Implementation of a CAM Assay Using Fibrosarcoma Spheroids
Abstract
1. Introduction
2. Results
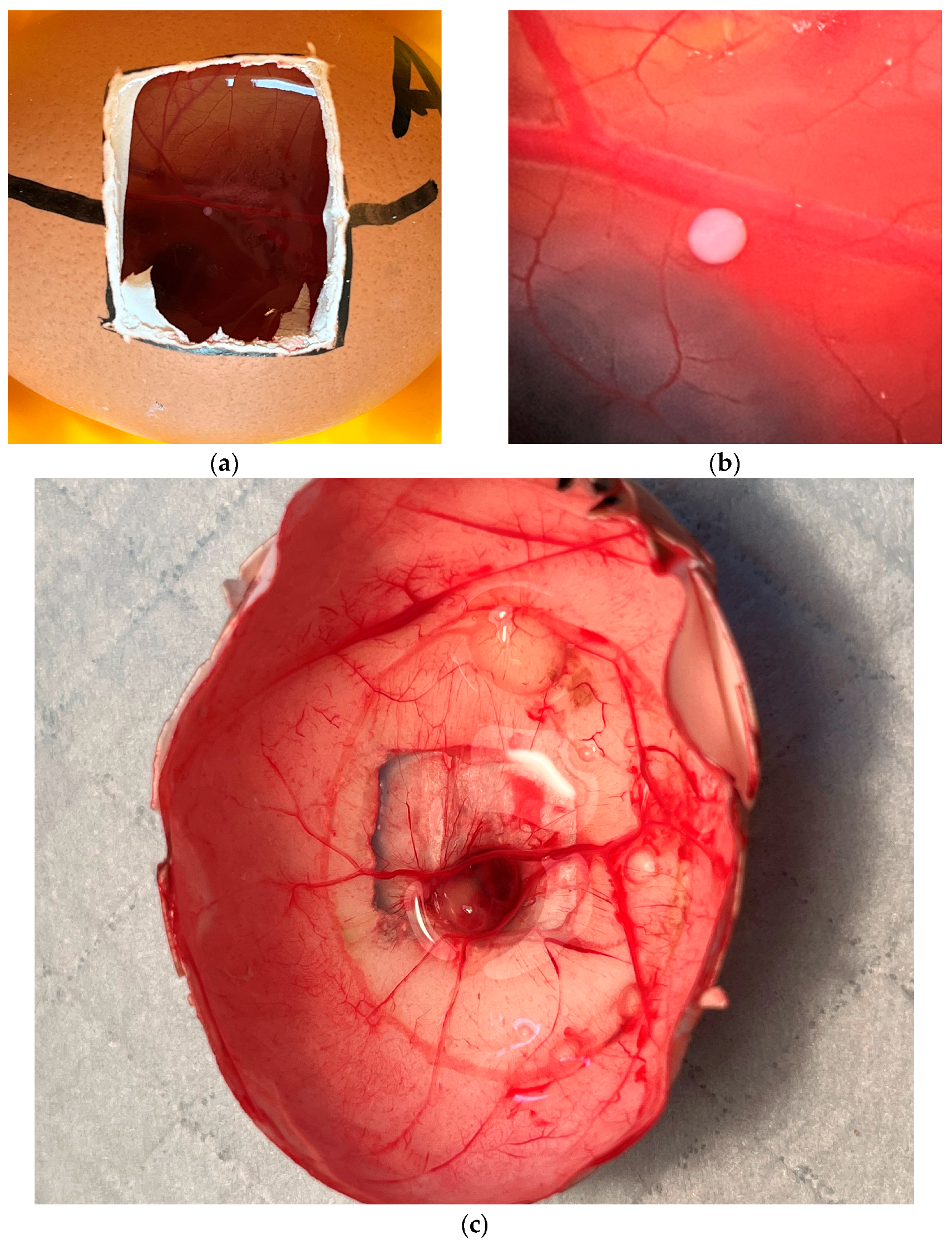
2.1. Egg Development and Tumor Growth
2.2. Tumor Area
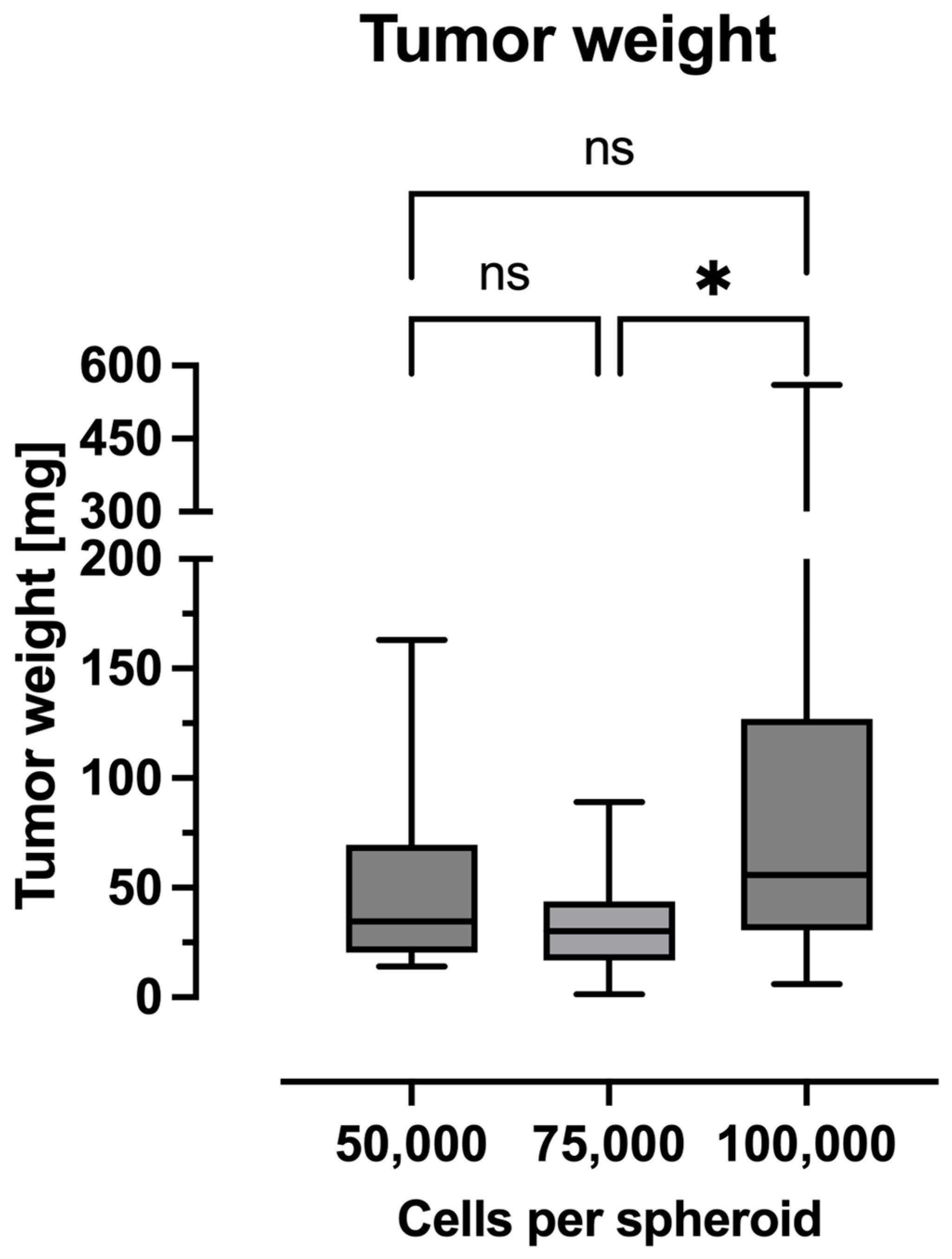
2.3. Tumor Weight
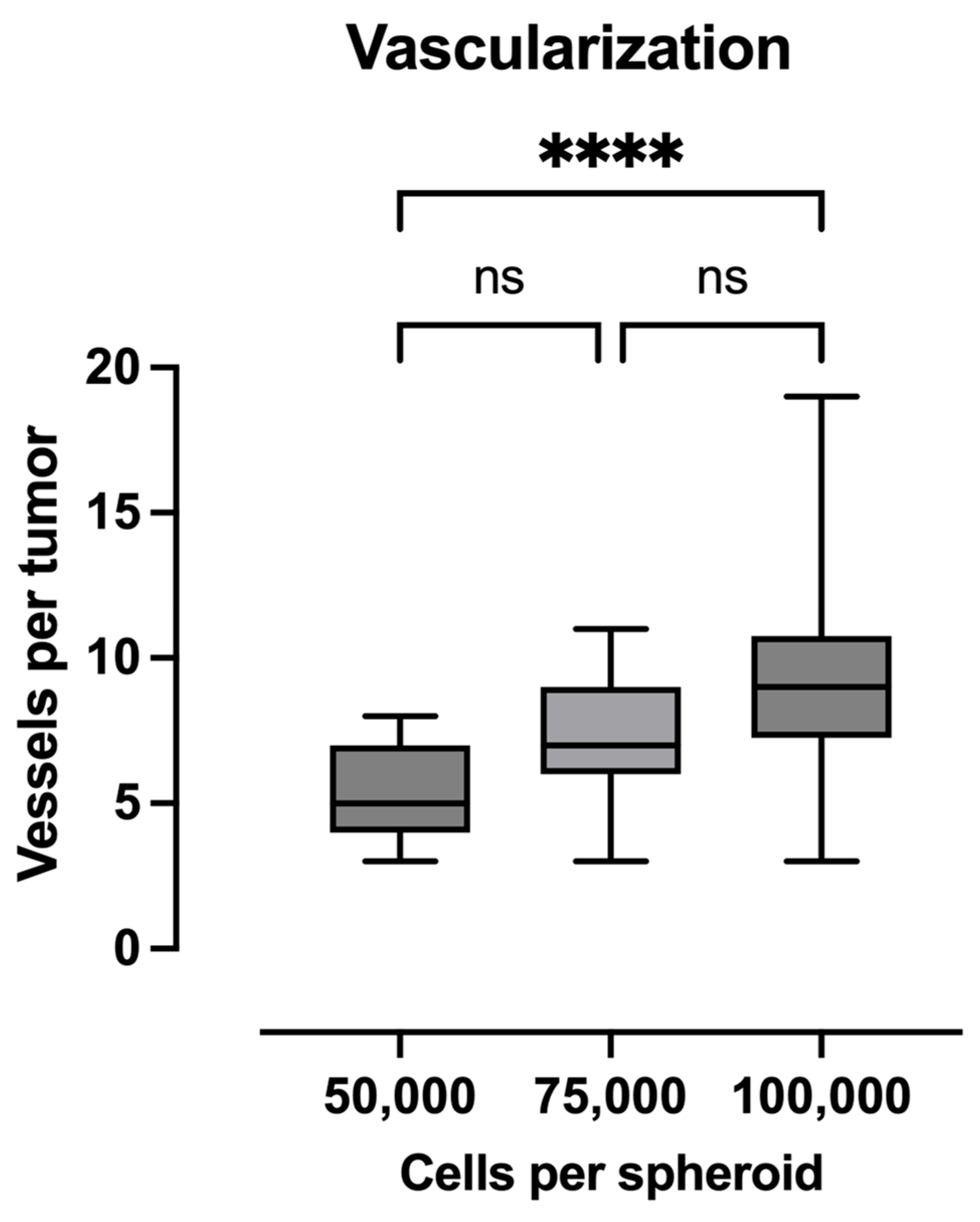
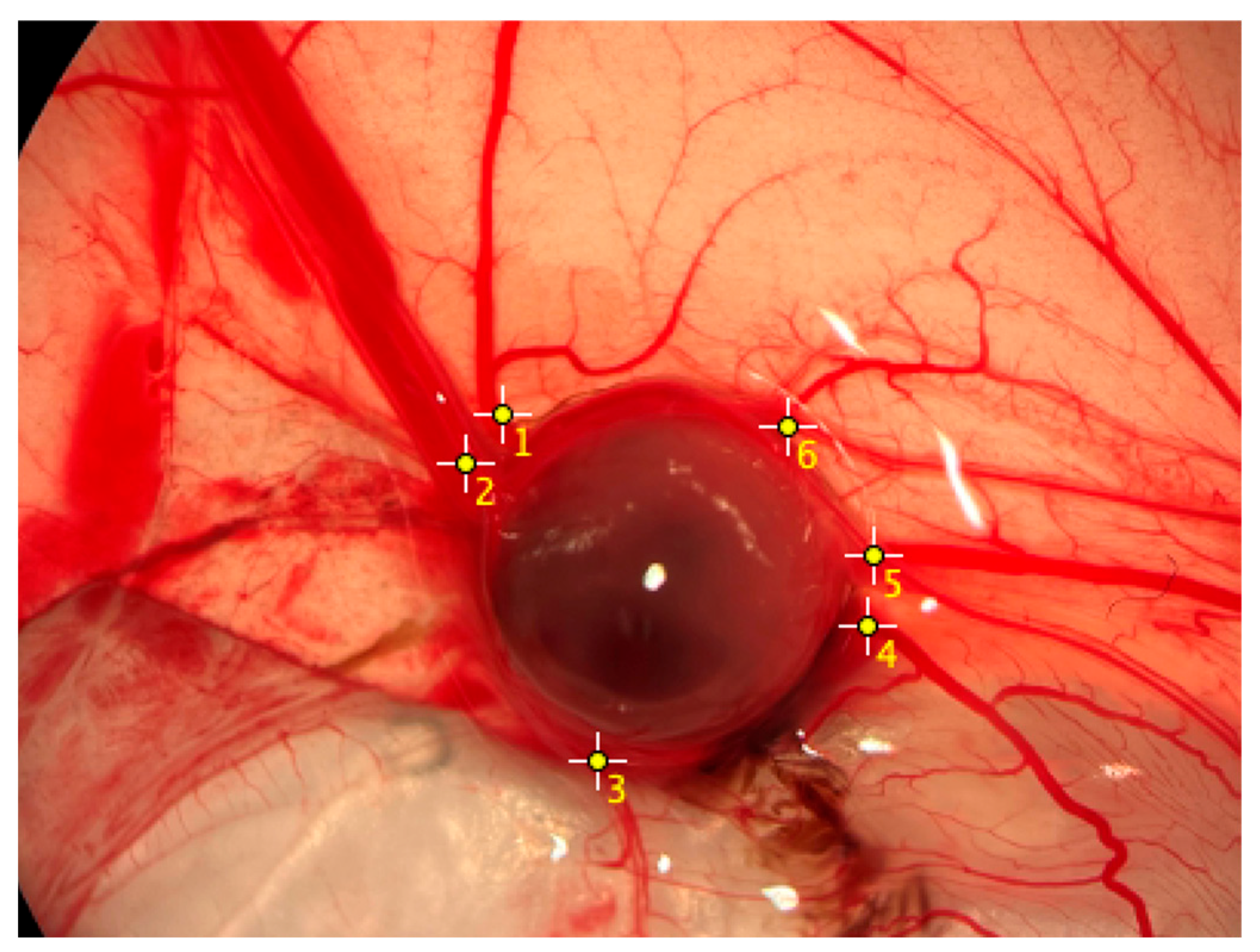
2.4. Quantification of Vascularization
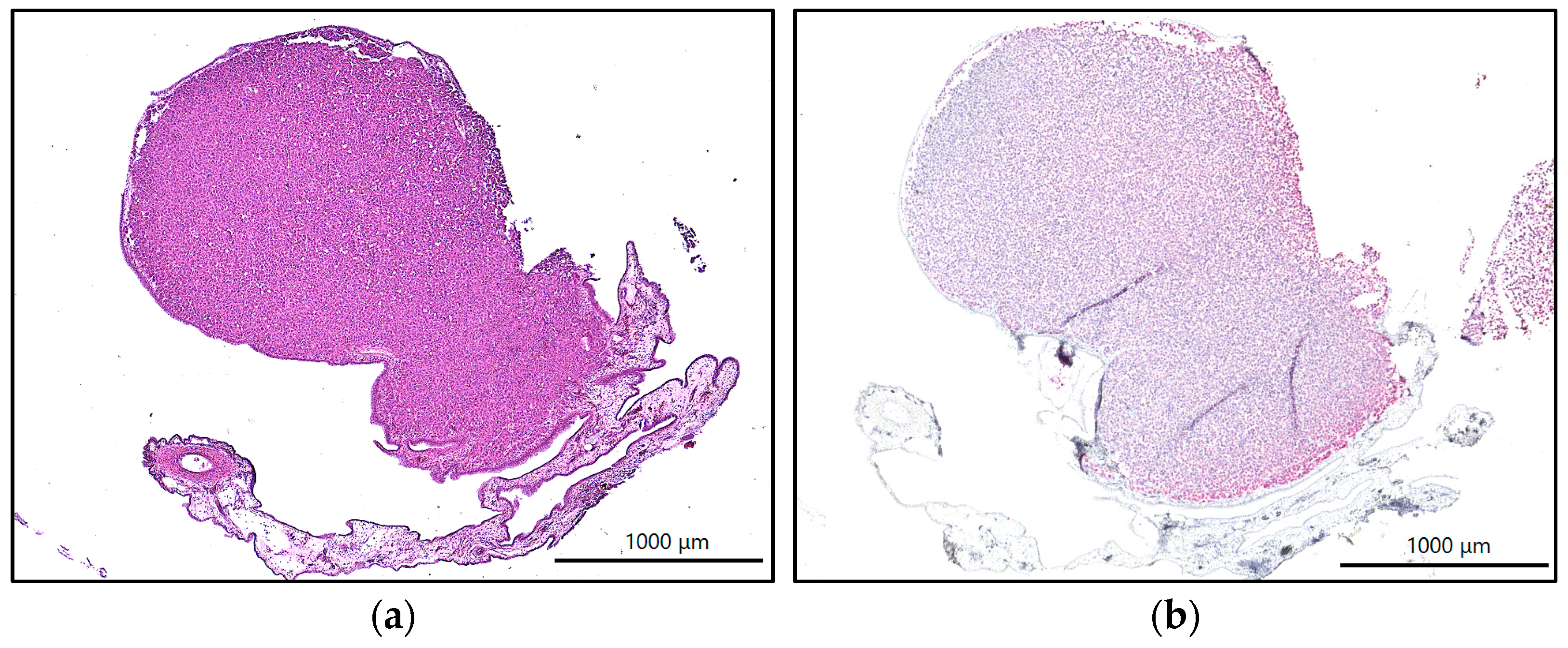
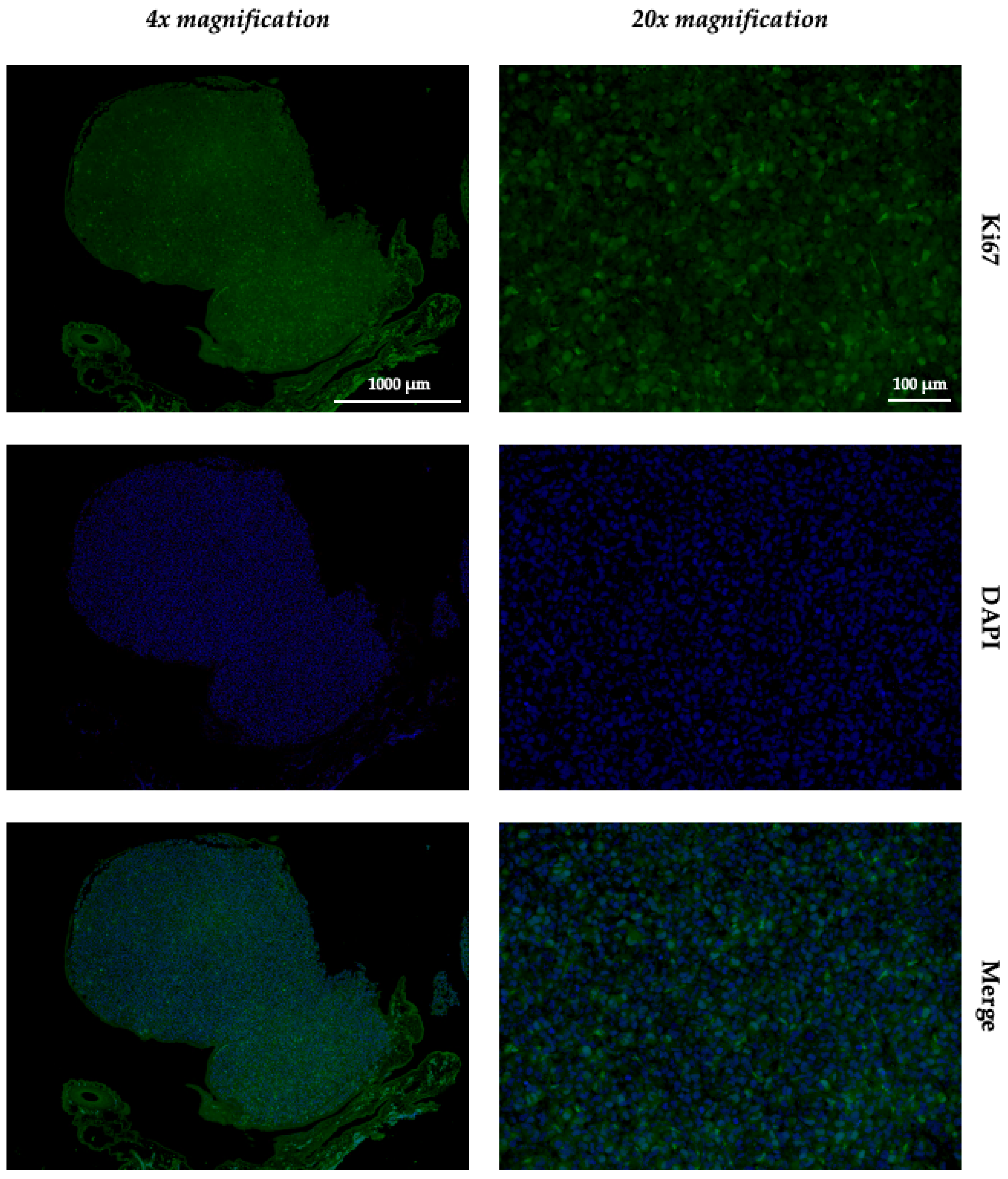
2.5. Immunohistochemistry and Immunofluorescence
3. Discussion
4. Materials and Methods
4.1. Experimental Groups and Study Design
4.2. Cell Line and Spheroid Formation
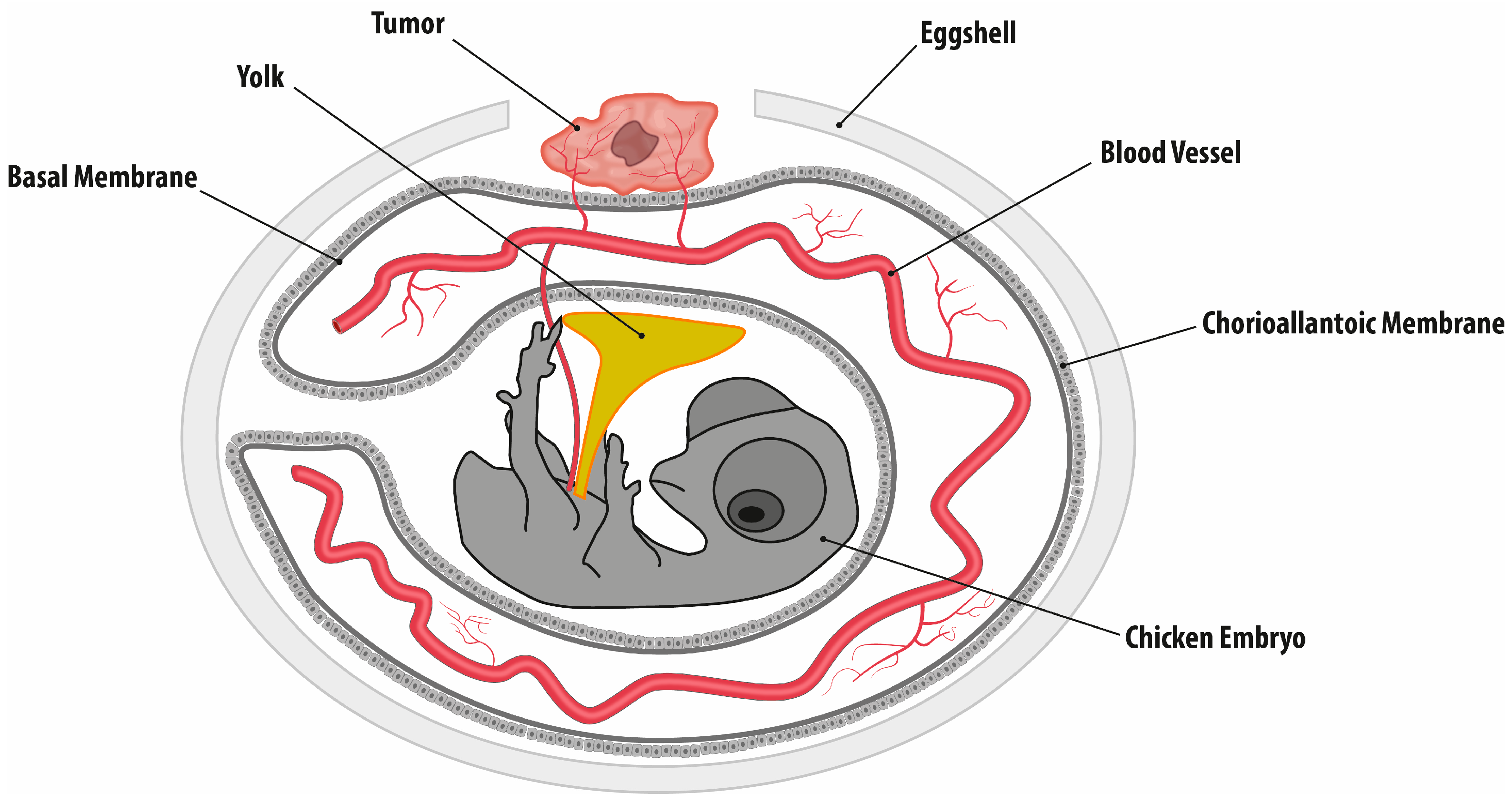
4.3. Chorioallantoic Membrane Assay
4.4. Weight and Tumor Surface Measurements
4.5. Vascularization
4.6. Histology, Immunohistochemistry, and Immunofluorescence
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 95% CI | 95% confidence interval |
| CAM | chorioallantoic membrane |
| CTGF | connective tissue growth factor |
| HE | hematoxylin and eosin |
| MMP | matrix metalloproteinases |
| Ns | no significance |
| 3Rs | replacement, reduction, and refinement |
| VEGF | vascular endothelial growth factor |
References
- Augsburger, D.; Nelson, P.J.; Kalinski, T.; Udelnow, A.; Knösel, T.; Hofstetter, M.; Qin, J.W.; Wang, Y.; Gupta, A.S.; Bonifatius, S.; et al. Current diagnostics and treatment of fibrosarcoma -perspectives for future therapeutic targets and strategies. Oncotarget 2017, 8, 104638–104653. [Google Scholar] [CrossRef] [PubMed]
