Antioxidants as Modulators of NETosis: Mechanisms, Evidence, and Therapeutic Potential
Abstract
1. Introduction
2. Mechanisms of ROS in NETosis Activation
2.1. Via NOX2 or Mitochondrial Activity
2.2. Via NOX-Independent NETosis
2.3. Via MPO Oxidation Pathway
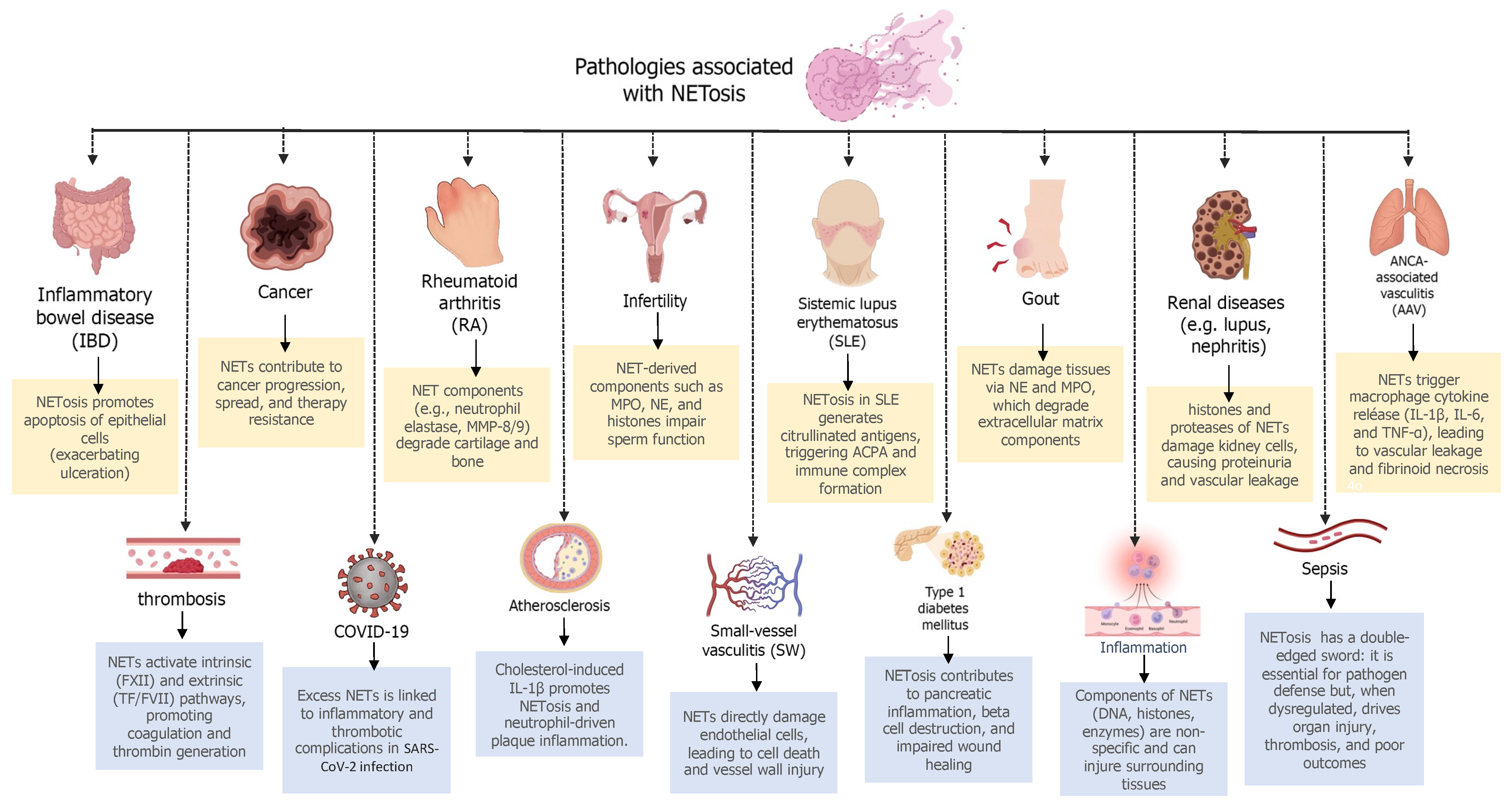
3. Evidence and Therapeutic Applications of NETosis Inhibition
4. Antioxidants as NETosis Inhibitors
5. Synergistic Strategies: Antioxidants in Combination Therapies
6. Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular Mechanisms of NETosis. Annu. Rev. Cell Dev. Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef]
- Huang, J.; Hong, W.; Wan, M.; Zheng, L. Molecular mechanisms and therapeutic target of NETosis in diseases. MedComm (2020) 2022, 3, e162. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, D.; Palaniyar, N. How Do ROS Induce NETosis? Oxidative DNA Damage, DNA Repair, and Chromatin Decondensation. Biomolecules 2024, 14, 1307. [Google Scholar] [CrossRef]
- Liang, X.; Liu, L.; Wang, Y.; Guo, H.; Fan, H.; Zhang, C.; Hou, L.; Liu, Z. Autophagy-driven NETosis is a double-edged—Review. Biomed. Pharmacother. 2020, 126, 110065. [Google Scholar] [CrossRef]
- Cao, J.F.; Chen, J. Pseudomonas plecoglossicida infection induces neutrophil autophagy-driven NETosis in large yellow croaker Larimichthys crocea. Front. Immunol. 2024, 15, 1521080. [Google Scholar] [CrossRef]
- Burgener, S.S.; Schroder, K. Neutrophil Extracellular Traps in Host Defense. Cold Spring Harb. Perspect. Biol. 2020, 12, a037028. [Google Scholar] [CrossRef]
- Schonrich, G.; Raftery, M.J. Neutrophil Extracellular Traps Go Viral. Front. Immunol. 2016, 7, 366. [Google Scholar] [CrossRef]
- Dos Santos Ramos, A.; Viana, G.C.S.; de Macedo Brigido, M.; Almeida, J.F. Neutrophil extracellular traps in inflammatory bowel diseases: Implications in pathogenesis and therapeutic targets. Pharmacol. Res. 2021, 171, 105779. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, G.; Tan, J.; Xiao, X.; Yang, L.; Saw, P.E. Targeting NETosis: Nature’s alarm system in cancer progression. Cancer Drug Resist. 2024, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Yang, R.; Wen, H.; Dong, Q.; Chen, Z.; Xiang, Y.; Wu, H. Myricetin reduces neutrophil extracellular trap release in a rat model of rheumatoid arthritis, which is associated with a decrease in disease severity. Innate Immun. 