Abstract
Otorhinolaryngological (ORL) cancers, including malignancies of the oral cavity, pharynx, and larynx, show significant challenges in oncology. Cisplatin, a platinum-based chemotherapy drug, remains a cornerstone of treatment but is often limited by systemic toxicity and resistance. A comprehensive literature review was conducted using recent studies and clinical trials focused on nanotechnology-based cisplatin delivery systems. The analysis covered various types of nanocarriers, their mechanisms, and advantages. Additionally, the limitations of nanotechnology-based cisplatin delivery systems were discussed. Findings indicate that lipid-based nanoparticles, polymeric nanoparticles, inorganic nanoparticles, and extracellular vesicles have demonstrated improved drug targeting, bioavailability, and reduced systemic toxicity in preclinical and clinical studies. Nanocarriers also offer potential for overcoming drug resistance and enabling combination therapy. However, challenges related to biocompatibility, scalability, and regulatory approval remain significant barriers to widespread clinical adoption. Nanotechnology offers a novel and promising approach to optimizing cisplatin delivery for ORL cancers. While preclinical studies demonstrate significant potential, further research and clinical validation are essential to translate these advancements into routine clinical practice. Addressing manufacturing and regulatory challenges will be critical for future research.
1. Introduction
Otorhinolaryngological (ORL) cancers, which include malignancies of the oral cavity, pharynx, and larynx, account for a significant proportion of global cancer cases. Approximately 7.7% of new cases of cancer diagnosed in 2020 were ORL cancers, with esophagus cancer having the highest incidence (3.1%) and also the highest death rate (5.5%) [1]. These cancers are often associated with risk factors such as tobacco use, alcohol consumption, human papillomavirus (HPV) infection, and environmental pollutants [2]. Despite advances in early detection and therapeutic strategies, ORL cancers continue to present high morbidity and mortality rates, particularly in advanced stages where treatment options become more limited.
The tumor microenvironment influences cancer progression and therapeutic responses [3]. Three critical factors that significantly affect tumor behavior and drug delivery are stromal density [4], hypoxia [5], and immune cell infiltration [6]. The interrelationships between these factors are essential for elucidating the complexity of tumor biology and improving treatment efficacy [7].
Stromal density—including different cell types, such as cancer-associated fibroblasts (CAFs) and immune cells—contributes to tumor progression and therapy resistance. Increased stroma can lead to alterations in the extracellular matrix, impacting drug diffusion and efficacy. The presence of CAFs has been associated with a more aggressive tumor phenotype. Tumor-associated macrophages (TAMs) can constitute a significant component of the tumor stroma and have been shown to contribute to both local tumor proliferation and systemic metastasis, particularly in glioblastoma [8,9]. Furthermore, the density of immune infiltrates is correlated with prognosis, indicating that manipulating stromal components may enhance therapeutic outcomes [10,11].
Hypoxia, a common feature in solid tumors, influences tumor behavior by promoting angiogenesis, immune evasion, and drug resistance. The hypoxic microenvironment can trigger adaptive responses, leading to the upregulation of escape mechanisms, such as the expression of immune checkpoints, such as PD-L1, on immune cells like macrophages and myeloid-derived suppressor cells (MDSCs), facilitating immune tolerance [12,13]. Furthermore, hypoxia is linked to metabolic changes that promote tumor cell survival and increase local immunosuppression, decreasing T cell activity and promoting a state of exhaustion that significantly reduces the efficacy of various therapeutic strategies [12,14].
The dynamic interplay between hypoxia and immune cell infiltration further complicates therapeutic strategies. Targeting pathways involved in hypoxia can enhance T cell infiltration and boost immunotherapy efficacy, particularly in tumors that traditionally show poor immune responses [15,16]. Studies show that reducing hypoxic stress has been shown to restore immune cell function and improve outcomes in conditions such as melanoma and bladder cancer [16,17].
Cisplatin, a platinum-based chemotherapeutic agent, remains one of the most effective drugs for treating ORL cancers [18,19]. It functions primarily by forming DNA adducts, which interfere with DNA replication and transcription, ultimately leading to apoptosis in rapidly dividing cancer cells [20,21]. However, despite its efficacy, cisplatin is associated with several drawbacks. Its nonspecific distribution results in severe systemic toxicities [22], including nephrotoxicity [23], ototoxicity [24], neurotoxicity [25], and myelosuppression [26]. These adverse effects often limit the maximum tolerable dose and lead to dose reductions or treatment discontinuation, negatively impacting therapeutic outcomes.
Another significant challenge in cisplatin-based chemotherapy is the development of resistance. Tumor cells develop various mechanisms to escape cisplatin-induced cytotoxicity, such as enhanced DNA repair mechanisms, increased drug efflux, and inhibition of apoptotic pathways [10,27,28]. These resistance mechanisms significantly reduce cisplatin’s effectiveness and contribute to treatment failure in ORL cancers. Consequently, improving cisplatin delivery while mitigating resistance and toxicity has become a major focus of oncological research.
Nanotechnology has emerged as a promising approach to addressing these challenges. By engineering nanoparticles as drug carriers, researchers have explored methods to improve cisplatin delivery, increase tumor specificity, and reduce systemic toxicity. Various nanoparticle-based delivery systems, including lipid-based, polymeric, inorganic, and biological nanocarriers, have demonstrated significant potential in preclinical and early clinical studies. These nanocarriers offer advantages such as controlled drug release, enhanced tumor penetration, and the ability to bypass resistance mechanisms, making them attractive candidates for optimizing ORL cancer therapy [29,30,31].
