Colonizing Bacteria Aggravate Inflammation, Cytotoxicity and Immune Defense During Influenza A Virus Infection
Abstract
1. Introduction
2. Results
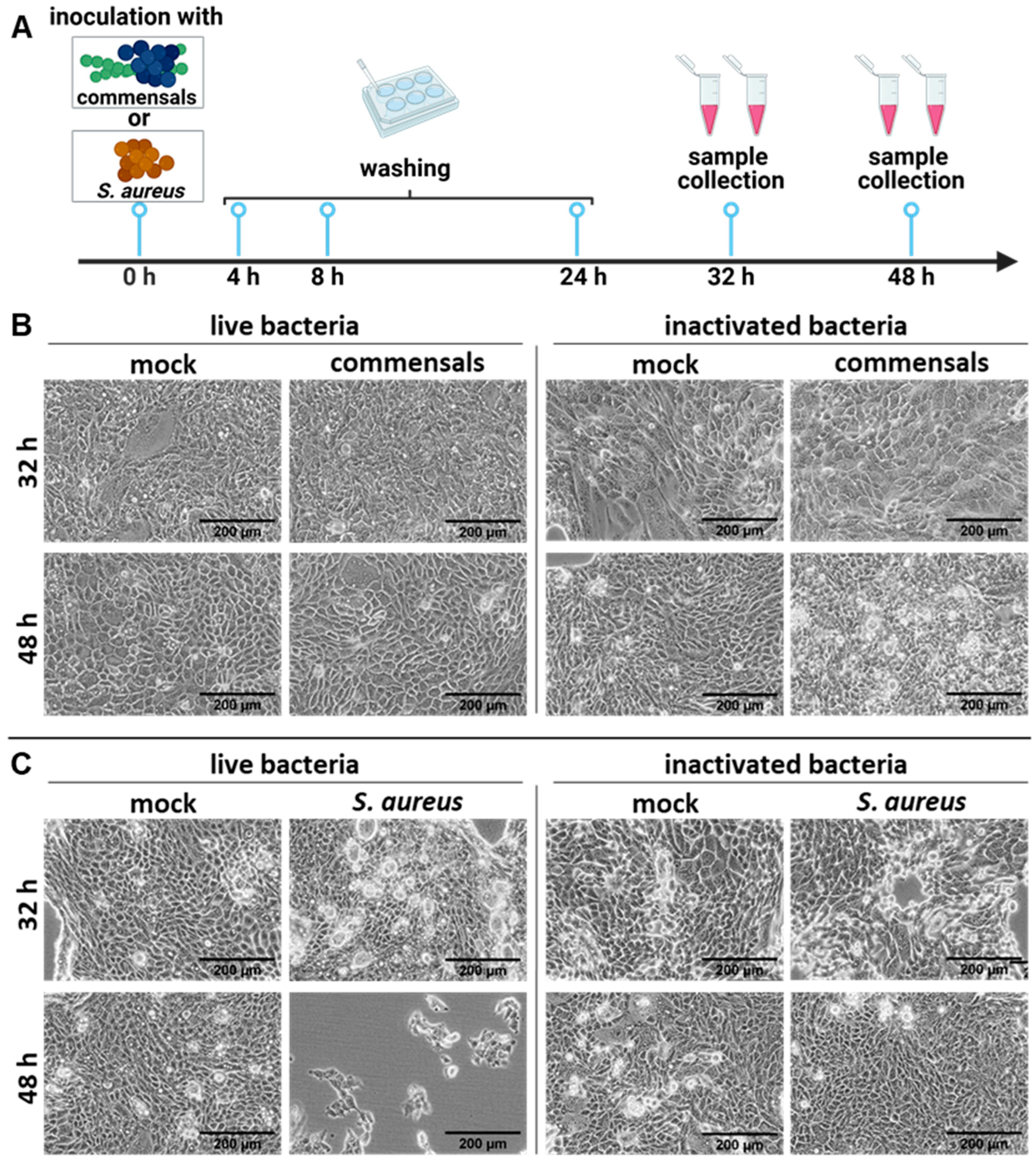
2.1. S. aureus Harms the Integrity of the Epithelial Cell Layer Compared to the Commensal Bacteria S. epidermidis and S. salivarius
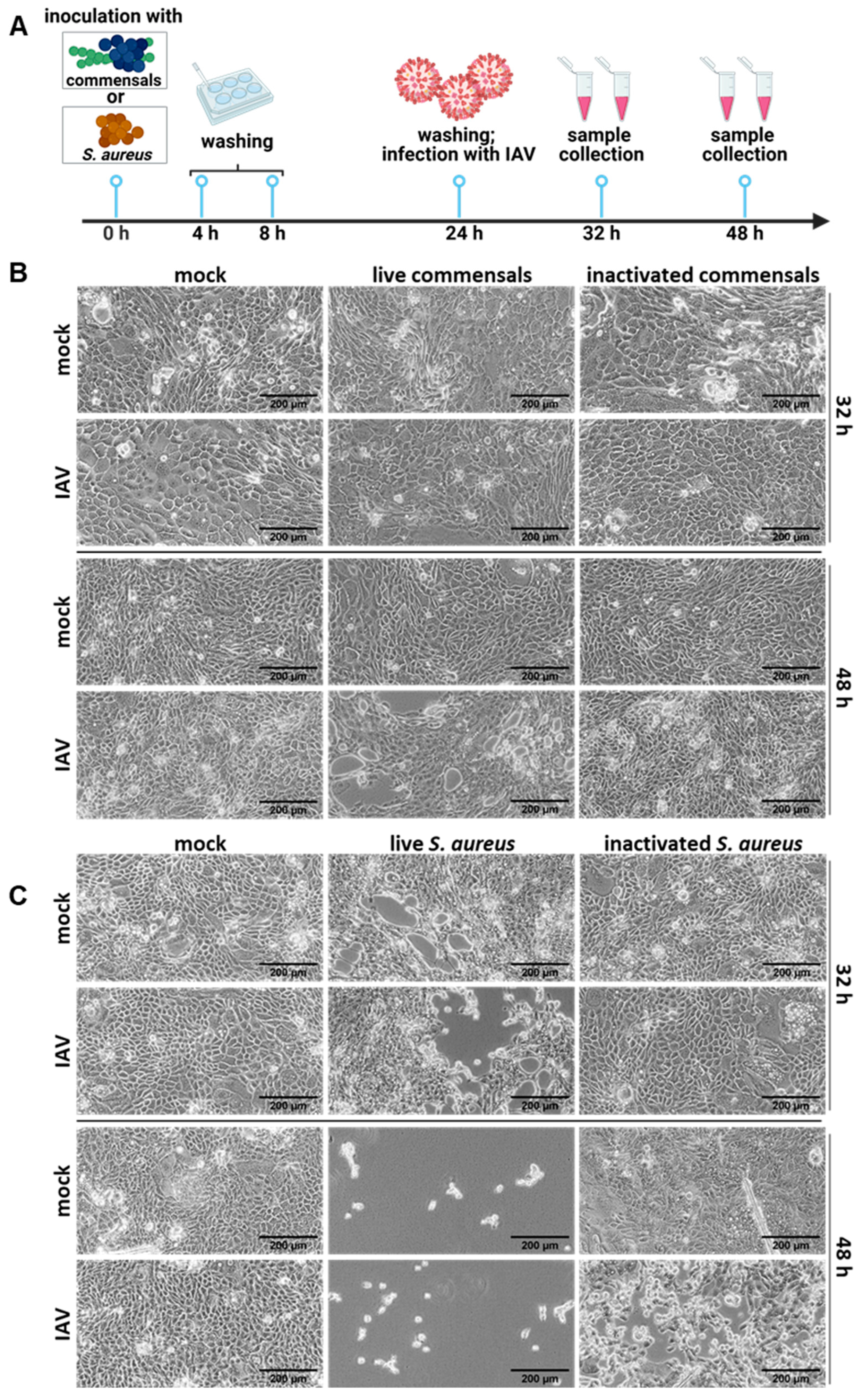
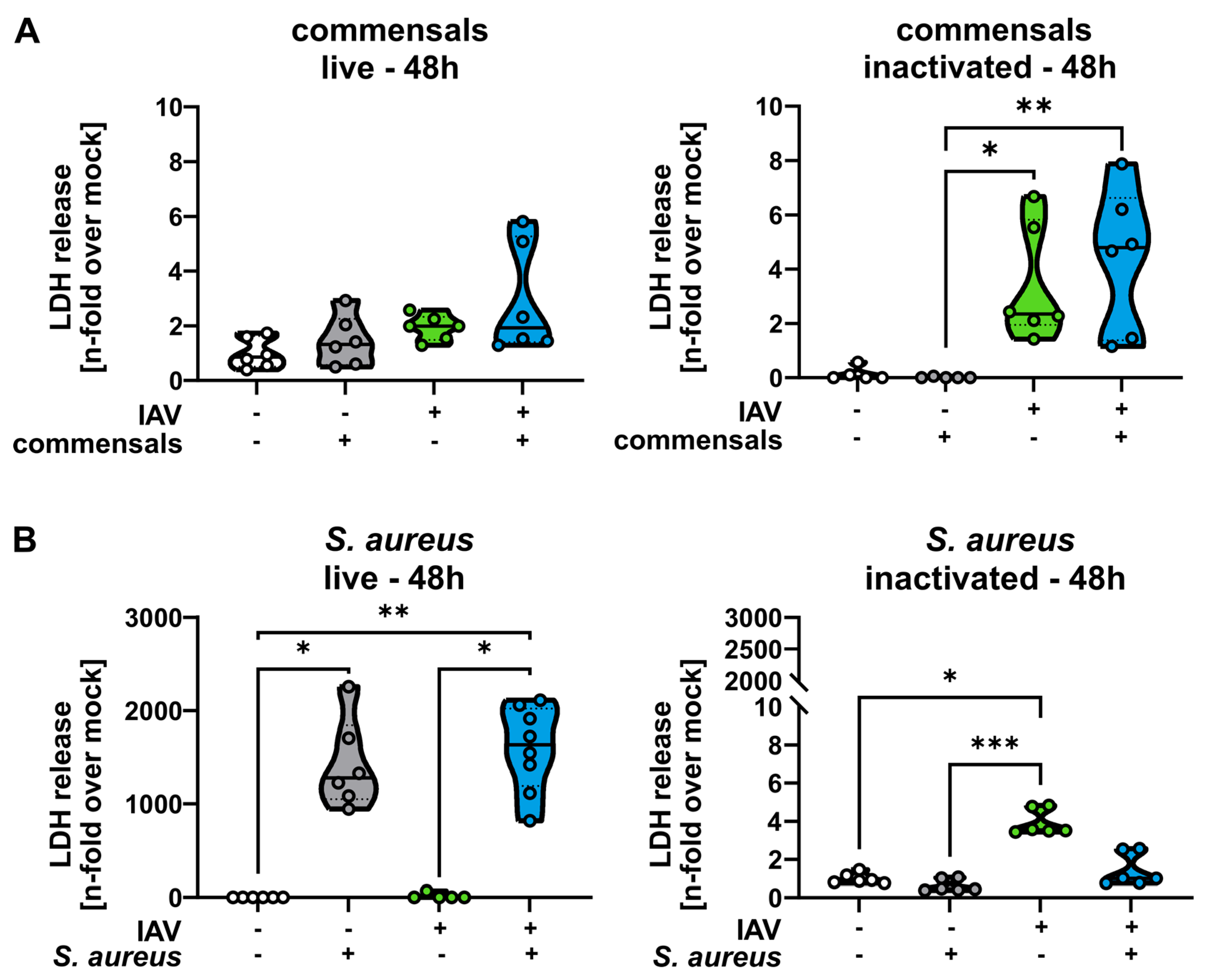
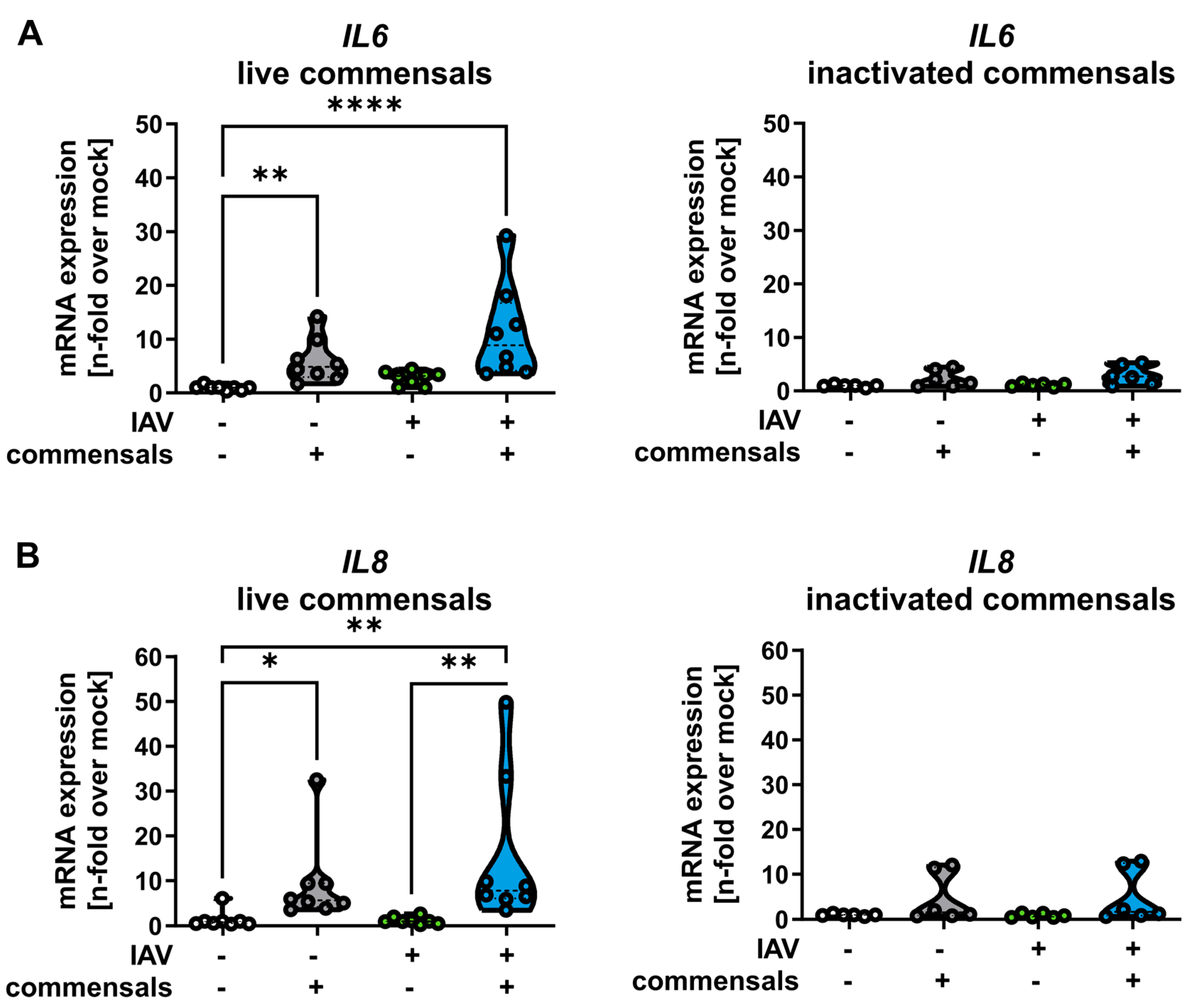
2.2. Colonization of the Epithelial Layer with Commensal Bacteria Does Not Protect from an Influenza Virus Infection, but Causes Inflammation and Enhances Viral Titers
2.3. Inactivated Colonizing Commensal Bacteria Act as Immune Training for Human Monocyte-Derived Macrophages
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Virus Propagation
4.3. Bacterial Culture
4.4. Bacterial Inactivation
4.5. Cell Culture and Viral Infection
4.6. Light Microscopy
4.7. Determination of Extracellular Bacterial Titers
4.8. Determination of Intracellular Bacterial Titers
4.9. RNA Extraction
4.10. cDNA Synthesis
4.11. qRT-PCR
4.12. Lactate Dehydrogenase (LDH)-Assay
4.13. Monocyte Isolation, Differentiation, and Infection
4.14. Protein Analysis
4.15. Flow Cytometry
4.16. Immunofluorescence
4.17. Statistics and Data Visualization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus epidermidis—the ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef]
- Delorme, C.; Abraham, A.L.; Renault, P.; Guédon, E. Genomics of Streptococcus salivarius, a major human commensal. Infect. Genet. Evol. 2015, 33, 381–392. [Google Scholar] [CrossRef]
