Abstract
Glass ionomer cements (GICs) have been clinically attractive dental restorative materials for many years and are widely used as luting, lining, and restorative materials. However, these materials still have limitations in terms of weak physio-mechanical properties. The aim of the study was to evaluate the effect of zirconium oxide nanoparticles (nano-ZrO2 particles) on the physical and mechanical properties of two commercially available GICs. Four groups were prepared for each material: the control group (without nanoparticles) and three groups modified by the incorporation of nanoparticles at 2, 5, and 7 weight% (wt%). Firstly, the morphology and size of the nanoparticles were evaluated via scanning electron microscopy (SEM) and X-ray diffraction (XRD). Secondly, flexural strength, flexural modulus, Vickers hardness, water sorption, and solubility were evaluated. The main effect plots revealed that the addition of nano-ZrO2 particles enhances flexural strength, flexural modulus, and water sorption of GICs at a 7 wt% concentration and Vickers hardness at a 2 wt% concentration. The SEM analysis clearly shows that the cracks became narrower with the addition of nano-ZrO2 particles, whereas these cracks were completely closed at 7% nano-ZrO2 particles. The findings of the study appear promising, and it is anticipated that the optimization of nano-ZrO2 particles may aid the development of improved materials for load-bearing restorations.
1. Introduction
Dental caries is the most prevalent chronic disease worldwide. Pain, apical periodontitis, abscess, and infection are some serious consequences of untreated dental caries. The commonly used restorative materials for caries management include dental amalgam, resin composite, and glass ionomer cements (GICs). All these materials have their own strengths and weaknesses [1]. In 1972, Wilson and Kent introduced GIC materials, which are also known as polyalkenoates [2]. They are considered smart biomaterials due to certain distinctive properties like biocompatibility, chemical bonding to teeth, dimensional stability, excellent marginal integrity, fluoride release and rechargeability, low coefficient of thermal expansion, resistance to microleakage, and less setting shrinkage [3,4,5]. However, brittleness and poor mechanical properties are the major drawbacks of the GICs. Due to their inferior mechanical properties, they cannot be used in high-stress-bearing areas. To improve the performance of these materials, several attempts have been made by researchers to utilize their beneficial effects. In 1983, Simmons made the earliest attempt by adding silver powder into a GIC powder component [6]. Cho et al. [7] incorporated resins into GICs and compared them with conventional GIC materials. They observed a low sensitivity toward moisture. The fracture toughness and microhardness of GICs were increased after the addition of hydroxyapatite by Yap et al. [8] and Lucas et al. [9]. Nevertheless, due to the inherent and unresolved conflict between strength and toughness in glass ionomer cements, many of these approaches failed to perform adequately in the oral cavity [10].
For many decades, in the field of health science, nanotechnology has become one of the most active research areas, and many revolutionary developments have been made [11]. The importance of these materials gained interest by researchers when they realized that the size of the particles was one of the important factors that dictate the properties of the materials [11]. Recently, many experimental studies have been carried out after incorporating a variety of nanoparticles (NPs) in GICs, such as titanium dioxide nanotubes [12] and their NPs, [13,14] aluminum [15], and nano-ZrO2 particles [16]. To increase strength, Gjorgievska et al. [17] incorporated TiO2, Al2O3, and ZrO2 NPs into conventional GICs [18]. Surface porosities were reduced by adding these NPs when evaluated via scanning electron microscopy [18].
Zirconia is a natural white color with three crystal forms: (1) a monoclinic phase (m-ZrO2) stable at room temperature, (2) tetragonal (t-ZrO2) phase (1100–2370 °C), and (3) cubic (c-ZrO2) phase (above 2370 °C) [19]. Moreover, zirconia exhibits antibacterial, antifungal, antioxidant, and anticancer properties [4,20]. Due to these properties, it has been widely used in dental applications like dental implants and the construction of crowns, bridges, and inserts [4]. Alobiedy et al. [4] reinforced conventional GICs with nano-ZrO2 particles and found that the mechanical strength was increased, except for the wear rate. In most of the literature, researchers utilize commercially available nano-ZrO2 particles and incorporate them into restorative materials. To the best of our knowledge, no study has yet reported the synthesis of ZrO2 NPs in the laboratory and investigated the effect of nano-ZrO2 at three different concentrations. Therefore, the present study aimed to synthesize and characterize the ZrO2 NPs and incorporate them into conventional GICs to evaluate their physio-mechanical properties, such as flexural strength, flexural modulus, microhardness, and water sorption and solubility with and without nano-ZrO2 particles at three different concentrations after 24 h of immersion in distilled water.
2. Results
2.1. Characterization of ZrO2 Nanoparticles
X-Ray Diffraction (XRD)
For particle size, the following equation was used, which is known as the Debye–Scherrer Formula.
where k is a constant and is assumed to be 0.9 for spherical particles; λ is wavelength of X-ray used—in this case, the value is 0.15406 nm; β is the FWHM (the full width of the selected peak at half maxima); and θ is the angle of the peak (Figure 1). The total number of peaks observed = 08, whereas the average particle size was found to be 13.7 nm. The XRD displayed a very strong peak at ~32° that corresponded to the 011 plane of ZrO2. Next, a peak at ~34° was due to the 002 plane of ZrO2, showing the monoclinic form of zirconia. Another smaller peak at ~49 corresponds to ZrO2 (020). Peaks through ~59–60° are those of 121 planes of ZrO2, indicating the tetragonal form of zirconium oxide nanoparticles. These findings are similar to the characterization studies published previously [21,22,23].