This review aims to provide a comprehensive analysis of the advancements in nanotechnology-based cisplatin delivery for ORL cancers. It will discuss the mechanisms of cisplatin action and resistance, different nanocarrier systems, their advantages and challenges, and the current landscape of preclinical and clinical studies. By highlighting the potential of nanotechnology in improving ORL cancer treatment, this review seeks to pave the way for future research and clinical translation of these innovative approaches.
2. Materials and Methods
This narrative review was designed to summarize and interpret recent advances in nanotechnology-based cisplatin delivery for ORL cancers, emphasizing depth of discussion over exhaustive coverage. Between January and February 2025, we performed targeted searches in PubMed, Scopus, and Google Scholar using combinations of the terms “cisplatin”, “nanocarriers”, “nanotechnology”, “lipid-based nanoparticles”, “polymeric nanoparticles”, “extracellular vesicles”, and “head and neck cancer”. To maintain focus, Google Scholar screening was limited to the first 200 results per keyword combination. We included peer-reviewed original research articles, early-phase clinical trials, and selected reviews published in English that provided quantitative data on drug delivery mechanisms, therapeutic efficacy, toxicity reduction, or resistance modulation in ORL cancer models. Editorials, letters, conference abstracts, non-English papers, and studies lacking substantive delivery- or outcome-related data were excluded. Titles and abstracts were first screened for relevance, followed by full-text review of articles meeting these criteria. The final selection was organized by nanocarrier class (lipid-based, polymeric, inorganic, biological), and within each class, studies were compared narratively in terms of targeting strategy (passive vs. active), in vitro and in vivo efficacy, immunological effects, and translational stage.
3. Mechanisms of Cisplatin Action and Resistance
Cisplatin is a platinum-based chemotherapeutic agent widely used to treat various cancers, including those of the oral and oropharyngeal regions, due to its efficacy in inducing DNA damage leading to apoptosis in malignant cells. The primary mechanism of action involves the formation of DNA cross-links, particularly at the N7 position of guanine residues, which disrupts DNA replication and transcription, triggering cellular signaling pathways that lead to programmed cell death [32]. However, cancer cells often develop resistance to cisplatin, complicating treatment regimens and leading to poor clinical outcomes. Understanding the mechanisms underlying cisplatin resistance is crucial for improving therapeutic strategies and outcomes in ORL cancers. The mechanisms of cisplatin resistance can be broadly categorized into several molecular mechanisms. These include alterations in drug uptake and efflux, enhanced DNA repair processes, and changes in apoptosis signaling pathways. Additionally, genetic and epigenetic changes in cancer cells can establish new resistance mechanisms. Mutations and alterations in gene expression profiles may decrease cisplatin-related damage responses [33]. Overall, the interplay of these mechanisms signifies a complex landscape of cisplatin resistance within ORL cancers, necessitating the exploration of combination therapies to enhance treatment efficacy. For instance, the copper transporter CTR1 has been identified as a critical factor in mediating cisplatin uptake (Figure 1A); reduced expression of CTR1 has been associated with decreased drug accumulation in cells, contributing significantly to resistance (Figure 1B) [34]. Additionally, increased expression of ATP-binding cassette (ABC) transporters facilitates the efflux of cisplatin from cancer cells, which also diminishes its intracellular concentration and effectiveness (Figure 1B) [33]. Furthermore, enhanced DNA repair capabilities, particularly through nucleotide excision repair pathways, enable cancer cells to overcome cisplatin-induced damage [33]. Another significant factor contributing to resistance is the role of glutathione and reactive oxygen species (ROS) in modulating cellular responses to cisplatin. High levels of glutathione can neutralize the oxidative stress induced by cisplatin, thereby protecting cancer cells and increasing their chance of survival [35]. In ORL cancers, studies have elucidated the production of ROS as a response to cisplatin treatment, suggesting that the modulation of oxidative stress could serve as a potential therapeutic target to enhance cisplatin’s effectiveness against resistant cancers [36]. Importantly, recent investigations have highlighted the influence of microRNAs (miRNAs) in modulating cisplatin resistance. Specific miRNAs can regulate the expression of genes involved in drug resistance pathways, facilitating the survival of cancer cells in the presence of cisplatin [37]. For example, miR-643 was shown to be transferred from cisplatin-resistant cells to sensitive ones, imparting resistance properties and complicating treatment options further [37].
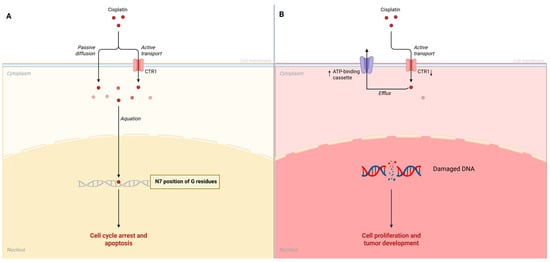
Figure 1.
(A) Cisplatin mechanism of action. (B) Cisplatin resistance mechanisms in cancer cells.
