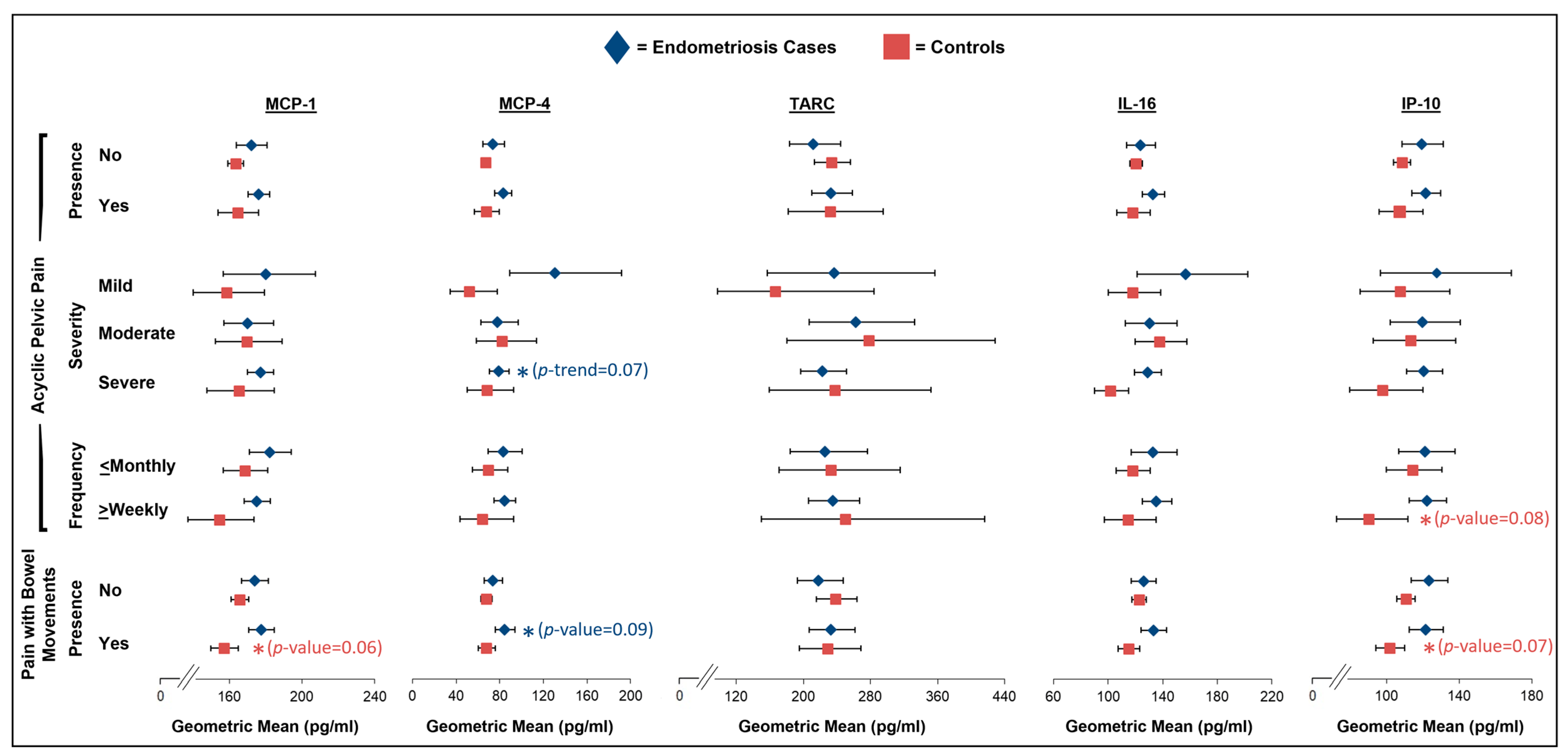
2.3. Acyclic Pelvic Pain
2.4. Pain with Bowel Movements
3. Discussion
4. Materials and Methods
4.1. Pain Symptom Assessment
4.2. Blood Collection
4.3. Cytokine and Chemokine Measurement
4.4. Assay Quality Control
4.5. Recalibration of Inflammatory Marker Values
4.6. Covariates
4.7. Analytic Study Population
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A2A | Adolescence to Adulthood cohort |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| CRP | C-reactive protein |
| MCP | Monocyte chemotactic protein |
| TARC | Thymus and activation-regulated chemokine |
| IP | Interferon gamma-induced protein |
| GM | Geometric mean |
| CI | Confidence intervals |
| rASRM | Revised American Society for Reproductive Medicine |
| IQR | Interquartile range |
| BMI | Body mass index |
| OR | Odds ratio |
| BWH | Brigham and Women’s Hospital |
| BCH | Boston Children’s Hospital |
| WERF | World Endometriosis Research Foundation |
| EPHect | Endometriosis Phenome and Biobanking Harmonization Project |
| NRS | Numeric rating scale |
| QC | Quality control |
| CV | Coefficient of variation |
References
- Armour, M.; Parry, K.; Manohar, N.; Holmes, K.; Ferfolja, T.; Curry, C.; MacMillan, F.; Smith, C.A. The prevalence and academic impact of dysmenorrhea in 21,573 young women: A systematic review and meta-analysis. J Women’s Health 2019, 28, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Mathias, S.D.; Kuppermann, M.; Liberman, R.F.; Lipschutz, R.C.; Steege, J.F. Chronic pelvic pain: Prevalence, health-related quality of life, and economic correlates. Obstet. Gynecol. 1996, 87, 321–327. [Google Scholar] [CrossRef]
- Lamvu, G.; Carrillo, J.; Ouyang, C.; Rapkin, A. Chronic Pelvic Pain in Women: A Review. JAMA 2021, 325, 2381–2391. [Google Scholar] [CrossRef]
- Ghiasi, M.; Chang, C.; Shafrir, A.L.; Vitonis, A.F.; Sasamoto, N.; Vazquez, A.I.; DiVasta, A.D.; Upson, K.; Sieberg, C.B.; Terry, K.L.; et al. Subgroups of pelvic pain are differentially associated with endometriosis and inflammatory comorbidities: A latent class analysis. Pain 2024, 165, 2119–2129. [Google Scholar] [CrossRef]
- Stanford, E.J.; Koziol, J.; Feng, A. The prevalence of interstitial cystitis, endometriosis, adhesions, and vulvar pain in women with chronic pelvic pain. J. Minim. Invasive Gynecol. 2005, 12, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Mowers, E.L.; Lim, C.S.; Skinner, B.; Mahnert, N.; Kamdar, N.; Morgan, D.M.; As-Sanie, S. Prevalence of endometriosis during abdominal or laparoscopic hysterectomy for chronic pelvic pain. Obstet. Gynecol. 2016, 127, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, M.; Kulkarni, M.T.; Missmer, S.A. Is Endometriosis More Common and More Severe Than It Was 30 Years Ago? J. Minim. Invasive Gynecol. 2020, 27, 452–461. [Google Scholar] [CrossRef]
