Oxidative Stress and Endothelial Dysfunction: The Pathogenesis of Pediatric Hypertension
Abstract
1. Introduction
2. Reactive Oxygen Species
3. Primary Pediatric Hypertension
3.1. Obesity
3.2. Other Primary Sources of Oxidative Stress
4. Secondary Pediatric Hypertension
4.1. Renal Disease
4.1.1. Uric Acid
4.1.2. Lipid Oxidation
4.1.3. Inflammation
4.1.4. Nitric Oxide Balance and Asymmetric Dimethylarginine
4.2. Coarctation of the Aorta
4.3. Gestational and Neonatal Stressors
4.4. Type 1 Diabetes Mellitus
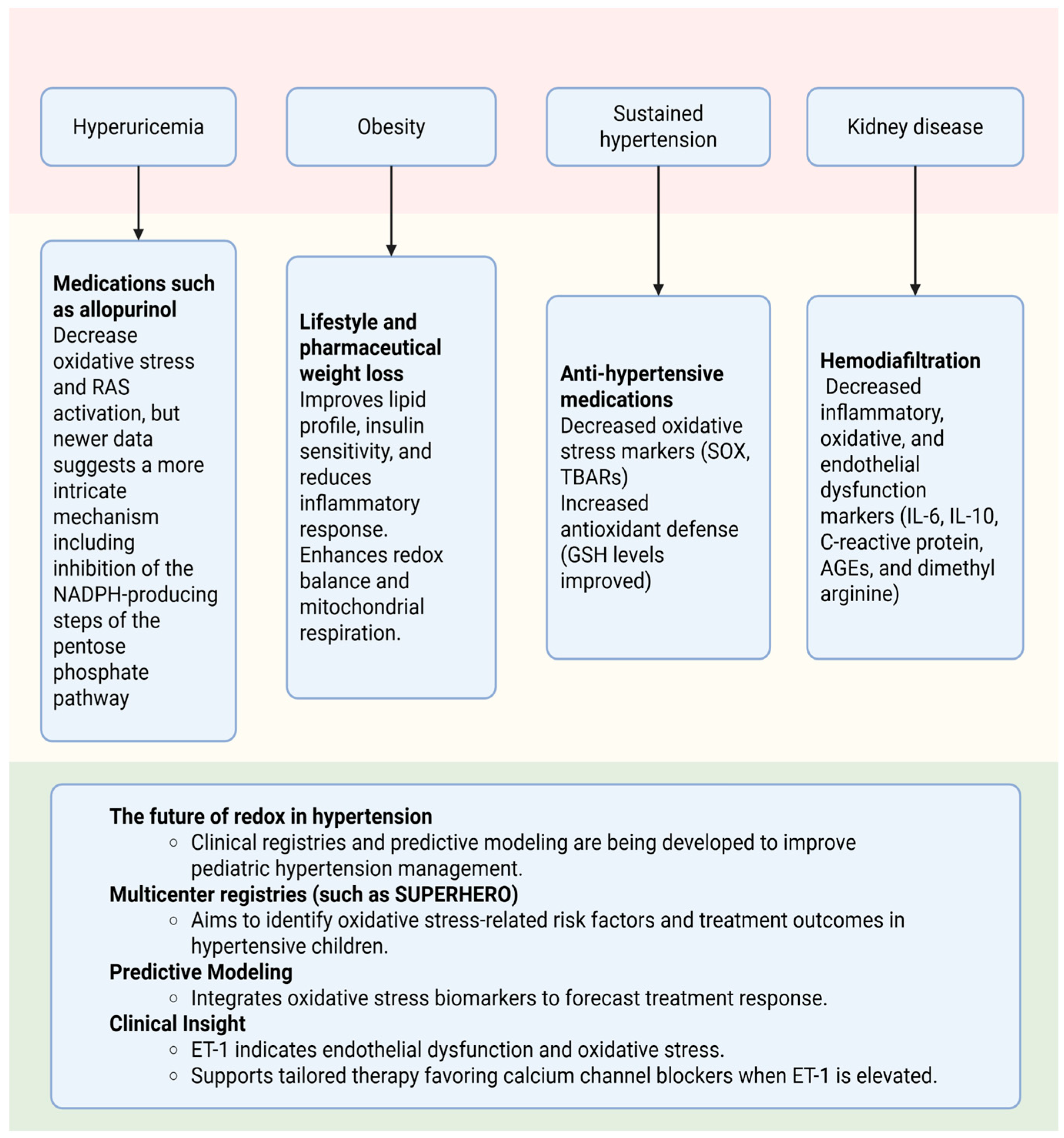
5. The Influence of ROS on the Treatment of Hypertension
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Flynn, J.T.; Kaelber, D.; Baker-Smith, C.; Blowey, D.; Carroll, A.; Daniels, S.; de Ferranti, S.; Dionne, J.; Falkner, B.; Flinn, S.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904, Erratum in Pediatrics 2017, 140, e20173035. https://doi.org/10.1542/peds.2017-3035; Erratum in Pediatrics 2018, 142, e20181739. https://doi.org/10.1542/peds.2018-1739. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.; Samuel, J.; Samuels, J. Prevalence of Hypertension in Children: Applying the New American Academy of Pediatrics Clinical Practice Guideline. Hypertension 2019, 73, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kelishadi, R.; Hong, Y.M.; Khadilkar, A.; Nawarycz, T.; Krzywińska-Wiewiorowska, M.; Aounallah-Skhiri, H.; Motlagh, M.E.; Kim, H.S.; Khadilkar, V.; et al. Impact of the 2017 American Academy of Pediatrics Guideline on Hypertension Prevalence Compared With the Fourth Report in an International Cohort. Hypertension 2019, 74, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Avesani, M.; Calcaterra, G.; Sabatino, J.; Pelaia, G.; Cattapan, I.; Barillà, F.; Martino, F.; Pedrinelli, R.; Bassareo, P.P.; Di Salvo, G. Pediatric Hypertension: A Condition That Matters. Children 2024, 11, 518. [Google Scholar] [CrossRef]
- Ahern, D.; Dixon, E. Pediatric Hypertension: A Growing Problem. Prim. Care Clin. Off. Pract. 2015, 42, 143–150. [Google Scholar] [CrossRef]
- Song, P.; Zhang, Y.; Yu, J. Global Prevalence of Hypertension in Children A Systematic Review and Meta-Analysis. JAMA Pediatr. 2019, 173, 1154–1163. [Google Scholar] [CrossRef]
- Hansen, M.L.; Gunn, P.W.; Kaelber, D.C. Underdiagnosis of Hypertension in Children and Adolescents. JAMA 2007, 298, 874–879. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y. Tracking of Blood Pressure From Childhood to Adulthood: A Systematic Review and Meta–Regression Analysis. Circulation 2008, 117, 3171–3180. [Google Scholar] [CrossRef]
- Theodore, R.F.; Broadbent, J.; Nagin, D.; Ambler, A.; Hogan, S.; Ramrakha, S.; Cutfield, W.; Williams, M.J.A.; Harrington, H.; Moffitt, T.E.; et al. Childhood to Early-Midlife Systolic Blood Pressure Trajectories. Hypertension 2015, 66, 1108–1115. [Google Scholar] [CrossRef]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics 2004, 114, 555–576. [Google Scholar] [CrossRef]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension Guidelines for the Management of High Blood Pressure in Children and Adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef] [PubMed]
- Umemura, S.; Arima, H.; Arima, S.; Asayama, K.; Dohi, Y.; Hirooka, Y.; Horio, T.; Hoshide, S.; Ikeda, S.; Ishimitsu, T.; et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens. Res. 2019, 42, 1235–1481. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.C.; Benoit, G.; Dionne, J.; Feber, J.; Cloutier, L.; Zarnke, K.B.; Padwal, R.S.; Rabi, D.M.; Fournier, A. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, and Assessment of Risk of Pediatric Hypertension. Can. J. Cardiol. 2016, 32, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ma, J.; Song, Y.; Dong, B.; Wang, Z.; Yang, Z.; Wang, X.; Prochaska, J.J. National Blood Pressure Reference for Chinese Han Children and Adolescents Aged 7 to 17 Years. Hypertension 2017, 70, 897–906. [Google Scholar] [CrossRef]
- LASH Guidelines Task Force Steering and Writing Committee; Sánchez, R.; Coca, A.; De Salazar, D.I.M.; Alcocer, L.; Aristizabal, D.; Barbosa, E.; Brandao, A.A.; Diaz-Velazco, M.E.; Hernández-Hernández, R.; et al. 2024 Latin American Society of Hypertension Guidelines on the Management of Arterial Hypertension and Related Comorbidities in Latin America. J. Hypertens. 2025, 43, 1–34. [Google Scholar] [CrossRef]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef]
- Hertiš Petek, T.; Petek, T.; Močnik, M.; Marčun Varda, N. Systemic Inflammation, Oxidative Stress and Cardiovascular Health in Children and Adolescents: A Systematic Review. Antioxidants 2022, 11, 894. [Google Scholar] [CrossRef]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Túri, S.; Friedman, A.; Bereczki, C.; Papp, F.; Kovàcs, J.; Karg, E.; Németh, I. Oxidative Stress in Juvenile Essential Hypertension. J. Hypertens. 2003, 21, 145–152. [Google Scholar] [CrossRef]
- Ostrow, V.; Wu, S.; Aguilar, A.; Bonner, R.; Suarez, E.; De Luca, F. Association between Oxidative Stress and Masked Hypertension in a Multi-Ethnic Population of Obese Children and Adolescents. J. Pediatr. 2011, 158, 628–633.e1. [Google Scholar] [CrossRef]
- Morandi, A.; Corradi, M.; Piona, C.; Fornari, E.; Puleo, R.; Maffeis, C. Systemic Anti-Oxidant Capacity Is Inversely Correlated with Systolic Blood Pressure and Pulse Pressure in Children with Obesity. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Kos, M.; Nađ, T.; Stupin, A.; Drenjančević, I.; Kolobarić, N.; Šušnjara, P.; Mihaljević, Z.; Damašek, M.; Pušeljić, S.; Jukić, I. Juvenile Primary Hypertension Is Associated with Attenuated Macro- and Microvascular Dilator Function Independently of Body Weight. J. Hypertens. 2024, 42, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, R.; Du, X.; Song, W.; Sun, X.; Liu, Z.; Lu, C. Assessment of Oxidative Balance Score with Hypertension and Arterial Stiffness in Children and Adolescents: NHANES 2001–2018. Eur. J. Nutr. 2025, 64, 177. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Taranta-Janusz, K.; Wasilewska, A.; Kossakowska, A.; Zalewska, A. A Case-Control Study of Salivary Redox Homeostasis in Hypertensive Children. Can Salivary Uric Acid Be a Marker of Hypertension? J. Clin. Med. 2020, 9, 837. [Google Scholar] [CrossRef]
- Paripović, D.; Kotur-Stevuljević, J.; Vukašinović, A.; Ilisić, T.; Miloševski-Lomić, G.; Peco-Antić, A. The Influence of Oxidative Stress on Cardiac Remodeling in Obese Adolescents. Scand. J. Clin. Lab. Investig. 2018, 78, 595–600. [Google Scholar] [CrossRef]