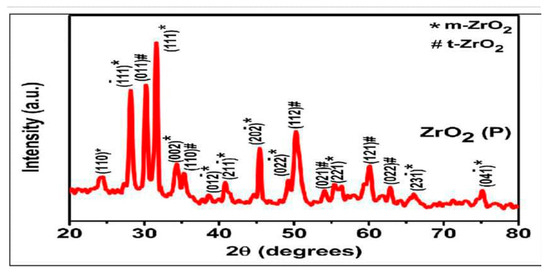
Figure 1.
The graph demonstrates XRD pattern of nano-ZrO2 particles with monoclinic and tetragonal phases.
The SEM images of ZrO2 and after the addition in GIC-1 and GIC-2 powder at different concentrations are given in Figure 2a–d and Figure 3a–d. The two-way ANOVA revealed that the flexural strength was affected by the material type (p = 0.018), and ZrO2 concentration (p = 0.007). The flexural modulus data were not affected by the material type (p = 0.182) and ZrO2 concentration (p = 0.371). The material type did not cause any significant influence on the surface hardness data (p = 0.419). However, the same characteristic was substantially influenced by the concentration of ZrO2 (p = 0.000). The water sorption showed a statistically significant difference regarding the ZrO2 concentration (p = 0.004), whereas it was not affected by the material type (p = 0.027). However, water solubility did not significantly affect both the material type (p = 0.190) and ZrO2 concentration (p = 0.948). Table 1 represents a positive impact of nano-ZrO2 particles on the mean flexural strength and mean Vickers microhardness GIC 1, whereas the mean flexural modulus was not affected by the incorporation of nano-ZrO2 particles. The means of water sorption and solubility after the incorporation of nano-ZrO2 particles in GICs are given in Table 2. Water sorption was found to be reduced at a 7% concentration in GIC 1, whereas no impact was observed at any concentration for water solubility. The effect of the material type and nano-ZrO2 particles on flexural strength, flexural modulus, and surface hardness data as a main effect graph is shown in Figure 4, whereas the main effects graphs of the water sorption and solubility are depicted in Figure 5.
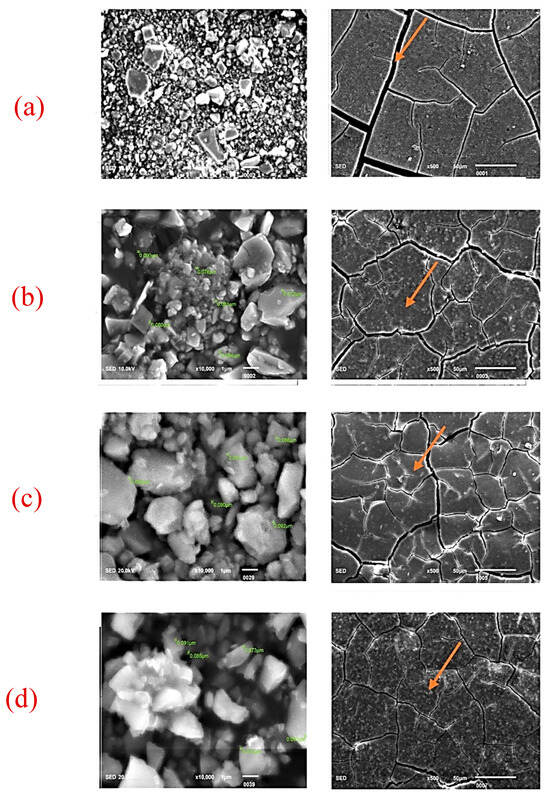
Figure 2.
SEM images for GC GOLD LABEL 2 GIC (powder and disk). (a) Control (0%), (b) 2% nano-ZrO2 particles, (c) 5% nano-ZrO2 particles, and (d) 7% nano-ZrO2 particles. The disc form clearly depicts that the cracks were wider in control group (orange arrows), which then became narrower with the addition of nano-ZrO2 particles, whereas these cracks were completely closed on 7% nano-ZrO2 particles.
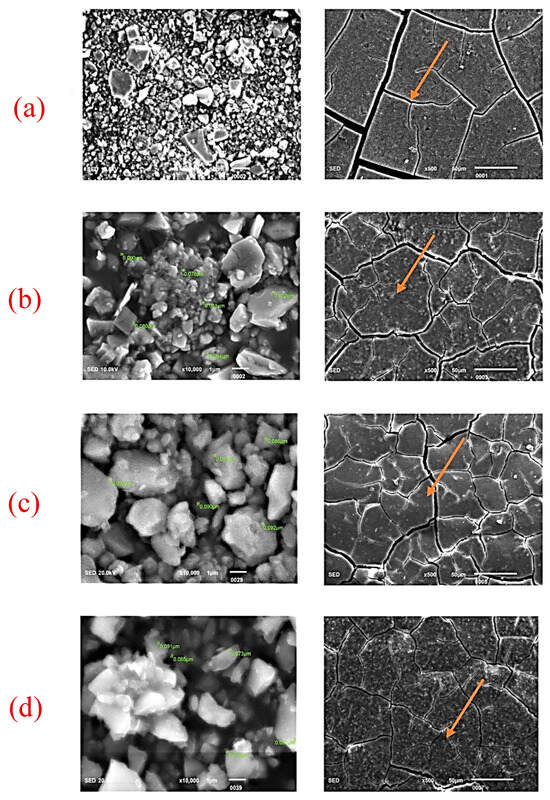
Figure 3.
SEM images for SHOFU GIC (powder and disk). (a) Control (0%), (b) 2% nano-ZrO2 particles, (c) 5% nano-ZrO2 particles, and (d) 7% nano-ZrO2 particles. The disc form clearly depicts that the control group shows cracks, which were wider but were not improved by the addition of nano-ZrO2 particles (Figure 3a–d) (orange arrow).

Table 1.
Mean (standard deviation SD) of flexural strength, flexural modulus, and surface hardness of two GICs after the addition of nano-ZrO2 particles at varying concentrations.

