Binding Capacity and Adsorption Stability of Uremic Metabolites to Albumin-Modified Magnetic Nanoparticles
Abstract
1. Introduction
2. Results
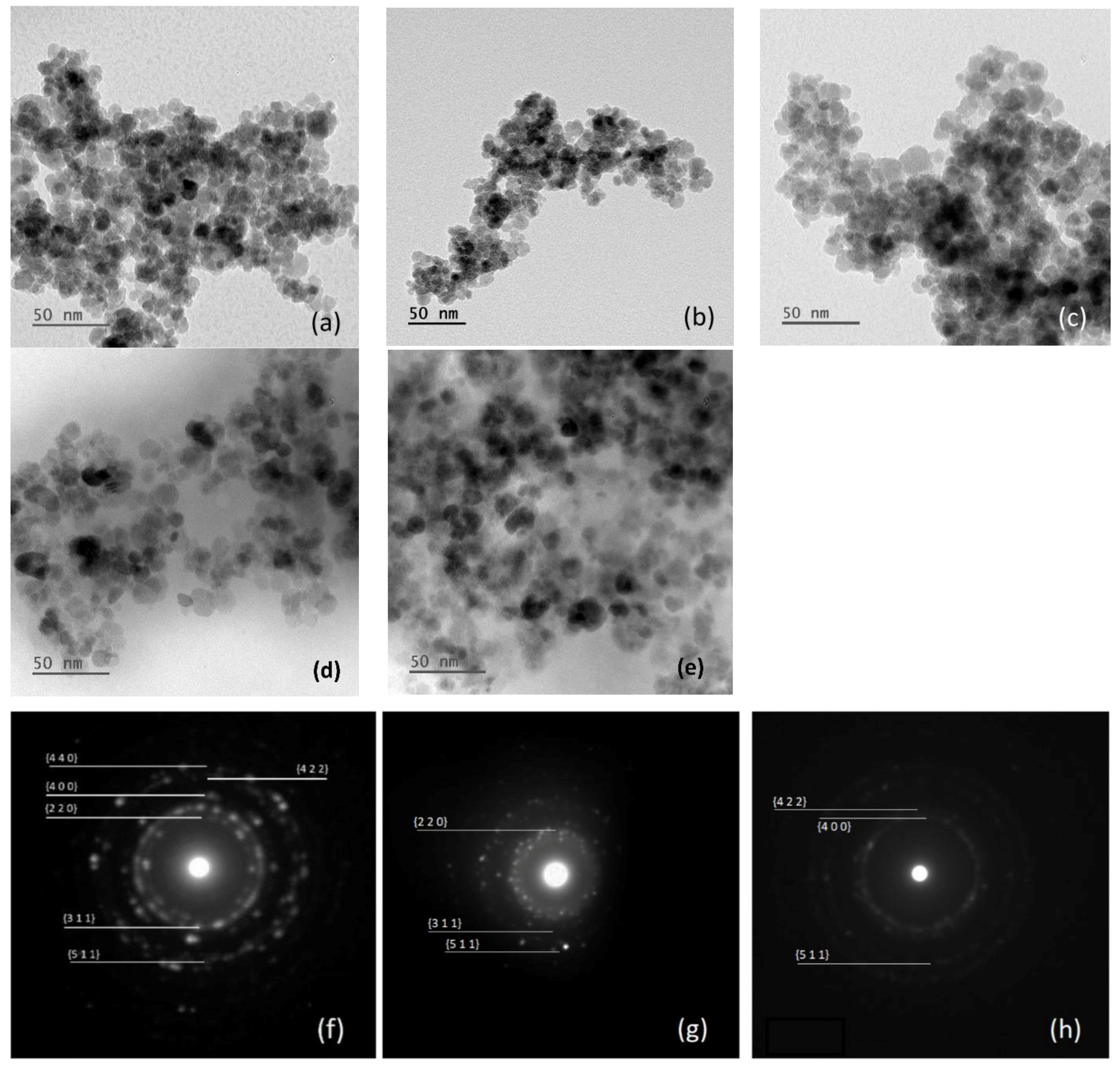
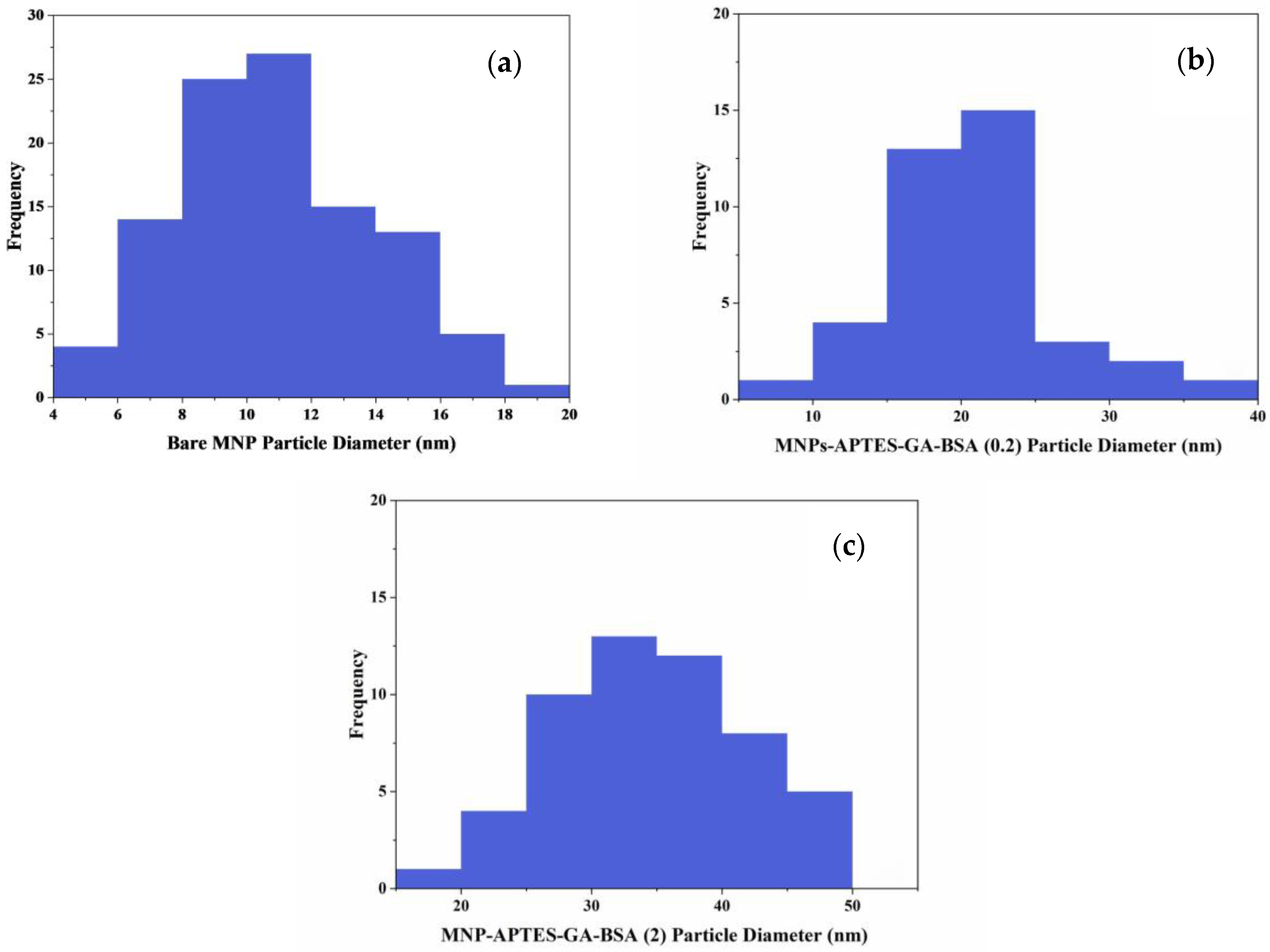
2.1. Particle and Film Dimensions
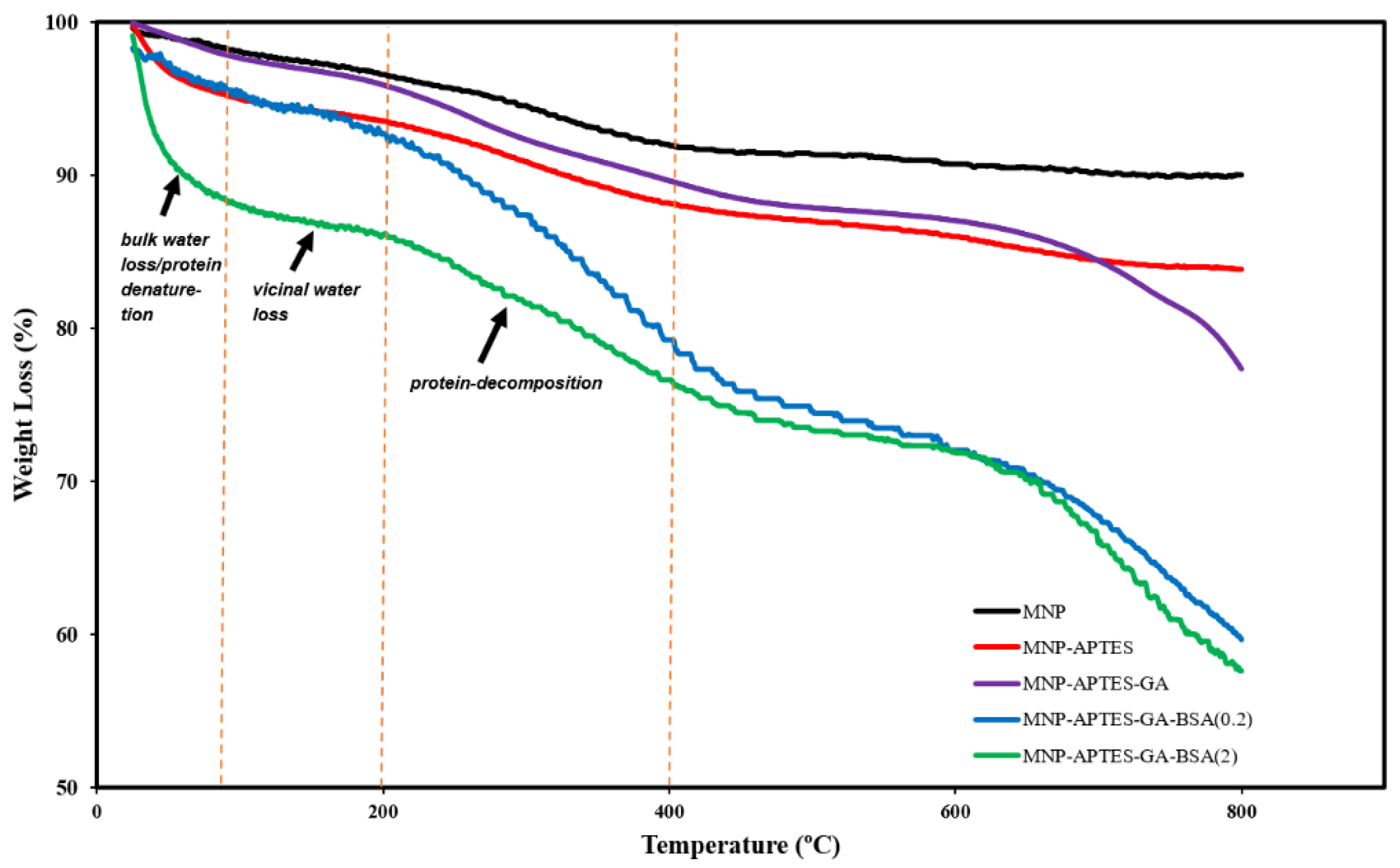
2.2. Relative Mass of Alb-MNP Films
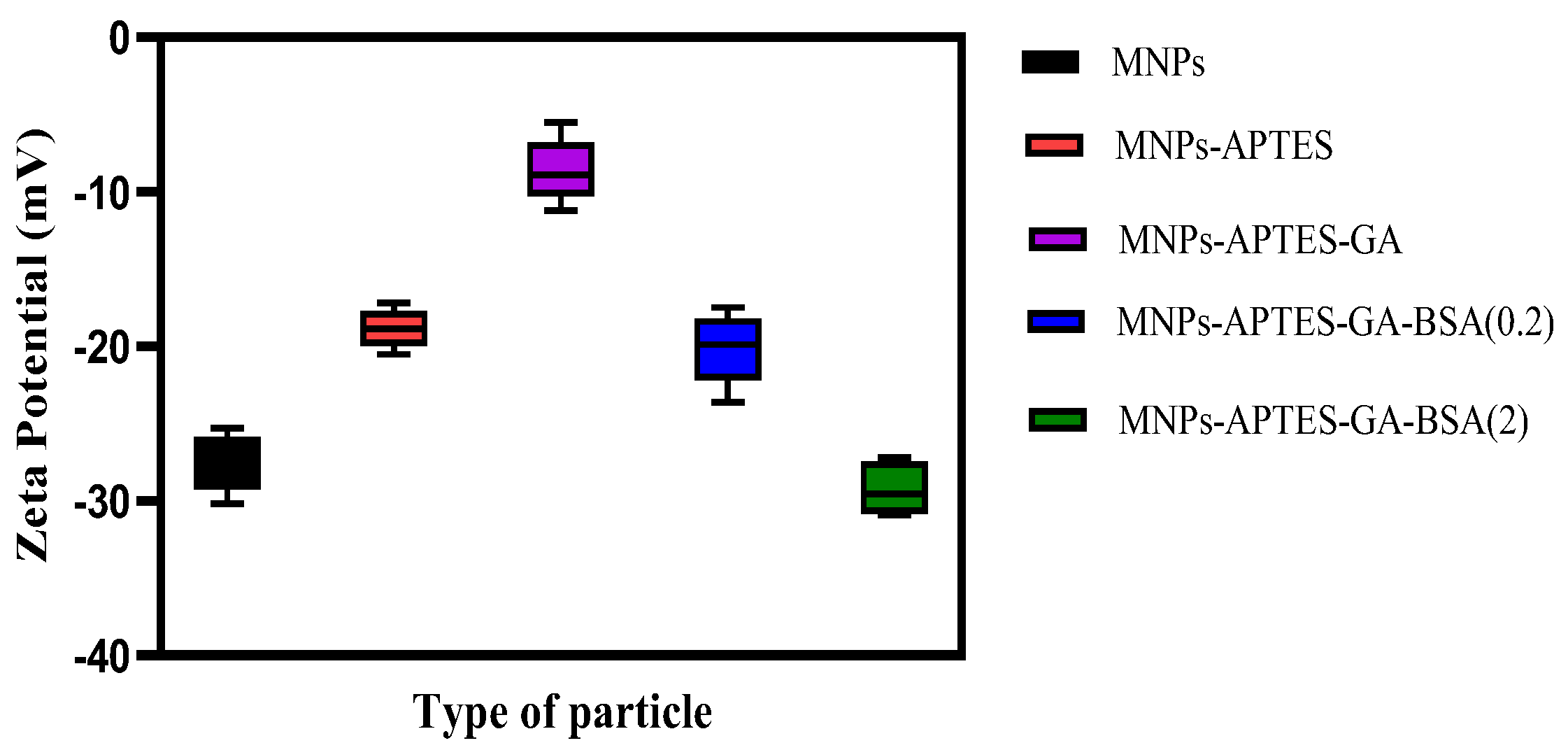
2.3. Surface Charge Analysis
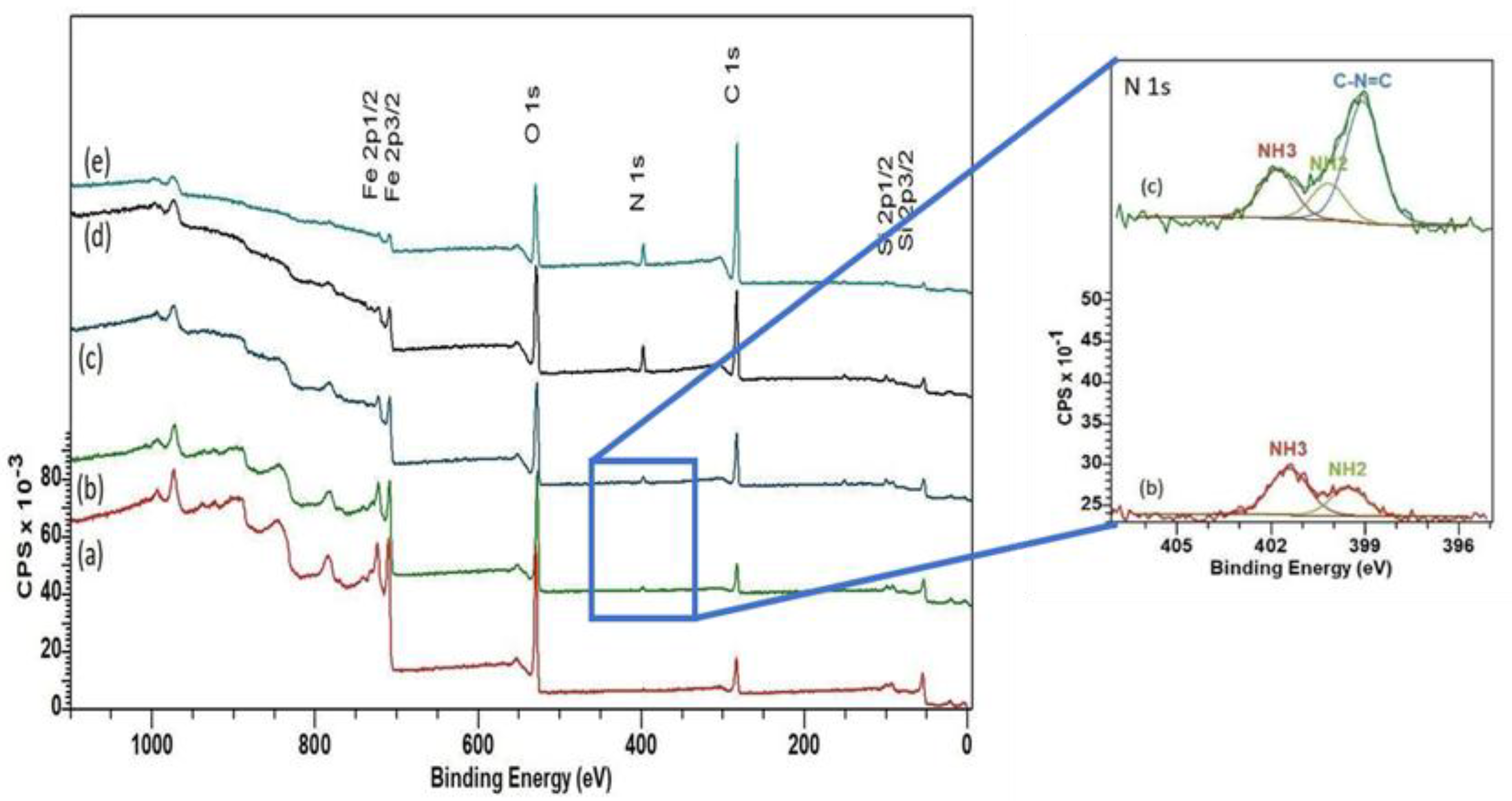
2.4. Film Bonding Chemistry
3. Discussion
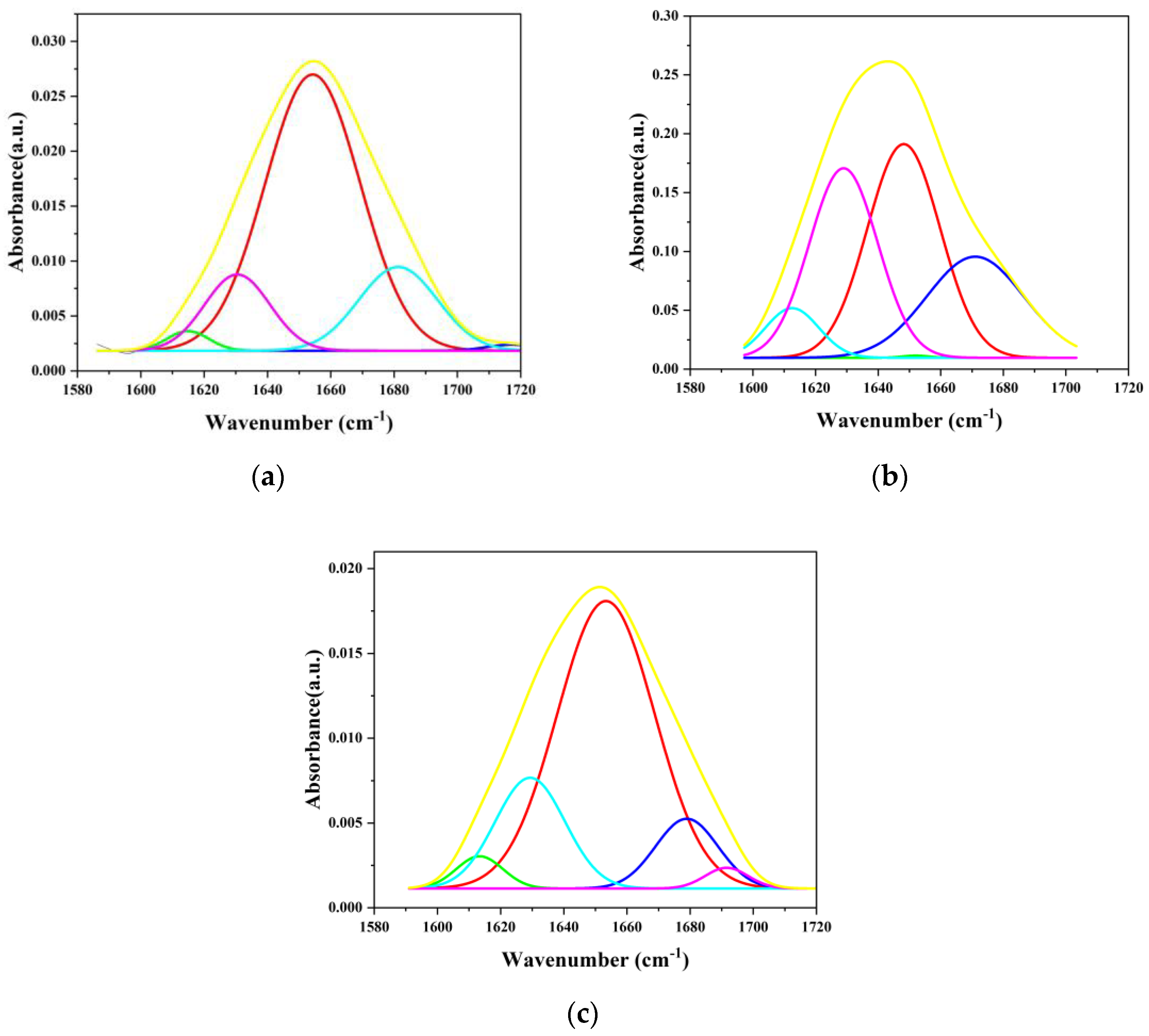
3.1. Effect of Film Formation on Albumin Secondary Structure
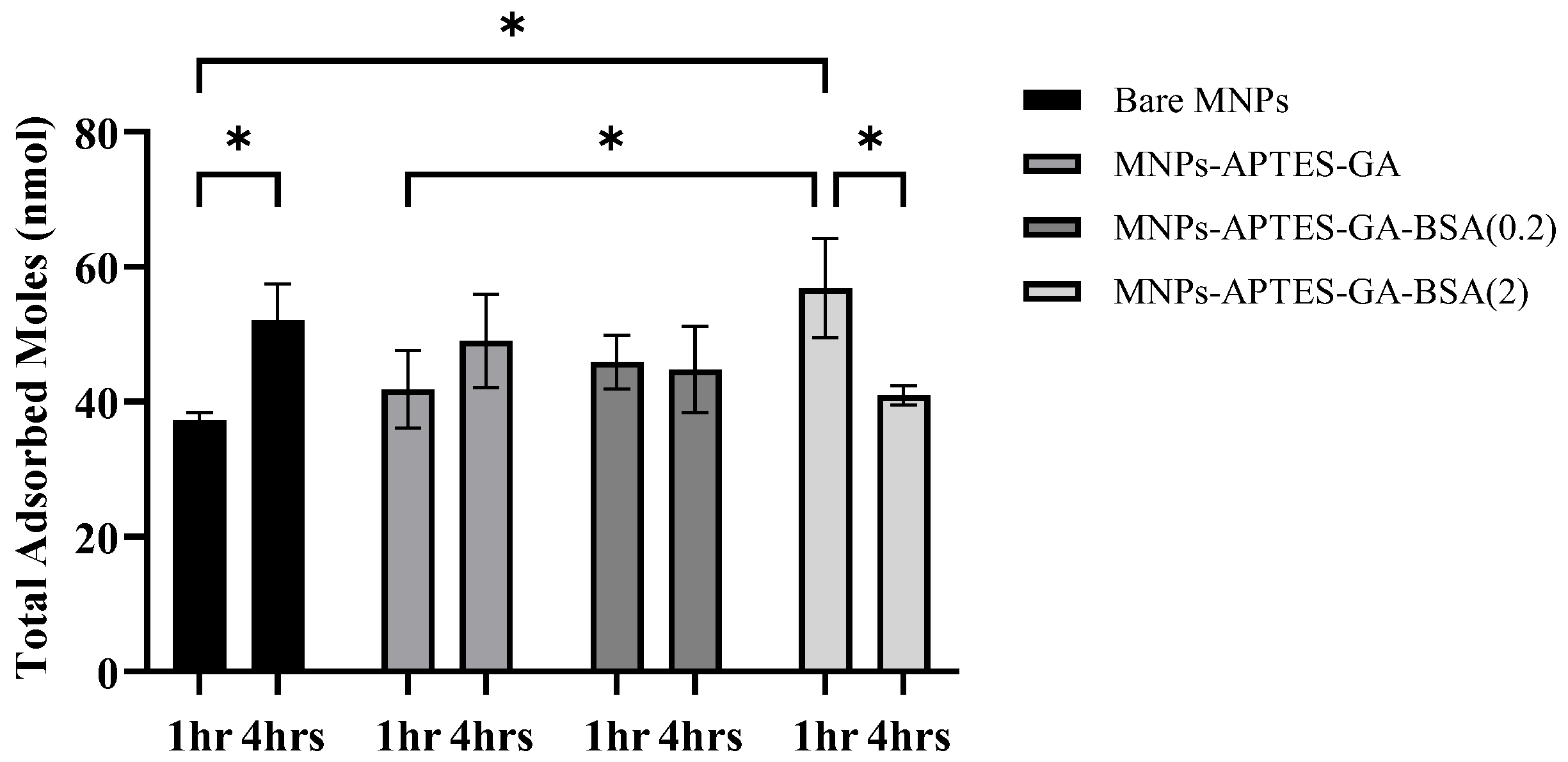
3.2. Uremic Metabolite Adsorbed Amounts
3.3. Uremic Metabolite Adsorption Selectivity
3.4. Uremic Metabolite Binding Characteristics of Albumin vs. Cyclodextrin Films
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Iron Oxide Nanoparticles
4.3. Functionalization with APTES and GA
4.4. Formation of BSA-Functionalized MNPs
4.5. Particle Characterization
4.6. Uremic Metabolite Solution
4.7. MNP Incubation with Uremic Toxin Solution
4.8. Analysis of Adsorbed Metabolites
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PBUT | Protein-bound uremic toxin |
| CKD | Chronic kidney disease |
| UM | Uremic metabolite |
| HD | Hemodialysis |
| MNP | Magnetic nanoparticle |
| p(MPC-co-PMβCD) | (Methacryloyloxy)ethyl phosphorylcholine-co-β-cyclodextrin |
| TGA | Thermogravimetric analysis |
| XPS | X-ray photoelectron spectroscopy |
| FTIR | Fourier-transform infrared spectroscopy |
| TEM | Transmission electron microscopy |
| FSD | Fourier self-deconvolution |
| PBS | Phosphate-buffered saline |
| HPLC | High-performance liquid chromatography |
| DI | Direct injection |
| MS | Mass spectrometry |
| MS/MS | Tandem mass spectrometry |
| MRM | Multiple reaction monitoring |
| BSA | Bovine serum albumin |
| Alb-MNP | Albumin-modified MNPs |
| (-NH2-) | Amide-I |
| (-NH-) | Amide-II |
| CD | Cyclodextrin |
References
- Jadoul, M.; Aoun, M.; Imani, M.M. The major global burden of chronic kidney disease. Lancet Glob. Health 2024, 12, e342–e343. [Google Scholar] [CrossRef] [PubMed]
- Antini, C.; Caixeta, R.; Luciani, S.; Hennis, A.J. Diabetes Mellitus and Cardiovascular Disease: Exploring Epidemiology, Pathophysiology, and Treatment Strategies. Int. J. Epidemiol. 2024, 53, dyad182. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Sidor, N.A.; Tonial, N.C.; Che, A.; Urquhart, B.L. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets. Toxins 2021, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-R.; Xin, M.-Y.; Li, K.; Wang, H.; Rao, Z.; Liu, T.-X.; Wu, X.-A. An LC-MS/MS analytical method for the determination of uremic toxins in patients with end-stage renal disease. J. Pharm. Biomed. Anal. 2020, 191, 113551. [Google Scholar] [CrossRef]