- Śladowska-Kozłowska, J.; Litwin, M.; Niemirska, A.; Płudowski, P.; Wierzbicka, A.; Skorupa, E.; Wawer, Z.T.; Janas, R. Oxidative Stress in Hypertensive Children before and after 1 Year of Antihypertensive Therapy. Pediatr. Nephrol. 2012, 27, 1943–1951. [Google Scholar] [CrossRef]
- Orlando, A.; Viazzi, F.; Giussani, M.; Nava, E.; Cazzaniga, E.; Bonino, B.; Palestini, P.; Parati, G.; Genovesi, S. Endothelin-1/Nitric Oxide Balance and HOMA Index in Children with Excess Weight and Hypertension: A Pathophysiological Model of Hypertension. Hypertens. Res. 2019, 42, 1192–1199. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The Thioredoxin Superfamily in Oxidative Protein Folding. Antioxid. Redox Signal. 2014, 21, 457–470. [Google Scholar] [CrossRef]
- Delaunay-Moisan, A.; Ponsero, A.; Toledano, M.B. Reexamining the Function of Glutathione in Oxidative Protein Folding and Secretion. Antioxid. Redox Signal. 2017, 27, 1178–1199. [Google Scholar] [CrossRef]
- Chatterjee, A. Reduced Glutathione: A Radioprotector or a Modulator of DNA-Repair Activity? Nutrients 2013, 5, 525–542. [Google Scholar] [CrossRef]
- Muri, J.; Heer, S.; Matsushita, M.; Pohlmeier, L.; Tortola, L.; Fuhrer, T.; Conrad, M.; Zamboni, N.; Kisielow, J.; Kopf, M. The Thioredoxin-1 System Is Essential for Fueling DNA Synthesis during T-Cell Metabolic Reprogramming and Proliferation. Nat. Commun. 2018, 9, 1851. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Cidlowski, J.A. Apoptosis and Glutathione: Beyond an Antioxidant. Cell Death Differ. 2009, 16, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Masutani, H.; Ueda, S.; Yodoi, J. The Thioredoxin System in Retroviral Infection and Apoptosis. Cell Death Differ. 2005, 12, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jia, Z.; Trush, M.A. Defining ROS in Biology and Medicine. React. Oxyg. Species 2016, 1, 9–21. [Google Scholar] [CrossRef]
- Ning, B.; Hang, S.; Zhang, W.; Mao, C.; Li, D. An Update on the Bridging Factors Connecting Autophagy and Nrf2 Antioxidant Pathway. Front. Cell Dev. Biol. 2023, 11, 1232241. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Y.; Saredy, J.; Wang, X.; Drummer Iv, C.; Shao, Y.; Saaoud, F.; Xu, K.; Liu, M.; Yang, W.Y.; et al. ROS Systems Are a New Integrated Network for Sensing Homeostasis and Alarming Stresses in Organelle Metabolic Processes. Redox Biol. 2020, 37, 101696. [Google Scholar] [CrossRef]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen Peroxide Sensing, Signaling and Regulation of Transcription Factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef]
- Ma, W.-X.; Li, C.-Y.; Tao, R.; Wang, X.-P.; Yan, L.-J. Reductive Stress-Induced Mitochondrial Dysfunction and Cardiomyopathy. Oxidative Med. Cell. Longev. 2020, 2020, 5136957. [Google Scholar] [CrossRef]
- Falkner, B.; Lurbe, E. Primordial Prevention of High Blood Pressure in Childhood: An Opportunity Not to Be Missed. Hypertension 2020, 75, 1142–1150. [Google Scholar] [CrossRef]
- Hassan, M.A.; Zhou, W.; Ye, M.; He, H.; Gao, Z. The Effectiveness of Physical Activity Interventions on Blood Pressure in Children and Adolescents: A Systematic Review and Network Meta-Analysis. J. Sport Health Sci. 2024, 13, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; He, X.; Liu, Q.; Ren, Y.; Xu, S.; Chen, L.; Wang, F.; Bi, Y.; Peng, Z. The Impact of Dietary and Sleep Rhythms on Blood Pressure in Children and Adolescents: A Cross-Sectional Study. Hypertens. Res. 2024, 47, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.K.; Lutzker, L.; Holm, S.; Margolis, H.; Neophytou, A.; Eisen, E.; Costello, S.; Tyner, T.; Holland, N.; Tindula, G.; et al. Traffic-Related Air Pollution Is Associated with Glucose Dysregulation, Blood Pressure, and Oxidative Stress in Children. Environ. Res. 2021, 195, 110870. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Kim, S.H. Obesity and Hypertension in Children and Adolescents. Clin. Hypertens. 2024, 30, 23. [Google Scholar] [CrossRef]
- Correia-Costa, L.; Sousa, T.; Morato, M.; Cosme, D.; Afonso, J.; Areias, J.C.; Schaefer, F.; Guerra, A.; Afonso, A.C.; Azevedo, A.; et al. Oxidative Stress and Nitric Oxide Are Increased in Obese Children and Correlate with Cardiometabolic Risk and Renal Function. Br. J. Nutr. 2016, 116, 805–815. [Google Scholar] [CrossRef]
- Charakida, M.; Jones, A.; Falaschetti, E.; Khan, T. Childhood Obesity and Vascular Phenotypes: A Population Study. J. Am. Coll. Cardiol. 2012, 60, 2643–2650. [Google Scholar] [CrossRef]
- Lo, M.-H.; Lin, I.-C.; Lu, P.-C.; Huang, C.-F.; Chien, S.-J.; Hsieh, K.-S.; Tain, Y.-L. Evaluation of Endothelial Dysfunction, Endothelial Plasma Markers, and Traditional Metabolic Parameters in Children with Adiposity. J. Formos. Med. Assoc. 2019, 118, 83–91. [Google Scholar] [CrossRef]
- Li, Y.; Haseler, E.; McNally, R.; Sinha, M.; Chowienczyk, P. A Meta-Analysis of the Haemodynamics of Primary Hypertension in Children and Adults. J. Hypertens. 2023, 41, 212–219. [Google Scholar] [CrossRef]
- Monostori, P.; Baráth, Á.; Fazekas, I.; Hódi, E.; Máté, A.; Farkas, I.; Hracskó, Z.; Varga, I.S.; Sümegi, V.; Gellén, B.; et al. Microvascular Reactivity in Lean, Overweight, and Obese Hypertensive Adolescents. Eur. J. Pediatr. 2010, 169, 1369–1374. [Google Scholar] [CrossRef]
- Kerr, J.A.; Patton, G.C.; Cini, K.I.; Abate, Y.H.; Abbas, N.; Magied, A.H.A.A.A.; ElHafeez, S.A.; Abd-Elsalam, S.; Abdollahi, A.; Abdoun, M.; et al. Global, Regional, and National Prevalence of Child and Adolescent Overweight and Obesity, 1990–2021, with Forecasts to 2050: A Forecasting Study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 785–812. [Google Scholar] [CrossRef]
- Sunil, B.; Ashraf, A.P. Dyslipidemia in Pediatric Type 2 Diabetes Mellitus. Curr. Diab. Rep. 2020, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Bendor, C.D.; Bardugo, A.; Pinhas-Hamiel, O.; Afek, A.; Twig, G. Cardiovascular Morbidity, Diabetes and Cancer Risk among Children and Adolescents with Severe Obesity. Cardiovasc. Diabetol. 2020, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; Do Carmo, J.M.; Da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-Induced Hypertension: Interaction of Neurohumoral and Renal Mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Liu, Y.-H.; Wu, D.-W.; Su, H.-M.; Chen, S.-C. Dyslipidemia Increases the Risk of Incident Hypertension in a Large Taiwanese Population Follow-Up Study. Nutrients 2022, 14, 3277. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Xia, N. The Interplay Between Adipose Tissue and Vasculature: Role of Oxidative Stress in Obesity. Front. Cardiovasc. Med. 2021, 8, 650214. [Google Scholar] [CrossRef]