4. Nanotechnology in Cisplatin Delivery
The deployment of nanotechnology in the delivery of cisplatin for treating oral and laryngeal cancers has attracted significant interest due to its potential to enhance drug efficacy and overcome various forms of drug resistance often associated with these malignancies. Nanoparticle-based drug delivery systems, particularly for cisplatin, have gained considerable attention in cancer therapeutics due to their potential to enhance targeting efficiency and reduce systemic toxicity. The strategies employed in nanoparticle formulations broadly fall into two categories: passive targeting and active targeting, each with distinct mechanisms and implications for internalization pathways. Passive targeting relies on the enhanced permeability and retention (EPR) effect, a phenomenon observed in tumor vasculature, which allows nanoparticles to accumulate preferentially in tumor tissues. The leaky blood vessels and compromised lymphatic drainage mechanisms common in tumors facilitate the extravasation of nanoparticles from the bloodstream into the tumor microenvironment [38]. This method benefits from the natural tendency of nanocarriers to passively accumulate in neoplastic tissues based on size and surface characteristics without the need for specific targeting ligands. Focusing on cisplatin, studies have demonstrated that encapsulating cisplatin within polymeric nanoparticles can enhance its delivery and lower its nephrotoxicity, a significant side effect of conventional cisplatin therapy [39]. In contrast, active targeting involves the functionalization of nanoparticles with specific ligands that bind to overexpressed receptors on tumor cells. This method enhances specificity in drug delivery, minimizing effects on healthy tissues while maximizing therapeutic efficacy [40]. Active targeting can dramatically increase cellular internalization rates, as targeting ligands facilitate receptor-mediated endocytosis pathways, thereby promoting efficient uptake of the drug within cancer cells.
The internalization pathways in active targeting often involve clathrin-mediated endocytosis or caveolae-mediated endocytosis, depending on the specific receptor–ligand interactions initiated by the nanoparticles. This enhanced internalization can facilitate a more efficient drug release profile, ensuring therapeutic concentrations of cisplatin are achieved within the target cells [41].
The internalization of nanoparticles can significantly influence their therapeutic outcome. For passively targeted nanoparticles, internalization occurs primarily through pinocytosis or diffusion, driven by nanoparticle size, shape, and surface charge [42]. However, for actively targeted nanoparticles, specific endocytosis pathways play a critical role.
By utilizing nanocarriers, researchers aim to enhance the targeted delivery of cisplatin, minimize its adverse effects, and improve therapeutic outcomes for patients with ORL cancers. Nanoparticles offer a versatile platform for drug delivery, enabling the encapsulation of cisplatin within various matrices including liposomes, polymeric nanoparticles, and metal-based nanoparticles. For instance, studies have shown that liposomal nanoparticles containing cisplatin exhibit enhanced cytotoxic activity compared to free cisplatin, significantly improving the drug’s efficacy against various cancers.
The lipid-based formulations improve the bioavailability of the drug by facilitating its entry into tumor cells while simultaneously reducing exposure to non-target tissues [43]. Furthermore, cisplatin-loaded methoxy-poly(ethylene glycol)-block-poly(L-glutamic acid) nanoparticles have displayed promising delivery characteristics, prompting enhanced antitumor effects due to their ability to modulate the intracellular release conditions of the drug [44]. A key advancement in nanotechnology relates to its ability to overcome multidrug resistance (MDR) mechanisms that tumor cells employ to evade chemotherapeutic agents like cisplatin. Recent findings suggest that metallofullerene nanoparticles may reactivate endocytosis pathways in resistant cancer cells, allowing for higher intracellular concentrations of cisplatin (Figure 2) [45]. Incorporating such mechanisms into the design of cisplatin delivery systems can potentially reverse resistance and restore sensitivity in tumors that have begun to lose responsiveness to standard therapies. To create a cisplatin delivery system for ORL cancers, the use of folic acid-conjugated nanoparticles has emerged as a promising strategy. These nanoparticles utilize the overexpression of folate receptors in certain tumor types to enhance cellular uptake, thereby increasing the efficacy of cisplatin while minimizing off-target effects (Figure 2) [46].
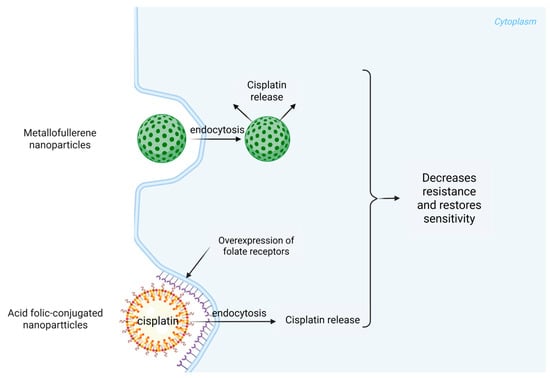
Figure 2.
Cisplatin delivery routes with metallofullerene and acid folic-conjugated nanoparticles.
Biodegradable polymeric nanoparticles allow for a controlled release of the drug, facilitating sustained therapeutic concentrations within the tumor microenvironment, a factor critical for achieving optimum treatment efficacy in ORL cancers [47]. This strategic release profile aims not only to maintain drug action over an extended period but also to reduce normal tissue toxicity by preventing high plasma concentration peaks that are typical with conventional drug administration methods. Furthermore, combining cisplatin with other therapeutic agents delivered via nanoparticles has shown synergistic effects against resistant cancer phenotypes. Studies have noted that co-delivering cisplatin with PARP (Poly (ADP-ribose) polymerase) inhibitors in layered nanoparticles can enhance therapeutic outcomes in ovarian cancer models, a strategy that may likewise be applicable in ORL cancer settings [48].
Nanoparticle-mediated delivery of cisplatin presents a promising avenue to enhance immune activation alongside chemotherapy. Three main strategies appear to dominate the current literature: the use of immunostimulatory agents, enhancing immune cell infiltration, and the development of synergistic treatment combinations.
