New Frontiers of Biomarkers in Metastatic Colorectal Cancer: Potential and Critical Issues
Abstract
1. Introduction
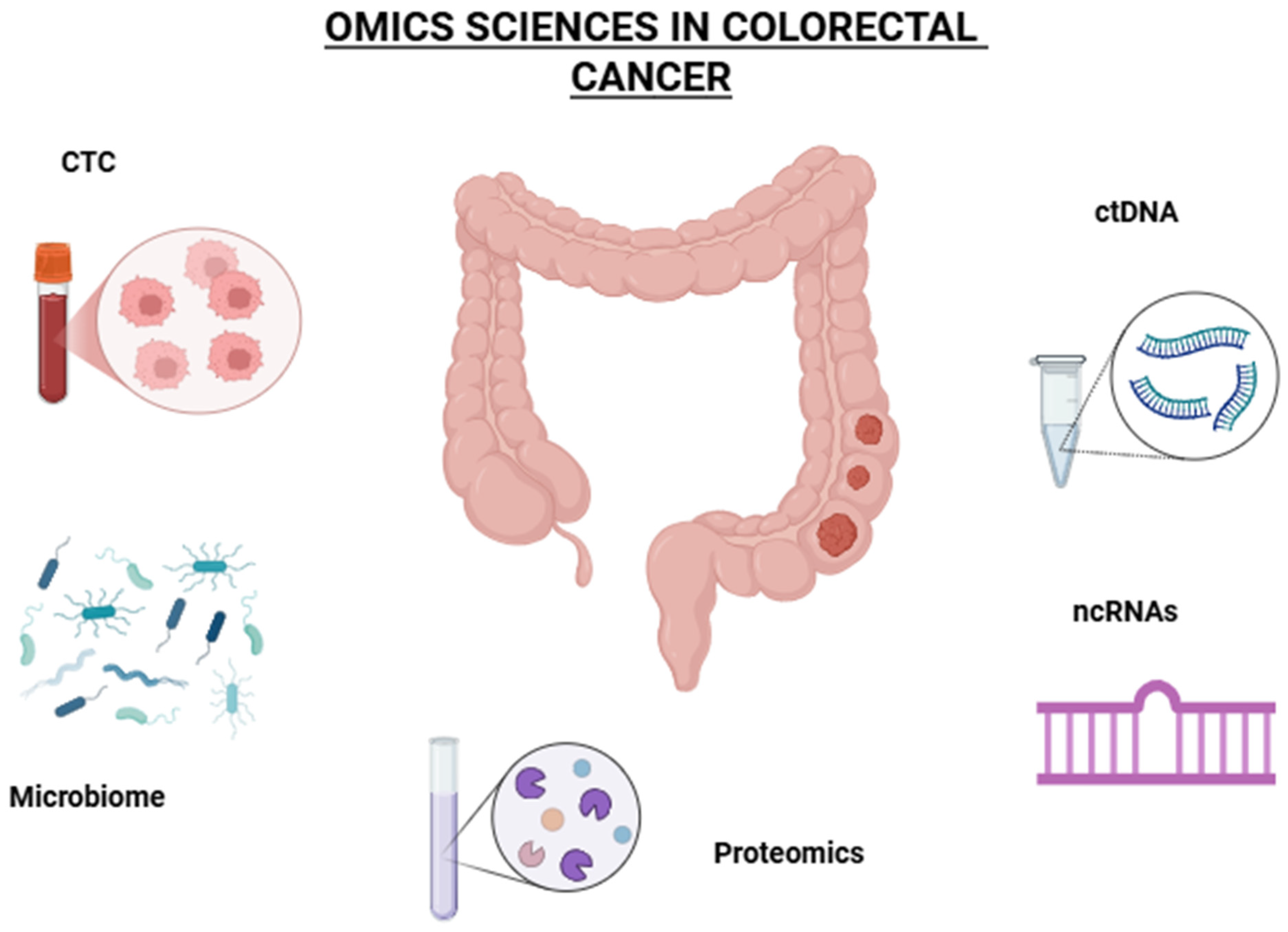
2. Omics Sciences
- More accurate diagnostics: Combining genomic and epigenomic data enhances the identification of cancer subtypes.
- Personalized treatment strategies: Multi-omics enables tailored therapies by identifying patient-specific molecular targets.
- Improved drug development: Systems biology approaches help predict drug responses and resistance patterns.
2.1. Genomics
2.1.1. MMR Genes
2.1.2. RAS and BRAF
2.1.3. PI3K/AKT Pathway
2.1.4. Conclusions
2.2. Circulating Tumor DNA
2.3. Transcriptomic
- Identify gene expression patterns that distinguish different cancer types and subtypes;
- Discover biomarkers for diagnosis, prognosis and treatment response;
- Understand tumor heterogeneity and the role of the tumor microenvironment;
- Explore mechanisms of drug resistance and metastasis.