- Daneshamouz, S.; Eduok, U.; Abdelrasoul, A.; Shoker, A. Protein-bound uremic toxins (PBUTs) in chronic kidney disease (CKD) patients: Production pathway, challenges and recent advances in renal PBUTs clearance. NanoImpact 2021, 21, 100299. [Google Scholar] [CrossRef]
- Li, S.; Sharaf, M.G.; Rowe, E.M.; Serrano, K.; Devine, D.V.; Unsworth, L.D. Hemocompatibility of β-Cyclodextrin-Modified (Methacryloyloxy)ethyl Phosphorylcholine Coated Magnetic Nanoparticles. Biomolecules 2023, 13, 1165. [Google Scholar] [CrossRef]
- Yang, F.; Liao, M.; Wang, P.; Yang, Z.; Liu, Y. The Cost-Effectiveness of Kidney Replacement Therapy Modalities: A Systematic Review of Full Economic Evaluations. Appl. Health Econ. Health Policy 2021, 19, 163–180. [Google Scholar] [CrossRef]
- Klefter, R.; Nielsen, B. Savings in Dialysis Treatment? Artif. Organs 2002, 26, 49–54. [Google Scholar] [CrossRef]
- Vanholder, R.; Annemans, L.; Bello, A.K.; Bikbov, B.; Gallego, D.; Gansevoort, R.T.; Lameire, N.; Luyckx, V.A.; Noruisiene, E.; Oostrom, T. Fighting the unbearable lightness of neglecting kidney health: The decade of the kidney. Clin. Kidney J. 2021, 14, 1719–1730. [Google Scholar] [CrossRef]
- Vanholder, R.; Snauwaert, E.; Verbeke, F.; Glorieux, G. Future of Uremic Toxin Management. Toxins 2024, 16, 463. [Google Scholar] [CrossRef]
- Ma, Y.; Li, S.; Tonelli, M.; Unsworth, L.D. Adsorption-based strategies for removing uremic toxins from blood. Microporous Mesoporous Mater. 2021, 319, 111035. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, Q.; Yang, Y.; Li, Y.; Lin, W. Recent trends in therapeutic application of engineered blood purification materials for kidney disease. Biomater. Res. 2022, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.M.; El-Safty, S.; Tounsi, A.; Shenashen, M. A review of magnetic nanoparticles used in nanomedicine. APL Mater. 2024, 12, 010601. [Google Scholar] [CrossRef]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.P.; Gómez-Pastora, J. Magnetic Nanoparticles: A Review on Synthesis, Characterization, Functionalization, and Biomedical Applications. Small 2024, 20, 2304848. [Google Scholar] [CrossRef]
- Salmanpour, Z.; Abolmaali, S.S.; Farahavr, G.; Salmanpour, M.; Tamaddon, A.M. Magnetic nanoparticles and their hybrid biomaterials for drug delivery and theranostic applications in cardiovascular diseases. J. Drug Deliv. Sci. Technol. 2024, 96, 105676. [Google Scholar] [CrossRef]
- Wang, L.; Han, K.; Jiang, X.; Mao, C.; Li, X.; Zhou, M. Recent Advances in Blood Toxin Removal Technology. Sustain. Mater. Technol. 2024, 39, e00828. [Google Scholar] [CrossRef]
- Li, J.; Han, L.; Xie, J.; Liu, S.; Jia, L. Multi-sites polycyclodextrin adsorbents for removal of protein-bound uremic toxins combining with hemodialysis. Carbohydr. Polym. 2020, 247, 116665. [Google Scholar] [CrossRef]
- Niestanak, V.D.; Unsworth, L.D. Detailing Protein-Bound Uremic Toxin Interaction Mechanisms with Human Serum Albumin in the Pursuit of Designing Competitive Binders. Int. J. Mol. Sci. 2023, 24, 7452. [Google Scholar] [CrossRef]
