Bisulfite Pretreatment Improves Enzymatic Digestibility of Oil Palm Empty Fruit Bunch and Poplar Through Changing Its Structure and Lignin Distribution
Abstract
1. Introduction
2. Results and Discussion
2.1. Improved Enzymatic Digestibility of EFB and Poplar by Bisulfite Pretreatment
2.2. Changes in Chemical Compositions of EFB and Poplar by Bisulfite Pretreatment
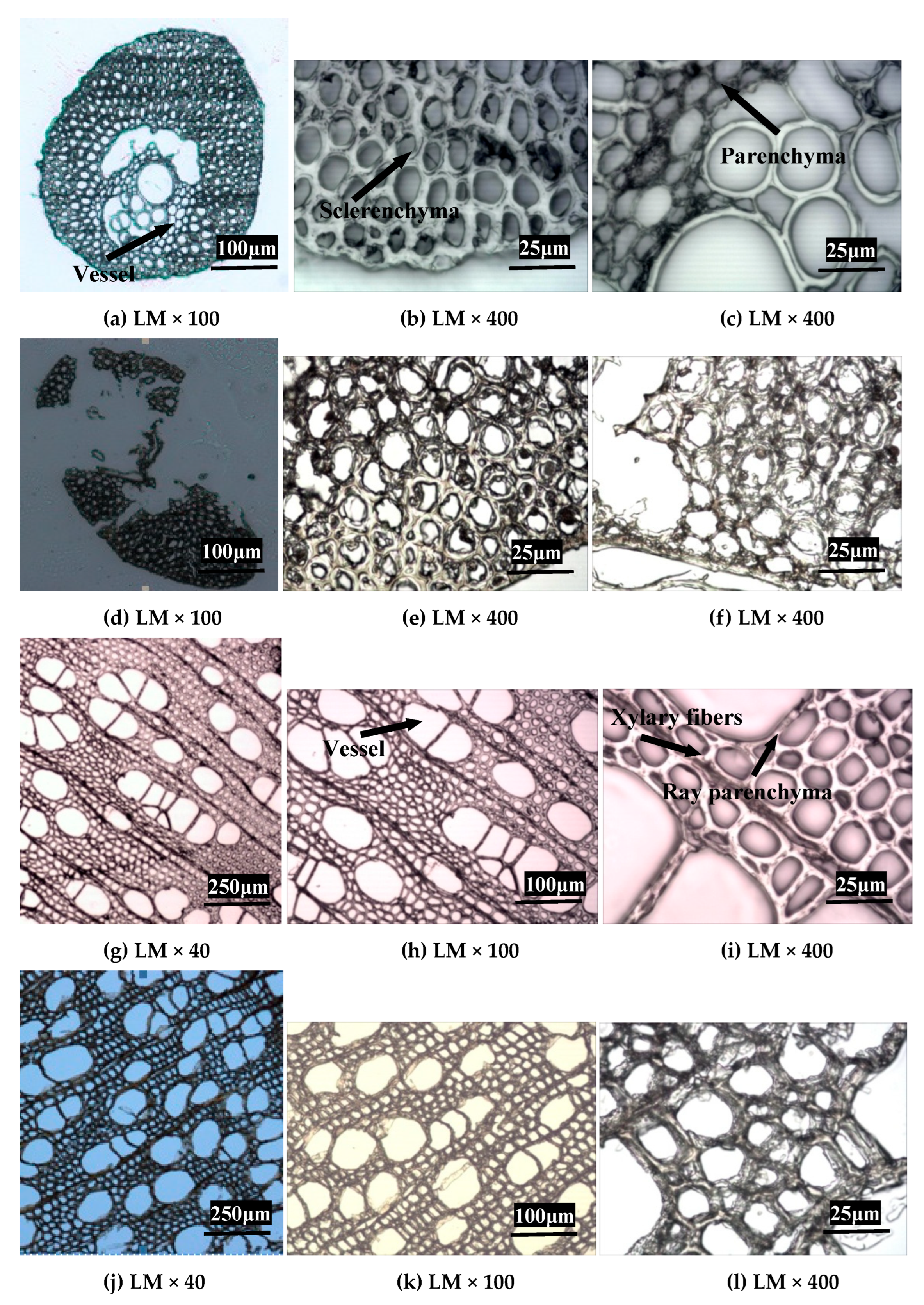
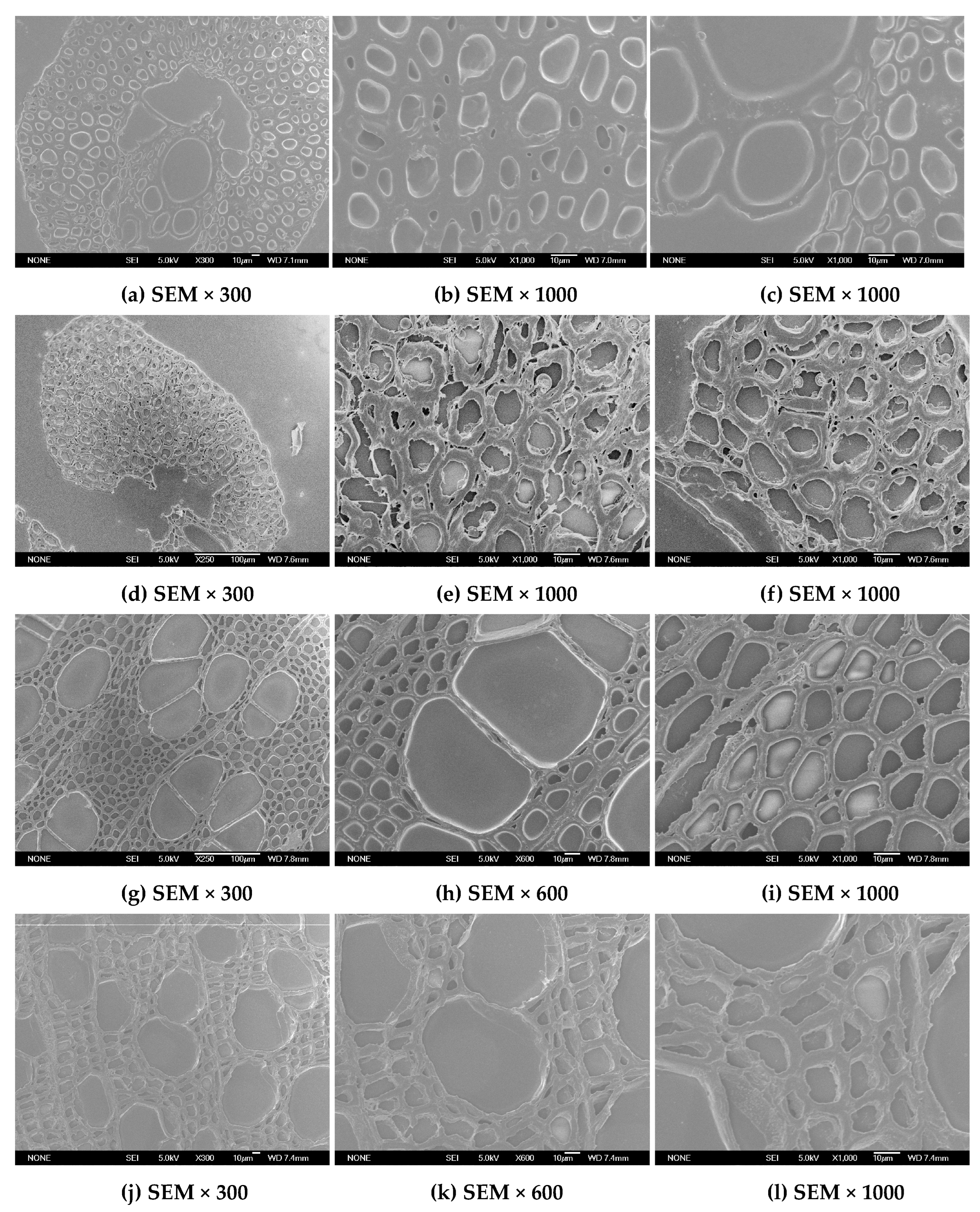
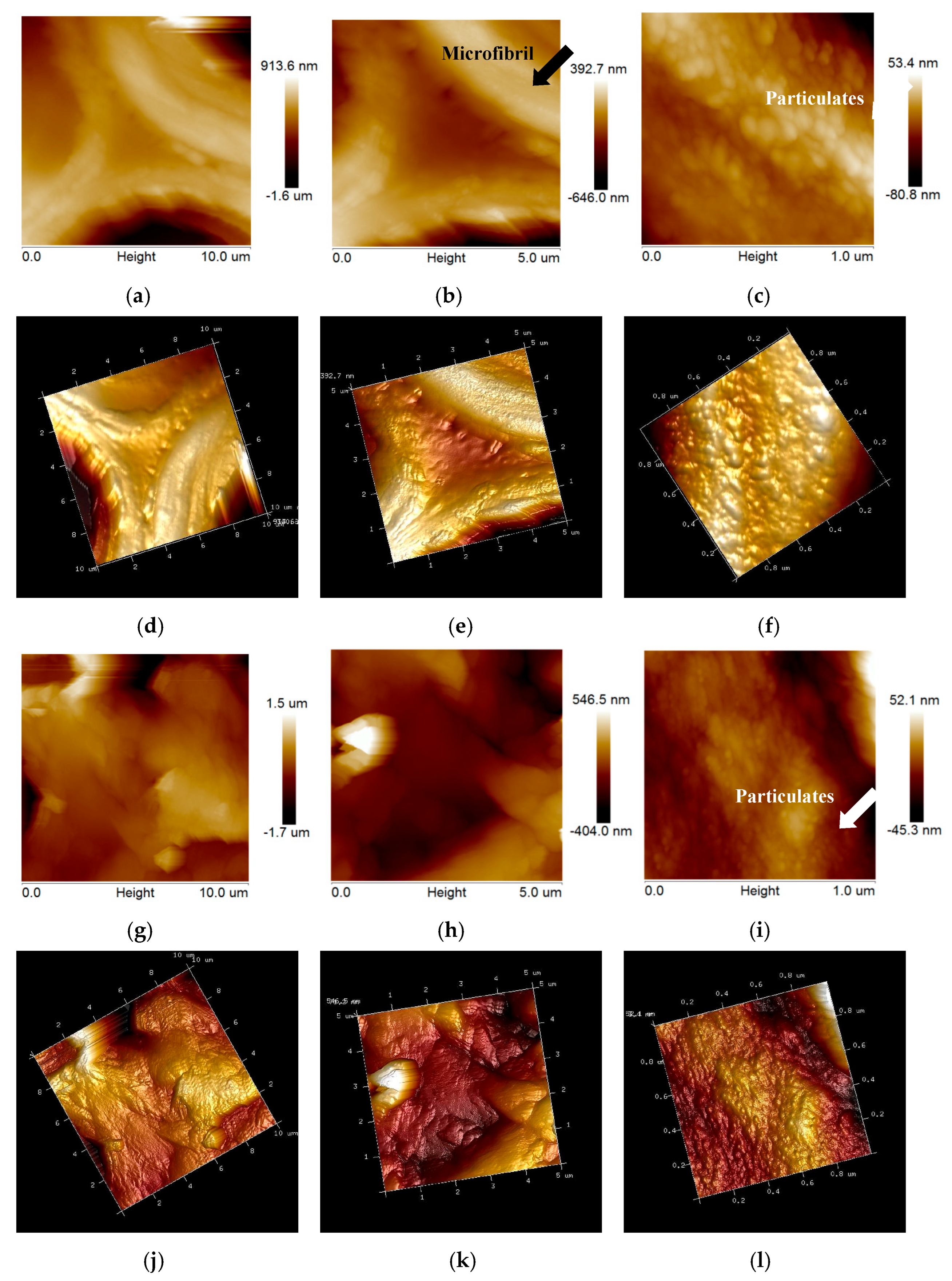
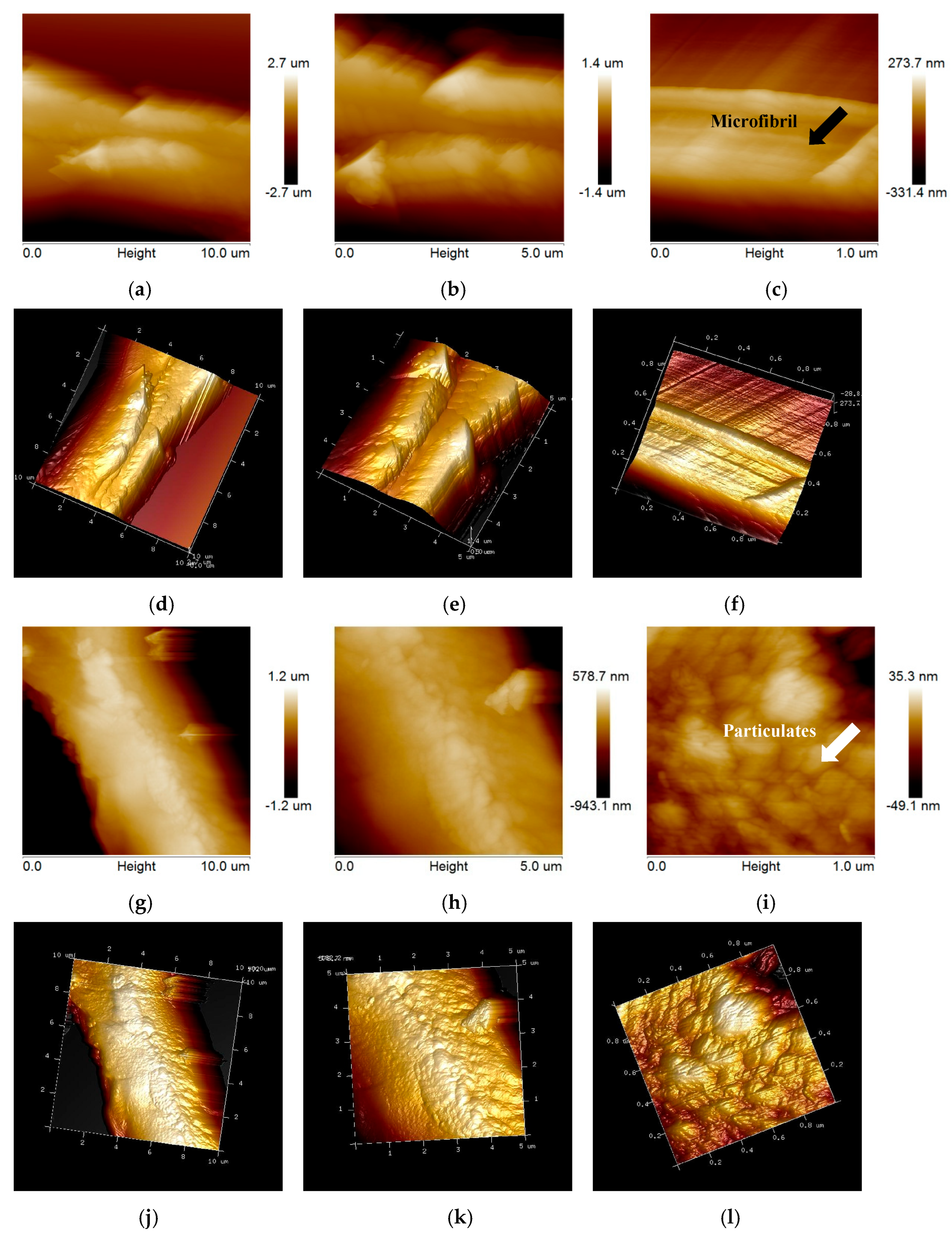
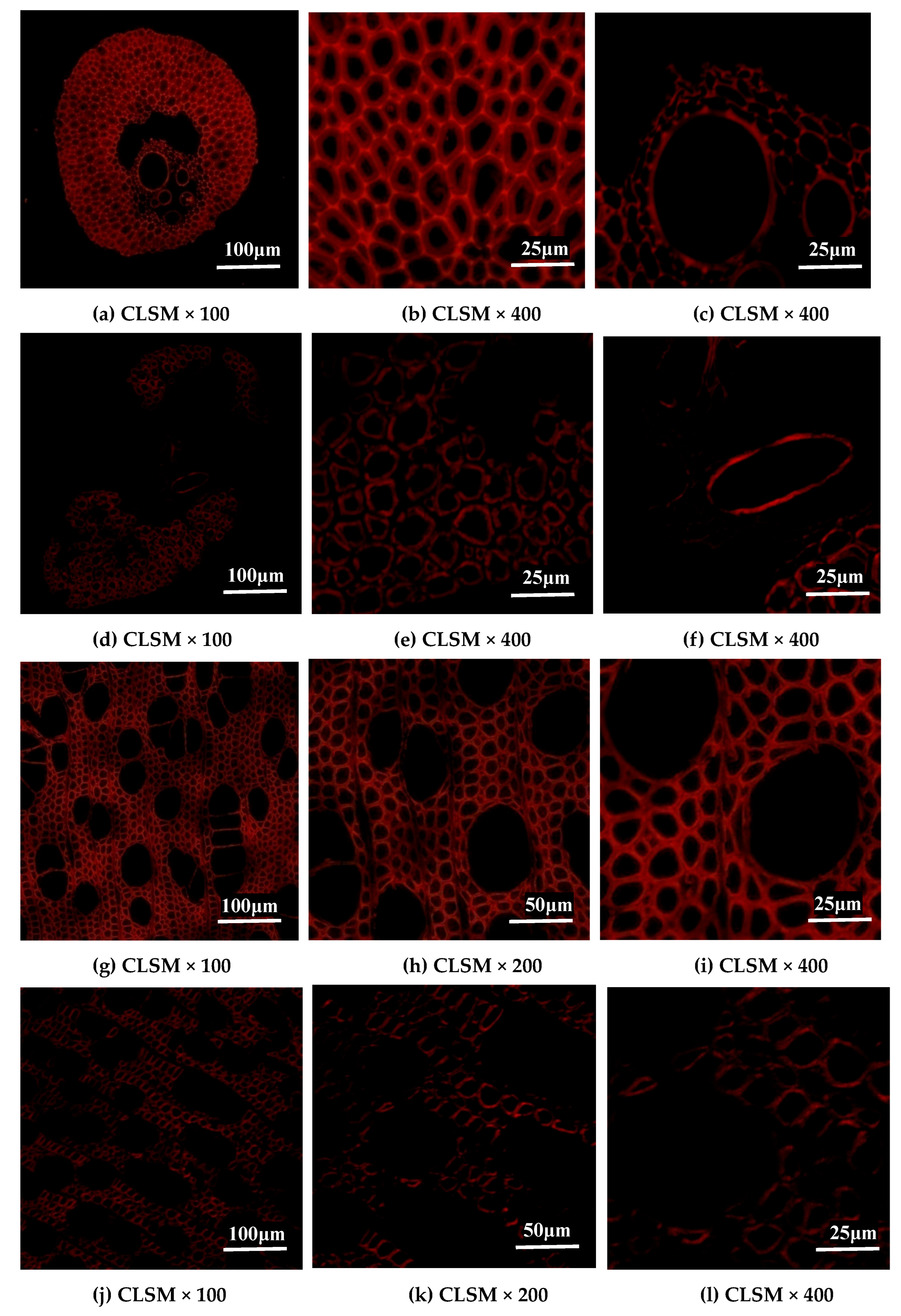
2.3. Changes in Anatomical and Ultrastructure Features of EFB and Poplar Cell Walls by Bisulfite Pretreatment
2.3.1. Changes in Anatomical Structure
2.3.2. Changes in Ultrastructure
2.4. Changes in Lignin Distribution of EFB and Poplar Cell Walls by Bisulfite Pretreatment
3. Materials and Methods
3.1. Materials
3.2. Bisulfite Pretreatment of EFB and Enzymatic Hydrolysis of Pretreated Samples
3.3. Assays of Chemical Compositions
3.4. Determination of Fiber Morphology
3.5. Observation of Anatomy and Ultrastructure
3.5.1. Light Microscopy (LM) Observation
3.5.2. Scanning Electron Microscopy (SEM) Observation
3.5.3. Atomic Force Microscopy (AFM) Observation
3.6. Lignin Distribution Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiong, Y.W.; Yap, C.L.; Gan, S.; Yap, W.S.P. One-pot conversion of oil palm empty fruit bunch and mesocarp fiber biomass to levulinic acid and upgrading to ethyl levulinate via indium trichloride-ionic liquids. J. Clean. Prod. 2017, 168, 1251–1261. [Google Scholar] [CrossRef]
- Darus, L.; Susana, S.; Sihombing, H.; Utami, A.R.I.; Mel, M. Enzymatic hydrolysis enhancement of oil palm empty fruit bunch by Peracetic-Sulfuric acid pretreatment. Chem. Eng. J. 2022, 429, 132452. [Google Scholar] [CrossRef]