- Laufer, M.R.; Goitein, L.; Bush, M.; Cramer, D.W.; Emans, S.J. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding to conventional therapy. J. Pediatr. Adolesc. Gynecol. 1997, 10, 199–202. [Google Scholar] [CrossRef]
- Janssen, E.B.; Rijkers, A.C.M.; Hoppenbrouwers, K.; Meuleman, C.; D’Hooghe, T.M. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: A systematic review. Hum. Reprod. Update 2013, 19, 570–582. [Google Scholar] [CrossRef]
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Lebovic, D.I.; Mueller, M.D.; Taylor, R.N. Immunobiology of endometriosis. Fertil. Steril. 2001, 75, 1–10. [Google Scholar] [CrossRef]
- Kyama, C.M.; Debrock, S.; Mwenda, J.M.; D’Hooghe, T.M. Potential involvement of the immune system in the development of endometriosis. Reprod. Biol. Endocrinol. RBE 2003, 1, 123. [Google Scholar] [CrossRef]
- Herington, J.L.; Bruner-Tran, K.L.; Lucas, J.A.; Osteen, K.G. Immune interactions in endometriosis. Expert Rev. Clin. Immunol. 2011, 7, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef]
- Harada, T.; Iwabe, T.; Terakawa, N. Role of cytokines in endometriosis. Fertil. Steril. 2001, 76, 1–10. [Google Scholar] [CrossRef]
- Greaves, E.; Cousins, F.L.; Murray, A.; Esnal-Zufiaurre, A.; Fassbender, A.; Horne, A.W.; Saunders, P.T.K. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am. J. Pathol. 2014, 184, 1930–1939. [Google Scholar] [CrossRef]
- Bacci, M.; Capobianco, A.; Monno, A.; Cottone, L.; Di Puppo, F.; Camisa, B.; Mariani, M.; Brignole, C.; Ponzoni, M.; Ferrari, S.; et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am. J. Pathol. 2009, 175, 547–556. [Google Scholar] [CrossRef]
- Coxon, L.; Horne, A.W.; Vincent, K. Pathophysiology of endometriosis-associated pain: A review of pelvic and central nervous system mechanisms. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 53–67. [Google Scholar] [CrossRef]
- Greaves, E.; Temp, J.; Esnal-Zufiaurre, A.; Mechsner, S.; Horne, A.W.; Saunders, P.T.K. Estradiol is a critical mediator of macrophage-nerve cross talk in peritoneal endometriosis. Am. J. Pathol. 2015, 185, 2286–2297. [Google Scholar] [CrossRef] [PubMed]
- Schrepf, A.; Bradley, C.S.; O’Donnell, M.; Luo, Y.; Harte, S.E.; Kreder, K.; Lutgendorf, S. Toll-like Receptor 4 and comorbid pain in Interstitial Cystitis/Bladder Pain Syndrome: A Multidisciplinary Approach to the Study of Chronic Pelvic Pain research network study. Brain Behav. Immun. 