2024, 30, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Zambrano, F.; Schuppe, H.C.; Wagenlehner, F.; Taubert, A.; Ulrich, G.; Sanchez, R.; Hermosilla, C. Determination of leucocyte extracellular traps (ETs) in seminal fluid (ex vivo) in infertile patients-A pilot study. Andrologia 2019, 51, e13356. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, F.; Carrau, T.; Gartner, U.; Seipp, A.; Taubert, A.; Felmer, R.; Sanchez, R.; Hermosilla, C. Leukocytes coincubated with human sperm trigger classic neutrophil extracellular traps formation, reducing sperm motility. Fertil. Steril. 2016, 106, 1053–1060.e1. [Google Scholar] [CrossRef]
- Wang, M.; Ishikawa, T.; Lai, Y.; Nallapothula, D.; Singh, R.R. Diverse Roles of NETosis in the Pathogenesis of Lupus. Front. Immunol. 2022, 13, 895216. [Google Scholar] [CrossRef]
- Liu, L.; Shan, L.; Wang, H.; Schauer, C.; Schoen, J.; Zhu, L.; Lu, C.; Wang, Z.; Xue, Y.; Wu, H.; et al. Neutrophil Extracellular Trap-Borne Elastase Prevents Inflammatory Relapse in Intercritical Gout. Arthritis Rheumatol. 2023, 75, 1039–1047. [Google Scholar] [CrossRef]
- Salazar-Gonzalez, H.; Zepeda-Hernandez, A.; Melo, Z.; Saavedra-Mayorga, D.E.; Echavarria, R. Neutrophil Extracellular Traps in the Establishment and Progression of Renal Diseases. Medicina 2019, 55, 431. [Google Scholar] [CrossRef]
- Ge, S.; Zhu, X.; Xu, Q.; Wang, J.; An, C.; Hu, Y.; Yang, F.; Wang, X.; Yang, Y.; Chen, S.; et al. Neutrophils in ANCA-associated vasculitis: Mechanisms and implications for management. Front. Pharmacol. 2022, 13, 957660. [Google Scholar] [CrossRef]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef]
- Serrano-Gonzalo, I.; Menendez-Jandula, B.; Franco-Garcia, E.; Arevalo-Vargas, I.; Lahoz-Gil, C.; Latre, P.; Roca-Esteve, S.; Kohler, R.; Lopez de Frutos, L.; Giraldo, P. Neutrophil extracellular traps and macrophage activation contibute to thrombosis and post-covid syndrome in SARS-CoV-2 infection. Front. Immunol. 2025, 16, 1507167. [Google Scholar] [CrossRef]
- Yalcinkaya, M.; Fotakis, P.; Liu, W.; Endo-Umeda, K.; Dou, H.; Abramowicz, S.; Xiao, T.; Libby, P.; Wang, N.; Tall, A.R.; et al. Cholesterol accumulation in macrophages drives NETosis in atherosclerotic plaques via IL-1beta secretion. Cardiovasc. Res. 2023, 119, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Safi, R.; Kallas, R.; Bardawil, T.; Mehanna, C.J.; Abbas, O.; Hamam, R.; Uthman, I.; Kibbi, A.G.; Nassar, D. Neutrophils contribute to vasculitis by increased release of neutrophil extracellular traps in Behcet’s disease. J. Dermatol. Sci. 2018, 92, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, A.; Popp, S.K.; Fukuda, R.; Parish, C.R.; Bosi, E.; Simeonovic, C.J. The Contribution of Neutrophils and NETs to the Development of Type 1 Diabetes. Front. Immunol. 