Table 2.
Mean (standard deviation SD) of water sorption and solubility of two GICs after the addition of nano-ZrO2 particles at varying concentrations.
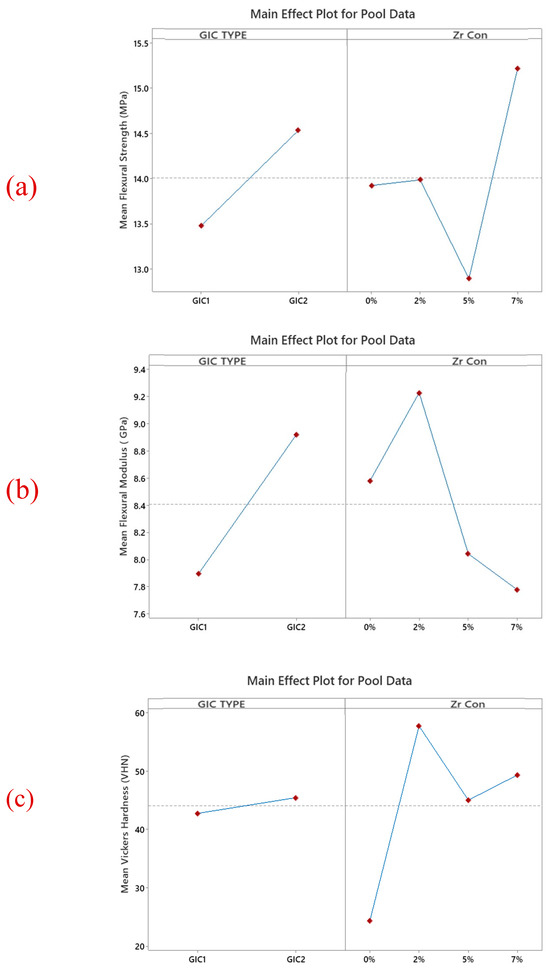
Figure 4.
The main effects of the combined (a) flexural strength, (b) flexural modulus, and (c) surface hardness data, highlighting the effect of material type and nano-ZrO2 concentration. In general, GIC 2 appears to be stronger than GIC 1. Moreover, the addition of 7% nano ZrO2 shows an increase in the flexural strength and modulus and a decrease in the surface hardness of GICs.
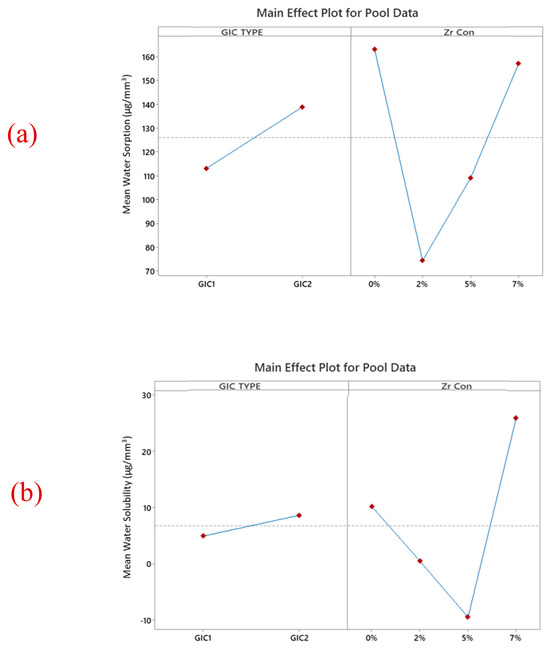
Figure 5.
The main effects graphs of combined (a) water sorption (b) water solubility data highlight the effect of material type and nano-ZrO2 concentration. In general, GIC 1 has lower water sorption and solubility than GIC 2. Moreover, the addition of 7% nano ZrO2 shows decreased water sorption, whereas water solubility was increased.
3. Discussion
3.1. Flexural Strength
The ISO 9917-2 [24] protocol was followed to measure the flexural strength in this study [25]. We found that the incorporation of nano-ZrO2 particles enhanced the flexural strength of GIC 1 used in the current study. These smaller NPs act as additional bonding sites between the larger particles by occupying the empty spaces to reinforce the cement [26]. The crystalline nature of monoclinic and tetragonal nano-ZrO2 particles depicted by XRD around the amorphous matrix of the GIC might be the reason for the increase in strength [26]. The formation of a poly salt bridge by nano-ZrO2 particles between the GIC matrix improves the flexural strength of GIC 2 [27]. The results obtained from the current study were comparable to the results presented by Kutuz et al. [27] and Sajjad et al. [28]. They concluded that the effect of nano-ZrO2 particles was concentration-dependent, as the highest strength was found at 7%, followed by 5% and then 2% nano-ZrO2 particles in comparison with the control group. This was explained by preventing the crack propagation of the GIC when a local compression stress was developed due to a change in volume after tetragonal zirconia was transformed to monoclinic zirconia, increasing the fracture resistance of the material [29]. Nevertheless, this phase transformation in XRD was found to be consistent with as-received nanoparticles without undergoing any changes. In addition, when the authors calculated the particle size of the crystal by using XRD, it was found to be 13.7 nm, falling within the correct definition of nano-ZrO2 particles. Moreover, the control group of GIC 2 showed greater flexural strength when compared with GIC 1. The addition of nano-ZrO2 particles did not increase the flexural strength of GIC 2. Similar results were found when TiO2 NPs (3 and 5%) were added to conventional GICs by Garcia-Contreras et al. in 2015 [30]. They explained that this finding might be due to the non-uniform and non-homogeneous distribution of NPs in the GIC powder.