- Folpe, A.L. Fibrosarcoma: A review and update. Histopathology 2014, 64, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.D.; Shah, S.J.; Kane, S.M. Fibrosarcoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/pubmed/32809594 (accessed on 8 March 2025).
- Bahrami, A.; Folpe, A.L. Adult-type Fibrosarcoma: A Reevaluation of 163 Putative Cases Diagnosed at a Single Institution Over a 48-year Period. Am. J. Surg. Pathol. 2010, 34, 1504–1513. [Google Scholar] [CrossRef]
- von Mehren, M.; Kane, J.M.; Agulnik, M.; Bui, M.M.; Carr-Ascher, J.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; Gonzalez, R.J.; et al. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 815–833. [Google Scholar] [CrossRef]
- Gronchi, A.; Palmerini, E.; Quagliuolo, V.; Martin-Broto, J.; Lopez Pousa, A.; Grignani, G.; Brunello, A.; Blay, J.-Y.; Tendero, O.; Diaz Beveridge, R.; et al. Neoadjuvant Chemotherapy in High-Risk Soft Tissue Sarcomas: Final Results of a Randomized Trial From Italian (ISG), Spanish (GEIS), French (FSG), and Polish (PSG) Sarcoma Groups. J. Clin. Oncol. 2020, 38, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM) assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef]
- Harper, K.; Yatsyna, A.; Charbonneau, M.; Brochu-Gaudreau, K.; Perreault, A.; Jeldres, C.; McDonald, P.P.; Dubois, C.M. The chicken chorioallantoic membrane tumor assay as a relevant in vivo model to study the impact of hypoxia on tumor progression and metastasis. Cancers 2021, 13, 1093. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis. Histochem. Cell Biol. 2008, 130, 1119–1130. [Google Scholar] [CrossRef]