2015, 49, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Schrepf, A.; Kaplan, C.; Harris, R.E.; Williams, D.A.; Clauw, D.J.; As-Sani, S.; Till, S.; Clemens, J.Q.; Rodriguez, L.V.; Van Bokhoven, A.; et al. Stimulated whole-blood cytokine/chemokine responses are associated with interstitial cystitis/bladder pain syndrome phenotypes and features of nociplastic pain: A multidisciplinary approach to the study of chronic pelvic pain research network study. Pain 2023, 164, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, H.; Hernandes, C.; Piccinato, C.A.; Podgaec, S. Interleukin in endometriosis-associated infertility-pelvic pain: Systematic review and meta-analysis. Reproduction 2019, 158, 1–12. [Google Scholar] [CrossRef]
- Scholl, B.; Bersinger, N.A.; Kuhn, A.; Mueller, M.D. Correlation between symptoms of pain and peritoneal fluid inflammatory cytokine concentrations in endometriosis. Gynecol. Endocrinol. 2009, 25, 701–706. [Google Scholar] [CrossRef]
- Riley, C.F.; Moen, M.H.; Videm, V. Inflammatory markers in endometriosis: Reduced peritoneal neutrophil response in minimal endometriosis. Acta Obstet. Gynecol. Scand. 2007, 86, 877–881. [Google Scholar] [CrossRef]
- Overton, C.; Fernandez-Shaw, S.; Hicks, B.; Barlow, D.; Starkey, P. Peritoneal fluid cytokines and the relationship with endometriosis and pain. Hum. Reprod. 1996, 11, 380–386. [Google Scholar] [CrossRef]
- Akoum, A.; Al-Akoum, M.; Lemay, A.; Maheux, R.; Leboeuf, M. Imbalance in the peritoneal levels of interleukin 1 and its decoy inhibitory receptor type II in endometriosis women with infertility and pelvic pain. Fertil. Steril. 2008, 89, 1618–1624. [Google Scholar] [CrossRef]
- Michaud, N.; Al-Akoum, M.; Gagnon, G.; Girard, K.; Blanchet, P.; Rousseau, J.A.; Akoum, A. Decreased concentrations of soluble interleukin-1 receptor accessory protein levels in the peritoneal fluid of women with endometriosis. J. Reprod. Immunol. 2011, 92, 68–73. [Google Scholar] [CrossRef]
- Santulli, P.; Borghese, B.; Chouzenoux, S.; Streuli, I.; Borderie, D.; De Ziegler, D.; Weill, B.; Chapron, C.; Batteux, F. Interleukin-19 and interleukin-22 serum levels are decreased in patients with ovarian endometrioma. Fertil. Steril. 2013, 99, 219–226.e212. [Google Scholar] [CrossRef]
- Manero, M.G.; Alcazar, J.L. Interleukin-8 serum levels do not correlate with pelvic pain in patients with ovarian endometriomas. Fertil. Steril. 2010, 94, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Rahmawati, N.Y.; Ahsan, F.; Santoso, B.; Mufid, A.F.; Sa’adi, A.; Dwiningsih, S.R.; Tunjungseto, A.; Widyanugraha, M.Y.A. IL-8 and IL-12p70 are associated with pelvic pain among infertile women with endometriosis. Pain Med. 2023, 24, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Babah, O.A.; Ojewunmi, O.O.; Onwuamah, C.K.; Udenze, I.C.A.; Osuntoki, A.A.; Afolabi, B.B. Serum concentrations of IL-16 and its genetic polymorphism rs4778889 affect the susceptibility and severity of endometriosis in Nigerian women. BMC Women’s Health 2023, 23, 253. [Google Scholar] [CrossRef] [PubMed]