- Risanto, L.; Adi, D.T.N.; Fajriutami, T.; Teramura, H.; Fatriasari, W.; Hermiati, E.; Kahar, P.; Kondo, A.; Ogino, C. Pretreatment with dilute maleic acid enhances the enzymatic digestibility of sugarcane bagasse and oil palm empty fruit bunch fiber. Bioresour. Technol. 2023, 369, 128382. [Google Scholar] [CrossRef] [PubMed]
- Polprasert, S.; Choopakar, O.; Elefsiniotis, P. Bioethanol production from pretreated palm empty fruit bunch (PEFB) using sequential enzymatic hydrolysis and yeast fermentation. Biomass Bioenergy 2021, 149, 106088. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Koo, B.; Lee, J.W. Structural changes in biomass (yellow poplar and empty fruit bunch) during hydrothermal and oxalic acid pretreatments and their effects on enzymatic hydrolysis efficiency. Ind. Crop. Prod. 2022, 178, 114569. [Google Scholar] [CrossRef]
- Sukhang, S.; Choojit, S.; Reungpeerakul, T.; Sangwichien, C. Bioethanol production from oil palm empty fruit bunch with SSF and SHF processes using Kluyveromyces marxianus yeast. Cellulose 2020, 27, 301–314. [Google Scholar] [CrossRef]
- Rehman, S.; Islam, M.K.; Khanzada, N.K.; Zhuang, H.; Wang, H.; Chaiprapat, S.; Leu, S.Y. Sustainability index accounting food and carbon benefits on circular 2,3-butanediol biorefinery with oil palm empty fruit bunches. Appl. Energy 2021, 303, 17667. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Cai, Z.; Zhang, J.; Guan, D.; Ji, D.; Gao, W. Effect of ammonia fiber expansion combined with NaOH pretreatment on the resource efficiency of herbaceous and woody lignocellulosic biomass. ACS Omega 2022, 7, 18761–18769. [Google Scholar] [CrossRef]
- Tan, L.P.; Yu, Y.C.; Li, X.Z.; Zhao, J.; Qu, Y.B.; Choo, Y.M.; Loh, S.K. Pretreatment of empty fruit bunch from oil palm for fuel ethanol production and proposed biorefinery process. Bioresour. Technol. 2013, 135, 275–282. [Google Scholar] [CrossRef]