- Subauste, M.C.; Kupriyanova, T.A.; Conn, E.M.; Ardi, V.C.; Quigley, J.P.; Deryugina, E.I. Evaluation of metastatic and angiogenic potentials of human colon carcinoma cells in chick embryo model systems. Clin. Exp. Metastasis 2009, 26, 1033–1047. [Google Scholar] [CrossRef]
- Shanbhag, S.; Rashad, A.; Nymark, E.H.; Suliman, S.; de Lange Davies, C.; Stavropoulos, A.; Bolstad, A.I.; Mustafa, K. Spheroid Coculture of Human Gingiva-Derived Progenitor Cells with Endothelial Cells in Modified Platelet Lysate Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 739225. [Google Scholar] [CrossRef]
- Fischer, D.; Fluegen, G.; Garcia, P.; Ghaffari-Tabrizi-Wizsy, N.; Gribaldo, L.; Huang, R.Y.-J.; Rasche, V.; Ribatti, D.; Rousset, X.; Pinto, M.T.; et al. The CAM Model—Q&A with Experts. Cancers 2023, 15, 191. [Google Scholar] [CrossRef]
- Rolver, M.G.; Elingaard-Larsen, L.O.; Pedersen, S.F. Assessing Cell Viability and Death in 3D Spheroid Cultures of Cancer Cells. J. Vis. Exp. 2019, 148, e59714. [Google Scholar] [CrossRef]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ohata, H.; Sato, A.; Yamawaki, K.; Enomoto, T.; Okamoto, K. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci. 2017, 108, 283–289. [Google Scholar] [CrossRef]