2022, 13, 930553. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, D.; Palaniyar, N. ROS and DNA repair in spontaneous versus agonist-induced NETosis: Context matters. Front. Immunol. 2022, 13, 1033815. [Google Scholar] [CrossRef]
- Azzouz, D.; Palaniyar, N. Mitochondrial ROS and base excision repair steps leading to DNA nick formation drive ultraviolet induced-NETosis. Front. Immunol. 2023, 14, 1198716. [Google Scholar] [CrossRef]
- Donkel, S.J.; Wolters, F.J.; Ikram, M.A.; de Maat, M.P.M. Circulating Myeloperoxidase (MPO)-DNA complexes as marker for Neutrophil Extracellular Traps (NETs) levels and the association with cardiovascular risk factors in the general population. PLoS ONE 2021, 16, e0253698. [Google Scholar] [CrossRef]
- Hu, Z.; Hua, X.; Mo, X.; Chang, Y.; Chen, X.; Xu, Z.; Tao, M.; Hu, G.; Song, J. Inhibition of NETosis via PAD4 alleviated inflammation in giant cell myocarditis. iScience 2023, 26, 107162. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Moran, G.; Uberti, B.; Quiroga, J. Role of Cellular Metabolism in the Formation of Neutrophil Extracellular Traps in Airway Diseases. Front. Immunol. 2022, 13, 850416. [Google Scholar] [CrossRef]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef]
- Parker, H.; Winterbourn, C.C. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front. Immunol. 2012, 3, 424. [Google Scholar] [CrossRef] [PubMed]
- Kremserova, S.; Kocurkova, A.; Chorvatova, M.; Klinke, A.; Kubala, L. Myeloperoxidase Deficiency Alters the Process of the Regulated Cell Death of Polymorphonuclear Neutrophils. Front. Immunol. 2022, 13, 707085. [Google Scholar] [CrossRef]
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014, 8, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra140. [Google Scholar] [CrossRef] [PubMed]
- Chirivi, R.G.S.; van Rosmalen, J.W.G.; van der Linden, M.; Euler, M.; Schmets, G.; Bogatkevich, G.; Kambas, K.; Hahn, J.; Braster, Q.; Soehnlein, O.; et al. Therapeutic ACPA inhibits NET formation: A potential therapy for neutrophil-mediated inflammatory diseases. Cell Mol. Immunol. 2021, 18, 1528–1544. [Google Scholar] [CrossRef]