One innovative approach involves the use of immunostimulatory nanoparticles, particularly those that combine cisplatin with immune adjuvants. For instance, Hernández-Gil et al. developed iron oxide nanoparticles loaded with a Pt(IV) prodrug of cisplatin and polyinosinic-polycytidylic acid, a double-stranded RNA (dsRNA) that functions as an immunomodulator. This combination improves the cytotoxic effects of the cisplatin prodrug and enhances the innate immune response, promoting a more effective antitumor environment [49]. This aligns with findings from other studies indicating that adding immune-modulating factors to theranostic nanoparticles can boost the infiltration of T and B lymphocytes within tumors, thereby heightening the potential for an effective immune response [50].
Enhancing immune cell infiltration is crucial for effective tumor control. Nanoparticles can be designed to actively target the tumor microenvironment, promoting the accumulation of cytotoxic T cells and other immune effectors. Hoffmann et al. describe therapies utilizing nanoparticles for head and neck cancers, where cisplatin delivery is paired with immune activators, leading to enhanced immune cell presence and improved therapeutic outcomes [51]. Similarly, the design of transformable nanoparticles that elicit immunogenic cell death through reactive oxygen species (ROS) generation—alongside cisplatin—demonstrates a synergistic approach to boosting both direct tumor killing and immune activation [52].
Another strategy described in the literature is targeting cisplatin with agents that modulate the immune microenvironment. Cytosolic DNA sensing, the cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) signaling pathway, has been identified as a crucial mediator of immune responses to cisplatin, as it not only inhibits cancer cell proliferation but also enhances the infiltration of CD8+ T cells and dendritic cells in tumor settings [53,54]. Utilizing nanoparticles loaded with cisplatin and designed to activate this pathway could result in superior anticancer efficacy due to the dual action on tumor cells and the immune system alike [55].
Furthermore, combining cisplatin with other immunotherapeutic agents may provide additive or synergistic effects. Co-delivery systems combining cisplatin with checkpoint inhibitors or additional chemotherapeutics have shown promise in overcoming resistance mechanisms associated with cisplatin therapy [56,57]. Such approaches are expected to create a more favorable tumor microenvironment for immune activation and decrease the chances of tumor recurrence.
Moreover, the integration of RNA interference (RNAi) techniques with cisplatin nanocarriers has successfully silenced genes responsible for drug resistance, presenting yet another layer of complexity and effectiveness in overcoming therapeutic barriers in cancer treatment [58,59,60]. Nanotechnology also allows for the engineering of smart drug delivery systems that respond to environmental stimuli. For instance, redox-responsive mesoporous silica nanoparticles can release cisplatin more effectively in the reductive environment typically found in tumor tissues, thereby enhancing the targeted action of the drug [61,62]. Such systems reflect a significant advancement in the precision of chemotherapeutic delivery, positioning nanotechnology as a cornerstone of future cancer therapies. The current research highlights the critical role of nanotechnology in the transformation of cisplatin delivery systems for ORL cancers. With ongoing studies aimed at refining the properties and functionalities of nanocarriers, the focus remains on achieving an optimum balance of efficacy, safety, and patient quality of life. As nanotechnology continues to advance, the prospects for improved treatment regimens utilizing cisplatin grow more promising, potentially setting new standards in cancer therapeutics (Figure 2).
5. Current Clinical and Preclinical Studies
The application of nanotechnology for the delivery of cisplatin in oral and oropharyngeal cancers represents a significant advancement in therapeutic strategies aimed at improving the efficacy and reducing the adverse effects of this important anticancer drug. Recent clinical and preclinical studies have underscored the role that nanoparticle formulations can play in enhancing the pharmacokinetics of cisplatin, thereby improving its tumor-targeting capabilities while minimizing systemic toxicity (Table 1).
Research indicates that nanoparticles can significantly enhance drug solubility and stability, which are often limitations with conventional cisplatin formulations. For instance, studies have demonstrated that nanoliposomes encapsulating cisplatin improve tumor targeting and enhance drug penetration into cancer cells, effectively addressing issues related to MDR [63,64]. Such formulations can facilitate intracellular delivery by exploiting the enhanced permeability and retention (EPR) effect, resulting in more effective cisplatin delivery directly to the tumor site [36,65]. These strategies can potentially reduce the required dosage of cisplatin, thereby decreasing side effects associated with higher systemic exposure.
Furthermore, targeting drug delivery systems that utilize surface modifications of nanoparticles can tailor the release profile of cisplatin. Studies have shown that co-encapsulation systems, which combine cisplatin with other therapeutic agents, can enhance chemotherapeutic efficacy through synergistic mechanisms. For example, one study highlighted the benefits of co-delivering cisplatin with agents that reverse MDR, such as paclitaxel, through a targeted nanosystem which resulted in increased apoptosis of resistant cancer cells [31]. The use of biodegradable polymeric nanoparticles also supports sustained drug release, thereby prolonging therapeutic effects and minimizing toxic peaks commonly associated with traditional administration routes [43].
Another critical aspect of using nanotechnology in cisplatin therapy for ORL cancers is its potential to modulate cellular pathways that increases the resistance. Targeting signaling pathways associated with apoptosis can enhance the sensitivity of cancer cells to cisplatin. Research indicates that inhibiting or downregulating proteins involved in the apoptotic process, such as SIRT2, can contribute to a reversion of resistance in ovarian cancer cells, thereby allowing cisplatin to exert its cytotoxic effects more effectively [10,35]. Advances in understanding tumor biology also facilitate the creation of nanoparticles that can specifically target cancerous tissues, guided by tumor-specific antigens or receptors, further improving treatment selectivity and efficacy [47,66].