- Lee, K.M.; Hong, J.Y.; Tey, W.Y. Combination of ultrasonication and deep eutectic solvent in pretreatment of lignocellulosic biomass for enhanced enzymatic saccharification. Cellulose 2021, 28, 1513–1526. [Google Scholar] [CrossRef]
- Tan, L.P.; Sun, W.; Li, X.Z.; Zhao, J.; Qu, Y.B.; Choo, Y.M.; Loh, S.K. Bisulfite pretreatment changes the structure and properties of oil palm empty fruit bunch to improve enzymatic hydrolysis and bioethanol production. Biotechnol. J. 2015, 10, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Refahi, Y.; Zoghlami, A.; Viné, T.; Terryn, C.; Päes, G. Plant cell wall enzymatic deconstruction: Bridging the gap between micro and nano scales. Bioresour. Technol. 2024, 414, 131551. [Google Scholar] [CrossRef]
- Kirui, A.; Zhao, W.; Deligey, F.; Yang, H.; Kang, X.; Mentink-Vigier, F.; Wang, T. Carbohydrate-aromatic interface and molecular architecture of lignocellulose. Nat. Commun. 2022, 13, 538. [Google Scholar] [CrossRef]
- Shamsudin, S.; Shah, U.K.M.; Zainudin, H.; Abd-Aziz, S.; Mustapa Kamal, S.M.; Shirai, Y.; Ali Hassan, M. Effect of steam pretreatment on oil palm empty fruit bunch for the production of sugars. Biomass Bioenergy 2012, 36, 280–288. [Google Scholar] [CrossRef]
- Fatriasari, W.; Anita, S.H.; Risanto, L. Microwave assisted acid pretreatment of oil palm empty fruit bunches (EFB) to enhance its fermentable sugar production. Waste Biomass Valorization 2016, 8, 379–391. [Google Scholar] [CrossRef]
- Fatriasari, W.; Ulwan, W.; Aminingsih, T.; Sari, F.P.; Fitria; Suryanegara, L.; Iswanto, A.H.; Ghozali, M.; Kholida, L.N.; Hussin, M.H.; et al. Optimization of maleic acid pretreatment of oil palm empty fruit bunches (OPEFB) using response surface methodology to produce reducing sugars. Ind. Crop. Prod. 2021, 171, 113971. [Google Scholar] [CrossRef]