- Cunha, R.S.D.; Azevedo, C.A.B.; Falconi, C.A.; Ruiz, F.F.; Liabeuf, S.; Carneiro-Ramos, M.S.; Stinghen, A.E.M. The Interplay between Uremic Toxins and Albumin, Membrane Transporters and Drug Interaction. Toxins 2022, 14, 177. [Google Scholar] [CrossRef]
- Li, S.; Tonelli, M.; Unsworth, L.D. Indoxyl and p-cresol sulfate binding with human serum albumin. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128042. [Google Scholar] [CrossRef]
- Baki, A.; Remmo, A.; Löwa, N.; Wiekhorst, F.; Bleul, R. Albumin-Coated Single-Core Iron Oxide Nanoparticles for Enhanced Molecular Magnetic Imaging (MRI/MPI). Int. J. Mol. Sci. 2021, 22, 6235. [Google Scholar] [CrossRef] [PubMed]
- Al-Abboodi, A.; Alsaady, H.M.; Banoon, S.; Al-Saady, M. Conjugation strategies on functionalized iron oxide nanoparticles as a malaria vaccine delivery system. Bionatura 2021, 3, 2009–2016. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, B.; Dai, X.; Wang, X.; Gao, F.; Zhang, X.; Tang, J. Glutaraldehyde Mediated Conjugation of Amino-Coated Magnetic Nanoparticles with Albumin Protein for Nanothermotherapy. J. Nanosci. Nanotechnol. 2010, 10, 7117–7120. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Sharaf, M.G.; Pawar, A.; Milley, A.; Unsworth, L.D. Hemocompatibility of Albumin-Modified Magnetic Nanoparticles. Int. J. Mol. Sci. 2024, 25, 11975. [Google Scholar] [CrossRef]
- Zhang, M.; Li, L.; Lei, L.; Kang, K.; Xiao, C. Effectively Decontaminating Protein-Bound Uremic Toxins in Human Serum Albumin Using Cationic Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2022, 14, 55354–55364. [Google Scholar] [CrossRef]
- Saito, K.; Fujigaki, S.; Heyes, M.P.; Shibata, K.; Takemura, M.; Fujii, H.; Wada, H.; Noma, A.; Seishima, M. Mechanism of increases in L-kynurenine and quinolinic acid in renal insufficiency. Am. J. Physiol.-Ren. Physiol. 2000, 279, F565–F572. [Google Scholar] [CrossRef]
- Vanholder, R.C.; De Smet, R.V.; Ringoir, S. Assessment of Urea and Other Uremic Markers for Quantification of Dialysis Efficacy. Clin. Chem. 1992, 38, 1429–1436. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; European Uremic Toxin Work Group. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef]
- Li, S.; Sharaf, M.G.; Zhang, L.; Wishart, D.S.; Tonelli, M.; Unsworth, L.D. Adsorption Dynamics of Uremic Toxins to Novel Modified Magnetic Nanoparticles. Macromol. Biosci. 2024, 24, 2300133. [Google Scholar] [CrossRef]
- Itoh, Y.; Ezawa, A.; Kikuchi, K.; Tsuruta, Y.; Niwa, T. Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal. Bioanal. Chem. 2012, 403, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Canepa, A.; Filho, J.C.D.; Gutierrez, A.; Carrea, A.; Forsberg, A.M.; Nilsson, E.; Verrina, E.; Perfumo, F.; Bergström, J. Free amino acids in plasma, red blood cells, polymorphonuclear leukocytes. and muscle in normal and uraemic children. Nephrol. Dial. Transplant. 2002, 17, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D.; Swendseid, M.E.; Shinaberger, J.H.; Umezawa, C.Y. The free and bound amino acids removed by hemodialysis. ASAIO J. 1973, 19, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, M.; Jones, M.R.; Kopple, J.D. Amino acid losses during hemodialysis with infusion of amino acids and glucose. Kidney Int. 1982, 21, 500–506. [Google Scholar] [CrossRef]