- Bourlev, V.; Moberg, C.; Ilyasova, N.; Davey, E.; Kunovac Kallak, T.; Olovsson, M. Vasoactive intestinal peptide is upregulated in women with endometriosis and chronic pelvic pain. Am. J. Reprod. Immunol. 2018, 80, e12857. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Hediger, M.L.; Peterson, C.M.; Croughan, M.; Sundaram, R.; Stanford, J.; Chen, Z.; Fujimoto, V.Y.; Varner, M.W.; Trumble, A.; et al. Incidence of endometriosis by study population and diagnostic method: The ENDO study. Fertil. Steril. 2011, 96, 360–365. [Google Scholar] [CrossRef]
- Bickel, M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 1993, 64, 456–460. [Google Scholar]
- Baggiolini, M.; Walz, A.; Kunkel, S.L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 1989, 84, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Velasco, I.; Acién, P.; Campos, A.; Acién, M.I.; Ruiz-Maciá, E. Interleukin-6 and other soluble factors in peritoneal fluid and endometriomas and their relation to pain and aromatase expression. J. Reprod. Immunol. 2010, 84, 199–205. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Gracia-Zepeda, E.A.; Combadiere, C.; Rothenberg, M.E.; Sarafi, M.N.; Lavigne, F.; Hamid, Q.; Murphy, P.M.; Luster, A.D. Human monocyte chemoattractant protein (MCP)-4 is a novel CC chemokine with activities on monocytes, eosinophils, and basophils induced in allergic and nonallergic inflammation that signals through the CC chemokine receptors (CCR)-2 and -3. J. Immunol. 1996, 157, 5613–5626. [Google Scholar] [CrossRef]
- Shin, S.; Chung, Y.-J.; Moon, S.W.; Choi, E.J.; Kim, M.-R.; Chung, Y.-J.; Lee, S.H. Single-cell profiling indentifies distinct hormonal, immunologic, and inflammatory signatures of endometriosis-constituting cells. J. Pathol. 2023, 261, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Cruikshank, W.W.; Kornfeld, H.; Center, D.M. Interleukin-16. J. Leukoc. Biol. 2000, 67, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011, 22, 121–130. [Google Scholar] [CrossRef]
- Koga, K.; Osuga, Y.; Yoshino, O.; Hirota, Y.; Yano, T.; Tsutsumi, O.; Taketani, Y. Elevated interleukin-16 levels in the peritoneal fluid of women with endometriosis may be a mechanism for inflammatory reactions associated with endometriosis. Fertil. Steril. 2005, 83, 878–882. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, J.; Deng, L.; Chen, Z.; Chen, L.I. Peritoneal fluid and serum concentration of interleukin-16 in women with endometriosis. Acta Obstet. Gynecol. Scand. 2005, 84, 297–298. [Google Scholar] [CrossRef]