- Oberbach, A.; Schlichting, N.; Hagl, C.; Lehmann, S.; Kullnick, Y.; Friedrich, M.; Köhl, U.; Horn, F.; Kumbhari, V.; Löffler, B.; et al. Four decades of experience of prosthetic valve endocarditis reflect a high variety of diverse pathogens. Cardiovasc. Res. 2023, 119, 410–428. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Q.; Meng, H.; Lv, H.; Liu, Y.; Liu, J.; Wang, H.; He, L.; Qin, J.; Wang, Y.; et al. Staphylococcus epidermidis Contributes to Healthy Maturation of the Nasal Microbiome by Stimulating Antimicrobial Peptide Production. Cell Host Microbe 2020, 27, 68–78.e5. [Google Scholar] [CrossRef]
- Janek, D.; Zipperer, A.; Kulik, A.; Krismer, B.; Peschel, A. High Frequency and Diversity of Antimicrobial Activities Produced by Nasal Staphylococcus Strains against Bacterial Competitors. PLoS Pathog. 2016, 12, e1005812. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.; Grahnen, H.; Jonsson, G.; Wikner, S. Early Establishment of Streptococcus salivarius in the Mouths of Infants. J. Dent. Res. 1970, 49, 415–418. [Google Scholar]
- Burton, J.P.; Wescombe, P.A.; Moore, C.J.; Chilcott, C.N.; Tagg, J.R. Safety assessment of the oral cavity probiotic Streptococcus salivarius K12. Appl. Environ. Microbiol. 2006, 72, 3050–3053. [Google Scholar] [CrossRef]
- Di Pierro, F.; Donato, G.; Fomia, F.; Adami, T.; Careddu, D.; Cassandro, C.; Albera, R. Preliminary pediatric clinical evaluation of the oral probiotic Streptococcus salivarius K12 in preventing recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes and recurrent acute otitis media. Int. J. Gen. Med. 2012, 5, 991–997. [Google Scholar] [CrossRef][Green Version]