2.4. Proteomics
2.5. Metabolomics
2.6. Immune Markers
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, V.; Kalita, J.; Pal, M. Predictive and Prognostic Biomarkers in Colorectal Cancer: A Systematic Review of Recent Advances and Challenges. Biomed. Pharmacother. 2017, 87, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Koncina, E.; Haan, S.; Rauh, S.; Letellier, E. Prognostic and Predictive Molecular Biomarkers for Colorectal Cancer: Updates and Challenges. Cancers 2020, 12, 319. [Google Scholar] [CrossRef]
- Erstad, D.J.; Tumusiime, G.; Cusack, J.C. Prognostic and Predictive Biomarkers in Colorectal Cancer: Implications for the Clinical Surgeon. Ann. Surg. Oncol. 2015, 22, 3433–3450. [Google Scholar] [CrossRef]
- Ren, H.; Shen, X.; Xie, M.; Guo, X. Construction of a Prognostic Score Model for Breast Cancer Based on Multi-Omics Analysis of Study on Bone Metastasis. Transl. Cancer Res. 2024, 13, 2419–2436. [Google Scholar] [CrossRef]
- Donisi, C.; Pretta, A.; Pusceddu, V.; Ziranu, P.; Lai, E.; Puzzoni, M.; Mariani, S.; Massa, E.; Madeddu, C.; Scartozzi, M. Immunotherapy and Cancer: The Multi-Omics Perspective. IJMS 2024, 25, 3563. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Hosen, M.d.I.; Ahmed, M.; Shekhar, H.U. Onco-Multi-OMICS Approach: A New Frontier in Cancer Research. BioMed Res. Int. 2018, 2018, 9836256. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, B.; Hamidpour, S.K.; Tayanloo-Beik, A.; Goodarzi, P.; Aghayan, H.R.; Adibi, H.; Larijani, B. Machine Learning: A New Prospect in Multi-Omics Data Analysis of Cancer. Front. Genet. 2022, 13, 824451. [Google Scholar] [CrossRef]
- Ullah, I.; Yang, L.; Yin, F.-T.; Sun, Y.; Li, X.-H.; Li, J.; Wang, X.-J. Multi-Omics Approaches in Colorectal Cancer Screening and Diagnosis, Recent Updates and Future Perspectives. Cancers 2022, 14, 5545. [Google Scholar] [CrossRef]
- Raufaste-Cazavieille, V.; Santiago, R.; Droit, A. Multi-Omics Analysis: Paving the Path toward Achieving Precision Medicine in Cancer Treatment and Immuno-Oncology. Front. Mol. Biosci. 2022, 9, 962743. [Google Scholar] [CrossRef]
- Dalal, N.; Jalandra, R.; Sharma, M.; Prakash, H.; Makharia, G.K.; Solanki, P.R.; Singh, R.; Kumar, A. Omics Technologies for Improved Diagnosis and Treatment of Colorectal Cancer: Technical Advancement and Major Perspectives. Biomed. Pharmacother. 2020, 131, 110648. [Google Scholar] [CrossRef]
- Liang, A.; Kong, Y.; Chen, Z.; Qiu, Y.; Wu, Y.; Zhu, X.; Li, Z. Advancements and Applications of Single-Cell Multi-Omics Techniques in Cancer Research: Unveiling Heterogeneity and Paving the Way for Precision Therapeutics. Biochem. Biophys. Rep. 2024, 37, 101589. [Google Scholar] [CrossRef] [PubMed]
- Pettini, F.; Visibelli, A.; Cicaloni, V.; Iovinelli, D.; Spiga, O. Multi-Omics Model Applied to Cancer Genetics. IJMS 2021, 22, 5751. [Google Scholar] [CrossRef] [PubMed]
- Sardo, E.; Napolitano, S.; Della Corte, C.M.; Ciardiello, D.; Raucci, A.; Arrichiello, G.; Troiani, T.; Ciardiello, F.; Martinelli, E.; Martini, G. Multi-Omic Approaches in Colorectal Cancer beyond Genomic Data. JPM 2022, 12, 128. [Google Scholar] [CrossRef]
- Medici, B.; Riccò, B.; Caffari, E.; Zaniboni, S.; Salati, M.; Spallanzani, A.; Garajovà, I.; Benatti, S.; Chiavelli, C.; Dominici, M.; et al. Early Onset Metastatic Colorectal Cancer: Current Insights and Clinical Management of a Rising Condition. Cancers 2023, 15, 3509. [Google Scholar] [CrossRef]
- Yaeger, R.; Chatila, W.K.; Lipsyc, M.D.; Hechtman, J.F.; Cercek, A.; Sanchez-Vega, F.; Jayakumaran, G.; Middha, S.; Zehir, A.; Donoghue, M.T.A.; et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 2018, 33, 125–136.e3. [Google Scholar] [CrossRef]