- Skapino, E.; Gonzalez-Gayan, L.; Seral-Cortes, M.; Sabroso-Lasa, S.; Llorente-Cereza, M.T.; Leis, R.; Aguilera, C.M.; Gil-Campos, M.; Moreno, L.A.; Bueno-Lozano, G. Independent Effect of Body Fat Content on Inflammatory Biomarkers in Children and Adolescents: The GENOBOX Study. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103811. [Google Scholar] [CrossRef]
- Nigro, E.; Scudiero, O.; Ludovica Monaco, M.; Polito, R.; Schettino, P.; Grandone, A.; Perrone, L.; Miraglia Del Giudice, E.; Daniele, A. Adiponectin Profile and Irisin Expression in Italian Obese Children: Association with Insulin-Resistance. Cytokine 2017, 94, 8–13. [Google Scholar] [CrossRef]
- Cadenas-Sanchez, C.; Cabeza, R.; Idoate, F.; Osés, M.; Medrano, M.; Villanueva, A.; Arenaza, L.; Sanz, A.; Ortega, F.B.; Ruiz, J.R.; et al. Effects of a Family-Based Lifestyle Intervention Plus Supervised Exercise Training on Abdominal Fat Depots in Children With Overweight or Obesity: A Secondary Analysis of a Nonrandomized Clinical Trial. JAMA Netw. Open 2022, 5, e2243864. [Google Scholar] [CrossRef]
- Gupta, A.; Uribarri, J. Dietary Advanced Glycation End Products and Their Potential Role in Cardiometabolic Disease in Children. Horm. Res. Paediatr. 2016, 85, 291–300. [Google Scholar] [CrossRef]
- Cepas, V.; Collino, M.; Mayo, J.C.; Sainz, R.M. Redox Signaling and Advanced Glycation Endproducts (AGEs) in Diet-Related Diseases. Antioxidants 2020, 9, 142. [Google Scholar] [CrossRef]
- Tsukahara, H.; Sekine, K.; Uchiyama, M.; Kawakami, H.; Hata, I.; Todoroki, Y.; Hiraoka, M.; Kaji, M.; Yorifuji, T.; Momoi, T.; et al. Formation of Advanced Glycosylation End Products and Oxidative Stress in Young Patients with Type 1 Diabetes. Pediatr. Res. 2003, 54, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Dello Russo, M.; Sirangelo, I.; Lauria, F.; Formisano, A.; Iannuzzi, C.; Hebestreit, A.; Pala, V.; Siani, A.; Russo, P. Dietary Advanced Glycation End Products (AGEs) and Urinary Fluorescent AGEs in Children and Adolescents: Findings from the Italian I.Family Project. Nutrients 2024, 16, 1831. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Lee, J.; Lim, J.; Cho, S.; An, S.; Lee, M.; Yoon, N.; Seo, M.; Lim, S.; Park, S. Soluble RAGE Attenuates AngII-Induced Endothelial Hyperpermeability by Disrupting HMGB1-Mediated Crosstalk between AT1R and RAGE. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Montagnani, M.; Koh, K.K.; Quon, M.J. Cardiovascular Actions of Insulin. Endocr. Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef]
- De Nigris, V.; Pujadas, G.; La Sala, L.; Testa, R.; Genovese, S.; Ceriello, A. Short-Term High Glucose Exposure Impairs Insulin Signaling in Endothelial Cells. Cardiovasc. Diabetol. 2015, 14, 114. [Google Scholar] [CrossRef]
- Kim, J.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal Relationships Between Insulin Resistance and Endothelial Dysfunction: Molecular and Pathophysiological Mechanisms. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef]
- Ozgen, I.T.; Tascilar, M.E.; Bilir, P.; Boyraz, M.; Guncikan, M.N.; Akay, C.; Dundaroz, R. Oxidative Stress in Obese Children and Its Relation with Insulin Resistance. J. Pediatr. Endocrinol. Metab. 2012, 25, 261–266. [Google Scholar] [CrossRef]
- Hudish, L.I.; Reusch, J.E.B.; Sussel, L. β Cell Dysfunction during Progression of Metabolic Syndrome to Type 2 Diabetes. J. Clin. Investig. 2019, 129, 4001–4008. [Google Scholar] [CrossRef]
- Dabelea, D.; Mayer-Davis, E.J.; Andrews, J.S.; Dolan, L.M.; Pihoker, C.; Hamman, R.F.; Greenbaum, C.; Marcovina, S.; Fujimoto, W.; Linder, B.; et al. Clinical Evolution of Beta Cell Function in Youth with Diabetes: The SEARCH for Diabetes in Youth Study. Diabetologia 2012, 55, 3359–3368. [Google Scholar] [CrossRef]
- Gehrmann, W.; Elsner, M.; Lenzen, S. Role of Metabolically Generated Reactive Oxygen Species for Lipotoxicity in Pancreatic β-cells. Diabetes Obes. Metab. 2010, 12, 149–158. [Google Scholar] [CrossRef]
- Hierons, S.J.; Abbas, K.; Sobczak, A.I.S.; Cerone, M.; Smith, T.K.; Ajjan, R.A.; Stewart, A.J. Changes in Plasma Free Fatty Acids in Obese Patients before and after Bariatric Surgery Highlight Alterations in Lipid Metabolism. Sci. Rep. 2022, 12, 15337. [Google Scholar] [CrossRef] [PubMed]
- Kleiboeker, B.; Lodhi, I.J. Peroxisomal Regulation of Energy Homeostasis: Effect on Obesity and Related Metabolic Disorders. Mol. Metab. 2022, 65, 101577. [Google Scholar] [CrossRef] [PubMed]
- Karpe, F.; Dickmann, J.R.; Frayn, K.N. Fatty Acids, Obesity, and Insulin Resistance: Time for a Reevaluation. Diabetes 2011, 60, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Stancill, J.S.; Broniowska, K.A.; Oleson, B.J.; Naatz, A.; Corbett, J.A. Pancreatic β-Cells Detoxify H2O2 through the Peroxiredoxin/Thioredoxin Antioxidant System. J. Biol. Chem. 2019, 294, 4843–4853. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H. Oxidative Stress in Pancreatic Beta Cell Regeneration. Oxidative Med. Cell. Longev. 2017, 2017, 1930261. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic Reticulum Stress: Molecular Mechanism and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef]
- Hu, X.; Hu, C.; Liu, J.; Wu, Z.; Duan, T.; Cao, Z. 1,25-(OH)D Protects Pancreatic Beta Cells against HO-Induced Apoptosis through Inhibiting the PERK-ATF4-CHOP Pathway. Acta Biochim. Biophys. Sin. 2020, 53, 46–53. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, Y.; Chen, X.; Wang, L.; Chen, C.; Qiu, C. Liraglutide Ameliorates Beta-Cell Function, Alleviates Oxidative Stress and Inhibits Low Grade Inflammation in Young Patients with New-Onset Type 2 Diabetes. Diabetol. Metab. Syndr. 2018, 10, 91. [Google Scholar] [CrossRef]
- Bhardwaj, R.L.; Parashar, A.; Parewa, H.P.; Vyas, L. An Alarming Decline in the Nutritional Quality of Foods: The Biggest Challenge for Future Generations’ Health. Foods 2024, 13, 877. [Google Scholar] [CrossRef]
- Leyvraz, M.; Chatelan, A.; Da Costa, B.R.; Taffé, P.; Paradis, G.; Bovet, P.; Bochud, M.; Chiolero, A. Sodium Intake and Blood Pressure in Children and Adolescents: A Systematic Review and Meta-Analysis of Experimental and Observational Studies. Int. J. Epidemiol. 2018, 47, 1796–1810. [Google Scholar] [CrossRef]