- Passali, D.; Passali, G.C.; Vesperini, E.; Cocca, S.; Visconti, I.C.; Ralli, M.; Bellussi, L.M. The efficacy and tolerability of Streptocococcus salivarius 24SMB and Streptococcus oralis 89a administered as nasal spray in the treatment of recurrent upper respiratory tract infections in children. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 67–72. [Google Scholar]
- Deinhardt-Emmer, S.; Sachse, S.; Geraci, J.; Fischer, C.; Kwetkat, A.; Dawczynski, K.; Tuchscherr, L.; Löffler, B. Virulence patterns of Staphylococcus aureus strains from nasopharyngeal colonization. J. Hosp. Infect. 2018, 100, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Otto, M. Staphylococcus aureus colonisation and strategies for decolonisation. Lancet Microbe 2024, 5, e606–e608. [Google Scholar] [CrossRef] [PubMed]
- von Eiff, C.; Becker, K.; Machka, K.; Stammer, H.; Peters, G. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 2001, 344, 11–16. [Google Scholar] [CrossRef]
- Troeman, D.P.R.; Hazard, D.; Timbermont, L.; Malhotra-Kumar, S.; van Werkhoven, C.H.; Wolkewitz, M.; Ruzin, A.; Goossens, H.; Bonten, M.J.M.; Harbarth, S.; et al. Postoperative Staphylococcus aureus Infections in Patients With and Without Preoperative Colonization. JAMA Netw. Open 2023, 6, e2339793. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Kahl, B.C.; Becker, K.; Loffler, B. Clinical Significance and Pathogenesis of Staphylococcal Small Colony Variants in Persistent Infections. Clin. Microbiol. Rev. 2016, 29, 401–427. [Google Scholar] [CrossRef] [PubMed]