- Brannon, A.R.; Vakiani, E.; Sylvester, B.E.; Scott, S.N.; McDermott, G.; Shah, R.H.; Kania, K.; Viale, A.; Oschwald, D.M.; Vacic, V.; et al. Comparative sequencing analysis reveals high genomic concordance between matched primary and metastatic colorectal cancer lesions. Genome Biol. 2014, 15, 454. [Google Scholar] [CrossRef]
- Vakiani, E.; Janakiraman, M.; Shen, R.; Sinha, R.; Zeng, Z.; Shia, J.; Cercek, A.; Kemeny, N.; D’Angelica, M.; Viale, A.; et al. Comparative Genomic Analysis of Primary Versus Metastatic Colorectal Carcinomas. JCO 2012, 30, 2956–2962. [Google Scholar] [CrossRef]
- Kather, J.N.; Halama, N.; Jaeger, D. Genomics and Emerging Biomarkers for Immunotherapy of Colorectal Cancer. Semin. Cancer Biol. 2018, 52, 189–197. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Kopetz, S. Predictive and Prognostic Markers in Colorectal Cancer. Curr. Oncol. Rep. 2011, 13, 206–215. [Google Scholar] [CrossRef]
- Zlobec, I.; Lugli, A. Prognostic and Predictive Factors in Colorectal Cancer. Postgrad. Med. J. 2008, 84, 403–411. [Google Scholar] [CrossRef]
- Walther, A.; Johnstone, E.; Swanton, C.; Midgley, R.; Tomlinson, I.; Kerr, D. Genetic Prognostic and Predictive Markers in Colorectal Cancer. Nat. Rev. Cancer 2009, 9, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Prior, I.A.; Hood, F.E.; Hartley, J.L. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020, 80, 2969–2974. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.-Y.; Oliner, K.S.; Siena, S.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Panitumumab–FOLFOX4 Treatment and RAS Mutations in Colorectal Cancer. N. Engl. J. Med. 2013, 369, 1023–1034. [Google Scholar] [CrossRef]
- De Roock, W.; Claes, B.; Bernasconi, D.; De Schutter, J.; Biesmans, B.; Fountzilas, G.; Kalogeras, K.T.; Kotoula, V.; Papamichael, D.; Laurent-Puig, P.; et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol. 2010, 11, 753–762. [Google Scholar] [CrossRef]
- Yaeger, R.; Cowell, E.; Chou, J.F.; Gewirtz, A.N.; Borsu, L.; Vakiani, E.; Solit, D.B.; Rosen, N.; Capanu, M.; Ladanyi, M.; et al. RAS mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer 2015, 121, 1195–1203. [Google Scholar] [CrossRef]
- Vidal, J.; Muinelo, L.; Dalmases, A.; Jones, F.; Edelstein, D.; Iglesias, M.; Orrillo, M.; Abalo, A.; Rodríguez, C.; Brozos, E.; et al. Plasma ctDNA RAS Mutation Analysis for the Diagnosis and Treatment Monitoring of Metastatic Colorectal Cancer Patients. Ann. Oncol. 2017, 28, 1325–1332. [Google Scholar] [CrossRef]
- Patelli, G.; Mauri, G.; Tosi, F.; Amatu, A.; Bencardino, K.; Bonazzina, E.; Pizzutilo, E.G.; Villa, F.; Calvanese, G.; Agostara, A.G.; et al. Circulating Tumor DNA to Drive Treatment in Metastatic Colorectal Cancer. Clin. Cancer Res. 2023, 29, 4530–4539. [Google Scholar] [CrossRef]
- Osumi, H.; Shinozaki, E.; Yamaguchi, K.; Zembutsu, H. Clinical Utility of Circulating Tumor DNA for Colorectal Cancer. Cancer Sci. 2019, 110, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Schøler, L.V.; Reinert, T.; Ørntoft, M.-B.W.; Kassentoft, C.G.; Árnadóttir, S.S.; Vang, S.; Nordentoft, I.; Knudsen, M.; Lamy, P.; Andreasen, D.; et al. Clinical Implications of Monitoring Circulating Tumor DNA in Patients with Colorectal Cancer. Clin. Cancer Res. 2017, 23, 5437–5445. [Google Scholar] [CrossRef]
- Reece, M.; Saluja, H.; Hollington, P.; Karapetis, C.S.; Vatandoust, S.; Young, G.P.; Symonds, E.L. The Use of Circulating Tumor DNA to Monitor and Predict Response to Treatment in Colorectal Cancer. Front. Genet. 2019, 10, 1118. [Google Scholar] [CrossRef]