3.2. Flexural Modulus
In the current study, the highest flexural modulus was found in GIC 1 compared to GIC 2. It is interesting to note that a higher modulus was observed when nano-ZrO2 particles were added to GIC 1 because larger GIC glass particles were occupied by nano-ZrO2 particles and acted as additional bonding sites for the polyacrylic polymer, thus reinforcing the GIC matrix [31]. The strength of GICs at an early stage of setting can be affected by the nature, concentration, and molecular weight of the polycarboxylic acid; the chemical composition and microstructure of the glass; and the powder-to-liquid ratio [32]. In the current study, the flexural modulus of GIC 2 significantly declined after the incorporation of nano-ZrO2 particles, which might be because some larger-sized NPs leached out from the matrix, and thus their capability to bear load was decreased [33]. However, it is uncertain whether this decline was due to an unknown instrument error or an error in sample preparation or storage. It might also be due to the batch of GICs used to assess the flexural modulus in this study. The flexural modulus was not evaluated in the past with nano-ZrO2 particles, and therefore, there is a dearth of literature that requires more future exploration.
3.3. Vickers Microhardness
After 24 h of immersion in distilled water, the mean VHN of experimental groups was significantly higher than that of the control group (0%) of both the GICs. It is noteworthy that the VHN for GIC 1 is lowest (46.00VHN) at 7% and highest (55.24VHN) at 5% nano-ZrO2 particles. Within the matrix, the homogenous dispersion of nano-ZrO2 particles might be a reason for the increase in the hardness of the experimental groups. Moreover, an increase in the interaction between the NPs and the acid also leads to an increase in the hardness of the material [34]. On the contrary, in the current study, as the weight percentages of the NPs increase (7 wt% in GIC 1 and 5% weight in GIC 2), it leads to a reduction in the surface hardness of the GICs. This could be due to the agglomeration of the NPs, which were stuck to one another, leading to a decrease in surface hardness and roughness [35]. Similar results were also found when 3% (w/w) TiO2 NPs were added in GIC. Due to the addition of NPs, fewer glass particles were at the GIC surface, resulting in a greater amount of acid reacting with NPs. The hardness, however, decreases at higher concentrations (5% and 7% w/w) of TiO2 NPs due to the weakening of the bulk material [35]. The lower hardness values at 3% and 5% of nano-ZrO2 particles could be attributable to the formation of weak spots in the cement matrix due to voids generated by the aggregates [36]. Moreover, agglomerate accumulation on the surface, ineffective reaction between cement liquid and NPs, and the formation of voids can all decrease the hardness of the cement [36]. In this respect, useful information can be best provided by microscopic studies at the indentation sites.
3.4. Water Sorption and Solubility
Water sorption and solubility were determined using the International Organization for Standardization’s (ISO 9917-1:2007) [37]. In the present study, the water sorption and solubility of GIC 1 are significantly lower than the GIC 2 group. Both water sorption and solubility of GIC with nano-ZrO2 particles were significantly reduced at all the concentrations compared to unmodified GIC, except for solubility in GIC 1, which increased at all the concentrations. This reduction could be due to fewer porosities, which, if present, would seem shallower than those found in the unmodified cement [38]. The increase in solubility for GIC 2 at a 7% nano-ZrO2 concentration might be because the bond between the tooth and the restorative interface will degrade when water is incorporated at the early stage of setting. This phenomenon occurs due to two effects: degradation and lamination. Moreover, the presence of more hygroscopic fillers blended with different sizes of ultrafine, highly reactive glass particles at a 7% concentration could also be the reason for higher values of solubility. Similar values were noticed in the study conducted by Lima et al. [39]. The manual mixing of the cement, composition of the cement, and control of poor porosities are also factors supporting higher solubility. Mustafa et al. [40] presented similar results when they evaluated a resin-modified composite in comparison with a high-viscosity conventional GIC material. This difference can be explained by the more stable polymeric structure of the resin-modified composite [40]. However, there is a lack of literature regarding the water sorption and solubility of GICs. Therefore, to investigate these characteristics of restorative materials further, long-term studies are needed. Interestingly, due to different sample dimensions that affect the diffusion of water into the cement matrix, the water sorption and solubility values obtained in different studies were greatly varied. Matrix stability is decreased by smaller specimen sizes. In the current study, 10 mm × 1 mm dimensions were used for sample preparation, which is similar to the study conducted by Sunbul HA et al. [41]. However, 15 mm × 2 mm dimensions were formulated by Alrahlah et al. [42]. Regarding the water solubility of GIC 1, a statistically insignificant increase in the values was found between the control group and the modified groups with nano-ZrO2 particles. This increase in solubility may be caused by clumping of the NPs, resulting in a decrease in filler–matrix interaction and leading to a decrease in the intrametric homogeneity of the specimens [43]. However, statistically significant reductions were found in the solubility of GIC 2 among the modified and unmodified groups. Such differences could mean that water molecules that bind to the GIC structure after an acid–base reaction were unequal in all groups. This results in the trapping of the water molecules in the matrix, filler, or matrix–filler interface rather than being tightly bound to the structure. After drying in the desiccator, this loosely bonded water was vaporized from the samples [44]. The present study displayed negative solubility values with a large standard deviation. Many factors contribute to these negative solubility values. However, the potential reason for the negative solubility was attributed to incomplete dehydration of these materials. The negative values do not indicate that no solubility occurred, but may hint at their solubility. Previously, Toledano et al. [44] in 2006, Keyf et al. [45] in 2007, and Sinthawornkul et al. [46] in 2017 also reported negative values. The conventional GIC takes up water as the acid–base reaction progresses, and this water becomes an integral part of its structure. Therefore, the longer the rate of the reaction, the greater the water uptake into the cement structure [45]. This gain in water is retained in the cement matrix and weighted as the “final mass”. This would lead to negative solubility values and a large standard deviation of the samples. This gained water participates in a continued acid–base reaction and is not lost by the desiccation, resulting in the negative solubility values and large standard deviation of the control group in GIC 1 [45]. The incorporation of nano-ZrO2 particles improved both the water sorption and solubility due to many factors. In conventional GICs, over time, matrix hydrolysis results in the deterioration of cement [47]. Solubility can be reduced by adding these nanofillers, as they are water-insoluble. This aligns with the work of Dehis et al. [48], who added TiO2NPs into heat-cured and microwave-cured acrylic resin denture bases and compared them with conventional GICs. They found a significant reduction in water sorption and solubility. The inconsistent findings in the current study and previously conducted studies were due to different concentrations of the NPs, their size, shape, and nature, as well as methodological differences like different sizes of the samples, storage time, and solution; formulation of the water sorption; and solubility.