- Tosta, E. The seven constitutive respiratory defense barriers against SARS-CoV-2 infection. Rev. Soc. Bras. Med. Trop. 2021, 54, e04612021. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- GBD 2017 Lower Respiratory Infections Collaborators. Quantifying risks and interventions that have affected the burden of lower respiratory infections among children younger than 5 years: An analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2020, 20, 60–79. [Google Scholar] [CrossRef]
- Tanner, A.R.; Dorey, R.B.; Brendish, N.J.; Clark, T.W. Influenza vaccination: Protecting the most vulnerable. Eur. Respir. Rev. 2021, 30, 200258. [Google Scholar] [CrossRef]
- Li, H.; Wang, A.; Zhang, Y.; Wei, F. Diverse roles of lung macrophages in the immune response to influenza A virus. Front. Microbiol. 2023, 14, 1260543. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Liu, P.F.; Liu, Y.T.; Kuo, S.; Zhang, X.Q.; Schooley, R.T.; Rohde, H.; Gallo, R.L.; Huang, C.M. Nasal commensal Staphylococcus epidermidis counteracts influenza virus. Sci. Rep. 2016, 6, 27870. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; Sequeira, R.P.; Clarke, T.B. The microbiota protects against respiratory infection via GM-CSF signaling. Nat. Commun. 2017, 8, 1512. [Google Scholar] [CrossRef]
- Kikukawa, H.; Nagao, T.; Ota, M.; Takashima, S.; Kitaguchi, K.; Yanase, E.; Maeda, S.; Hara, K.Y. Production of a selective antibacterial fatty acid against Staphylococcus aureus by Bifidobacterium strains. Microbiome Res. Rep. 2023, 2, 4. [Google Scholar] [CrossRef]