- Shimo, T.; Nakanishi, T.; Nishida, T.; Asano, M.; Sasaki, A.; Kanyama, M.; Kuboki, T.; Matsumura, T.; Takigawa, M. Involvement of CTGF, a hypertrophic chondrocyte-specific gene product, in tumor angiogenesis. Oncology 2001, 61, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Zijlstra, A.; Partridge, J.J.; Kupriyanova, T.A.; Madsen, M.A.; Papagiannakopoulos, T.; Quigley, J.P. Unexpected effect of matrix metalloproteinase down-regulation on vascular intravasation and metastasis of human fibrosarcoma cells selected in vivo for high rates of dissemination. Cancer Res. 2005, 65, 10959–10969. [Google Scholar] [CrossRef]
- Zijlstra, A.; Seandel, M.; Kupriyanova, T.A.; Partridge, J.J.; Madsen, M.A.; Hahn-Dantona, E.A.; Quigley, J.P.; Deryugina, E.I. Proangiogenic role of neutrophil-like inflammatory heterophils during neovascularization induced by growth factors and human tumor cells. Blood 2006, 107, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Bailey, A.P.; Sartin, A.; Makey, I.; Brady, A.L. Ethanol stimulates tumor progression and expression of vascular endothelial growth factor in chick embryos. Cancer 2005, 103, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Shoji, C.; Kikuchi, K.; Yoshida, H.; Miyachi, M.; Yagyu, S.; Tsuchiya, K.; Nakaya, T.; Hosoi, H.; Iehara, T. In ovo chorioallantoic membrane assay as a xenograft model for pediatric rhabdomyosarcoma. Oncol. Rep. 2023, 49, 76. [Google Scholar] [CrossRef]