- Suhartini, S.; Rohma, N.A.; Elviliana; Hidayat, N.; Sunyoto, N.M.S.; Mardawati, E.; Kasbawati; Mascruhin, N.; Idrus, S.; Fitria; et al. Comparison of acid and alkaline pre-treatment on methane production from empty palm oil fruit bunches (OPEFB): Effect on characteristics, digester performance, and correlation of kinetic parameters. Renew. Energy 2023, 215, 119009. [Google Scholar] [CrossRef]
- Katahira, R.; Sluiter, J.B.; Schell, J.D.; Davis, M.F. Degradation of carbohydrates during dilute sulfuric acid pretreatment can interfere with lignin measurements in solid residues. J. Agric. Food Chem. 2013, 61, 3286–3292. [Google Scholar] [CrossRef]
- Tian, S.; Zhu, W.; Gleisner, R.; Pan, X.J.; Zhu, J.Y. Comparisons of SPORL and dilute acid pretreatments for sugar and ethanol productions from aspen. Biotechnol. Progr. 2011, 27, 419–427. [Google Scholar] [CrossRef]
- de Carvalho, D.M.; Martínez-Abad, A.; Evtuguin, D.V.; Colodette, J.L.; Lindström, M.E.; Vilaplana, F.; Sevastyanova, O. Isolation and characterization of acetylated glucuronoarabinoxylan from sugarcane bagasse and straw. Carbohydr. Polym. 2017, 156, 223–234. [Google Scholar] [CrossRef]
- Tan, L.P.; Wang, M.M.; Li, X.Z.; Li, H.X.; Zhao, J.; Qu, Y.B.; Choo, Y.M.; Loh, S.K. Fractionation of oil palm empty fruit bunch by bisulfite pretreatment for the production of bioethanol and high value products. Bioresour. Technol. 2016, 200, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Jana, B.S.; Christian, G.; Tiina, N.; Takayuki, U.; Stefan, S. How resilient is wood xylan to enzymatic degradation in a matrix with kraft lignin? Biomacromolecules 2024, 25, 3532–3541. [Google Scholar]