- Fraunholz, M.; Sinha, B. Intracellular staphylococcus aureus: Live-in and let die. Front. Cell Infect. Microbiol. 2012, 2, 43. [Google Scholar] [CrossRef]
- Kanmani, P.; Clua, P.; Vizoso-Pinto, M.G.; Rodriguez, C.; Alvarez, S.; Melnikov, V.; Takahashi, H.; Kitazawa, H.; Villena, J. Respiratory Commensal Bacteria Corynebacterium pseudodiphtheriticum Improves Resistance of Infant Mice to Respiratory Syncytial Virus and Streptococcus pneumoniae Superinfection. Front. Microbiol. 2017, 8, 1613. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus aureus toxins. Curr. Opin. Microbiol. 2014, 17, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Krismer, B.; Weidenmaier, C.; Zipperer, A.; Peschel, A. The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat. Rev. Microbiol. 2017, 15, 675–687. [Google Scholar] [CrossRef]
- Tuchscherr, L.; Pöllath, C.; Siegmund, A.; Deinhardt-Emmer, S.; Hoerr, V.; Svensson, C.M.; Thilo Figge, M.; Monecke, S.; Löffler, B. Clinical S. aureus Isolates Vary in Their Virulence to Promote Adaptation to the Host. Toxins 2019, 11, 135. [Google Scholar] [CrossRef]
- Deinhardt-Emmer, S.; Haupt, K.F.; Garcia-Moreno, M.; Geraci, J.; Forstner, C.; Pletz, M.; Ehrhardt, C.; Löffler, B. Staphylococcus aureus Pneumonia: Preceding Influenza Infection Paves the Way for Low-Virulent Strains. Toxins 2019, 11, 734. [Google Scholar] [CrossRef]