- Schools, A.; De Vries, P.; Thiemann, R.; Hazejager, W.; Visser, S.; Oe, P. Biochemical and neurophysiological parameters in hemodialyzed patients with chronic renal failure. Clin. Chim. Acta 1989, 185, 91–107. [Google Scholar] [CrossRef]
- Landon, J.; Fawcett, J.; Wynn, V. Blood pyruvate concentration measured by a specific method in control subjects. J. Clin. Pathol. 1962, 15, 579–584. [Google Scholar] [CrossRef]
- Bain, M.A.; Faull, R.; Fornasini, G.; Milne, R.W.; Evans, A.M. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol. Dial. Transplant. 2006, 21, 1300–1304. [Google Scholar] [CrossRef]
- Taki, K.; Tsuruta, Y.; Niwa, T. Indoxyl sulfate and atherosclerotic risk factors in hemodialysis patients. Am. J. Nephrol. 2007, 27, 30–35. [Google Scholar] [CrossRef]
- Toshimitsu, N.; Naohito, T.; Hideo, Y. RNA metabolism in uremic patients: Accumulation of modified ribonucleosides in uremic serum. Kidney Int. 1998, 53, 1801–1806. [Google Scholar]
- Mikhaylova, M.; Kim, D.K.; Berry, C.C.; Zagorodni, A.; Toprak, M.; Curtis, A.S.; Muhammed, M. BSA immobilization on amine-functionalized superparamagnetic iron oxide nanoparticles. Chem. Mater. 2004, 16, 2344–2354. [Google Scholar] [CrossRef]
- Rahdar, S.; Rahdar, A.; Ahmadi, S.; Trant, J.F. Adsorption of bovine serum albumin (BSA) by bare magnetite nanoparticles with surface oxidative impurities that prevent aggregation. Can. J. Chem. 2019, 97, 577–583. [Google Scholar] [CrossRef]
- Csach, K.; Juríková, A.; Miškuf, J.; Koneracká, M.; Závišová, V.; Kubovčíková, M.; Kopčanský, P. Thermogravimetric Study of the Decomposition of BSA-Coated Magnetic Nanoparticles. Acta Phys. Pol. A 2012, 121, 1293–1295. [Google Scholar] [CrossRef]
- Mazario, E.; Forget, A.; Belkahla, H.; Lomas, J.; Decorse, P.; Chevillot-Biraud, A.; Verbeke, P.; Wilhelm, C.; Ammar, S.; Chahine, J.E.H. Functionalization of Iron Oxide Nanoparticles with HSA Protein for Thermal Therapy. IEEE Trans. Magn. 2017, 53, 5200305. [Google Scholar] [CrossRef]
- Gonzalez-Moragas, L.; Yu, S.-M.; Carenza, E.; Laromaine, A.; Roig, A. Protective Effects of Bovine Serum Albumin on Superparamagnetic Iron Oxide Nanoparticles Evaluated in the Nematode Caenorhabditis elegans. ACS Biomater. Sci. Eng. 2015, 1, 1129–1138. [Google Scholar] [CrossRef]
- Chubarov, A.S. Serum Albumin for Magnetic Nanoparticles Coating. Magnetochemistry 2022, 8, 13. [Google Scholar] [CrossRef]