- Sokolenko, E.A.; Berchner-Pfannschmidt, U.; Ting, S.C.; Schmid, K.W.; Bechrakis, N.E.; Seitz, B.; Tsimpaki, T.; Kraemer, M.M.; Fiorentzis, M. Optimisation of the chicken chorioallantoic membrane assay in uveal melanoma research. Pharmaceutics 2022, 14, 13. [Google Scholar] [CrossRef]
- Doege, A.; Steens, R.; Dünker, N.; Busch, M.A. Retinoblastoma Cell Growth In Vitro and Tumor Formation In Ovo-Influence of Different Culture Conditions. Methods Protoc. 2022, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Fergelot, P.; Bernhard, J.-C.; Soulet, F.; Kilarski, W.W.; Léon, C.; Courtois, N.; Deminière, C.; Herbert, J.M.J.; Antczak, P.; Falciani, F.; et al. The experimental renal cell carcinoma model in the chick embryo. Angiogenesis 2013, 16, 181–194. [Google Scholar] [CrossRef]
- Kilarski, W.W.; Samolov, B.; Petersson, L.; Kvanta, A.; Gerwins, P. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat. Med. 2009, 15, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Larger, E.; Marre, M.; Corvol, P.; Gasc, J.-M. Hyperglycemia-Induced Defects in Angiogenesis in the Chicken Chorioallantoic Membrane Model. Diabetes 2004, 53, 752–761. [Google Scholar] [CrossRef]
- Annese, T.; Tamma, R.; Ribatti, D. IKOSA® CAM Assay Application to Quantify Blood Vessels on Chick Chorioallantoic Membrane (CAM). In Tumor Angiogenesis Assays: Methods and Protocols; Ribatti, D., Ed.; Springer: New York, NY, USA, 2023; pp. 129–139. [Google Scholar] [CrossRef]