- Zipperer, A.; Konnerth, M.C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N.A.; Slavetinsky, C.; Marschal, M.; et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 2016, 535, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Khongthong, S.; Roekngam, N.; Theapparat, Y.; Sunpaweravong, S.; Faroongsarng, D.; Otto, M. Probiotic for pathogen-specific Staphylococcus aureus decolonisation in Thailand: A phase 2, double-blind, randomised, placebo-controlled trial. Lancet Microbe 2023, 4, e75–e83. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Larsen, J.; Li, M.; Walter, A.; Slavetinsky, C.; Both, A.; Sanchez Carballo, P.M.; Stegger, M.; Lehmann, E.; Liu, Y.; et al. Staphylococcus epidermidis clones express Staphylococcus aureus-type wall teichoic acid to shift from a commensal to pathogen lifestyle. Nat. Microbiol. 2021, 6, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Gordon, A.; Shedden, K.; Kuan, G.; Ng, S.; Balmaseda, A.; Foxman, B. The respiratory microbiome and susceptibility to influenza virus infection. PLoS ONE 2019, 14, e0207898. [Google Scholar] [CrossRef]
- Yildiz, S.; Pereira Bonifacio Lopes, J.P.; Bergé, M.; González-Ruiz, V.; Baud, D.; Kloehn, J.; Boal-Carvalho, I.; Schaeren, O.P.; Schotsaert, M.; Hathaway, L.J.; et al. Respiratory tissue-associated commensal bacteria offer therapeutic potential against pneumococcal colonization. eLife 2020, 9, e53581. [Google Scholar] [CrossRef]