The nanoparticles utilized in the current study met the specific requirements and adhered to the standards as they were synthesized in our own laboratory rather than purchased commercially. This study evaluated two commercially available GICs at three different concentrations of nano-ZrO2 particles following a storage period of 24 h. However, the base materials, GICs, can be prepared in the laboratory by using raw materials and can be explored in future studies, which might produce stronger and more cost-effective materials than commercially available GICs. A more systematic and controlled approach is mandatory to optimize GIC reinforcement, as it will negate the compositional difference of commercially available GICs. Moreover, the long-term suitability of clinical applications of these materials can be predicted by aging GICs over an extended period, and a 24 h period is not sufficient. Nevertheless, to establish clinical correlation, further studies should be conducted by incorporating broader concentrations of nano-ZrO2 particles. In this work, researchers used hand-mixed GICs in which the probability of the inclusion of porosities due to air entrapment is increased, which results in an increase in the likelihood of inaccurate data. Therefore, future studies should be conducted using mechanically mixed GICs.
4. Materials and Methods
4.1. Materials
Two commercially available GICs were purchased from manufacturers for the sole purpose of this study and were used without further purification or modification. The details of each GIC are given in Table 3.

Table 3.
Composition of glass ionomer cements used in the study.
4.2. Methods
4.2.1. Nano-ZrO2 Particles Preparation
The nano-ZrO2 particles were prepared through co-precipitation method. The methodology outlined by Foo YT et al. was followed [49].
4.2.2. Characterization of Nano-ZrO2 Particles
Elemental morphology and average particle size of the nanocrystalline powder were evaluated via scanning electron microscope (JSM IT100, JEOL Akishima, Tokyo, JAPAN) at accelerating voltage of 10–20 kV at 10,000 magnification. The crystal phase of the specimens was determined via X-ray diffraction (XRD) technique using a Shimadzu-6000 diffractometer Shimadzu Corporation Kyoto Japan utilizing Cu Kα (0.154 nm) radiation operating at 40 kV and 40 mA, recorded over a 2θ range of 10° to 80°.
4.2.3. Incorporation of Nano-ZrO2 Particles into Glass Ionomer Cements
In an Eppendorf tube, nano-ZrO2 particles in powdered form were mixed with powder components of two different conventional GICs and then thoroughly mixed using a vortex.
4.2.4. Specimens’ Preparation for Physio-Mechanical Properties
Seventy-two (n = 72) specimens of different sizes and shapes for different types of physio-mechanical evaluation were prepared using stainless steel molds. To determine flexural strength and flexural modulus, twenty-four (n = 24) bar-shaped specimens were used (2 × 2 × 25 mm); for Vickers microhardness, twenty-four (n = 24) disc-shaped specimens (4 × 6 mm) were used; and for water sorption and solubility, twenty-four (n = 24) disc-shaped samples (15 × 1 mm) were prepared (Figure 6). Samples were prepared at room temperature in 70% relative humidity. Powder and liquid were mixed according to the manufacturer’s recommendation and then placed in their respective stainless-steel molds. Before being removed from the mold, the specimens were left to dry at room temperature for 20 min. Prior to testing, they were coated with a protective varnish (G-C Dental Industrial Corp., Tokyo, Japan) and stored at 37 °C for 24 h in distilled water.
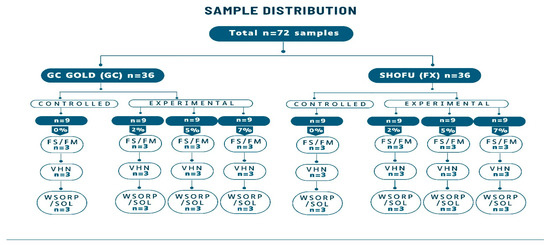
Figure 6.
Sample distribution for GIC 1 and GIC 2. FS: flexural strength, FM: flexural modulus, VHN: Vickers hardness, WSORP/SOL: water sorption and solubility.
4.2.5. Flexural Strength Evaluation (FS)
Using a universal testing machine (Testometric M500-25CT Testometric Ltd. Rochdale, United Kingdom) three point bending test was performed (ISO 4049:2009) [50] with a crosshead speed of 0.500 mm/min and preload of 0.1 N. FS (σ) was calculated using Equation (2) [51].
where F—the maximum load (N), l—the distance between the supports (20 mm), b—specimen’s width (2.00 mm), and h—the specimen’s height (2.00 mm)
4.2.6. Flexural Modulus Evaluation (FM)
Load/displacement values produced by flexural strength testing were used to determine the flexural modulus in accordance with Equation (3) [52].
where E—flexural modulus; p1—load in the elastic region of the stress–strain plot; l—distance between supports; h and b—the specimen’s height and width; d—deflection at p1.
4.2.7. Vickers Hardness Evaluation (VHN)
Vickers micro-hardness tester (INDENTEC ZHV1-M) Indentec Hardness Testing, Brierley Hill, United Kingdom was used to measure the hardness. To make indentations on GIC surfaces, a 200 g load was applied for 15 s. Mean value was calculated by making three indentations with a 100 µm distance on the center of each sample.