- Gan, X.L.; Lin, Y.H.; Zhang, Y.; Yu, T.H.; Hu, L.N. Association of an interleukin-16 gene polymorphism with the risk and pain phenotype of endometriosis. DNA Cell Biol. 2010, 29, 663–667. [Google Scholar] [CrossRef]
- Sawyer, S.M.; Azzopardi, P.S.; Wickremarathne, D.; Patton, G.C. The age of adolescence. Lancet Child Adolesc. Helath 2018, 2, 223–228. [Google Scholar] [CrossRef]
- Greene, R.; Stratton, P.; Cleary, S.D.; Ballweg, M.L.; Sinaii, N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil. Steril. 2009, 91, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; D’Hooghe, T.; de Cicco Nardone, F.; De Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T. Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2011, 96, 366–373.e8. [Google Scholar] [CrossRef]
- Rahmioglu, N.; Fassbender, A.; Vitonis, A.F.; Tworoger, S.S.; Hummelshoj, L.; D’Hooghe, T.M.; Adamson, G.D.; Giudice, L.C.; Becker, C.M.; Zondervan, K.T.; et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: III. Fluid biospecimen collection, processing, and storage in endometriosis research. Fertil. Steril. 2014, 102, 1233–1243. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Cardon, L.R.; Kennedy, S.H. What makes a good case-control study? Design issues for complex traits such as endometriosis. Hum. Reprod. 2002, 17, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- DiVasta, A.D.; Vitonis, A.F.; Laufer, M.R.; Missmer, S.A. Spectrum of symptoms in women diagnosed with endometriosis during adolescence vs adulthood. Am. J. Obstet. Gynecol. 2018, 218, e321–e324. [Google Scholar] [CrossRef] [PubMed]
- Vitonis, A.F.; Vincent, K.; Rahmioglu, N.; Fassbender, A.; Buck Louis, G.M.; Hummelshoj, L.; Giudice, L.C.; Stratton, P.; Adamson, D.; Becker, C.M.; et al. World Endometriosis Research Foundation Endometriosis Phenome and biobanking harmonization project: II. Clinical and covariate phenotype data collection in endometriosis research. Fertil. Steril. 2014, 102, 1244–1253. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Eliassen, A.H.; Hendrickson, S.J.; Brinton, L.A.; Buring, J.E.; Campos, H.; Dai, Q.; Dorgan, J.F.; Franke, A.A.; Gao, Y.-T.; Goodman, M.T.; et al. Circulating Carotenoids and Risk of Breast Cancer: Pooled Analysis of Eight Prospective Studies. J. Natl. Cancer Inst. 2012, 104, 1905–1916. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Laufer, M.R.; Stratton, P.; Hummelshoj, L.; Missmer, S.A.; Zondervan, K.T.; Adamson, G.D. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: I. Surgical phenotype data collection in endometriosis research. Fertil. Steril. 2014, 102, 1213–1222. [Google Scholar] [CrossRef]
- Rosner, B. Percentage points for a generalized ESD many-outlier procedure. Technometrics 1983, 25, 165–172. [Google Scholar] [CrossRef]