- Gnutti, A.; Signoroni, A.; Leonardi, R.; Corsini, M.; Presta, M.; Mitola, S. A tool for the quantification of radial neo-vessels in chick chorioallantoic membrane angiogenic assays. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milano, Italy, 25–29 August 2015; pp. 763–766. [Google Scholar] [CrossRef]
- Bai, C.; Yang, M.; Fan, Z.; Li, S.; Gao, T.; Fang, Z. Associations of chemo- and radio-resistant phenotypes with the gap junction, adhesion and extracellular matrix in a three-dimensional culture model of soft sarcoma. J. Exp. Clin. Cancer Res. 2015, 34, 58. [Google Scholar] [CrossRef]
- Fruehauf, S.; Veldwijk, M.; Berlinghoff, S.; Basara, N.; Baum, C.; Flasshove, M.; Hegewisch-Becker, S.; Kröger, N.; Licht, T.; Moritz, T.; et al. Gene Therapy for Sarcoma. Cells Tissues Organs 2002, 172, 133–144. [Google Scholar] [CrossRef]
- Becerikli, M.; Merwart, B.; Lam, M.; Suppelna, P.; Rittig, A.; Mirmohammedsadegh, A.; Stricker, I.; Theiss, C.; Singer, B.; Jacobsen, F.; et al. EPHB4 tyrosine-kinase receptor expression and biological significance in soft tissue sarcoma. Int. J. Cancer 2015, 136, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.; Greve, B.; Bölling, T.; Eich, H.T.; Willich, N.; Harrach, S.; Hintelmann, H.; Lenz, G.; Mesters, R.M.; Kessler, T.; et al. Radiation synergizes with antitumor activity of CD13-targeted tissue factor in a HT1080 xenograft model of human soft tissue sarcoma. PLoS ONE 2020, 15, e0229271. [Google Scholar] [CrossRef]