4.2.8. Water Sorption and Solubility Evaluation
After 24 h of distilled water immersion, the samples were transferred to a desiccator for 22 h and maintained at 37 °C. After this, they were kept at room temperature for an additional 2 h. Baselines dry mass (m1) (mg) was weighed at the end of 24 h period. The samples were then stored in 25 mL of distilled water at 37 °C for 24 h. After 24 h, the samples were removed from the distilled water. A paper towel was used to remove excess water, and the specimens were air-dried for 15 s at 23 °C before being re-weighed (m2). The specimens were transferred back to a desiccator, which was placed in an incubator for 22 h at 37 °C and then 2 h at 23 °C. This cycle was repeated until a constant mass was achieved (m3) [52,53]. The means were calculated as per Equations (4) and (5) [50,53].
Water sorption and solubility calculations were performed in micrograms per cubic millimeter (µg/mm3).
4.2.9. Statistical Analysis
Minitab statistical software version 19 (Minitab 203 Ltd., Coventry, UK) was used to analyze the data. To evaluate the effect of material type (GIC 1 and GIC 2) and ZrO2 (0, 2, 5, and 7% w/w) on the flexural strength and modulus, micro-hardness, water sorption, and solubility, two-way analysis of variance (ANOVA) was used. To determine the differences between the means of surface hardness, flexural strength, modulus, and water sorption and solubility for the different concentrations of nano ZrO2 particles, one-way analysis of variance (ANOVA) and post hoc Tukey’s test were conducted for each GIC. For the comparison of all the groups, α = 0.05 was set as significance level, whereas to estimate the precision of the observed effects for the means, 95% confidence intervals were used. To highlight the effect of material type and ZrO2 concentrations, main effect graphs were made.
5. Conclusions
In this work, we developed nano-ZrO2 particles and incorporated them into conventional GICs. This addition enhanced the flexural strength and flexural modulus of GICs, most likely due to the reinforcement of the nano-ZrO2 particles by improving force transfer at the filler–matrix interface. The Vickers hardness was greatly enhanced at all concentrations for both the GICs, but at 7 wt% nano-ZrO2 particles, hardness was decreased due to the nanoparticles’ agglomeration. There was an increase in the water solubility of both the GICs at a 7% concentration after 24 h of water immersion as, at higher filler loading, there is a chance of incomplete matrix formation, which led to the microstructural disruption. Reduced porosity and improved matrix densification result in a significant reduction in water sorption.
Author Contributions
Conceptualization, S.F.M. and F.A.; methodology, M.A.A., F.A., and S.J.M.; software, F.A.; validation, N.K., M.A.A., and S.F.M.; formal analysis, F.A., P.J.P., and N.K.; investigation, F.A.; resources, F.A., S.J.M., and M.A.A.; data curation, F.A.; writing—original draft preparation, F.A.; writing—review and editing, N.K., S.F.M., M.A.A., and P.J.P.; visualization, F.A.; supervision, N.K., S.F.M., M.A.A., and P.J.P.; project administration, F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The internal review board of the University of Karachi, Pakistan, approved the present in vitro study (ASRB. No. 05986/Sc.).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FS | Flexural strength. |
| FM | Flexural modulus. |
| VHN | Vickers microhardness. |
| NPs | Nanoparticles. |
References
- Heng, C. Tooth Decay Is the Most Prevalent Disease. Fed. Pract. 2016, 33, 31–33. [Google Scholar] [PubMed]
- Croll, T.P.; Nicholson, J.W. Glass ionomer cements in pediatric dentistry: Review of the literature. Pediatr. Dent. 2002, 24, 423–429. [Google Scholar] [PubMed]
- Berg, J.H.; Croll, T.P. Glass ionomer restorative cement systems: An update. Pediatr. Dent. 2015, 37, 116–124. [Google Scholar]
- Alobiedy, A.N.; Al-Helli, A.H.; Al-Hamaoy, A.R. Effect of adding micro and nano-carbon particles on conventional glass ionomer cement mechanical properties. Ain. Shams Eng. J. 2019, 10, 785–789. [Google Scholar] [CrossRef]
- Gupta, A.A.; Mulay, S.; Mahajan, P.; Raj, A.T. Assessing the effect of ceramic additives on the physical, rheological and mechanical properties of conventional glass ionomer luting cement—An in-vitro study. Heliyon 2019, 5, 02094. [Google Scholar] [CrossRef]