- Li, X.; Xiao, S.; Filipczak, N.; Yalamarty, S.S.K.; Shang, H.; Zhang, J.; Zheng, Q. Role and Therapeutic Targeting Strategies of Neutrophil Extracellular Traps in Inflammation. Int. J. Nanomed. 2023, 18, 5265–5287. [Google Scholar] [CrossRef]
- Jaboury, S.; Wang, K.; O’Sullivan, K.M.; Ooi, J.D.; Ho, G.Y. NETosis as an oncologic therapeutic target: A mini review. Front. Immunol. 2023, 14, 1170603. [Google Scholar] [CrossRef]
- Yan, M.; Gu, Y.; Sun, H.; Ge, Q. Neutrophil extracellular traps in tumor progression and immunotherapy. Front. Immunol. 2023, 14, 1135086. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Zheng, Z.; Guo, Y.; He, G.; Wang, Y.; Fu, S.; Zheng, C.; Deng, X. Neutrophil Extracellular Traps in Breast Cancer: Roles in Metastasis and Beyond. J. Cancer 2024, 15, 3272–3283. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, Y.S.; Lee, B.S.; Han, C.H.; Kim, S.G.; Kim, C.H. NLRP3 inflammasome activation and NETosis positively regulate each other and exacerbate proinflammatory responses: Implications of NETosis inhibition for acne skin inflammation treatment. Cell Mol. Immunol. 2024, 21, 466–478. [Google Scholar] [CrossRef]
- Chen, X.; Cuffari, B.J.; Dubljevic, V.; Shirali, A.; Zhou, J.; Campbell, J.A.; Suits, S.C.; O’Sullivan, K.M.; Hansen, J.E. Inhibition of NETosis by a Nuclear-Penetrating Anti-DNA Autoantibody. Immunohorizons 2022, 6, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Chamardani, T.M.; Amiritavassoli, S. Inhibition of NETosis for treatment purposes: Friend or foe? Mol. Cell Biochem. 2022, 477, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.H.; Feng, L.; Su, X.; Brassard, A.; Dhoparee-Doomah, I.; Ferri, L.E.; Spicer, J.D.; Cools-Lartigue, J.J. Neutrophil Extracellular Traps in Cancer Therapy Resistance. Cancers 2022, 14, 1359. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Sanchez, G.; Godinez-Mendez, L.A.; Fafutis-Morris, M.; Delgado-Rizo, V. Effect of Antioxidant Supplementation on NET Formation Induced by LPS In Vitro; the Roles of Vitamins E and C, Glutathione, and N-acetyl Cysteine. Int. J. Mol. Sci. 2023, 24, 13162. [Google Scholar] [CrossRef]
- Hallberg, L.A.E.; Barlous, K.; Hawkins, C.L. Antioxidant Strategies to Modulate NETosis and the Release of Neutrophil Extracellular Traps during Chronic Inflammation. Antioxidants 2023, 12, 478. [Google Scholar] [CrossRef]