The incorporation of natural compounds within nanocarriers is an emerging area of interest, as such combinations can decrease the nephrotoxic effects frequently associated with cisplatin therapy. For example, honokiol, which has been shown to possess protective effects against cisplatin-induced toxicity, can be delivered using nanoparticles to enhance both its bioavailability and chemosensitivity in treated cancer cells [67,68]. This dual action not only preserves the therapeutic benefits of cisplatin but also addresses its deleterious side effects, signifying an important step forward in personalized cancer therapy. Moreover, antioxidant micronutrients such as beta-carotene serve as oral carcinogenesis inhibitors, and in synergy with vitamin E, vitamin C, and chemotherapeutic drugs, they not only induce clinical regression of oral leukoplakia but also significantly enhance the overall efficacy of cancer therapy [69].
Preclinical investigations are focusing on various types of nanoparticles, including gold nanoparticles and mesoporous silica nanoparticles. These novel systems have been shown to not only deliver cisplatin but also allow for imaging and diagnostic functionalities [13,70]. Such multifaceted nanoparticles can be engineered to respond to specific physiological conditions, such as pH changes typical of the tumor microenvironment, ensuring that cisplatin is released precisely where it is needed most [62,71].
While several preclinical studies demonstrate promising outcomes in utilizing nanotechnology for cisplatin delivery, transitioning these findings into clinical practice remains a vital objective. The challenges surrounding this transition include rigorous evaluation of safety, long-term effects, and the complexity of manufacturing reproducibly. Ongoing trials and accumulating evidence from early-phase studies are gradually making a pathway toward the clinical implementation [61] of these innovative nanoparticle-based delivery systems [72].

Table 1.
Advantages of nanotechnology-based delivery systems of cisplatin in ORL and other cancers.
Table 1.
Advantages of nanotechnology-based delivery systems of cisplatin in ORL and other cancers.
| Aspect | Nanotechnology-Based Approach | Nanocarrier Type | Study Model | Type of Cancer | Advantages | References |
|---|---|---|---|---|---|---|
| Drug Bioavailability and Pharmacokinetics | Nanoparticles encapsulating cisplatin | Lipid-based nanoparticles | In vitro, in vivo | ORL cancer, ovarian cancer | Protect cisplatin from degradation, prolong systemic circulation, and improve bioavailability at the tumor site, particularly in OSCC treatment. | [44,73,74] |
| Specificity and Targeting | Targeted delivery via functionalized nanoparticles (e.g., folic acid conjugation) | Polymeric particles, mesoporous silica particles | In vitro | Cervical cancer (HeLa cell line) | Enhanced drug delivery to cancer cells that overexpress receptors, improving efficacy while minimizing off-target effects and reducing systemic toxicity. | [46,75] |
| Overcoming Drug Resistance | Nanoparticle-based systems evading efflux pumps | Polymeric particles, mesoporous silica particles | In vitro | Oral cancer | Prevent drug resistance by facilitating drug accumulation inside cancer cells, bypassing efflux mechanisms like drug efflux pumps. | [29,43] |
| Controlled Release Systems | Redox-responsive mesoporous silica nanoparticles | Mesoporous silica nanoparticles | In vitro | Cervical cancer (HeLa cell line) | Release cisplatin in reducing environments of cancer cells, enhancing cytotoxicity against malignant cells while minimizing systemic exposure. | [46] |
| Biocompatibility and Pharmacokinetic Modulation | Custom nanoparticle systems (e.g., solid lipid nanoparticles) | Lipid nanoparticles, polymeric nanoparticles | In vitro | Various ORL cancer, ovarian cancer cell | Modify drug release profiles, enhance solubility and stability, and improve oral bioavailability, ensuring more efficient chemotherapy in oral cancer. | [47,74] |
| Combination Therapies | Combination of cisplatin with other therapeutic agents (antioxidants, immune modulators) | Organic nanoparticles, polymeric nanoparticles, lipid-based nanoparticles | In vitro, in vivo | Oral cancer, laryngeal cancers | Enhance overall cytotoxicity and counteract chemoresistance by integrating other therapeutic agents within nanoparticles, boosting the effectiveness of cisplatin. | [62,76,77] |
| Multifunctional Platforms for Diagnostics and Therapy | Nanoparticles integrating imaging agents | Gold nanoparticles, inorganic nanoparticles, polymeric nanoparticles | In vitro | Laryngeal cancer | Enable real-time monitoring of treatment efficacy, allowing adaptive strategies based on tumor response, optimizing treatment outcomes. | [43,78] |
| Stimuli-Responsive Release Mechanisms | Tumor-specific signal-responsive nanocarriers (e.g., pH-sensitive or enzyme-responsive systems) | Polymeric nanoparticles, drug-delivery platforms | In vitro | Various ORL cancers | Enable precise drug release within the tumor, enhancing therapeutic efficacy and minimizing systemic toxicity by evading healthy tissues. | [79] |
As shown in Table 2, lipid-based nanoparticles lead the way in late-stage clinical development, whereas other platforms such as mesoporous silica and extracellular vesicles remain largely at the preclinical stage.

Table 2.
Comparative features of major nanocarrier platforms for targeted cisplatin delivery.