- Tie, J.; Cohen, J.D.; Lahouel, K.; Lo, S.N.; Wang, Y.; Kosmider, S.; Wong, R.; Shapiro, J.; Lee, M.; Harris, S.; et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N. Engl. J. Med. 2022, 386, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Kasi, P.M.; Aushev, V.N.; Ensor, J.; Langer, N.; Wang, C.G.; Cannon, T.L.; Berim, L.D.; Feinstein, T.; Grothey, A.; McCollom, J.W.; et al. Circulating Tumor DNA (ctDNA) for Informing Adjuvant Chemotherapy (ACT) in Stage II/III Colorectal Cancer (CRC): Interim Analysis of BESPOKE CRC Study. JCO 2024, 42, 9. [Google Scholar] [CrossRef]
- Yukami, H.; Mishima, S.; Kotani, D.; Oki, E.; Taniguchi, H.; Nakamura, Y.; Kato, T.; Takemasa, I.; Yamanaka, T.; Shirasu, H.; et al. P-120 Prospective Observational Study Monitoring Circulating Tumor DNA in Resectable Colorectal Cancer Patients Undergoing Radical Surgery: GALAXY Study in CIRCULATE-Japan (Trial in Progress). Ann. Oncol. 2020, 31, S128–S129. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yoshino, T. Clinical Utility of Analyzing Circulating Tumor DNA in Patients with Metastatic Colorectal Cancer. Oncologist 2018, 23, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Pietrantonio, F.; Lonardi, S.; Mussolin, B.; Rua, F.; Crisafulli, G.; Bartolini, A.; Fenocchio, E.; Amatu, A.; Manca, P.; et al. Circulating Tumor DNA to Guide Rechallenge with Panitumumab in Metastatic Colorectal Cancer: The Phase 2 CHRONOS Trial. Nat. Med. 2022, 28, 1612–1618. [Google Scholar] [CrossRef]
- Tie, J.; Kinde, I.; Wang, Y.; Wong, H.L.; Roebert, J.; Christie, M.; Tacey, M.; Wong, R.; Singh, M.; Karapetis, C.S.; et al. Circulating Tumor DNA as an Early Marker of Therapeutic Response in Patients with Metastatic Colorectal Cancer. Ann. Oncol. 2015, 26, 1715–1722. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Lapke, N.; Wang, C.-W.; Lin, P.-Y.; You, J.F.; Yeh, C.Y.; Tsai, W.-S.; Hung, H.Y.; Chiang, S.-F.; Chen, H.-C.; et al. Targeted Sequencing of Circulating Tumor DNA to Monitor Genetic Variants and Therapeutic Response in Metastatic Colorectal Cancer. Mol. Cancer Ther. 2018, 17, 2238–2247. [Google Scholar] [CrossRef]
- Tie, J.; Wang, Y.; Cohen, J.; Li, L.; Hong, W.; Christie, M.; Wong, H.L.; Kosmider, S.; Wong, R.; Thomson, B.; et al. Circulating Tumor DNA Dynamics and Recurrence Risk in Patients Undergoing Curative Intent Resection of Colorectal Cancer Liver Metastases: A Prospective Cohort Study. PLoS Med. 2021, 18, e1003620. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Kiavue, N.; Ychou, M.; Cabel, L.; Stern, M.-H.; Madic, J.; Saliou, A.; Rampanou, A.; Decraene, C.; Bouché, O.; et al. Circulating Tumor Cells and Circulating Tumor DNA Detection in Potentially Resectable Metastatic Colorectal Cancer: A Prospective Ancillary Study to the Unicancer Prodige-14 Trial. Cells 2019, 8, 516. [Google Scholar] [CrossRef]
- Loupakis, F.; Sharma, S.; Derouazi, M.; Murgioni, S.; Biason, P.; Rizzato, M.D.; Rasola, C.; Renner, D.; Shchegrova, S.; Koyen Malashevich, A.; et al. Detection of Molecular Residual Disease Using Personalized Circulating Tumor DNA Assay in Patients with Colorectal Cancer Undergoing Resection of Metastases. JCO Precis. Oncol. 2021, 5, 1166–1177. [Google Scholar] [CrossRef]
- Thomsen, C.B.; Hansen, T.F.; Andersen, R.F.; Lindebjerg, J.; Jensen, L.H.; Jakobsen, A. Early Identification of Treatment Benefit by Methylated Circulating Tumor DNA in Metastatic Colorectal Cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920918472. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Mock, A.; Braun, M.; Scholl, C.; Fröhling, S.; Erkut, C. Transcriptome Profiling for Precision Cancer Medicine Using Shallow Nanopore cDNA Sequencing. Sci. Rep. 2023, 13, 2378. [Google Scholar] [CrossRef] [PubMed]