- Menegazzo, L.; Scattolini, V.; Cappellari, R.; Bonora, B.M.; Albiero, M.; Bortolozzi, M.; Romanato, F.; Ceolotto, G.; Vigili de Kreutzeberg, S.; Avogaro, A.; et al. The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo. Acta Diabetol. 2018, 55, 593–601. [Google Scholar] [CrossRef]
- Ohinata, H.; Phimarn, W.; Mizuno, M.; Obama, T.; Fukuhara, K.; Makiyama, T.; Watanabe, Y.; Itabe, H. Suppressive effect of resveratrol, catechin and their conformationally constrained analogs on neutrophil extracellular trap formation by HL-60-derived neutrophils. J. Clin. Biochem. Nutr. 2024, 75, 17–23. [Google Scholar] [CrossRef]
- Bilski, R.; Nuszkiewicz, J. Antioxidant Therapies as Emerging Adjuncts in Rheumatoid Arthritis: Targeting Oxidative Stress to Enhance Treatment Outcomes. Int. J. Mol. Sci. 2025, 26, 2873. [Google Scholar] [CrossRef]
- de Souza Andrade, M.M.; Leal, V.N.C.; Fernandes, I.G.; Gozzi-Silva, S.C.; Beserra, D.R.; Oliveira, E.A.; Teixeira, F.M.E.; Yendo, T.M.; Sousa, M.; Teodoro, W.R.; et al. Resveratrol Downmodulates Neutrophil Extracellular Trap (NET) Generation by Neutrophils in Patients with Severe COVID-19. Antioxidants 2022, 11, 1690. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Pinegin, B.V. Effects of the antioxidants Trolox, Tiron and Tempol on neutrophil extracellular trap formation. Immunobiology 2016, 221, 208–219. [Google Scholar] [CrossRef]
- Bekeschus, S.; Winterbourn, C.C.; Kolata, J.; Masur, K.; Hasse, S.; Broker, B.M.; Parker, H.A. Neutrophil extracellular trap formation is elicited in response to cold physical plasma. J. Leukoc. Biol. 2016, 100, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xia, W. Proanthocyanidin Regulates NETosis and Inhibits the Growth and Proliferation of Liver Cancer Cells—In Vivo, In Vitro and In Silico Investigation. Cell Biochem. Biophys. 2025, 83, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Schorn, C.; Janko, C.; Krenn, V.; Zhao, Y.; Munoz, L.E.; Schett, G.; Herrmann, M. Bonding the foe—NETting neutrophils immobilize the pro-inflammatory monosodium urate crystals. Front. Immunol. 2012, 3, 376. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, T.; Li, X.; Gao, L.; Wang, K.; Cheng, M.; Zeng, Z.; Chen, L.; Shen, Y.; Wen, F. DNA of neutrophil extracellular traps promote NF-kappaB-dependent autoimmunity via cGAS/TLR9 in chronic obstructive pulmonary disease. Signal Transduct. Target. Ther. 2024, 9, 163. [Google Scholar] [CrossRef]