- Ketonen, J.; Mervaala, E. Effects of Dietary Sodium on Reactive Oxygen Species Formation and Endothelial Dysfunction in Low-Density Lipoprotein Receptor-Deficient Mice on High-Fat Diet. Heart Vessels 2008, 23, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Mwene-Batu, P.; Lemogoum, D.; De Le Hoye, L.; Bisimwa, G.; Hermans, M.P.; Minani, J.; Amani, G.; Mateso, G.-Q.; Cikomola, J.C.; Dramaix, M.; et al. Association between Severe Acute Malnutrition during Childhood and Blood Pressure during Adulthood in the Eastern Democratic Republic of the Congo: The Lwiro Cohort Study. BMC Public Health 2021, 21, 847. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Mehndiratta, M.; Aaradhana; Shah, D.; Gupta, P. Oxidative Stress, Mitochondrial Dysfunction, and Premature Ageing in Severe Acute Malnutrition in Under-Five Children. Indian J. Pediatr. 2022, 89, 558–562, Erratum in Indian J. Pediatr. 2022, 89, 635. https://doi.org/10.1007/s12098-022-04136-w. [Google Scholar] [CrossRef] [PubMed]
- Berlic, M.; Korošec, M.; Remec, Ž.I.; Čuk, V.; Battelino, T.; Repič Lampret, B. Effect of Antioxidant-Rich Kindergarten Meals on Oxidative Stress Biomarkers in Healthy 5–6-Year-Old Children: A Randomized Controlled Trial. Eur. J. Pediatr. 2024, 183, 3085–3094. [Google Scholar] [CrossRef]
- Avloniti, A.; Chatzinikolaou, A.; Deli, C.; Vlachopoulos, D.; Gracia-Marco, L.; Leontsini, D.; Draganidis, D.; Jamurtas, A.; Mastorakos, G.; Fatouros, I. Exercise-Induced Oxidative Stress Responses in the Pediatric Population. Antioxidants 2017, 6, 6. [Google Scholar] [CrossRef]
- Llorente-Cantarero, F.J.; Aguilar-Gómez, F.J.; Leis, R.; Bueno, G.; Rupérez, A.I.; Anguita-Ruiz, A.; Vázquez-Cobela, R.; Mesa, M.D.; Moreno, L.A.; Gil, Á.; et al. Relationship between Physical Activity, Oxidative Stress, and Total Plasma Antioxidant Capacity in Spanish Children from the GENOBOX Study. Antioxidants 2021, 10, 320. [Google Scholar] [CrossRef]
- De Ciuceis, C.; Rizzoni, D.; Palatini, P. Microcirculation and Physical Exercise In Hypertension. Hypertension 2023, 80, 730–739. [Google Scholar] [CrossRef]
- Dong, G.-H.; Qian, Z.; Trevathan, E.; Zeng, X.-W.; Vaughn, M.G.; Wang, J.; Zhao, Y.; Liu, Y.-Q.; Ren, W.-H.; Qin, X.-D. Air Pollution Associated Hypertension and Increased Blood Pressure May Be Reduced by Breastfeeding in Chinese Children: The Seven Northeastern Cities Chinese Children’s Study. Int. J. Cardiol. 2014, 176, 956–961. [Google Scholar] [CrossRef]
- Huang, M.; Chen, J.; Yang, Y.; Yuan, H.; Huang, Z.; Lu, Y. Effects of Ambient Air Pollution on Blood Pressure Among Children and Adolescents: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e017734. [Google Scholar] [CrossRef]
- Guarnieri, M.; Balmes, J.R. Outdoor Air Pollution and Asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Broussard, J.L.; Ehrmann, D.A.; Van Cauter, E.; Tasali, E.; Brady, M.J. Impaired Insulin Signaling in Human Adipocytes After Experimental Sleep Restriction: A Randomized, Crossover Study. Ann. Intern. Med. 2012, 157, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tian, L.; Ma, D.; Wu, P.; Tang, Y.; Cui, X.; Xu, Z. Autonomic Nervous Function and Low-Grade Inflammation in Children with Sleep-Disordered Breathing. Pediatr. Res. 2022, 91, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hakim, F.; Kheirandish-Gozal, L.; Gozal, D. Inflammatory Pathways in Children with Insufficient or Disordered Sleep. Respir. Physiol. Neurobiol. 2011, 178, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Ingelfinger, J.R. Adverse Childhood Experiences and Their Relevance to Hypertension in Children and Youth. In Pediatric Hypertension; Flynn, J.T., Ingelfinger, J.R., Brady, T., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–12. ISBN 978-3-319-31420-4. [Google Scholar]
- Horn, S.R.; Leve, L.D.; Levitt, P.; Fisher, P.A. Childhood Adversity, Mental Health, and Oxidative Stress: A Pilot Study. PLoS ONE 2019, 14, e0215085. [Google Scholar] [CrossRef]
- Jenkins, N.D.M.; Rogers, E.M.; Banks, N.F.; Tomko, P.M.; Sciarrillo, C.M.; Emerson, S.R.; Taylor, A.; Teague, T.K. Childhood Psychosocial Stress Is Linked with Impaired Vascular Endothelial Function, Lower SIRT1, and Oxidative Stress in Young Adulthood. Am. J. Physiol.-Heart Circ. Physiol. 2021, 321, H532–H541. [Google Scholar] [CrossRef]
- Flynn, J.; Zhang, Y.; Solar-Yohay, S.; Shi, V. Clinical and Demographic Characteristics of Children with Hypertension. Hypertension 2012, 60, 1047–1054. [Google Scholar] [CrossRef]
- Gupta-Malhotra, M.; Banker, A.; Shete, S.; Hashmi, S.S.; Tyson, J.E.; Barratt, M.S.; Hecht, J.T.; Milewicz, D.M.; Boerwinkle, E. Essential Hypertension vs. Secondary Hypertension Among Children. Am. J. Hypertens. 2015, 28, 73–80. [Google Scholar] [CrossRef]
- Nugent, J.T.; Young, C.; Funaro, M.C.; Jiang, K.; Saran, I.; Ghazi, L.; Wilson, F.P.; Greenberg, J.H. Prevalence of Secondary Hypertension in Otherwise Healthy Youths with a New Diagnosis of Hypertension: A Meta-Analysis. J. Pediatr. 2022, 244, 30–37.e10. [Google Scholar] [CrossRef]
- Yang, W.-C.; Wu, H.-P. Clinical Analysis of Hypertension in Children Admitted to the Emergency Department. Pediatr. Neonatol. 2010, 51, 44–51. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, P.; Liu, X.; Gong, C.; Li, C.; Yuan, Y.; Zheng, H.; Xu, X.; Dong, H.; Kong, Q.; et al. Characteristics of Pediatric Inpatients with Primary and Secondary Hypertension. Pediatr. Investig. 2021, 5, 28–32. [Google Scholar] [CrossRef]
- Safdar, O.; AlJehani, R.; Aljuhani, M.; AlGhamdi, H.; Asiri, A.; AlGhofaily, O.; Hisan, F.; Altabsh, G. Hypertension in Pediatric Patients Admitted to Inpatient Ward at King Abdulaziz Universty Hospital in Saudi Arabia: Prevalence, Causes, and Outcomes. J. Fam. Med. Prim. Care 2020, 9, 4031. [Google Scholar] [CrossRef] [PubMed]
- Haseler, E.; Singh, C.; Newton, J.; Melhem, N.; Sinha, M.D. Demographics of Childhood Hypertension in the UK: A Report from the Southeast England. J. Hum. Hypertens. 2022, 37, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, Y.; Qin, Y.; Pang, Y. Clinical Characteristics and Factors Associated With Hypertension in 205 Hospitalized Children: A Single-Center Study in Southwest China. Front. Pediatr. 2021, 9, 620158. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Meyers, K.E.; Vidi, S.R. Secondary Forms of Hypertension in Children: Overview. In Pediatric Hypertension; Flynn, J., Ingelfinger, J.R., Redwine, K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–20. ISBN 978-3-319-31420-4. [Google Scholar]
- Patel, P.A.; Cahill, A.M. Renovascular Hypertension in Children. CVIR Endovasc. 2021, 4, 10. [Google Scholar] [CrossRef]
- Friederich-Persson, M.; Thörn, E.; Hansell, P.; Nangaku, M.; Levin, M.; Palm, F. Kidney Hypoxia, Attributable to Increased Oxygen Consumption, Induces Nephropathy Independently of Hyperglycemia and Oxidative Stress. Hypertension 2013, 62, 914–919. [Google Scholar] [CrossRef]