- Chen, S.; Zhang, X.; Ling, Z.; Xu, F. Characterization of the micromorphology and topochemistry of poplar wood during mild ionic liquid pretreatment for improving enzymatic saccharification. Molecules 2017, 22, 115. [Google Scholar] [CrossRef]
- Ling, Z.; Ji, Z.; Ding, D.; Gao, G.; Xu, F. Microstructural and topochemical characterization of thermally modified poplar (Populus cathayaha) cell wall. Bioresources 2015, 11, 786–799. [Google Scholar] [CrossRef]
- Chen, X.; Santos, A.C.F.D.; Gutierrez, D.M.R.; Zhang, S.; Aston, J.E.; Thompson, D.N.; Ladisch, M.R.; Mosier, N.S. Understanding the influence of water-soluble compounds from unpretreated corn stover pellets on enzymatic hydrolysis of cellulose. ACS Sustain. Chem. Eng. 2023, 11, 17616–17624. [Google Scholar] [CrossRef]
- Jiang, X.; Zhai, R.; Leng, Y.; Deng, Q.; Jin, M. Understanding the toxicity of lignin-derived phenolics towards enzymatic saccharification of lignocellulose for rationally developing effective in-situ mitigation strategies to maximize sugar production from lignocellulosic biorefinery. Bioresour. Technol. 2022, 349, 126813. [Google Scholar] [CrossRef]
- Song, G.; Madadi, M.; Meng, X.; Sun, C.; Aghbashlo, M.; Sun, F.; Ragauskas, A.J.; Tabatabaei, M.; Ashori, A. Double in-situ lignin modification in surfactant-assisted glycerol organosolv pretreatment of sugarcane bagasse towards efficient enzymatic hydrolysis. Chem. Eng. J. 2024, 481, 148713. [Google Scholar] [CrossRef]
- Morita, K.; Takenaka, M.; Tomita, K.; Ishii, J.; Kawaguchi, H.; Murakami, D.; Amo, H.; Fujii, M.; Maruyama, T.; Matsumoto, T.; et al. Nanoscopic lignin mapping on cellulose nanofibers via scanning transmission electron microscopy and atomic force microscopy. Cellulose 2023, 30, 11357. [Google Scholar] [CrossRef]