- Leung, H.H.L.; Perdomo, J.; Ahmadi, Z.; Yan, F.; McKenzie, S.E.; Chong, B.H. Inhibition of NADPH oxidase blocks NETosis and reduces thrombosis in heparin-induced thrombocytopenia. Blood Adv. 2021, 5, 5439–5451. [Google Scholar] [CrossRef]
- Almasi, N.; Torok, S.; Al-Awar, A.; Veszelka, M.; Kiraly, L.; Borzsei, D.; Szabo, R.; Varga, C. Voluntary Exercise-Mediated Protection in TNBS-Induced Rat Colitis: The Involvement of NETosis and Prdx Antioxidants. Antioxidants 2023, 12, 1531. [Google Scholar] [CrossRef]
- Chen, H.; Xu, X.; Tang, Q.; Ni, L.; Cao, S.; Hao, Y.; Wang, L.; Hu, X. (+)-Borneol inhibits the generation of reactive oxygen species and neutrophil extracellular traps induced by phorbol-12-myristate-13-acetate. Front. Pharmacol. 2022, 13, 1023450. [Google Scholar] [CrossRef]
- Khan, M.A.; D’Ovidio, A.; Tran, H.; Palaniyar, N. Anthracyclines Suppress Both NADPH Oxidase- Dependent and -Independent NETosis in Human Neutrophils. Cancers 2019, 11, 1328. [Google Scholar] [CrossRef]
- Dodo, K.; Kuboki, E.; Shimizu, T.; Imamura, R.; Magarisawa, M.; Takahashi, M.; Tokuhiro, T.; Yotsumoto, S.; Asano, K.; Nakao, S.; et al. Development of a Water-Soluble Indolylmaleimide Derivative IM-93 Showing Dual Inhibition of Ferroptosis and NETosis. ACS Med. Chem. Lett. 2019, 10, 1272–1278. [Google Scholar] [CrossRef]
- Rochael, N.C.; Guimaraes-Costa, A.B.; Nascimento, M.T.; DeSouza-Vieira, T.S.; Oliveira, M.P.; Garcia e Souza, L.F.; Oliveira, M.F.; Saraiva, E.M. Classical ROS-dependent and early/rapid ROS-independent release of Neutrophil Extracellular Traps triggered by Leishmania parasites. Sci. Rep. 2015, 5, 18302. [Google Scholar] [CrossRef]
- Remijsen, Q.; Kuijpers, T.W.; Wirawan, E.; Lippens, S.; Vandenabeele, P.; Vanden Berghe, T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011, 18, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Munafo, D.B.; Johnson, J.L.; Brzezinska, A.A.; Ellis, B.A.; Wood, M.R.; Catz, S.D. DNase I inhibits a late phase of reactive oxygen species production in neutrophils. J. Innate Immun. 2009, 1, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, J.F.; Wenskus, J.; Lenz, M.; Rhode, H.; Trochimiuk, M.; Appl, B.; Pagarol-Raluy, L.; Bornigen, D.; Bang, C.; Reinshagen, K.; et al. DNases improve effectiveness of antibiotic treatment in murine polymicrobial sepsis. Front. Immunol. 2023, 14, 1254838. [Google Scholar] [CrossRef] [PubMed]