- Gherghina, M.-E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric Acid and Oxidative Stress—Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef]
- Kimura, Y.; Tsukui, D.; Kono, H. Uric Acid in Inflammation and the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 12394. [Google Scholar] [CrossRef]
- Rodenbach, K.E.; Schneider, M.F.; Furth, S.L.; Moxey-Mims, M.M.; Mitsnefes, M.M.; Weaver, D.J.; Warady, B.A.; Schwartz, G.J. Hyperuricemia and Progression of CKD in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort Study. Am. J. Kidney Dis. 2015, 66, 984–992. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Roem, J.L.; Hooper, S.R.; Furth, S.L.; Weaver, D.J.; Warady, B.A.; Schneider, M.F. Longitudinal Changes in Uric Acid Concentration and Their Relationship with Chronic Kidney Disease Progression in Children and Adolescents. Pediatr. Nephrol. 2023, 38, 489–497. [Google Scholar] [CrossRef]
- Feig, D.I.; Johnson, R.J. Hyperuricemia in Childhood Primary Hypertension. Hypertension 2003, 42, 247–252. [Google Scholar] [CrossRef]
- Scheepers, L.E.J.M.; Boonen, A.; Pijnenburg, W.; Bierau, J.; Staessen, J.A.; Stehouwer, C.D.A.; Thijs, C.; Arts, I.C.W. Associations of Plasma Uric Acid and Purine Metabolites with Blood Pressure in Children: The KOALA Birth Cohort Study. J. Hypertens. 2017, 35, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Valle, M.; Martos, R.; Cañete, M.D.; Valle, R.; Van Donkelaar, E.L.; Bermudo, F.; Cañete, R. Association of Serum Uric Acid Levels to Inflammation Biomarkers and Endothelial Dysfunction in Obese Prepubertal Children: Uric Acid and Inflammation in Children. Pediatr. Diabetes 2015, 16, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, S.; Raghavamenon, A.; Garelnabi, M.O.; Santanam, N. Oxidized Low-Density Lipoprotein. In Free Radicals and Antioxidant Protocols; Uppu, R.M., Murthy, S.N., Pryor, W.A., Parinandi, N.L., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 610, pp. 403–417. ISBN 978-1-58829-710-5. [Google Scholar]
- Wen, Y.; Leake, D.S. Low Density Lipoprotein Undergoes Oxidation Within Lysosomes in Cells. Circ. Res. 2007, 100, 1337–1343. [Google Scholar] [CrossRef]
- Shroff, R.; Speer, T.; Colin, S.; Charakida, M.; Zewinger, S.; Staels, B.; Chinetti-Gbaguidi, G.; Hettrich, I.; Rohrer, L.; O’Neill, F.; et al. HDL in Children with CKD Promotes Endothelial Dysfunction and an Abnormal Vascular Phenotype. J. Am. Soc. Nephrol. 2014, 25, 2658–2668. [Google Scholar] [CrossRef]
- Kaseda, R.; Jabs, K.; Hunley, T.E.; Jones, D.; Bian, A.; Allen, R.M.; Vickers, K.C.; Yancey, P.G.; Linton, M.F.; Fazio, S.; et al. Dysfunctional High-Density Lipoproteins in Children with Chronic Kidney Disease. Metabolism 2015, 64, 263–273. [Google Scholar] [CrossRef]
- Speer, T.; Rohrer, L.; Blyszczuk, P.; Shroff, R.; Kuschnerus, K.; Kränkel, N.; Kania, G.; Zewinger, S.; Akhmedov, A.; Shi, Y.; et al. Abnormal High-Density Lipoprotein Induces Endothelial Dysfunction via Activation of Toll-like Receptor-2. Immunity 2013, 38, 754–768. [Google Scholar] [CrossRef]
- Drożdż, D.; Kwinta, P.; Sztefko, K.; Kordon, Z.; Drożdż, T.; Łątka, M.; Miklaszewska, M.; Zachwieja, K.; Rudziński, A.; Pietrzyk, J.A. Oxidative Stress Biomarkers and Left Ventricular Hypertrophy in Children with Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2016, 2016, 7520231. [Google Scholar] [CrossRef]
- Nagy, L.; Tontonoz, P.; Alvarez, J.G.A.; Chen, H.; Evans, R.M. Oxidized LDL Regulates Macrophage Gene Expression through Ligand Activation of PPARγ. Cell 1998, 93, 229–240. [Google Scholar] [CrossRef]
- Rios, F.J.; Koga, M.M.; Pecenin, M.; Ferracini, M.; Gidlund, M.; Jancar, S. Oxidized LDL Induces Alternative Macrophage Phenotype through Activation of CD36 and PAFR. Mediat. Inflamm. 2013, 2013, 198193. [Google Scholar] [CrossRef]
- van Tits, L.J.H.; Stienstra, R.; van Lent, P.L.; Netea, M.G.; Joosten, L.A.B.; Stalenhoef, A.F.H. Oxidized LDL Enhances Pro-Inflammatory Responses of Alternatively Activated M2 Macrophages: A Crucial Role for Krüppel-like Factor 2. Atherosclerosis 2011, 214, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, S.; Stumpf, M.; Strehlow, K.; Schmid, A.; Schieffer, B.; Böhm, M.; Nickenig, G. Interleukin-6 Induces Oxidative Stress and Endothelial Dysfunction by Overexpression of the Angiotensin II Type 1 Receptor. Circ. Res. 2004, 94, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Karava, V.; Kondou, A.; Dotis, J.; Taparkou, A.; Farmaki, E.; Kollios, K.; Printza, N. Exploring Systemic Inflammation in Children with Chronic Kidney Disease: Correlates of Interleukin 6. Pediatr. Nephrol. 2024, 39, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Musiał, K.; Bargenda, A.; Drożdż, D.; Zwolińska, D. New Markers of Inflammation and Tubular Damage in Children with Chronic Kidney Disease. Dis. Markers 2017, 2017, 9389432. [Google Scholar] [CrossRef]
- Musiał, K.; Danuta, Z. Monocyte Chemoattractant Protein-1, Macrophage Colony Stimulating Factor, Survivin, and Tissue Inhibitor of Matrix Metalloproteinases-2 in Analysis of Damage and Repair Related to Pediatric Chronic Kidney Injury. Adv. Clin. Exp. Med. 2020, 29, 1083–1090. [Google Scholar] [CrossRef]
- Vianna, H.R.; Soares, C.M.B.M.; Silveira, K.D.; Elmiro, G.S.; Mendes, P.M.; De Sousa Tavares, M.; Teixeira, M.M.; Miranda, D.M.; Simões E Silva, A.C. Cytokines in Chronic Kidney Disease: Potential Link of MCP-1 and Dyslipidemia in Glomerular Diseases. Pediatr. Nephrol. 2013, 28, 463–469. [Google Scholar] [CrossRef]
- Williams, C.E.C.; Toner, A.; Wright, R.D.; Oni, L. A Systematic Review of Urine Biomarkers in Children with IgA Vasculitis Nephritis. Pediatr. Nephrol. 2021, 36, 3033–3044. [Google Scholar] [CrossRef]
- Xuan, X.; Pu, X.; Yang, Y.; Yang, J.; Li, Y.; Wu, H.; Xu, J. Plasma MCP-1 and TGF-Β1 Levels Are Associated with Kidney Injury in Children with Congenital Anomalies of the Kidney and Urinary Tract. Appl. Biochem. Biotechnol. 2024, 196, 6222–6233. [Google Scholar] [CrossRef]
- Litwin, M.; Michałkiewicz, J.; Gackowska, L. Primary Hypertension in Children and Adolescents Is an Immuno-Metabolic Disease with Hemodynamic Consequences. Curr. Hypertens. Rep. 2013, 15, 331–339. [Google Scholar] [CrossRef]
- Barbieri, S. Reactive Oxygen Species Mediate Cyclooxygenase-2 Induction during Monocyte to Macrophage Differentiation: Critical Role of NADPH Oxidase. Cardiovasc. Res. 2003, 60, 187–197. [Google Scholar] [CrossRef]