- Hawn, D.D.; DeKoven, B.M. Deconvolution as a correction for photoelectron inelastic energy losses in the core level XPS spectra of iron oxides. Surf. Interface Anal. 1987, 10, 63–74. [Google Scholar] [CrossRef]
- Maity, D.; Kale, S.; Kaul-Ghanekar, R.; Xue, J.-M.; Ding, J. Studies of magnetite nanoparticles synthesized by thermal decomposition of iron (III) acetylacetonate in tri(ethylene glycol). J. Magn. Magn. Mater. 2009, 321, 3093–3098. [Google Scholar] [CrossRef]
- Park, H.J.; McConnell, J.T.; Boddohi, S.; Kipper, M.J.; Johnson, P.A. Synthesis and characterization of enzyme–magnetic nanoparticle complexes: Effect of size on activity and recovery. Colloids Surf. B Biointerfaces 2011, 83, 198–203. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Sharma, I.; Pattanayek, S.K. Effect of surface energy of solid surfaces on the micro- and macroscopic properties of adsorbed BSA and lysozyme. Biophys. Chem. 2017, 226, 14–22. [Google Scholar] [CrossRef]
- Tworek, P.; Rakowski, K.; Szota, M.; Lekka, M.; Jachimska, B. Changes in Secondary Structure and Properties of Bovine Serum Albumin as a Result of Interactions with Gold Surface. Chemphyschem 2024, 25, e202300505. [Google Scholar] [CrossRef] [PubMed]
- Givens, B.E.; Xu, Z.; Fiegel, J.; Grassian, V.H. Bovine serum albumin adsorption on SiO2 and TiO2 nanoparticle surfaces at circumneutral and acidic pH: A tale of two nano-bio surface interactions. J. Colloid Interface Sci. 2017, 493, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Pattanayek, S.K. Effect of characteristics of shear force on secondary structures and viscosity of bovine serum albumin solution. Rheol. Acta 2018, 57, 801–812. [Google Scholar] [CrossRef]
- Alhazmi, H.; Albratty, M.; Meraya, A.; Najmi, A.; Alam, M.; Javed, S.; Ahsan, W. Spectroscopic characterization of the interactions of bovine serum albumin with medicinally important metal ions: Platinum (IV), iridium (III) and iron (II). Acta Biochim. Pol. 2021, 68, 99–107. [Google Scholar] [CrossRef]
- Shang, L.; Wang, Y.; Jiang, J.; Dong, S. pH-dependent protein conformational changes in albumin:gold nanoparticle bioconjugates: A spectroscopic study. Langmuir 2007, 23, 2714–2721. [Google Scholar] [CrossRef]
- Marcelino, A.M.C.; Gierasch, L.M. Roles of beta-turns in protein folding: From peptide models to protein engineering. Biopolymers 2008, 89, 380–391. [Google Scholar] [CrossRef]
- Yu, C.; Al-Saadi, A.; Shih, S.-J.; Qiu, L.; Tam, K.; Tsang, S. Immobilization of BSA on Silica-Coated Magnetic Iron Oxide Nanoparticle. J. Phys. Chem. C-J PHYS CHEM C 2008, 113, 537–543. [Google Scholar] [CrossRef]
- Miller, T.M.; Wiens, J.P.; Viggiano, A.A.; Ard, S.G.; Shuman, N.S. Thermal Electron Attachment to Pyruvic Acid, Thermal Detachment from the Parent Anion, and the Electron Affinity of Pyruvic Acid. J. Phys. Chem. A 2022, 126, 5545–5551. [Google Scholar] [CrossRef]