- Simmons, J.J. The miracle mixture. Glass ionomer and alloy powder. Tex. Dent. J. 1983, 100, 6–12. [Google Scholar]
- Cho, E.; Kopel, H.; White, S.N. Moisture susceptibility of resin-modified glass ionomer materials. Quintessence Int. 1995, 26, 351–358. [Google Scholar]
- Yap, A.; Cheang, P.; Chay, P. Mechanical properties of two restorative reinforced glass-ionomer cements. J. Oral Rehabil. 2002, 29, 682–688. [Google Scholar] [CrossRef]
- Lucas, M.E.; Arita, K.; Nishino, M. Toughness, bonding and fluoride-release properties of hydroxyapatite-added glass ionomer cement. Biomaterials 2003, 24, 3787–3794. [Google Scholar] [CrossRef]
- Tian, K.V.; Yang, B.; Yue, Y.; Bowron, D.T.; Mayers, J.; Donnan, R.S.; Dobó-Nagy, C.; Nicholson, J.W.; Fang, D.C.; Greer, A.L.; et al. Atomic and vibrational origins of mechanical toughness in bioactive cement during setting. Nat. Commun. 2015, 6, 8631. [Google Scholar] [CrossRef]
- Yakop, F.; Abd Ghafar, S.A.; Yong, Y.K.; Saiful Yazan, L.; Mohamad Hanafiah, R.; Lim, V.; Eshak, Z. Silver NPs Clinacanthus Nutans leaves extract induced apoptosis towards oral squamous cell carcinoma cell lines. Artif. Cells Nanomed. Biotechnol. 2018, 46, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, G.R.; Swamy, M.K. Potential applications of engineering NPs in medicine and biology: An update. J. Biol. Inorg. Chem. 2018, 23, 1185–1204. [Google Scholar] [CrossRef] [PubMed]
- Semyari, H.; Sattari, M.; Atai, M.; Pournasir, M. The effect of nanozirconia mixed with glass-ionomer on proliferation of epithelial cells and adhesive molecules. J. Periodontol. Implant. Dent. 2011, 3, 63–68. [Google Scholar]
- Dowling, A.H.; Schmitt, W.S.; Fleming, G.J.P. Modification of titanium dioxide particles to reinforce glass-ionomer restoratives. Dent. Mater. 2014, 30, 159–160. [Google Scholar] [CrossRef]
- Khademolhosseini, M.R.; Barounian, M.H.; Eskandari, A.; Aminzare, M.; Zahedi, A.M.; Ghahremani, D. Development of new Al2O3/TiO2 reinforced glass-ionomer cements (GICs) nanocomposites. J. Basic. Appl. Sci. Res. 2012, 2, 7526–7529. [Google Scholar]
- Cibim, D.D.; Saito, M.T.; Giovani, P.A.; Borges, A.F.S.; Pecorari, V.G.A.; Gomes, O.P.; Lisboa-Filho, P.N.; Niciti-Junior, F.H.; Puppin-Rontani, R.M.; Kantovitz, K.R. Novel nanotechnology of TiO2 improves physical-chemical and biological properties of glass ionomer cement. Int. J. Biomater. 2017, 2017, 7123919. [Google Scholar] [CrossRef]
- Gjorgievska, E.; Nicholson, J.W.; Grabić, D.; Guclu, Z.A.; Melitić, I.; Coleman, N.J. Assessment of the impact of the addition of NPs on the properties of glass-ionomer cements. Materials 2020, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Shinde, H.M.; Bhosale, T.T.; Gavade, N.L.; Babar, S.B.; Kamble, R.J.; Shirke, B.S.; Garadkar, K.M. Biosynthesis of ZrO2 NPs from Ficus benghalensis leaf extract for photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 14055–14064. [Google Scholar] [CrossRef]
- Jalill, R.D.A.; Jawad, M.M.H.M.; Abd, A.N. Plants extracts as green synthesis of nano-ZrO2 particles. J. Genet. Environ. Res. Conserv. 2017, 5, 623. [Google Scholar]
- Balaji, S.; Mandal, B.K.; Ranjan, S.; Dasgupta, N.; Chidambaram, R. Nano-zirconia–evaluation of its antioxidant and anticancer activity. J. Photochem. Photobiol. B 2017, 170, 125–133. [Google Scholar] [CrossRef]
- Shiekh, R.A.; Ab, R.I.; Masudi, S.A.; Luddin, N. Modification of glass ionomer cement by incorporating hydroxyapatite-silica nano-powder composite: Sol–gel synthesis and characterization. Ceram. Int. 2014, 40, 3165–3170. [Google Scholar] [CrossRef]
- Moheet, I.A.; Luddin, N.; Ab, R.I.; Masudi, S.A.; Kannan, T.P.; Abd Ghani, N.R. Evaluation of mechanical properties and bond strength of nano-hydroxyapatite-silica added glass ionomer cement. Ceram. Int. 2018, 44, 9899–9906. [Google Scholar] [CrossRef]
- Barandehfard, F.; Rad, M.K.; Hosseinnia, A.; Khoshroo, K.; Tahriri, M.; Jazayeri, H.E.; Moharamzadeh, K.; Tayebi, L. The addition of synthesized hydroxyapatite and fluorapatite nanoparticles to a glass-ionomer cement for dental restoration and its effects on mechanical properties. Ceram. Int. 2016, 42, 17866–17875. [Google Scholar] [CrossRef]
- ISO 9917-2:2017; Dentistry—Water-Based Cements—Part 2: Resin-Modified Cements. 3rd ed. International Organization for Standardization: Geneva, Switzerland, 2017.