- Swiatlowska, P.; Tipping, W.; Marhuenda, E.; Severi, P.; Fomin, V.; Yang, Z.; Xiao, Q.; Graham, D.; Shanahan, C.; Iskratsch, T. Hypertensive Pressure Mechanosensing Alone Triggers Lipid Droplet Accumulation and Transdifferentiation of Vascular Smooth Muscle Cells to Foam Cells. Adv. Sci. 2024, 11, 2308686. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yancey, P.; Castro, I.; Khan, W.; Motojima, M.; Ichikawa, I.; Fogo, A.B.; Linton, M.F.; Fazio, S.; Kon, V. Renal Dysfunction Potentiates Foam Cell Formation by Repressing ABCA1. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1277–1282, Erratum in Arterioscler. Thromb. Vasc. Biol. 2009, 29. https://doi.org/10.1161/01.atv.0000363770.34980.d7. [Google Scholar] [CrossRef] [PubMed]
- Thürmann, L.; Bauer, M.; Ferland, M.; Messingschlager, M.; Schikowski, T.; Von Berg, A.; Heinrich, J.; Herberth, G.; Lehmann, I.; Standl, M.; et al. Undiagnosed Pediatric Elevated Blood Pressure Is Characterized by Induction of Proinflammatory and Cytotoxic Mediators. Hypertension 2023, 80, 2425–2436. [Google Scholar] [CrossRef] [PubMed]
- Madhur, M.S.; Lob, H.E.; McCann, L.A.; Iwakura, Y.; Blinder, Y.; Guzik, T.J.; Harrison, D.G. Interleukin 17 Promotes Angiotensin II–Induced Hypertension and Vascular Dysfunction. Hypertension 2010, 55, 500–507. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Yang, P.; Song, X.; Li, Y. Elevated Th17 Cell Proportion, Related Cytokines and mRNA Expression Level in Patients with Hypertension-Mediated Organ Damage: A Case Control Study. BMC Cardiovasc. Disord. 2022, 22, 257. [Google Scholar] [CrossRef]
- Calcaterra, V.; Croce, S.; Vinci, F.; De Silvestri, A.; Cordaro, E.; Regalbuto, C.; Zuccotti, G.V.; Mameli, C.; Albertini, R.; Avanzini, M.A. Th17 and Treg Balance in Children with Obesity and Metabolically Altered Status. Front. Pediatr. 2020, 8, 591012. [Google Scholar] [CrossRef]
- Vallance, P.; Leiper, J. Cardiovascular Biology of the Asymmetric Dimethylarginine:Dimethylarginine Dimethylaminohydrolase Pathway. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1023–1030. [Google Scholar] [CrossRef]
- Wilcox, C.S. Asymmetric Dimethylarginine and Reactive Oxygen Species: Unwelcome Twin Visitors to the Cardiovascular and Kidney Disease Tables. Hypertension 2012, 59, 375–381. [Google Scholar] [CrossRef]
- Achan, V.; Broadhead, M.; Malaki, M.; Whitley, G.; Leiper, J.; MacAllister, R.; Vallance, P. Asymmetric Dimethylarginine Causes Hypertension and Cardiac Dysfunction in Humans and Is Actively Metabolized by Dimethylarginine Dimethylaminohydrolase. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1455–1459. [Google Scholar] [CrossRef]
- Brooks, E.R.; Langman, C.B.; Wang, S.; Price, H.E.; Hodges, A.L.; Darling, L.; Yang, A.Z.; Smith, F.A. Methylated Arginine Derivatives in Children and Adolescents with Chronic Kidney Disease. Pediatr. Nephrol. 2009, 24, 129–134. [Google Scholar] [CrossRef]
- Chien, S.-J.; Lin, I.-C.; Hsu, C.-N.; Lo, M.-H.; Tain, Y.-L. Homocysteine and Arginine-to-Asymmetric Dimethylarginine Ratio Associated With Blood Pressure Abnormalities in Children With Early Chronic Kidney Disease. Circ. J. 2015, 79, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-C.; Hsu, C.-N.; Huang, C.-F.; Lo, M.-H.; Chien, S.-J.; Tain, Y.-L. Urinary Arginine Methylation Index Associated with Ambulatory Blood Pressure Abnormalities in Children with Chronic Kidney Disease. J. Am. Soc. Hypertens. 2012, 6, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.-C.; Hsu, C.-N.; Lo, M.-H.; Chien, S.-J.; Tain, Y.-L. Low Urinary Citrulline/Arginine Ratio Associated with Blood Pressure Abnormalities and Arterial Stiffness in Childhood Chronic Kidney Disease. J. Am. Soc. Hypertens. 2016, 10, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-N.; Lu, P.-C.; Lo, M.-H.; Lin, I.-C.; Tain, Y.-L. The Association between Nitric Oxide Pathway, Blood Pressure Abnormalities, and Cardiovascular Risk Profile in Pediatric Chronic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 5301. [Google Scholar] [CrossRef]
- Baliga, M.M.; Klawitter, J.; Christians, U.; Hopp, K.; Chonchol, M.; Gitomer, B.Y.; Cadnapaphornchai, M.A.; Klawitter, J. Metabolic Profiling in Children and Young Adults with Autosomal Dominant Polycystic Kidney Disease. Sci. Rep. 2021, 11, 6629. [Google Scholar] [CrossRef]
- Klawitter, J.; Reed-Gitomer, B.Y.; McFann, K.; Pennington, A.; Klawitter, J.; Abebe, K.Z.; Klepacki, J.; Cadnapaphornchai, M.A.; Brosnahan, G.; Chonchol, M.; et al. Endothelial Dysfunction and Oxidative Stress in Polycystic Kidney Disease. Am. J. Physiol.-Ren. Physiol. 2014, 307, F1198–F1206. [Google Scholar] [CrossRef]
- Drożdż, D.; Łątka, M.; Drożdż, T.; Sztefko, K.; Kwinta, P. Thrombomodulin as a New Marker of Endothelial Dysfunction in Chronic Kidney Disease in Children. Oxidative Med. Cell. Longev. 2018, 2018, 1619293. [Google Scholar] [CrossRef]
- De Giorgis, T.; Marcovecchio, M.L.; Giannini, C.; Chiavaroli, V.; Chiarelli, F.; Mohn, A. Blood Pressure from Childhood to Adolescence in Obese Youths in Relation to Insulin Resistance and Asymmetric Dimethylarginine. J. Endocrinol. Investig. 2016, 39, 169–176. [Google Scholar] [CrossRef]
- Giannini, C. Asymmetric Dimethylarginine in Obese Youth: Relationship with 24-Hour Ambulatory Blood Pressure. Biomed. J. Sci. Tech. Res. 2020, 31, 24069–24075. [Google Scholar] [CrossRef]
- Doshi, A.R.; Chikkabyrappa, S. Coarctation of Aorta in Children. Cureus 2018, 10, e3690. [Google Scholar] [CrossRef]
- Joshi, G.; Skinner, G.; Shebani, S.O. Presentation of Coarctation of the Aorta in the Neonates and the Infant with Short and Long Term Implications. Paediatr. Child Health 2017, 27, 83–89. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Roberts, C.K.; Ehdaie, A.; Zhan, C.-D.; Vaziri, N.D. Effects of Aortic Coarctation on Aortic Antioxidant Enzymes and NADPH Oxidase Protein Expression. Life Sci. 2005, 76, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Barton, C.H.; Ni, Z.; Vaziri, N.D. Enhanced Nitric Oxide Inactivation in Aortic Coarctation-Induced Hypertension. Kidney Int. 2001, 60, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Skeffington, K.; Bond, A.; Bigotti, M.G.; Abdulghani, S.; Iacobazzi, D.; Kang, S.-L.; Heesom, K.; Wilson, M.; Stoica, S.; Martin, R.; et al. Changes in Inflammation and Oxidative Stress Signalling Pathways in Coarcted Aorta Triggered by Bicuspid Aortic Valve and Growth in Young Children. Exp. Ther. Med. 2020, 20, 1. [Google Scholar] [CrossRef]
- Martin, J.; Hamilton, B.; Osterman, M. Births in the United States, 2023; National Center for Health Statistics: Hyattsville, MA, USA, 2024. [Google Scholar]
- Ohuma, E.O.; Moller, A.-B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, Regional, and Global Estimates of Preterm Birth in 2020, with Trends from 2010: A Systematic Analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef]