| Characteristics | Endometriosis (n = 389) | No Endometriosis (n = 505) |
|---|---|---|
| Age at blood draw, years | ||
| Median (IQR) | 17 (16–21) | 24 (22–29) |
| Race, n (%) | ||
| White | 353 (91%) | 366 (72%) |
| Black | 9 (2%) | 33 (7%) |
| Other/unknown 1 | 27 (7%) | 106 (21%) |
| Body mass index (kg/m2), n (%) 2,3 | ||
| Underweight | 4 (1%) | 18 (4%) |
| Normal weight | 236 (61%) | 328 (65%) |
| Overweight | 110 (28%) | 106 (21%) |
| Obese | 39 (10%) | 52 (10%) |
| Smoking status, n (%) 2 | ||
| Never | 346 (95%) | 456 (92%) |
| Ever | 20 (5%) | 37 (8%) |
| Age at menarche, years | ||
| Median (IQR) | 12 (11–13) | 12 (11–13) |
| Menstrual cycles in the last 3 months, n (%) 4 | ||
| No | 135 (35%) | 78 (15%) |
| Yes | 250 (65%) | 427 (85%) |
| Menstrual cycle phase at blood draw, n (%) 2,5 | ||
| Follicular | 17 (49%) | 71 (42%) |
| Peri-ovulation | 6 (17%) | 24 (14%) |
| Luteal | 12 (34%) | 73 (43%) |
| Hormonal medication use in the last 30 days prior to the blood draw, n (%) 2 | ||
| Not using hormones | 48 (12%) | 219 (44%) |
| Using hormones | 340 (88%) | 278 (56%) |
| Pain medication use in the last 48 h prior to the blood draw, n (%) 2 | ||
| No | 269 (77%) | 401 (81%) |
| Yes | 81 (23%) | 96 (19%) |
| Steroid medication use in the last 48 h prior to the blood draw, n (%) 2,6 | ||
| No | 329 (94%) | 468 (94%) |
| Yes | 21 (6%) | 29 (6%) |
| rASRM stage, n (%) 2 | ||
| Stage I/II | 360 (95%) | -- |
| Stage III/IV | 18 (5%) | -- |
| Endometriosis subtype, n (%) 2 | ||
| Superficial peritoneal lesions only | 364 (95%) | -- |
| Endometrioma | 8 (2%) | -- |
| Deep infiltrating | 6 (2%) | -- |
| Endometrioma and Deep | 1 (0%) | -- |
| Age at first endometriosis symptoms, years | ||
| Median (IQR) | 13 (12–15) | -- |
| Time between first symptoms and surgical diagnosis, years | ||
| Median (IQR) | 2 (1–5) | -- |
| Number of doctors seen for symptoms before diagnosis | ||
| Median (IQR) | 2 (1–4) | -- |
| Inflammation Marker | Endometriosis | No Endometriosis | |||
|---|---|---|---|---|---|
| N | Geometric Mean (95% CI) | N | Geometric Mean (95% CI) | p-Value 2 | |
| CRP | 368 | 1.54 (1.34, 1.78) | 486 | 1.26 (1.11, 1.42) | 0.05 |
| IL-6 | 384 | 1.59 (1.49, 1.70) | 504 | 1.63 (1.55, 1.73) | 0.55 |
| IL-8 | 389 | 5.10 (4.96, 5.26) | 505 | 4.87 (4.75, 5.00) | 0.03 |
| IL-10 | 382 | 1.82 (1.75, 1.90) | 501 | 1.98 (1.91, 2.05) | 0.005 |
| MCP-1 | 389 | 173.7 (168.5, 179.0) | 504 | 165.3 (161.1, 169.7) | 0.03 |
| MCP-4 | 307 | 77.3 (71.7, 83.4) | 295 | 70.1 (64.9, 75.7) | 0.10 |
| TARC | 299 | 230.2 (210.2, 252.2) | 288 | 231.0 (210.5, 253.5) | 0.96 |
| IL-16 | 303 | 127.9 (122.0, 134.1) | 294 | 122.7 (117.0, 128.98) | 0.27 |