- Lajtha, A.; Oja, S.; Saransaari, P.; Schousboe, A. Handbook of Neurochemistry and Molecular Neurobiology: Amino Acids and Peptides in the Nervous System; Springer: New York, NY, USA, 2007. [Google Scholar]
- Dhondt, A.; Vanholder, R.; Van Biesen, W.; Lameire, N. The removal of uremic toxins. Kidney Int. 2000, 58, S47–S59. [Google Scholar] [CrossRef]
- Wei, J.; Li, R.; Zhang, P.; Jin, H.; Zhang, Z.; Li, Y.; Chen, Y. Efficient selective removal of uremic toxin precursor by olefin-linked covalent organic frameworks for nephropathy treatment. Nat. Commun. 2023, 14, 2805. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, J.; Ahmed, R.; Huang, G.; Reid, J.; Mandal, R.; Maksymuik, A.; Sitar, D.S.; Tappia, P.S.; Ramjiawan, B. A High-Performing Plasma Metabolite Panel for Early-Stage Lung Cancer Detection. Cancers 2020, 12, 622. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, L.; Johnson, M.; Mandal, R.; Wishart, D.S. Comprehensive Targeted Metabolomic Assay for Urine Analysis. Anal. Chem. 2020, 92, 10627–10634. [Google Scholar] [CrossRef]


| Uremic Toxin | Patient Concentration (Ave ± 1 SD, mg/L) | Experimental Concentration (mg/L) | Solution Composition (mol%) | Ref. |
|---|---|---|---|---|
| 3-Deoxyglucosone | 1.7 ± 1.0 | 1.7 | 0.131 | [28] |
| 4-Ethylphenyl sulfate | 0.242 ± 0.044 | 0.25 | 0.015 | [31] |
| Argininic acid | <0.077 | 0.077 | 0.005 | [28] |
| Asymmetric dimethylarginine | 0.385 ± 0.2884 | 0.385 | 0.024 | [29] |
| Creatinine | 136.0 ± 46.0 | 136 | 15.023 | [28] |
| Dimethyl glycine | 0.5768 | 0.59 | 0.071 | [28] |
| Guanidinopropionic acid | 0.288 ± 0.0183 | 0.29 | 0.028 | [28] |
| Hippuric acid | 247.0 ± 112 | 236 | 16.454 | [28] |
| Homocysteine | 8.1 ± 1.6 | 8.1 | 0.749 | [28] |
| Hypoxanthine | 2.0 ± 1.6 | 2 | 0.184 | [28] |
| Indole acetic acid | 2.03 ± 0.38 | 2.03 | 0.145 | [29] |
| Indoxyl glucuronide | 2.5 ± 0.3 | 2.5 | 0.101 | [29] |
| Indoxyl sulfate | 53.0 ± 91.5 | 53 | 3.106 | [28] |
| Kynurenine | 0.7~1.0 | 0.75 | 0.045 | [29] |
| L-Asparagine | 7.13 ± 3.7 | 7.13 | 0.674 | [32] |
| L-Tyrosine | 54.35 ± 16.3 | 54.35 | 3.747 | [33] |
| Methylhistidine | 0.08459 ± 0.05 | 0.085 | 0.006 | [33] |
| Orotic acid | 0.928 ± 0.489 | 0.928 | 0.074 | [4] |
| p-Cresol sulfate | 20.9 ± 12.2 | 20.9 | 1.387 | [29] |
| Phenylacetic acid | 467.2 ± 10.6 | 467.2 | 42.887 | [29] |
| Phenylalanine | 8.92 ± 1.81 | 9.25 | 0.7 | [34] |
| p-Hydroxy hippuric acid | 4.43 ± 2.79 | 4.25 | 0.272 | [35] |
| p-Hydroxyphenyl acetic acid | 2.43 ± 2.28 | 2.5 | 0.205 | [35] |
| Putrescine | 0.00942 ± 0.00759 | 0.00942 | 0.001 | [29] |
| Pyruvic acid | 11.7 ± 8.6 | 11.7 | 1.661 | [36] |
| Quinolinic acid | 0.0835 | 0.084 | 0.006 | [26] |
| Spermidine | 0.097 ± 0.045 | 0.096 | 0.008 | [29] |
| Spermine | 0.018 ± 0.0162 | 0.018 | 0.001 | [28] |
| Trimethylamine n-oxide | 7.49 ± 2.39 | 7.5 | 1.248 | [37] |
| Uric acid | 83 ± 13 | 83 | 6.169 | [38] |
| Uridine | 9.8 ± 11.4 | 9.8 | 0.501 | [28] |
| Xanthine | 1.5 ± 0.8 | 1.5 | 0.123 | [28] |