6. Challenges and Future Perspectives
While the integration of nanotechnology in cisplatin delivery shows significant promise for improving the treatment of ORL cancers, several challenges remain that need to be addressed before its widespread clinical adoption. One of the primary concerns is biocompatibility, which is the ability of the nanoparticle formulation to perform its intended function without showing significant adverse biological responses. Specifically, in the context of cisplatin-loaded nanoparticles, this includes minimizing cytotoxic effects on healthy tissues, avoiding undesired immune activation, and ensuring favorable pharmacokinetics and biodistribution [44]. Nanoparticles used for drug delivery must be safe for human use without inducing adverse immune responses or toxicity. The precise interaction of nanoparticles with biological tissues needs to be thoroughly understood to ensure they do not trigger inflammatory or allergic reactions [62].
Additionally, the scalability of nanoparticle manufacturing presents another challenge. Current production methods may not be suitable for large-scale, cost-effective manufacturing, which could hinder the widespread use of these treatments in clinical settings. Overcoming these manufacturing barriers will require the development of efficient, reproducible methods to produce nanoparticles in large quantities while maintaining their stability and therapeutic efficacy.
The clinical translation of nanoparticle systems for delivering cisplatin in ORL cancers is an area of growing interest, driven by the need to enhance therapeutic efficacy while minimizing systemic toxicity. Several nanocarrier approaches are currently being investigated in clinical settings, demonstrating promising developments.
Liposomal formulations of cisplatin, such as Lipoplatin, are being evaluated for their targeted delivery capabilities. A meta-analysis comparing liposomal cisplatin to conventional non-liposomal cisplatin in head and neck squamous cell carcinoma (HNSCC) suggests that the liposomal formulation may offer improved efficacy, although definitive conclusions regarding overall survival and progression-free survival are still needed [80]. Various clinical trials are ongoing that explore these formulations, highlighting the established safety and tolerability in patient populations [81,82].
In addition, polymeric systems, such as polysaccharide vesicles and PEGylated micelles, have shown potential in preclinical studies for their ability to co-deliver multiple anticancer agents alongside cisplatin. These formulations have been noted for their reduced toxicity profiles and improved pharmacodynamics, prompting future clinical evaluations [62,83]. Recent trials are focused on the use of hybrid nanocarriers based on poly lactic-co-glycolic acid (PLGA) and other approved polymers to deliver cisplatin in a targeted manner [84], with early results suggesting beneficial outcomes.
New strategies such as the integration of machine learning with molecular modeling are also being explored to improve the design and function of these nanocarriers, addressing challenges related to drug delivery efficiency and biological interactions. This innovative approach may pave the way for rationally designed nanocarrier systems that could integrate more effectively into the clinical setting [85].
While progress in the clinical translation of these nanoparticle formulations appears promising, it is crucial that ongoing and future trials evaluate their efficacy and safety in larger, diverse populations.
Future research should focus on optimizing nanoparticle formulations to improve their biocompatibility, drug release profiles, and targeting mechanisms.
Finally, while preclinical studies and early-phase trials have shown positive results, large-scale clinical trials are essential to establish the broader clinical applicability of nanotechnology-based cisplatin delivery systems. These trials will help determine the appropriate dosing, patient selection criteria, and long-term outcomes of this treatment approach, ultimately determining whether it can become a standard of care in the treatment of ORL cancers.
7. Conclusions
Nanotechnology represents a revolutionary approach that holds great promise for addressing the inherent limitations of traditional cisplatin therapy in the treatment of ORL cancers. By enhancing the precision, efficacy, and safety of cisplatin delivery, nanotechnology could significantly improve the outcomes for patients suffering from these aggressive cancers. However, to fully realize the potential of nanotechnology in clinical practice, further research and clinical validation are critical. Investigating more efficient nanoparticle formulations, conducting large-scale clinical trials, and navigating regulatory challenges will be essential steps in translating these technological advancements into routine clinical use. If successful, these innovations could usher in a new era of cancer treatment, improving survival rates and quality of life for patients with ORL cancers.