- Pleasance, E.; Bohm, A.; Williamson, L.M.; Nelson, J.M.T.; Shen, Y.; Bonakdar, M.; Titmuss, E.; Csizmok, V.; Wee, K.; Hosseinzadeh, S.; et al. Whole-genome and transcriptome analysis enhances precision cancer treatment options. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 939–949. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Zhao, Z.; Yang, M.; Chen, M.; Liu, C.; Ji, J.; Zhu, D. Single-Cell Transcriptome Analysis Reveals Tumor Immune Microenvironment Heterogenicity and Granulocytes Enrichment in Colorectal Cancer Liver Metastases. Cancer Lett. 2020, 470, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.S.; Pennel, K.A.F.; Leslie, H.; Legrini, A.; Cameron, A.J.; Melissourgou-Syka, L.; Quinn, J.A.; Van Wyk, H.C.; Hay, J.; Roseweir, A.K.; et al. Spatially Resolved Transcriptomics Deconvolutes Prognostic Histological Subgroups in Patients with Colorectal Cancer and Synchronous Liver Metastases. Cancer Res. 2023, 83, 1329–1344. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Chae, J.; Na, D.; Kang, W.; Lee, A.; Min, S.; Kang, J.; Choi, B.; Lee, J.; Sung, C.O.; et al. Unstable Genome and Transcriptome Dynamics during Tumor Metastasis Contribute to Therapeutic Heterogeneity in Colorectal Cancers. Clin. Cancer Res. 2019, 25, 2821–2834. [Google Scholar] [CrossRef]
- Moosavi, S.H.; Eide, P.W.; Eilertsen, I.A.; Brunsell, T.H.; Berg, K.C.G.; Røsok, B.I.; Brudvik, K.W.; Bjørnbeth, B.A.; Guren, M.G.; Nesbakken, A.; et al. De Novo Transcriptomic Subtyping of Colorectal Cancer Liver Metastases in the Context of Tumor Heterogeneity. Genome Med. 2021, 13, 143. [Google Scholar] [CrossRef]
- Kamal, Y.; Schmit, S.L.; Hoehn, H.J.; Amos, C.I.; Frost, H.R. Transcriptomic Differences between Primary Colorectal Adenocarcinomas and Distant Metastases Reveal Metastatic Colorectal Cancer Subtypes. Cancer Res. 2019, 79, 4227–4241. [Google Scholar] [CrossRef]
- Roessler, S.; Lin, G.; Forgues, M.; Budhu, A.; Hoover, S.; Simpson, R.M.; Wu, X.; He, P.; Qin, L.-X.; Tang, Z.-Y.; et al. Integrative Genomic and Transcriptomic Characterization of Matched Primary and Metastatic Liver and Colorectal Carcinoma. Int. J. Biol. Sci. 2015, 11, 88–98. [Google Scholar] [CrossRef]
- Lee, J.-R.; Kwon, C.H.; Choi, Y.; Park, H.J.; Kim, H.S.; Jo, H.-J.; Oh, N.; Park, D.Y. Transcriptome Analysis of Paired Primary Colorectal Carcinoma and Liver Metastases Reveals Fusion Transcripts and Similar Gene Expression Profiles in Primary Carcinoma and Liver Metastases. BMC Cancer 2016, 16, 539. [Google Scholar] [CrossRef]
- Woolston, A.; Khan, K.; Spain, G.; Barber, L.J.; Griffiths, B.; Gonzalez-Exposito, R.; Hornsteiner, L.; Punta, M.; Patil, Y.; Newey, A.; et al. Genomic and Transcriptomic Determinants of Therapy Resistance and Immune Landscape Evolution during Anti-EGFR Treatment in Colorectal Cancer. Cancer Cell 2019, 36, 35–50.e9. [Google Scholar] [CrossRef]
- Martinez-Romero, J.; Bueno-Fortes, S.; Martín-Merino, M.; Ramirez De Molina, A.; De Las Rivas, J. Survival Marker Genes of Colorectal Cancer Derived from Consistent Transcriptomic Profiling. BMC Genom. 2018, 19, 857. [Google Scholar] [CrossRef]
- Mendes, M.; Peláez-García, A.; López-Lucendo, M.; Bartolomé, R.A.; Calviño, E.; Barderas, R.; Casal, J.I. Mapping the Spatial Proteome of Metastatic Cells in Colorectal Cancer. Proteomics 2017, 17, 1700094. [Google Scholar] [CrossRef]
- Ma, Y.-S.; Huang, T.; Zhong, X.-M.; Zhang, H.-W.; Cong, X.-L.; Xu, H.; Lu, G.-X.; Yu, F.; Xue, S.-B.; Lv, Z.-W.; et al. Proteogenomic Characterization and Comprehensive Integrative Genomic Analysis of Human Colorectal Cancer Liver Metastasis. Mol. Cancer 2018, 17, 139. [Google Scholar] [CrossRef]
- Alves Martins, B.A.; De Bulhões, G.F.; Cavalcanti, I.N.; Martins, M.M.; De Oliveira, P.G.; Martins, A.M.A. Biomarkers in Colorectal Cancer: The Role of Translational Proteomics Research. Front. Oncol. 2019, 9, 1284. [Google Scholar] [CrossRef]