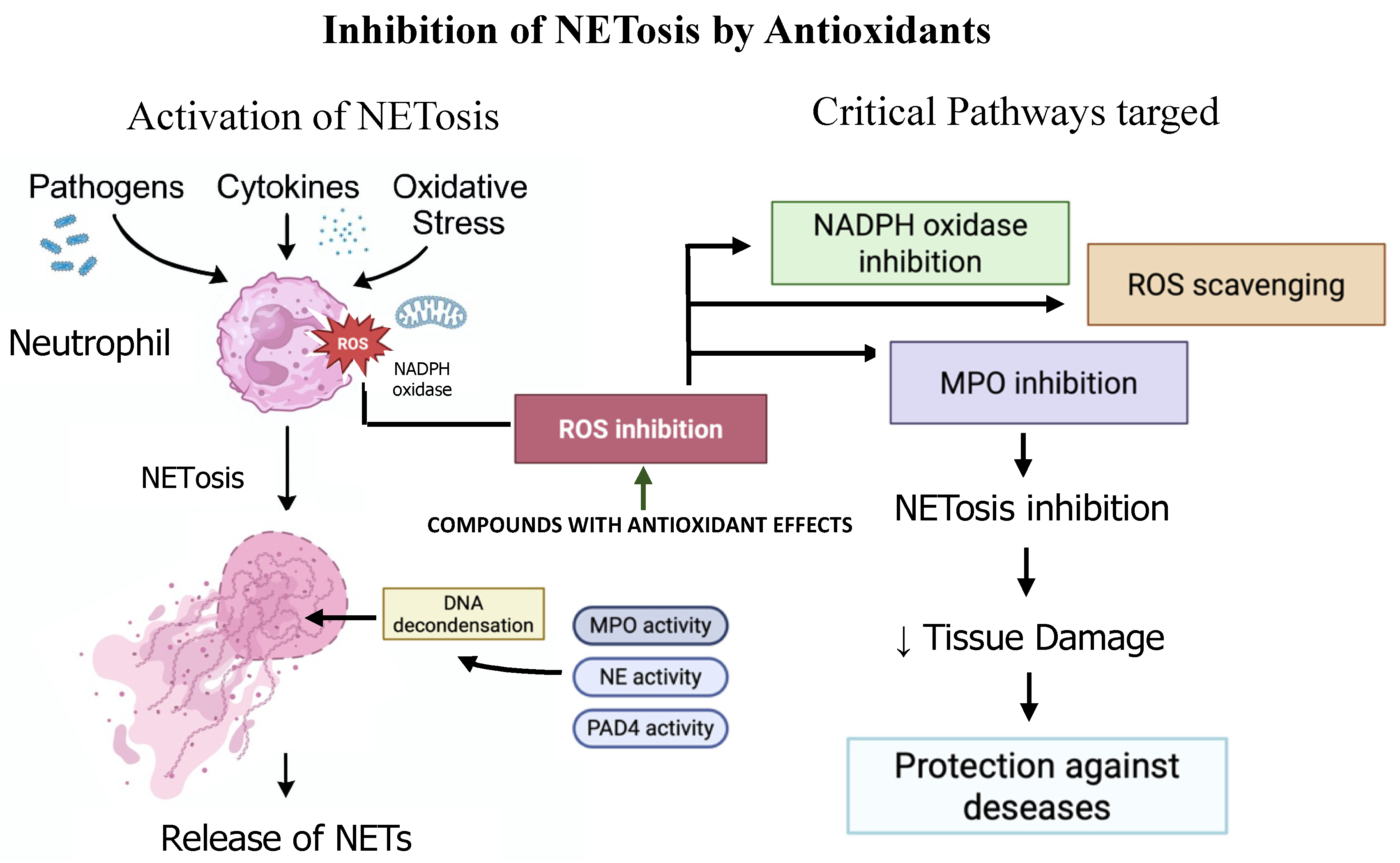
| Antioxidant Compound | Disease Models | Clinical Status | Mechanism of Action | Effect on NETosis | Reference |
|---|---|---|---|---|---|
| Vitamin C | Non-specific | Preclinical study | Enhances redox balance and scavenges ROS. | Dose-dependent reduction in NET formation; complete prevention at pharmacological concentrations. | [44] |
| Trolox, Tempol | Non-specific | Preclinical study | Scavenge ROS and inhibit enzymes responsible for ROS synthesis. | Inhibit ROS-dependent NET release. | [50] |
| Catalase | Pan-inflammation | Preclinical study | Enhances redox balance and scavenges ROS. | Inhibits NETosis via H2O2 scavenging. | [5,51] |
| Proanthocyanidin | Liver Cancer | Preclinical study | Significantly reducing ROS levels in neutrophils. | Blocks the oxidative stress pathways required for chromatin decondensation and NET release. | [52] |
| N-Acetyl Cysteine (NAC) | Non-specific | Preclinical study | Combined with GSH, it abolishes NET formation in LPS-stimulated neutrophils. | Reduces NETs by scavenging ROS and inhibiting MPO activity. | [44,45] |
| Resveratrol | Non-specific SARS-CoV-2 infection | Preclinical Study Preclinical study | Inhibits myeloperoxidase release, particularly when stimulated with PMA and POVPC, and reduces oxidative stress. | Suppresses DNA release from neutrophils. Decreases the neutrophil-activated status and the release of free DNA, inhibiting NET formation. | [47,49] |
| Glutathione (GSH) | Non-specific | Preclinical study | Improves intracellular antioxidant capacity and GSH/GSSG ratio. | Strong suppression of NET formation in serum and intracellular levels. | [44] |
| butylated hydroxytoluene (BHT) | Non-specific | Preclinical study | Enhances redox balance and scavenges ROS. | Reduces NET formation in vitro. | [53] |
| Metformin | Diabetes | Preclinical and clinical study | Inhibits PKC-NADPH oxidase pathway. | Reduces NET components and blunts NETosis in vitro. | [46] |
| Thiocyanate, Selenocyanate, Nitroxides | Chronic Inflammation | Preclinical study | Modulate HOCl production by MPO. | Prevent NETosis in PLB-985 neutrophils exposed to PMA and HOCl. | [45] |
| MitoTEMPO | Chronic obstructive pulmonary disease (COPD) | Preclinical and clinical study | Prevents NADPH oxidase activation. | Reduces NETosis in specific contexts (e.g., COPD mouse model). | [54] |