- Smieško, G.; Banović, P.; Gusman, V.; Simin, V.; Cimpean, A.M.; Lalošević, D. Molecular evaluation of chronic restrain stress in mice model of non metastatic fibrosarcoma. J. Mol. Histol. 2020, 51, 367–374. [Google Scholar] [CrossRef]
- Harati, K.; Emmelmann, S.; Behr, B.; Goertz, O.; Hirsch, T.; Kapalschinski, N.; Kolbenschlag, J.; Stricker, I.; Tannapfel, A.; Lehnhardt, M.; et al. Evaluation of the safety and efficacy of TRAIL and taurolidine use on human fibrosarcoma xenografts in vivo. Oncol. Lett. 2016, 11, 1955–1961. [Google Scholar] [CrossRef]
- Harati, K.; Slodnik, P.; Chromik, A.M.; Goertz, O.; Hirsch, T.; Kapalschinski, N.; Klein-Hitpass, L.; Kolbenschlag, J.; Uhl, W.; Lehnhardt, M.; et al. Resveratrol induces apoptosis and alters gene expression in human fibrosarcoma cells. Anticancer Res. 2015, 35, 767–774. [Google Scholar] [PubMed]
- Guder, W.K.; Hartmann, W.; Buhles, C.; Burdack, M.; Busch, M.; Dünker, N.; Hardes, J.; Dirksen, U.; Bauer, S.; Streitbürger, A. 5-ALA-mediated fluorescence of musculoskeletal tumors in a chick chorio-allantoic membrane model: Preclinical in vivo qualification analysis as a fluorescence-guided surgery agent in Orthopedic Oncology. J. Orthop. Surg. Res. 2022, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, M.; Grunewald, S.; Schaefer, C.; Zöllner, S.K.; Plaumann, P.; Busch, M.; Dünker, N.; Ketzer, J.; Kersting, J.; Bauer, S.; et al. Evaluation of the Effect of Photodynamic Therapy on CAM-Grown Sarcomas. Bioengineering 