- De Wit, M.; Fijneman, R.J.A.; Verheul, H.M.W.; Meijer, G.A.; Jimenez, C.R. Proteomics in Colorectal Cancer Translational Research: Biomarker Discovery for Clinical Applications. Clin. Biochem. 2013, 46, 466–479. [Google Scholar] [CrossRef]
- O’Dwyer, D.; Ralton, L.D.; O’Shea, A.; Murray, G.I. The Proteomics of Colorectal Cancer: Identification of a Protein Signature Associated with Prognosis. PLoS ONE 2011, 6, e27718. [Google Scholar] [CrossRef]
- Wong, G.Y.M.; Diakos, C.; Hugh, T.J.; Molloy, M.P. Proteomic Profiling and Biomarker Discovery in Colorectal Liver Metastases. IJMS 2022, 23, 6091. [Google Scholar] [CrossRef]
- Ghosh, D.; Yu, H.; Tan, X.F.; Lim, T.K.; Zubaidah, R.M.; Tan, H.T.; Chung, M.C.M.; Lin, Q. Identification of Key Players for Colorectal Cancer Metastasis by iTRAQ Quantitative Proteomics Profiling of Isogenic SW480 and SW620 Cell Lines. J. Proteome Res. 2011, 10, 4373–4387. [Google Scholar] [CrossRef]
- Tanaka, A.; Ogawa, M.; Zhou, Y.; Namba, K.; Hendrickson, R.C.; Miele, M.M.; Li, Z.; Klimstra, D.S.; Buckley, P.G.; Gulcher, J.; et al. Proteogenomic Characterization of Primary Colorectal Cancer and Metastatic Progression Identifies Proteome-Based Subtypes and Signatures. Cell Rep. 2024, 43, 113810. [Google Scholar] [CrossRef]
- Ji, H.; Greening, D.W.; Barnes, T.W.; Lim, J.W.; Tauro, B.J.; Rai, A.; Xu, R.; Adda, C.; Mathivanan, S.; Zhao, W.; et al. Proteome Profiling of Exosomes Derived from Human Primary and Metastatic Colorectal Cancer Cells Reveal Differential Expression of Key Metastatic Factors and Signal Transduction Components. Proteomics 2013, 13, 1672–1686. [Google Scholar] [CrossRef]
- Choi, D.; Choi, D.; Hong, B.; Jang, S.; Kim, D.; Lee, J.; Kim, Y.; Pyo Kim, K.; Gho, Y. Quantitative Proteomics of Extracellular Vesicles Derived from Human Primary and Metastatic Colorectal Cancer Cells. J. Extracell. Vesicle 2012, 1, 18704. [Google Scholar] [CrossRef]
- Tan, H.T.; Wu, W.; Ng, Y.Z.; Zhang, X.; Yan, B.; Ong, C.W.; Tan, S.; Salto-Tellez, M.; Hooi, S.C.; Chung, M.C.M. Proteomic Analysis of Colorectal Cancer Metastasis: Stathmin-1 Revealed as a Player in Cancer Cell Migration and Prognostic Marker. J. Proteome Res. 2012, 11, 1433–1445. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Zhao, W.; Deng, K.; Wang, Z.; Yang, C.; Ma, L.; Openkova, M.S.; Hou, Y.; Li, K. Metabolomics for Biomarker Discovery in the Diagnosis, Prognosis, Survival and Recurrence of Colorectal Cancer: A Systematic Review. Oncotarget 2017, 8, 35460–35472. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Ab-Rahim, S.; Suddin, L.S.; Ahmad Saman, M.S.; Mazlan, M. Metabolomics Profiling on Different Stages of Colorectal Cancer: A Systematic Review. MJMS 2018, 25, 16–34. [Google Scholar] [CrossRef]
- Bertini, I.; Cacciatore, S.; Jensen, B.V.; Schou, J.V.; Johansen, J.S.; Kruhøffer, M.; Luchinat, C.; Nielsen, D.L.; Turano, P. Metabolomic NMR Fingerprinting to Identify and Predict Survival of Patients with Metastatic Colorectal Cancer. Cancer Res. 2012, 72, 356–364. [Google Scholar] [CrossRef]
- Farshidfar, F.; Weljie, A.M.; Kopciuk, K.A.; Hilsden, R.; McGregor, S.E.; Buie, W.D.; MacLean, A.; Vogel, H.J.; Bathe, O.F. A Validated Metabolomic Signature for Colorectal Cancer: Exploration of the Clinical Value of Metabolomics. Br. J. Cancer 2016, 115, 848–857. [Google Scholar] [CrossRef]
- Di Donato, S.; Vignoli, A.; Biagioni, C.; Malorni, L.; Mori, E.; Tenori, L.; Calamai, V.; Parnofiello, A.; Di Pierro, G.; Migliaccio, I.; et al. A Serum Metabolomics Classifier Derived from Elderly Patients with Metastatic Colorectal Cancer Predicts Relapse in the Adjuvant Setting. Cancers 2021, 13, 2762. [Google Scholar] [CrossRef]