- Davies, E.L.; Bell, J.S.; Bhattacharya, S. Preeclampsia and Preterm Delivery: A Population-Based Case–Control Study. Hypertens. Pregnancy 2016, 35, 510–519. [Google Scholar] [CrossRef]
- Surmiak, P.; Wojnarowicz, O.; Szymkowiak, M. Malondialdehyde and Neutrophil Gelatinase-Associated Lipocalin as Markers of Oxidative Stress in Small for Gestational Age Newborns from Hypertensive and Preeclamptic Pregnancies. BioMed Res. Int. 2022, 2022, 9246233. [Google Scholar] [CrossRef]
- Crump, C.; Winkleby, M.A.; Sundquist, K.; Sundquist, J. Risk of Hypertension Among Young Adults Who Were Born Preterm: A Swedish National Study of 636,000 Births. Am. J. Epidemiol. 2011, 173, 797–803. [Google Scholar] [CrossRef]
- Li, S.; Xi, B. Preterm Birth Is Associated with Risk of Essential Hypertension in Later Life. Int. J. Cardiol. 2014, 172, e361–e363. [Google Scholar] [CrossRef]
- Abdel Ghany, E.A.G.; Alsharany, W.; Ali, A.A.; Youness, E.R.; Hussein, J.S. Anti-Oxidant Profiles and Markers of Oxidative Stress in Preterm Neonates. Paediatr. Int. Child Health 2016, 36, 134–140. [Google Scholar] [CrossRef]
- Jayet, P.-Y.; Rimoldi, S.F.; Stuber, T.; Salmòn, C.S.; Hutter, D.; Rexhaj, E.; Thalmann, S.; Schwab, M.; Turini, P.; Sartori-Cucchia, C.; et al. Pulmonary and Systemic Vascular Dysfunction in Young Offspring of Mothers With Preeclampsia. Circulation 2010, 122, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Flahault, A.; Paquette, K.; Fernandes, R.O.; Delfrate, J.; Cloutier, A.; Henderson, M.; Lavoie, J.-C.; Mâsse, B.; Nuyt, A.M.; Luu, T.M.; et al. Increased Incidence but Lack of Association Between Cardiovascular Risk Factors in Adults Born Preterm. Hypertension 2020, 75, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Zandstra, H.; Van Montfoort, A.P.A.; Dumoulin, J.C.M.; Zimmermann, L.J.I.; Touwslager, R.N.H. Increased Blood Pressure and Impaired Endothelial Function after Accelerated Growth in IVF/ICSI Children. Hum. Reprod. Open 2020, 2020, hoz037. [Google Scholar] [CrossRef] [PubMed]
- Asserhøj, L.L.; Mizrak, I.; Lebech Kjaer, A.S.; Clausen, T.D.; Hoffmann, E.R.; Greisen, G.; Main, K.M.; Madsen, P.L.; Pinborg, A.; Jensen, R.B. Blood Pressure and Lipid Profiles in Children Born after ART with Frozen Embryo Transfer. Hum. Reprod. Open 2024, 2024, hoae016. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Selamet Tierney, E.S.; Chen, A.C.; Ling, A.Y.; Fleischmann, R.R.; Baker, V.L. Vascular Health of Children Conceived via In Vitro Fertilization. J. Pediatr. 2019, 214, 47–53. [Google Scholar] [CrossRef]
- Downie, M.L.; Ulrich, E.H.; Noone, D.G. An Update on Hypertension in Children With Type 1 Diabetes. Can. J. Diabetes 2018, 42, 199–204. [Google Scholar] [CrossRef]
- Rönnback, M.; Fagerudd, J.; Forsblom, C.; Pettersson-Fernholm, K.; Reunanen, A.; Groop, P.-H. Altered Age-Related Blood Pressure Pattern in Type 1 Diabetes. Circulation 2004, 110, 1076–1082. [Google Scholar] [CrossRef]
- Alghazeer, R.; Alghazir, N.; Awayn, N.; Ahtiwesh, O.; Elgahmasi, S. Biomarkers of Oxidative Stress and Antioxidant Defense in Patients with Type 1 Diabetes Mellitus. Ibnosina J. Med. Biomed. Sci. 2018, 10, 198–204. [Google Scholar] [CrossRef]
- Grabia, M.; Socha, K.; Bossowski, A.; Markiewicz-Żukowska, R. Metabolic Syndrome as a Factor of Impairment of Antioxidant Defense System in Youth with T1DM. Int. J. Mol. Sci. 2023, 24, 9428. [Google Scholar] [CrossRef]
- Chiarelli, F.; De Martino, M.; Mezzetti, A.; Catino, M.; Morgese, G.; Cuccurullo, F.; Verrotti, A. Advanced Glycation End Products in Children and Adolescents with Diabetes: Relation to Glycemic Control and Early Microvascular Complications. J. Pediatr. 1999, 134, 486–491. [Google Scholar] [CrossRef]
- Shah, S.; Baez, E.A.; Felipe, D.L.; Maynard, J.D.; Hempe, J.M.; Chalew, S.A. Advanced Glycation Endproducts in Children with Diabetes. J. Pediatr. 2013, 163, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Le Bagge, S.; Righi, S.; Fotheringham, A.K.; Gallo, L.A.; McCarthy, D.A.; Leung, S.; Baskerville, T.; Nisbett, J.; Morton, A.; et al. Advanced Glycation End Products as Predictors of Renal Function in Youth with Type 1 Diabetes. Sci. Rep. 2021, 11, 9422. [Google Scholar] [CrossRef] [PubMed]
- Brunvand, L.; Heier, M.; Brunborg, C.; Hanssen, K.F.; Fugelseth, D.; Stensaeth, K.H.; Dahl-Jørgensen, K.; Margeirsdottir, H.D. Advanced Glycation End Products in Children with Type 1 Diabetes and Early Reduced Diastolic Heart Function. BMC Cardiovasc. Disord. 2017, 17, 133. [Google Scholar] [CrossRef] [PubMed]
- The U.S. Food and Drug Administration. FDA Approves Treatment for Chronic Weight Management in Pediatric Patients Aged 12 Years and Older; FDA: Silver Spring, MD, USA, 2022. [Google Scholar]
- VIVUS LLC. A Multicenter, Randomized, Double-Blind Study to Compare the Effects of VI-0521,Phentermine, and Placebo on Ambulatory Blood Pressure in Overweight or Obese Subjects; VIVUS LLC: Campbell, CA, USA, 2024. [Google Scholar]
- Feig, D.I.; Soletsky, B.; Johnson, R.J. Effect of Allopurinol on Blood Pressure of Adolescents With Newly Diagnosed Essential Hypertension: A Randomized Trial. JAMA 2008, 300, 924. [Google Scholar] [CrossRef]
- Soletsky, B.; Feig, D.I. Uric Acid Reduction Rectifies Prehypertension in Obese Adolescents. Hypertension 2012, 60, 1148–1156. [Google Scholar] [CrossRef]
- Sun, M.; Hines, N.; Scerbo, D.; Buchanan, J.; Wu, C.; Ten Eyck, P.; Zepeda-Orozco, D.; Taylor, E.B.; Jalal, D.I. Allopurinol Lowers Serum Urate but Does Not Reduce Oxidative Stress in CKD. Antioxidants 2022, 11, 1297. [Google Scholar] [CrossRef]
- Calò, L.A.; Dall’Amico, R.; Pagnin, E.; Bertipaglia, L.; Zacchello, G.; Davis, P.A. Oxidative Stress and Post-Transplant Hypertension in Pediatric Kidney-Transplanted Patients. J. Pediatr. 2006, 149, 53–57. [Google Scholar] [CrossRef]
- Al-Biltagi, M.; Tolba, O.A.; ElHafez, M.A.A.; Abo-Elezz, A.A.E.; El Kady, E.K.; Hazza, S.M.E. Oxidative Stress and Cardiac Dysfunction in Children with Chronic Renal Failure on Regular Hemodialysis. Pediatr. Nephrol. 2016, 31, 1329–1339. [Google Scholar] [CrossRef]
- Ağbaş, A.; Canpolat, N.; Çalışkan, S.; Yılmaz, A.; Ekmekçi, H.; Mayes, M.; Aitkenhead, H.; Schaefer, F.; Sever, L.; Shroff, R. Hemodiafiltration Is Associated with Reduced Inflammation, Oxidative Stress and Improved Endothelial Risk Profile Compared to High-Flux Hemodialysis in Children. PLoS ONE 2018, 13, e0198320. [Google Scholar] [CrossRef]