| Diphenyleneiodonium (DPI) | Thrombosis | Preclinical and clinical study | Inhibition of NOX2. | Identifies NOX2 inhibition as a potential new therapeutic target for antithrombotic treatment. | [55] |
| Peroxiredoxin (Prdx) | Inflammatory bowel disease (IBD) | Preclinical study | Upregulation in response to voluntary exercise. | Reduces inflammation and inhibits NETosis in TNBS (induce experimental colitis). | [56] |
| (+)-Borneol | Non-specific | Preclinical study | Inhibits ROS generation and NADPH oxidase activity. | Decreases ROS levels and inhibits NETosis triggered by PMA stimulation. | [57] |
| Anthracyclines (e.g., Epirubicin, Daunorubicin) | Non-specific | Preclinical study | Suppress both NADPH oxidase-dependent and -independent NETosis. | Inhibit NETosis without suppressing ROS necessary for antimicrobial functions. | [58] |
| Indolylmaleimide Derivative IM-93 | Non-specific | Preclinical study | Inhibits oxidative stress-induced necrotic cell death. | Inhibits both ferroptosis and NETosis. | [59] |
| Combination Therapies | Non-specific | Preclinical study | Enhances TAC and reduces ROS/RNS levels. | Most effective approach; achieves strongest suppression of NET formation using Vit E + Vit C + GSH + NAC. | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambrano, F.; Uribe, P.; Schulz, M.; Hermosilla, C.; Taubert, A.; Sánchez, R. Antioxidants as Modulators of NETosis: Mechanisms, Evidence, and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 5272. https://doi.org/10.3390/ijms26115272
Zambrano F, Uribe P, Schulz M, Hermosilla C, Taubert A, Sánchez R. Antioxidants as Modulators of NETosis: Mechanisms, Evidence, and Therapeutic Potential. International Journal of Molecular Sciences. 2025; 26(11):5272. https://doi.org/10.3390/ijms26115272
Chicago/Turabian StyleZambrano, Fabiola, Pamela Uribe, Mabel Schulz, Carlos Hermosilla, Anja Taubert, and Raúl Sánchez. 2025. "Antioxidants as Modulators of NETosis: Mechanisms, Evidence, and Therapeutic Potential" International Journal of Molecular Sciences 26, no. 11: 5272. https://doi.org/10.3390/ijms26115272
APA StyleZambrano, F., Uribe, P., Schulz, M., Hermosilla, C., Taubert, A., & Sánchez, R. (2025). Antioxidants as Modulators of NETosis: Mechanisms, Evidence, and Therapeutic Potential. International Journal of Molecular Sciences, 26(11), 5272. https://doi.org/10.3390/ijms26115272