- Fu, J.; Liu, X.; Cui, Z.; Zheng, Y.; Jiang, H.; Zhang, Y.; Li, Z.; Liang, Y.; Zhu, S.; Chu, P.K.; et al. Probiotic-based nanoparticles for targeted microbiota modulation and immune restoration in bacterial pneumonia. Natl. Sci. Rev. 2023, 10, nwac221. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Gaush, C.R.; Smith, T.F. Replication and plaque assay of influenza virus in an established line of canine kidney cells. Appl. Microbiol. 1968, 16, 588–594. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giebeler, L.; Ehrhardt, C.; Häder, A.; Lauf, T.; Deinhardt-Emmer, S.; Löffler, B. Colonizing Bacteria Aggravate Inflammation, Cytotoxicity and Immune Defense During Influenza A Virus Infection. Int. J. Mol. Sci. 2025, 26, 5364. https://doi.org/10.3390/ijms26115364
Giebeler L, Ehrhardt C, Häder A, Lauf T, Deinhardt-Emmer S, Löffler B. Colonizing Bacteria Aggravate Inflammation, Cytotoxicity and Immune Defense During Influenza A Virus Infection. International Journal of Molecular Sciences. 2025; 26(11):5364. https://doi.org/10.3390/ijms26115364
Chicago/Turabian StyleGiebeler, Liane, Christina Ehrhardt, Antje Häder, Thurid Lauf, Stefanie Deinhardt-Emmer, and Bettina Löffler. 2025. "Colonizing Bacteria Aggravate Inflammation, Cytotoxicity and Immune Defense During Influenza A Virus Infection" International Journal of Molecular Sciences 26, no. 11: 5364. https://doi.org/10.3390/ijms26115364
APA StyleGiebeler, L., Ehrhardt, C., Häder, A., Lauf, T., Deinhardt-Emmer, S., & Löffler, B. (2025). Colonizing Bacteria Aggravate Inflammation, Cytotoxicity and Immune Defense During Influenza A Virus Infection. International Journal of Molecular Sciences, 26(11), 5364. https://doi.org/10.3390/ijms26115364