2023, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- Puscz, F.; Dadras, M.; Dermietzel, A.; Jacobsen, F.; Lehnhardt, M.; Behr, B.; Hirsch, T.; Kueckelhaus, M. A chronic rejection model and potential biomarkers for vascularized composite allotransplantation. PLoS ONE 2020, 15, e0235266. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puscz, F.; Hatem, N.J.; Schmidt, S.V.; Reinkemeier, F.; Drysch, M.; Becerikli, M.; Steubing, Y.; Lehnhardt, M.; Wallner, C. Implementation of a CAM Assay Using Fibrosarcoma Spheroids. Int. J. Mol. Sci. 2025, 26, 5318. https://doi.org/10.3390/ijms26115318
Puscz F, Hatem NJ, Schmidt SV, Reinkemeier F, Drysch M, Becerikli M, Steubing Y, Lehnhardt M, Wallner C. Implementation of a CAM Assay Using Fibrosarcoma Spheroids. International Journal of Molecular Sciences. 2025; 26(11):5318. https://doi.org/10.3390/ijms26115318
Chicago/Turabian StylePuscz, Flemming, Noah Jozsef Hatem, Sonja Verena Schmidt, Felix Reinkemeier, Marius Drysch, Mustafa Becerikli, Yonca Steubing, Marcus Lehnhardt, and Christoph Wallner. 2025. "Implementation of a CAM Assay Using Fibrosarcoma Spheroids" International Journal of Molecular Sciences 26, no. 11: 5318. https://doi.org/10.3390/ijms26115318
APA StylePuscz, F., Hatem, N. J., Schmidt, S. V., Reinkemeier, F., Drysch, M., Becerikli, M., Steubing, Y., Lehnhardt, M., & Wallner, C. (2025). Implementation of a CAM Assay Using Fibrosarcoma Spheroids. International Journal of Molecular Sciences, 26(11), 5318. https://doi.org/10.3390/ijms26115318