- Boris, N.K.; Anna, I.C.; Aleksandr, S.K.; Olga Yu, F.; Valentina, S.B.; Sergei, A.V.; Anton, A.K.; Elena, V.G.; Elena, V.M.; Andrey, M.S.; et al. Fractionation of aspen wood to produce microcrystalline, microfibrillated and nanofibrillated celluloses, xylan and ethanollignin. Polymers 2023, 15, 2671. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, J.; Ji, Z.; Zhang, X.; Ramaswamy, S.; Xu, F.; Sun, R.C. Dilute acid pretreatment differentially affects the compositional and architectural features of Pinus bungeana Zucc. compression and opposite wood tracheid walls. Ind. Crop. Prod. 2014, 62, 196–203. [Google Scholar] [CrossRef]
- Gustafsson, J.; Lehto, J.H.; Tienvieri, T.; Ciovica, L.; Peltonen, J. Surface characteristics of thermomechanical pulps; the influence of defibration temperature and refining. Colloids Surf. A 2003, 225, 95–104. [Google Scholar] [CrossRef]
- Koljonen, K.; Österberg, M.; Kleen, M.; Fuhrmann, A.; Stenius, P. Precipitation of lignin and extractives on kraft pulp: Effect on surface chemistry, surface morphology and paper strength. Cellulose 2004, 11, 209–224. [Google Scholar] [CrossRef]
- Eibinger, M.; Bubner, P.; Ganner, T.; Plank, H.; Nidetzky, B. Surface structural dynamics of enzymatic cellulose degradation, revealed by combined kinetic and atomic force microscopy studies. FEBS J. 2014, 281, 275–290. [Google Scholar] [CrossRef]
- López-Serrano, M.; Fernández, M.D.; Pomar, F.; Pedreño, M.A.; Ros Barceló, A. Zinnia elegans uses the same peroxidase isoenzyme complement for cell wall lignification in both single-cell tracheary elements and xylem vessels. J. Exp. Bot. 2004, 55, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.A.; Singh, A.P.; Yoshinaga, A.; Takabe, K. Lignin distribution in mild compression wood of Pinus radiata. Can. J. Bot. 1999, 77, 41–50. [Google Scholar]
- Xu, F.; Zhong, X.C.; Sun, R.C.; Lu, Q. Anatomy, ultrastructure and lignin distribution in cell wall of Caragana korshinskii. Ind. Crop. Prod. 2006, 24, 186–193. [Google Scholar] [CrossRef]