| IP-10 | 383 | 119.3 (113.0, 125.8) | 505 | 109.9 (105.0, 115.1) | 0.04 |
| Luminex Assay Only | Ella Immunoassay and Luminex Recalibration 1 | Ella Immunoassay Only | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MCP-4 | TARC | IL-16 | IL-8 | MPC-1 | IP-10 | CRP | IL-1β | IL-6 | IL-10 | |
| Inflammation marker | ca/co | ca/co | ca/co | ca/co | ca/co | ca/co | ca/co | ca/co | ca/co | ca/co |
| Blood samples sent to laboratory (cases/controls) | 335/303 | 335/303 | 335/303 | 431/526 | 431/526 | 431/526 | 431/526 | 431/526 | 431/526 | 431/526 |
| Exclusions | ||||||||||
| Non-detectable levels | -- | 8/7 * | -- | * | -- | * | -- | ** | * | * |
| Outliers 2 | -- | 1/0 | 4/1 | -- | 0/1 | 6/0 | -- | -- | 5/1 | 8/5 |
| Intra-assay batch CV > 30% | -- | -- | -- | -- | -- | -- | 23/19 | -- | -- | -- |
| Baseline questionnaire completed 90 days before/after blood draw | 22/4 | 21/4 | 22/4 | 33/10 | 33/10 | 33/10 | 31/10 | 33/10 | 33/10 | 33/9 |
| Using immune-modulating drugs at time of blood draw | 6/4 | 6/4 | 6/4 | 9/11 | 9/11 | 9/11 | 9/11 | 9/11 | 9/11 | 8/11 |
| Final analytic sample | 307/295 | 299/288 | 303/294 | 389/505 | 389/504 | 383/505 | 368/486 | 389/505 | 384/504 | 382/501 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafrir, A.L.; Laliberte, A.; Wallace, B.; Vitonis, A.F.; Sieberg, C.B.; Ghiasi, M.; Magpantay, L.I.; Epeldegui, M.; Schrepf, A.; As-Sanie, S.; et al. Pelvic Pain Symptoms and Inflammation Among Adolescents and Adults with and Without Endometriosis. Int. J. Mol. Sci. 2025, 26, 5377. https://doi.org/10.3390/ijms26115377
Shafrir AL, Laliberte A, Wallace B, Vitonis AF, Sieberg CB, Ghiasi M, Magpantay LI, Epeldegui M, Schrepf A, As-Sanie S, et al. Pelvic Pain Symptoms and Inflammation Among Adolescents and Adults with and Without Endometriosis. International Journal of Molecular Sciences. 2025; 26(11):5377. https://doi.org/10.3390/ijms26115377
Chicago/Turabian StyleShafrir, Amy L., Ashley Laliberte, Britani Wallace, Allison F. Vitonis, Christine B. Sieberg, Marzieh Ghiasi, Larry I. Magpantay, Marta Epeldegui, Andrew Schrepf, Sawsan As-Sanie, and et al. 2025. "Pelvic Pain Symptoms and Inflammation Among Adolescents and Adults with and Without Endometriosis" International Journal of Molecular Sciences 26, no. 11: 5377. https://doi.org/10.3390/ijms26115377
APA StyleShafrir, A. L., Laliberte, A., Wallace, B., Vitonis, A. F., Sieberg, C. B., Ghiasi, M., Magpantay, L. I., Epeldegui, M., Schrepf, A., As-Sanie, S., Terry, K. L., & Missmer, S. A. (2025). Pelvic Pain Symptoms and Inflammation Among Adolescents and Adults with and Without Endometriosis. International Journal of Molecular Sciences, 26(11), 5377. https://doi.org/10.3390/ijms26115377