| Xanthosine | 96.6 ± 62.9 | 96.6 | 4.247 | [39] |
| Sample | MNPs | MNPs-APTES | MNPs-APTES-GA | MNPs-APTES-GA-BSA (0.2) | MNPs-APTES-GA-BSA (2) |
|---|---|---|---|---|---|
| Fe | 18.75 | 15.28 | 10.97 | 5.94 | 1.78 |
| C | 33.79 | 33.00 | 45.14 | 56.54 | 74.18 |
| O | 47.46 | 46.79 | 36.88 | 27.21 | 18.11 |
| N | - | 2.01 | 3.29 | 8.22 | 5.47 |
| Si | - | 2.93 | 3.71 | 2.10 | 0.46 |
| Secondary Structure | Pure BSA | MNPs-APTES-GA-BSA (0.2) | MNPs-APTES-GA-BSA (2) |
|---|---|---|---|
| β-sheets/turns | 10 ± 2 | 9 ± 2 | 17 ± 2 |
| α-helix | 65 ± 2 | 55 ± 2 | 60 ± 2 |
| Random chains | 5 ± 2 | 9 ± 2 | 7 ± 2 |
| Extended chains/β-sheets | 20 ± 2 | 21 ± 2 | 16 ± 2 |
| Dehydrated β-turns | - | 6 ± 2 |
| Bare MNPs | MNP-APTES-GA | MNP-APTES-GA-BSA | MNP-APTES-GA-BSA | |||||
|---|---|---|---|---|---|---|---|---|
| (0.2 mg/mL) | (2 mg/mL) | |||||||
| Uremic metabolites | 1 h | 4 h | 1 h | 4 h | 1 h | 4 h | 1 h | 4 h |
| 3-Deoxyglucosone | 0.30 | 0.31 | 0.32 | 0.20 | 0.23 | 0.24 | 0.19 | 0.36 |
| 4-Ethylphenyl Sulfate | 1.11 | 0.78 | 0.99 | 1.01 | 1.26 | 1.24 | 0.80 | 1.27 |
| Argininic Acid | - | - | - | - | - | - | - | - |
| Asymmetric Dimethylarginine | - | - | - | - | - | - | - | - |
| Creatinine | 1.82 | 1.06 | 1.18 | 0.87 | 1.19 | 1.27 | 0.95 | 1.18 |
| Dimethyl Glycine | 0.59 | 0.40 | 0.50 | 0.29 | 0.41 | 0.59 | 0.29 | 0.42 |
| Guanidinopropionic Acid | 0.72 | 0.73 | 0.67 | 0.49 | 0.64 | 0.82 | 0.40 | 0.72 |
| Hippuric Acid | 1.09 | 0.88 | 0.99 | 0.66 | 0.84 | 1.03 | 0.60 | 0.85 |
| Homocysteine | - | - | - | - | - | - | - | - |
| Hypoxanthine | 1.34 | 0.87 | 1.13 | 0.73 | 1.09 | 1.22 | 0.94 | 1.14 |
| Indole Acetic Acid | 1.00 | 0.84 | 0.89 | 0.73 | 0.98 | 1.04 | 0.58 | 0.87 |
| Indoxyl Glucuronide | 0.76 | 0.53 | 0.69 | 0.47 | 0.63 | 0.65 | 0.39 | 0.60 |
| Indoxyl Sulfate | 1.07 | 0.76 | 1.20 | 1.46 | 1.60 | 1.73 | 1.01 | 1.69 |
| Kynurenine | 1.80 | 1.63 | - | 0.21 | 0.64 | 0.12 | 0.94 | 0.38 |
| Methylhistidine | 4.10 | 2.44 | 3.63 | 1.93 | 2.02 | 2.95 | 1.76 | 3.20 |
| Orotic Acid | 1.14 | 0.95 | 1.08 | 0.86 | 0.93 | 1.04 | 0.67 | 1.15 |
| p-Cresol Sulfate | 0.35 | 0.27 | 0.33 | 0.29 | 0.31 | 0.33 | 0.23 | 0.34 |
| Phenylacetic Acid | - | - | - | - | - | - | - | - |
| Phenylalanine | 1.14 | 0.58 | 0.78 | 0.53 | 0.76 | 0.99 | 0.52 | 0.82 |
| p-Hydroxyhippuric Acid | 0.81 | 0.76 | 0.82 | 0.60 | 0.74 | 0.89 | 0.50 | 0.77 |
| Pyruvic Acid | 24.14 | 35.17 | 31.50 | 38.70 | 32.84 | 29.00 | 39.75 | 31.73 |
| Quinolinic Acid | 1.16 | 0.77 | 1.09 | 0.74 | 0.99 | 1.05 | 0.64 | 1.02 |
| Spermidine | - | - | - | - | - | - | - | - |
| Spermine | - | - | - | - | - | - | - | - |
| Trimethylamine N-Oxide | 0.97 | 0.56 | 0.73 | 0.55 | 0.70 | 0.83 | 0.49 | 0.74 |
| Uric Acid | 0.84 | 0.70 | 0.83 | 0.55 | 0.67 | 0.82 | 0.56 | 0.79 |
| Uridine | 1.01 | 0.61 | 0.80 | 0.59 | 0.74 | 0.87 | 0.52 | 0.78 |
| Xanthine | - | - | - | - | - | - | - | - |
| Xanthosine | 0.48 | 0.45 | 0.41 | 0.38 | 0.38 | 0.41 | 0.26 | 0.52 |