- Donaldson, L.A. Lignification and lignin topochemistry—An ultrastructural view. Phytochemistry 2001, 57, 859–873. [Google Scholar] [CrossRef]
- Kutscha, N.; McOrmond, R. The suitability of using fluorescence microscopy for studying lignification in Balsam Fir. Life Sci. Agric. Exp. Stn. Tech. Bull. 1972, 62, 3–15. [Google Scholar]
- De Micco, V.; Aronne, G. Anatomical features, monomer lignin composition and accumulation of phenolics in 1-year-old branches of the Mediterranean Cistusladanifer L. Bot. J. Linn. Soc. 2007, 155, 361–371. [Google Scholar] [CrossRef]
- Donaldson, B.L.; Hague, J.; Snell, R. Lignin distribution in coppice poplar, linseed and wheat straw. Holzforschung 2001, 55, 379–385. [Google Scholar] [CrossRef]
- Sluiter, A.D.; Hames, B.; Ruiz, R.; Scarlata, C.J.; Sluiter, J.; Templeton, D.; Crocker, D.P. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. Natl. Renew. Energy Lab. 2008, 1617, 1–16. [Google Scholar]
- Shi, S.L.; He, F.W. Analysis and Measurement of Pulp and Paper; China Light Industry Press Ltd.: Beijing, China, 2008; pp. 46–51. [Google Scholar]
- Yu, Z.; Gwak, K.S.; Treasure, T.; Jameel, H.; Chang, H.M.; Park, S. Effect of lignin chemistry on the enzymatic hydrolysis of woody biomass. ChemSusChem 2014, 7, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
| Yield (%) | Extractives (%) | Glucan (%) | Xylan (%) | Lignin (%) | Lignin (%) (% loss) | Xylan (%) (% loss) | Glucan (%) (% loss) | Glucose Yield b (g/g) | |
|---|---|---|---|---|---|---|---|---|---|
| Untreated EFB | — | 1.5 ± 0.3 | 34.7 ± 1.1 | 23.1 ± 1.3 | 22.1 ± 1.2 | — | — | — | 0.078 |
| Pretreated EFB | 65.8 ± 3.9 | 7.6 ± 1.9 | 51.8 ± 3.5 | 7.2 ± 2.1 | 20.5 ± 3.2 | 39.0 | 79.4 | 1.8 | 0.378 |