- Bresciani, E.; Barata, T.D.J.E.; Fagundes, T.C.; Adachi, A.; Terrin, M.M.; Navarro, M.F.D.L. Compressive and diametral tensile strength of glass ionomer cements. J. Appl. Oral Sci. 2004, 12, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M. Materials Selection in Mechanical Design; Butterworth-Heinemann: Oxford, UK, 2011; p. 40. [Google Scholar]
- Kutuk, Z.B.; Vural, U.K.; Cakir, F.Y.; Miletic, I.; Gurgan, S. Mechanical properties and water sorption of two experimental glass ionomer cements with hydroxyapatite or calcium fluorapatite formulation. Dent. Mater. J. 2019, 38, 471–479. [Google Scholar] [CrossRef]
- Sajjad, A.; Bakar, W.Z.W.; Mohamad, D.; Kannan, T.P. Characterization and enhancement of physicomechanical properties of glass ionomer cement by incorporating a novel nano zirconia silica hydroxyapatite composite synthesized via sol-gel. AIMS Mater. Sci. 2019, 6, 730–747. [Google Scholar] [CrossRef]
- Bariker, R.H.; Mandroli, S.P. An in-vitro evaluation of antibacterial effect of Amalgomer CR and Fuji VII against bacteria causing severe early childhood caries. J. Indian. Soc. Pedod. Prev. Dent. 2016, 34, 23–29. [Google Scholar]
- Garcia-Contreras, R.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Sakagami, H.; Morales-Luckie, R.A.; Nakajima, H. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J. Appl. Oral Sci. 2015, 23, 321–328. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Ansari, S.; Moshaverinia, M.; Roohpour, N.; Darr, J.A.; Rehman, I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 2008, 4, 432–440. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Ansari, S.; Movasaghi, Z.; Billington, R.W.; Darr, J.A.; Rehman, I. Modification of conventional glass-ionomer cements with N-vinylpyrrolidone containing polyacids, nano-hydroxy and fluoroapatite to improve mechanical properties. Dent. Mater. 2008, 24, 1381–1390. [Google Scholar] [CrossRef]
- Kumar, N. Exploring the Variability in Mechanical Property Testing of Dental Resin Composites. Doctoral’s Dissertation, University of Birmingham, Birmingham, UK, 2011. [Google Scholar]
- Elsaka, S.E.; Hamouda, I.M.; Swain, M.V. Titanium dioxide NPs addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. J. Dent. 2011, 39, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Prentice, L.H.; Tyas, M.J.; Burrow, M.F. The effect of ytterbium fluoride and barium sulphate NPs on the reactivity and strength of a glass-ionomer cement. Dent. Mater. J. 2006, 22, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Elshenawy, E.A.; El-Ebiary, M.A.; Kenawy, E.R.; El-Olimy, G.A. Modification of glass-ionomer cement properties by quaternized chitosan-coated nanoparticles. Odontology 2023, 111, 328–341. [Google Scholar] [CrossRef] [PubMed]
- ISO 9917-1:2007; Dentistry—Water-Based Cements—Part 1: Powder/Liquid Acid-Base Cements. 2nd ed. International Organization for Standardization: Geneva, Switzerland, 2007.
- Alshali, R.Z.; Salim, N.A.; Satterthwaite, J.D.; Silikas, N. Long-term sorption and solubility of bulk-fill and conventional resin-composites in water and artificial saliva. J. Dent. 2015, 43, 1511–1518. [Google Scholar] [CrossRef]
- Lima, R.B.; Faris, J.F.; Andrade, A.M.; Silva, F.D.; Duarte, R.M. Water sorption and solubility of glass ionomer cements indicated for atraumatic restorative treatment considering the time and the pH of the storage solution. RGO-Rev. Gaúcha De Odontol. 2018, 66, 29–34. [Google Scholar] [CrossRef]
- Mustafa, R.; Alshali, R.Z.; Silikas, N. The effect of desiccation on water sorption, solubility and hygroscopic volumetric expansion of dentine replacement materials. Dent. Mater. J. 2018, 34, 205–213. [Google Scholar] [CrossRef]
- Sunbul, H.A.; Silikas, N.; Watts, D.C. Resin-based composites show similar kinetic profiles for dimensional change and recovery with solvent storage. Dent. Mater. 2015, 31, 201–217. [Google Scholar] [CrossRef]
- Alrahlah, A.; Silikas, N.; Watts, D.C. Hygroscopic expansion kinetics of dental resin-composites. Dent. Mater. 2014, 30, 143–148. [Google Scholar] [CrossRef]
- Ferracane, J.L. Elution of leachable components from composites. J. Oral Rehabil. 1994, 21, 441–452. [Google Scholar] [CrossRef]
- Toledano, M.B.; Osorio, R.; Osorio, E.; Aguilera, F.S.; Romeo, A.; De La Higuera, B.; García-Godoy, F. Sorption and solubility testing of orthodontic bonding cements in different solutions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 76, 251–256. [Google Scholar] [CrossRef]
- Keyf, F.; Tuna, S.H.; ¸Sen, M.; Safrany, A. Water sorption and solubility of different luting and restorative dental cements. Turk. J. Med. Sci. 2007, 37, 47–55. [Google Scholar]
- Sinthawornkul, S.; Thunyakitpisal, P.; Thunyakitpisal, N.; Jiemsirilers, S. Comparison of Shear Bond Strength, Water Sorption and Solubility of 3 Glass Ionomer Cements for Direct Bonding of Orthodontic Brackets in vitro. J. Dent. Assoc. Thail. 2017, 67, 225–235. [Google Scholar]
- Fathi, U.A.; Maha, A.A.; Ahmad, Z.A. The Effect of the Incorporation of Titanium Dioxide NPs on the Mechanical and Physical Properties of Glass Ionomer Cement. JRMDS 2022, 10, 88–91. [Google Scholar]
- Dehis, W.; Eissa, S.; Elawady, A.; Elhotaby, M. Impact of nano-TiO2 particles on water sorption and solubility in different denture base materials. J. Arab. Soc. Med. Res. 2018, 13, 99–105. [Google Scholar] [CrossRef]
- Foo, Y.T.; Abdullah, A.Z.; Horri, B.A.; Salamatinia, B. Optimised Co-Precipitation synthesis condition for oxalate-derived zirconia NPs. Ceram. Int. 2019, 45, 22930–22939. [Google Scholar] [CrossRef]
- ISO 4049:2009; Dentistry—Polymer-Based Restorative Materials. 4th ed. International Organization for Standardization: Geneva, Switzerland, 2009.
- Curtis, A.R. The Influence of ‘Nanocluster’ Reinforcement on the Mechanical Properties of a Resin-Based Composite Material. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2009. [Google Scholar]
- Wang, Y.; Zhu, M.; Zhu, X.X. Functional fillers for dental resin composites. Acta Biomater. 2021, 122, 50–65. [Google Scholar] [CrossRef]
- Yilmaz, M.N.; Gul, P.; Kiziltunc, A. Water sorption and solubility of a high-viscous glass-ionomer cement after the application of different surface-coating agents. Eur. J. Gen. Dent. 2020, 9, 118–121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).