| Uremic Metabolites | Bare MNPs | MNP-APTES-GA | MNP-APTES-GA-BSA (0.2 mg/mL) | MNP-APTES-GA-BSA (2 mg/mL) | |
|---|---|---|---|---|---|
| 3-Deoxyglucosone | 1.7 | −60.0 | 5.2 | 47.1 | >30 |
| 4-Ethylphenyl Sulfate | −41.5 | 1.9 | −1.5 | 37.3 | 10 to 29 |
| Argininic Acid | - | - | - | - | −9 to 9 |
| Asymmetric Dimethylarginine | - | - | - | - | −29 to −10 |
| Creatinine | −71.9 | −36.0 | 6.2 | 19.2 | −49 to −30 |
| Dimethyl Glycine | −48.5 | −72.3 | 31.0 | 30.8 | <−50 |
| Guanidinopropionic Acid | 0.5 | −35.9 | 22.4 | 44.5 | |
| Hippuric Acid | −24.2 | −50.4 | 18.9 | 30.3 | |
| Homocysteine | - | - | - | - | |
| Hypoxanthine | −53.5 | −55.2 | 10.7 | 17.6 | |
| Indole Acetic Acid | −19.6 | −21.5 | 5.7 | 34.0 | |
| Indoxyl Glucuronide | −44.7 | −47.2 | 3.6 | 34.8 | |
| Indoxyl Sulfate | −42.2 | 17.9 | 7.3 | 40.5 | |
| Kynurenine | −10.6 | 100.0 | −425.0 | −146.1 | |
| Methylhistidine | −67.6 | −87.8 | 31.6 | 44.8 | |
| Orotic Acid | −20.1 | −25.4 | 10.9 | 41.9 | |
| p-Cresol Sulfate | −30.0 | −14.7 | 6.8 | 32.0 | |
| Phenylacetic Acid | - | - | - | - | |
| Phenylalanine | −96.0 | −46.4 | 23.6 | 36.3 | |
| p-Hydroxyhippuric Acid | −7.4 | −36.8 | 16.7 | 35.3 | |
| Pyruvic Acid | 31.4 | 18.6 | −13.2 | −25.3 | |
| Quinolinic Acid | −51.5 | −48.5 | 5.9 | 36.8 | |
| Spermidine | - | - | - | - | |
| Spermine | - | - | - | - | |
| Trimethylamine N-Oxide | −73.3 | −33.2 | 16.4 | 33.6 | |
| Uric Acid | −20.3 | −50.8 | 18.9 | 29.3 | |
| Uridine | −65.8 | −35.0 | 14.4 | 33.3 | |
| Xanthine | - | - | - | - | |
| Xanthosine | −5.9 | −7.2 | 6.3 | 48.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, I.; Milley, A.; Zhang, L.; Zheng, J.; Lockwood, E.; Wishart, D.S.; Tonelli, M.; Unsworth, L.D. Binding Capacity and Adsorption Stability of Uremic Metabolites to Albumin-Modified Magnetic Nanoparticles. Int. J. Mol. Sci. 2025, 26, 5366. https://doi.org/10.3390/ijms26115366
Sharma I, Milley A, Zhang L, Zheng J, Lockwood E, Wishart DS, Tonelli M, Unsworth LD. Binding Capacity and Adsorption Stability of Uremic Metabolites to Albumin-Modified Magnetic Nanoparticles. International Journal of Molecular Sciences. 2025; 26(11):5366. https://doi.org/10.3390/ijms26115366
Chicago/Turabian StyleSharma, Indu, Agatha Milley, Lun Zhang, Jiamin Zheng, Ethan Lockwood, David S. Wishart, Marcello Tonelli, and Larry D. Unsworth. 2025. "Binding Capacity and Adsorption Stability of Uremic Metabolites to Albumin-Modified Magnetic Nanoparticles" International Journal of Molecular Sciences 26, no. 11: 5366. https://doi.org/10.3390/ijms26115366
APA StyleSharma, I., Milley, A., Zhang, L., Zheng, J., Lockwood, E., Wishart, D. S., Tonelli, M., & Unsworth, L. D. (2025). Binding Capacity and Adsorption Stability of Uremic Metabolites to Albumin-Modified Magnetic Nanoparticles. International Journal of Molecular Sciences, 26(11), 5366. https://doi.org/10.3390/ijms26115366