| Untreated Poplar | — | 1.3 ± 0.1 | 43.7 ± 1.7 | 14.6 ± 1.0 | 23.1 ± 0.9 | — | — | — | 0.088 |
| Pretreated Poplar | 71.7 ± 4.1 | 10.6 ± 1.8 | 58.5 ± 3.2 | 2.6 ± 1.3 | 21.3 ± 3.9 | 33.8 | 87.3 | 4.0 | 0.312 |
| L (n) a (mm) | L (w) b (mm) | Width (µm) | Fine (%) | |
|---|---|---|---|---|
| Untreated EFB | 0.525 ± 0.001 | 0.674 ± 0.001 | 17.234 ± 0.036 | 24.35 ± 0.226 |
| Pretreated EFB | 0.425 ± 0.001 | 0.530 ± 0.006 | 16.283 ± 0.138 | 41.22 ± 0.184 |
| Untreated Poplar | 0.632 ± 0.014 | 0.721 ± 0.015 | 20.408 ± 0.654 | 14.89 ± 0.219 |
| Pretreated Poplar | 0.397 ± 0.001 | 0.482 ± 0.001 | 17.870 ± 0.099 | 60.12 ± 2.192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, L.; Li, X.; Lu, X.; Zhao, J. Bisulfite Pretreatment Improves Enzymatic Digestibility of Oil Palm Empty Fruit Bunch and Poplar Through Changing Its Structure and Lignin Distribution. Int. J. Mol. Sci. 2025, 26, 5334. https://doi.org/10.3390/ijms26115334
Tan L, Li X, Lu X, Zhao J. Bisulfite Pretreatment Improves Enzymatic Digestibility of Oil Palm Empty Fruit Bunch and Poplar Through Changing Its Structure and Lignin Distribution. International Journal of Molecular Sciences. 2025; 26(11):5334. https://doi.org/10.3390/ijms26115334
Chicago/Turabian StyleTan, Liping, Xuezhi Li, Xianqin Lu, and Jian Zhao. 2025. "Bisulfite Pretreatment Improves Enzymatic Digestibility of Oil Palm Empty Fruit Bunch and Poplar Through Changing Its Structure and Lignin Distribution" International Journal of Molecular Sciences 26, no. 11: 5334. https://doi.org/10.3390/ijms26115334
APA StyleTan, L., Li, X., Lu, X., & Zhao, J. (2025). Bisulfite Pretreatment Improves Enzymatic Digestibility of Oil Palm Empty Fruit Bunch and Poplar Through Changing Its Structure and Lignin Distribution. International Journal of Molecular Sciences, 26(11), 5334. https://doi.org/10.3390/ijms26115334