Author Contributions
Conceptualization, A.I.M. and E.R.B.; methodology, A.I.M. and K.G.; software, A.C.M.; validation, D.S., E.R.B., A.M.S. and A.C.M.; formal analysis, M.C.N.; investigation, A.I.M.; resources, A.O. and S.C.; data curation, A.C.M., A.O., K.G. and S.C.; writing—original draft preparation, A.I.M. and D.M.P.; writing—review and editing, M.C.N. and N.C.B.; visualization, D.S. and A.M.S.; supervision, N.C.B. and A.C.M.; project administration, D.M.P. and E.R.B.; funding acquisition, E.R.B. and A.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to acknowledge Victor Babes University of Medicine and Pharmacy, Timișoara, for their support in covering the costs of publication for this research paper.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lee, S.C.; Tang, I.P.; Avatar, S.P.; Ahmad, N.; Selva, K.S.; Tay, K.K.; Vikneswaran, T.; Tan, T.Y. Head and Neck Cancer: Possible Causes for Delay in Diagnosis and Treatment. Med. J. Malays. 2011, 66, 101–104. [Google Scholar]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020, 11, 940. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, M.; Wu, L.; Yang, H.; Yao, Y.; Yang, Q.; Du, J.; Liu, L.; Li, Y.; Bai, Y. Stromal Cells in the Tumor Microenvironment: Accomplices of Tumor Progression? Cell Death Dis. 2023, 14, 587. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Sig. Transduct. Target Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Babar, Q.; Saeed, A.; Tabish, T.A.; Sarwar, M.; Thorat, N.D. Targeting the Tumor Microenvironment: Potential Strategy for Cancer Therapeutics. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166746. [Google Scholar] [CrossRef]
- Seager, R.J.; Hajal, C.; Spill, F.; Kamm, R.D.; Zaman, M.H. Dynamic Interplay between Tumour, Stroma and Immune System Can Drive or Prevent Tumour Progression. Converg. Sci. Phys. Oncol. 2017, 3, 034002. [Google Scholar] [CrossRef]
- Marallano, V.; Ughetta, M.E.; Tejero, R.; Nanda, S.; Ramalingam, R.; Stalbow, L.; Sattiraju, A.; Huang, Y.; Ramakrishnan, A.; Shen, L.; et al. Hypoxia Drives Shared and Distinct Transcriptomic Changes in Two Invasive Glioma Stem Cell Lines. Sci. Rep. 2024, 14, 7246. [Google Scholar] [CrossRef]
- Noman, M.Z.; Hasmim, M.; Lequeux, A.; Xiao, M.; Duhem, C.; Chouaib, S.; Berchem, G.; Janji, B. Improving Cancer Immunotherapy by Targeting the Hypoxic Tumor Microenvironment: New Opportunities and Challenges. Cells 2019, 8, 1083. [Google Scholar] [CrossRef]
- Wang, L.; Mosel, A.J.; Oakley, G.G.; Peng, A. Deficient DNA Damage Signaling Leads to Chemoresistance to Cisplatin in Oral Cancer. Mol. Cancer Ther. 2012, 11, 2401–2409. [Google Scholar] [CrossRef]
- Bader, J.E.; Voss, K.; Rathmell, J.C. Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy. Mol. Cell 2020, 78, 1019–1033. [Google Scholar] [CrossRef]
- Lin, W.; Wu, S.; Chen, X.; Ye, Y.; Weng, Y.; Pan, Y.; Chen, Z.; Chen, L.; Qiu, X.; Qiu, S. Characterization of Hypoxia Signature to Evaluate the Tumor Immune Microenvironment and Predict Prognosis in Glioma Groups. Front. Oncol. 2020, 15, 796. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, C.; Wang, J.; Chen, L.; Chen, J.; Chen, T.; Zeng, Q. Application of Nanotechnology in the Diagnosis and Treatment of Bladder Cancer. J. Nanobiotechnol. 2021, 19, 393. [Google Scholar] [CrossRef]
- Khouzam, R.A.; Brodaczewska, K.; Filipiak-Duliban, A.; Zeinelabdin, N.A.; Buart, S.; Szczylik, C.; Kiéda, C.; Chouaib, S. Tumor Hypoxia Regulates Immune Escape/Invasion: Influence on Angiogenesis and Potential Impact of Hypoxic Biomarkers on Cancer Therapies. Front. Immunol. 2021, 11, 613114. [Google Scholar] [CrossRef]
- Cao, M.; Xiao, L.; Chen, S.; Huang, J. Characterization of Hypoxia-Responsive States in Ovarian Cancer to Identify Hot Tumors and Aid Adjuvant Therapy. Discov. Oncol. 2024, 15, 23. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Chen, C.; Zhao, S.; Zhang, X.; Zhang, J.; Yan, Y. Hypoxia Score for Predicting Prognosis and Tumor Response to Immunotherapy in Bladder Urothelial Carcinoma. Res. Sq. 2022. preprint. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Ai, M.; Liu, A.; Budhani, P.; Bartkowiak, T.; Sheng, J.; Ager, C.R.; Nicholas, C.; Jaiswal, A.R.; Sun, Y.; et al. Targeted Hypoxia Reduction Restores T Cell Infiltration and Sensitizes Prostate Cancer to Immunotherapy. J. Clin. Investig. 2018, 128, 5137–5149. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Mathai, M.L.; Zulli, A. Cisplatin for Cancer Therapy and Overcoming Chemoresistance. Heliyon 2022, 8, e10608. [Google Scholar] [CrossRef]
- Brown, A.; Kumar, S.; Tchounwou, P.B. Cisplatin-Based Chemotherapy of Human Cancers. J. Cancer Sci. Ther. 2019, 11, 97. [Google Scholar]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Makovec, T. Cisplatin and beyond: Molecular Mechanisms of Action and Drug Resistance Development in Cancer Chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef]