- González-Olmedo, C.; García-Verdejo, F.J.; Reguera-Teba, A.; Rosa-Garrido, C.; López-López, J.A.; Díaz-Beltrán, L.; García, P.M.; Luque-Caro, N.; Gálvez-Montosa, F.; Ortega-Granados, A.L.; et al. Metabolomics Signature as a Survival Predictor in Patients with Resectable Colorectal Liver Metastasis. Clin. Transl. Med. 2024, 14, e1541. [Google Scholar] [CrossRef]
- Nishiumi, S.; Kobayashi, T.; Ikeda, A.; Yoshie, T.; Kibi, M.; Izumi, Y.; Okuno, T.; Hayashi, N.; Kawano, S.; Takenawa, T.; et al. A Novel Serum Metabolomics-Based Diagnostic Approach for Colorectal Cancer. PLoS ONE 2012, 7, e40459. [Google Scholar] [CrossRef]
- Costantini, S.; Di Gennaro, E.; Capone, F.; De Stefano, A.; Nasti, G.; Vitagliano, C.; Setola, S.V.; Tatangelo, F.; Delrio, P.; Izzo, F.; et al. Plasma Metabolomics, Lipidomics and Cytokinomics Profiling Predict Disease Recurrence in Metastatic Colorectal Cancer Patients Undergoing Liver Resection. Front. Oncol. 2023, 12, 1110104. [Google Scholar] [CrossRef]
- Martín-Blázquez, A.; Díaz, C.; González-Flores, E.; Franco-Rivas, D.; Jiménez-Luna, C.; Melguizo, C.; Prados, J.; Genilloud, O.; Vicente, F.; Caba, O.; et al. Untargeted LC-HRMS-Based Metabolomics to Identify Novel Biomarkers of Metastatic Colorectal Cancer. Sci. Rep. 2019, 9, 20198. [Google Scholar] [CrossRef]
- Tommelein, J.; Verset, L.; Boterberg, T.; Demetter, P.; Bracke, M.; De Wever, O. Cancer-Associated Fibroblasts Connect Metastasis-Promoting Communication in Colorectal Cancer. Front. Oncol. 2015, 5, 63. [Google Scholar] [CrossRef]
- Peng, S.; Li, Y.; Huang, M.; Tang, G.; Xie, Y.; Chen, D.; Hu, Y.; Yu, T.; Cai, J.; Yuan, Z.; et al. Metabolomics Reveals That CAF-Derived Lipids Promote Colorectal Cancer Peritoneal Metastasis by Enhancing Membrane Fluidity. Int. J. Biol. Sci. 2022, 18, 1912–1932. [Google Scholar] [CrossRef]
- Yahaya, M.A.F.; Lila, M.A.M.; Ismail, S.; Zainol, M.; Afizan, N.A.R.N.M. Tumour-Associated Macrophages (TAMs) in Colon Cancer and How to Reeducate Them. J. Immunol. Res. 2019, 2019, 2368249. [Google Scholar] [CrossRef]
- Tan, S.-Y. Prognostic Significance of Cell Infiltrations of Immunosurveillance in Colorectal Cancer. WJG 2005, 11, 1210. [Google Scholar] [CrossRef]
- Erreni, M.; Mantovani, A.; Allavena, P. Tumor-Associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenviron. 2011, 4, 141–154. [Google Scholar] [CrossRef]
- Zhao, Y.; Ge, X.; He, J.; Cheng, Y.; Wang, Z.; Wang, J.; Sun, L. The Prognostic Value of Tumor-Infiltrating Lymphocytes in Colorectal Cancer Differs by Anatomical Subsite: A Systematic Review and Meta-Analysis. World J. Surg. Onc. 2019, 17, 85. [Google Scholar] [CrossRef]
- Lee, J.H. Prognostic Significance of Tumor-Infiltrating Lymphocytes for Patients with Colorectal Cancer. Arch. Surg. 2012, 147, 366. [Google Scholar] [CrossRef]
- Iseki, Y.; Shibutani, M.; Maeda, K.; Nagahara, H.; Fukuoka, T.; Matsutani, S.; Kashiwagi, S.; Tanaka, H.; Hirakawa, K.; Ohira, M. A New Method for Evaluating Tumor-Infiltrating Lymphocytes (TILs) in Colorectal Cancer Using Hematoxylin and Eosin (H-E)-Stained Tumor Sections. PLoS ONE 2018, 13, e0192744. [Google Scholar] [CrossRef]
- Deschoolmeester, V.; Baay, M.; Lardon, F.; Pauwels, P.; Peeters, M. Immune Cells in Colorectal Cancer: Prognostic Relevance and Role of MSI. Cancer Microenviron. 2011, 4, 377–392. [Google Scholar] [CrossRef]
- Bai, Z.; Zhou, Y.; Ye, Z.; Xiong, J.; Lan, H.; Wang, F. Tumor-Infiltrating Lymphocytes in Colorectal Cancer: The Fundamental Indication and Application on Immunotherapy. Front. Immunol. 2022, 12, 808964. [Google Scholar] [CrossRef]