- Maschietto, N.; Semplicini, L.; Ceolotto, G.; Cattelan, A.; Poser Dvm, H.; Iacopetti, I.; Gerardi, G.; De Benedictis, G.M.; Pilla, T.; Bernardini, D.; et al. Aortic Stenting in the Growing Sheep Causes Aortic Endothelial Dysfunction but Not Hypertension: Clinical Implications for Coarctation Repair: Maschietto et al. Congenit. Heart Dis. 2017, 12, 74–83. [Google Scholar] [CrossRef]
- Martins, J.D.; Zachariah, J.; Selamet Tierney, E.S.; Truong, U.; Morris, S.A.; Kutty, S.; De Ferranti, S.D.; Guarino, M.; Thomas, B.; Oliveira, D.; et al. Impact of Treatment Modality on Vascular Function in Coarctation of the Aorta: The LOVE-COARCT Study. J. Am. Heart Assoc. 2019, 8, e011536. [Google Scholar] [CrossRef] [PubMed]
- South, A.M.; Giammattei, V.C.; Bagley, K.W.; Bakhoum, C.Y.; Beasley, W.H.; Bily, M.B.; Biswas, S.; Bridges, A.M.; Byfield, R.L.; Campbell, J.F.; et al. The Study of the Epidemiology of Pediatric Hypertension Registry (SUPERHERO): Rationale and Methods. Am. J. Epidemiol. 2024, 193, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, H.; Li, Y.; Liu, Y.; Liu, Y.; Zhang, H.; Deng, Y.; Shi, L. A Multivariate Prediction Model for Amlodipine Therapeutic Efficacy in Pediatric Primary Hypertension. Front. Endocrinol. 2025, 16, 1542276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Q.; Liu, H.; Hong, F.; Tang, Q.; Hu, C.; Xu, T.; Lu, H.; Ye, L.; Zhu, Y.; et al. Systemic Inflammation Markers and the Prevalence of Hypertension in 8- to 17-Year-Old Children and Adolescents: A NHANES Cross-Sectional Study. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103727. [Google Scholar] [CrossRef]
- Marčun Varda, N.; Močnik, M.; Filipič, M.; Homšak, E.; Svetej, M.; Golob Jančič, S. Interleukin-2 Receptor as a Marker of Oxidative Stress in Paediatric Patients with Chronic Kidney Disease or Hypertension. Children 2025, 12, 569. [Google Scholar] [CrossRef]

| ROS Source | Principal Enzyme (s) or Complexes | Pathophysiologic Contexts in Pediatrics | Molecular Mechanism of ROS Generation | Downstream Vascular Effects |
|---|---|---|---|---|
| Mitochondrial Electron Leakage |
|
|
|
|
| NADPH Oxidase Activation |
|
|
|
|
| Xanthine Oxidoreductase System |
|
|
|
|
| AGE-RAGE Signaling Axis |
|
|
|
|
| Peroxisomal β-Oxidation |
|
|
|
|
| Arginine Methylation Pathway |
|
|
|
|
| Category | Risk Factor | Mechanism of Oxidative Stress | Associated Pediatric Conditions | Clinical Relevance |
|---|---|---|---|---|
| Metabolic | Obesity | Increased adiposity → visceral fat accumulation → mitochondrial overload and NADPH oxidase activation → ↑ superoxide and hydrogen peroxide production | Primary hypertension, metabolic syndrome, and insulin resistance | Drives ROS generation through excess nutrient metabolism and adipokine-mediated inflammation |
| Hyperglycemia | Glucose overload → ↑ mitochondrial respiration + ↑ diacylglycerol → PKC activation → NOX stimulation → ↑ ROS; AGE-RAGE signaling | Type 1 and Type 2 diabetes mellitus | Promotes endothelial dysfunction, protein glycation, and vascular remodeling | |
| Dyslipidemia | Oxidized LDL and abnormal HDL profiles → foam cell formation → ROS amplification and vascular inflammation | Obesity, CKD, and early cardiovascular disease | Alters lipid metabolism, enhances inflammatory responses, and impairs NO· signaling | |
| Dietary | High sodium intake | Endothelial sodium overload → NOX uncoupling → ROS production, instead, of nitric oxide | Primary hypertension | Increases vascular tone, reduces NO· bioavailability, linked to BP elevation |
| Micronutrient deficiency | Insufficient antioxidants (e.g., vitamins and E, and selenium) → impaired ROS neutralization | Malnutrition, stunting, and early-life growth restriction | Reduces redox buffering capacity, increasing vulnerability to oxidative insults | |
| Environmental | Air pollution (PM2.5, NO2, O3) | Inhaled particulates activate pulmonary macrophages → systemic cytokine release → endothelial ROS; direct ROS induction in lung and vasculature | Urban-dwelling children showing asthma and hypertension | Promotes systemic inflammation and vascular dysfunction |
| Tobacco smoke exposure (prenatal/secondhand) | Nicotine and free radicals → oxidative DNA damage, reduced antioxidant enzymes | Preterm birth, SIDS, and hypertension | Impairs fetal and pediatric vascular development | |
| Behavioral/Lifestyle | Physical inactivity | ↓ Mitochondrial biogenesis and antioxidant defense (e.g., Nrf2 pathway); ↑ basal inflammation | Obesity and metabolic syndrome | Diminishes adaptive redox response, worsens vascular stiffness |
| Sleep deprivation or apnea | Intermittent hypoxia → ↑ ROS via NOX and mitochondrial pathways; ↑ IL-6, TNF-α | Pediatric obstructive sleep apnea | Links to BP elevation, endothelial dysfunction, and sympathetic overactivity | |
| Developmental | Prematurity | Immature antioxidant systems + high oxygen exposure → ↑ lipid peroxidation and ROS | Bronchopulmonary dysplasia and early-onset hypertension | Contributes to long-term vascular programming and oxidative damage |
| Pre-eclampsia exposure (in utero) | Placental oxidative stress → fetal endothelial dysfunction + altered NO· signaling | Neonatal hypertension and later-life cardiometabolic risk | Early-life ROS exposure programs hypertension risk | |
| Psychosocial | Adverse childhood experiences (ACEs) | Chronic stress → ↑ cortisol and sympathetic tone → mitochondrial dysfunction + ↑ NOX activity | Hypertension, anxiety, and insulin resistance | Elevates systemic oxidative stress markers and accelerates vascular aging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Backston, K.; Morgan, J.; Patel, S.; Koka, R.; Hu, J.; Raina, R. Oxidative Stress and Endothelial Dysfunction: The Pathogenesis of Pediatric Hypertension. Int. J. Mol. Sci. 2025, 26, 5355. https://doi.org/10.3390/ijms26115355
Backston K, Morgan J, Patel S, Koka R, Hu J, Raina R. Oxidative Stress and Endothelial Dysfunction: The Pathogenesis of Pediatric Hypertension. International Journal of Molecular Sciences. 2025; 26(11):5355. https://doi.org/10.3390/ijms26115355
Chicago/Turabian StyleBackston, Kyle, Jordan Morgan, Samipa Patel, Riddhima Koka, Jieji Hu, and Rupesh Raina. 2025. "Oxidative Stress and Endothelial Dysfunction: The Pathogenesis of Pediatric Hypertension" International Journal of Molecular Sciences 26, no. 11: 5355. https://doi.org/10.3390/ijms26115355
APA StyleBackston, K., Morgan, J., Patel, S., Koka, R., Hu, J., & Raina, R. (2025). Oxidative Stress and Endothelial Dysfunction: The Pathogenesis of Pediatric Hypertension. International Journal of Molecular Sciences, 26(11), 5355. https://doi.org/10.3390/ijms26115355