- Loupakis, F.; Depetris, I.; Biason, P.; Intini, R.; Prete, A.A.; Leone, F.; Lombardi, P.; Filippi, R.; Spallanzani, A.; Cascinu, S.; et al. Prediction of Benefit from Checkpoint Inhibitors in Mismatch Repair Deficient Metastatic Colorectal Cancer: Role of Tumor Infiltrating Lymphocytes. Oncologist 2020, 25, 481–487. [Google Scholar] [CrossRef]
- Jakubowska, K.; Koda, M.; Kisielewski, W.; Kańczuga-Koda, L.; Famulski, W. Tumor-infiltrating Lymphocytes in Primary Tumors of Colorectal Cancer and Their Metastases. Exp. Ther. Med. 2019, 18, 4904–4912. [Google Scholar] [CrossRef]
- Ge, P.; Wang, W.; Li, L.; Zhang, G.; Gao, Z.; Tang, Z.; Dang, X.; Wu, Y. Profiles of Immune Cell Infiltration and Immune-Related Genes in the Tumor Microenvironment of Colorectal Cancer. Biomed. Pharmacother. 2019, 118, 109228. [Google Scholar] [CrossRef]
- An, S.; Kim, S.-K.; Kwon, H.Y.; Kim, C.S.; Bang, H.-J.; Do, H.; Kim, B.; Kim, K.; Kim, Y. Expression of Immune-Related and Inflammatory Markers and Their Prognostic Impact in Colorectal Cancer Patients. IJMS 2023, 24, 11579. [Google Scholar] [CrossRef]
- Idos, G.E.; Kwok, J.; Bonthala, N.; Kysh, L.; Gruber, S.B.; Qu, C. The Prognostic Implications of Tumor Infiltrating Lymphocytes in Colorectal Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 3360. [Google Scholar] [CrossRef]
| Omics Science | Role | Comment |
|---|---|---|
| Genomics | Identifies mutations and genetic predispositions to CRC | Traditional genomics studies have identified cancer-driving mutations (e.g., TP53, KRAS, EGFR) but fail to explain tumor heterogeneity. |
| Epigenomics | Reveals DNA methylation and histone modifications linked to tumor suppression or activation | |
| Transcriptomics | Examines RNA expression changes, highlighting regulatory ncRNAs like miRNAs | Transcriptomics and proteomics reveal that mRNA expression does not always correlate with protein abundance, requiring integrated analysis. |
| Proteomics | Detects CRC-related proteins and their modifications | Transcriptomics and proteomics reveal that mRNA expression does not always correlate with protein abundance, requiring integrated analysis. |
| Metabolomics | Assesses metabolic alterations in CRC cells, revealing dysbiosis associated with CRC and potential diagnostic markers | Metabolomics and microbiomics contribute to understanding cancer metabolism and tumor microenvironment interactions. |
| Microbiomics | Investigates the gut microbiota’s role | Studies integrating genomics and metabolomics have identified Fusobacterium nucleatum as a gut microbiome biomarker associated with CRC progression. |
| Lipidomics | Studies lipid metabolism changes as potential CRC markers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medici, B.; Benatti, S.; Dominici, M.; Gelsomino, F. New Frontiers of Biomarkers in Metastatic Colorectal Cancer: Potential and Critical Issues. Int. J. Mol. Sci. 2025, 26, 5268. https://doi.org/10.3390/ijms26115268
Medici B, Benatti S, Dominici M, Gelsomino F. New Frontiers of Biomarkers in Metastatic Colorectal Cancer: Potential and Critical Issues. International Journal of Molecular Sciences. 2025; 26(11):5268. https://doi.org/10.3390/ijms26115268
Chicago/Turabian StyleMedici, Bianca, Stefania Benatti, Massimo Dominici, and Fabio Gelsomino. 2025. "New Frontiers of Biomarkers in Metastatic Colorectal Cancer: Potential and Critical Issues" International Journal of Molecular Sciences 26, no. 11: 5268. https://doi.org/10.3390/ijms26115268
APA StyleMedici, B., Benatti, S., Dominici, M., & Gelsomino, F. (2025). New Frontiers of Biomarkers in Metastatic Colorectal Cancer: Potential and Critical Issues. International Journal of Molecular Sciences, 26(11), 5268. https://doi.org/10.3390/ijms26115268