- Aldossary, S.A. Review on Pharmacology of Cisplatin: Clinical Use, Toxicity and Mechanism of Resistance of Cisplatin. Biomed. Pharmacol. J. 2019, 12, 7–15. [Google Scholar] [CrossRef]
- Faig, J.; Haughton, M.; Taylor, R.C.; D’Agostino, R.B.J.; Whelen, M.J.; Porosnicu Rodriguez, K.A.; Bonomi, M.; Murea, M.; Porosnicu, M. Retrospective Analysis of Cisplatin Nephrotoxicity in Patients With Head and Neck Cancer Receiving Outpatient Treatment With Concurrent High-Dose Cisplatin and Radiotherapy. Am. J. Clin. Oncol. 2018, 41, 432. [Google Scholar] [CrossRef]
- Paken, J.; Govender, C.D.; Pillay, M.; Sewram, V. Cisplatin-Associated Ototoxicity: A Review for the Health Professional. J. Toxicol. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Verstappen, C.C.P.; Heimans, J.J.; Hoekman, K.; Postma, T.J. Neurotoxic Complications of Chemotherapy in Patients with Cancer. Drugs 2003, 63, 1549–1563. [Google Scholar] [CrossRef]
- Chen, W.; Peng, L.; Zeng, X.; Wen, W.; Sun, W. Predictors of Myelosuppression for Patients with Head and Neck Squamous Cell Carcinoma After Induction Chemotherapy. Clin. Med. Insights Oncol. 2024, 18, 11795549231219497. [Google Scholar] [CrossRef]
- Stewart, D.J. Mechanisms of Resistance to Cisplatin and Carboplatin. Crit. Rev. Oncol. Hematol. 2007, 63, 12–31. [Google Scholar] [CrossRef]
- Chen, S.-H.; Chang, J.-Y. New Insights into Mechanisms of Cisplatin Resistance: From Tumor Cell to Microenvironment. Int. J. Mol. Sci. 2019, 20, 4136. [Google Scholar] [CrossRef]
- Chang, P.-Y.; Peng, S.-F.; Lee, C.-Y.; Lu, C.-C.; Tsai, S.-C.; Shieh, T.-M.; Wu, T.-S.; Tu, M.-G.; Chen, M.Y.; Yang, J.-S. Curcumin-Loaded Nanoparticles Induce Apoptotic Cell Death through Regulation of the Function of MDR1 and Reactive Oxygen Species in Cisplatin-Resistant CAR Human Oral Cancer Cells. Int. J. Oncol. 2013, 43, 1141–1150. [Google Scholar] [CrossRef]
- Kumbar, V.M.; Muddapur, U.; Bin Muhsinah, A.; Alshehri, S.A.; Alshahrani, M.M.; Almazni, I.A.; Kugaji, M.S.; Bhat, K.; Peram, M.R.; Mahnashi, M.H.; et al. Curcumin-Encapsulated Nanomicelles Improve Cellular Uptake and Cytotoxicity in Cisplatin-Resistant Human Oral Cancer Cells. J. Funct. Biomater. 2022, 13, 158. [Google Scholar] [CrossRef]
- Fan, X.; Wang, T.; Ji, Z.; Li, Q.; Shen, H.; Wang, J. Synergistic Combination Therapy of Lung Cancer Using Lipid-Layered Cisplatin and Oridonin Co-Encapsulated Nanoparticles. Biomed. Pharmacother. 2021, 141, 111830. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Dasari, S.; Noubissi, F.K.; Ray, P.C.; Kumar, S. Advances in Our Understanding of the Molecular Mechanisms of Action of Cisplatin in Cancer Therapy. J. Exp. Pharmacol. 2021, 13, 303–328. [Google Scholar] [CrossRef]
- Lugones, Y.; Loren, P.; Salazar, L.A. Cisplatin Resistance: Genetic and Epigenetic Factors Involved. Biomolecules 2022, 12, 1365. [Google Scholar] [CrossRef]
- Schoeberl, A.; Gutmann, M.; Theiner, S.; Corte-Rodríguez, M.; Braun, G.; Vician, P.; Berger, W.; Koellensperger, G. The Copper Transporter CTR1 and Cisplatin Accumulation at the Single-Cell Level by LA-ICP-TOFMS. Front. Mol. Biosci. 2022, 9, 1055356. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, S.; Hu, H.; Zhang, J.; Cao, K.; Wang, Y.; Liu, Y. Reactions of Cisplatin With Thioredoxin-1 Regulate Intracellular Redox Homeostasis. Inorg. Chem. 2024, 63, 11779–11787. [Google Scholar] [CrossRef]
- Dasari, S.; Njiki, S.; Mbemi, A.; Yedjou, C.G.; Tchounwou, P.B. Pharmacological Effects of Cisplatin Combination With Natural Products in Cancer Chemotherapy. Int. J. Mol. Sci. 2022, 23, 1532. [Google Scholar] [CrossRef]
- Raji, G.R.; Poyyakkara, A.; Krishnan, A.K.; Maurya, A.K.; Changmai, U.; Shankar, S.S.; Sameer Kumar, V.B. Horizontal Transfer of miR-643 From Cisplatin-Resistant Cells Confers Chemoresistance to Recipient Drug-Sensitive Cells by Targeting APOL6. Cells 2021, 10, 1341. [Google Scholar] [CrossRef]
- Balyapan, H.; Ak, G. Nanoparticle Targeting Strategies in Cancer Therapy. In Recent Progress in Pharmaceutical Nanobiotechnology: A Medical Perspective; Bentham Science Publishers: Sharjah, United Arab Emirates, 2023; Volume 8, pp. 223–238. [Google Scholar]
- Davoudi, M.; Jadidi, Y.; Moayedi, K.; Farrokhi, V.; Afrisham, R. Ameliorative Impacts of Polymeric and Metallic Nanoparticles on Cisplatin-Induced Nephrotoxicity: A 2011–2022 Review. J. Nanobiotechnol. 2022, 20, 504. [Google Scholar] [CrossRef]
- Soni, A.; Bhandari, M.P.; Tripathi, G.K.; Bundela, P.; Khiriya, P.K.; Khare, P.S.; Kashyap, M.K.; Dey, A.; Vellingiri, B.; Suresh, S.; et al. Nano-biotechnology in Tumour and Cancerous Disease: A Perspective Review. J. Cell. Mol. Med. 2023, 27, 737–762. [Google Scholar] [CrossRef]
- Wu, J.; Hu, Y.; Ye, H.; Zhang, S.; Zhu, J.; Ji, D.; Zhang, Y.; Ding, Y.; Huang, Z. One Stone Two Birds: Redox-Sensitive Colocalized Delivery of Cisplatin and Nitric Oxide Through Cascade Reactions. JACS Au 2022, 2, 2339–2351. [Google Scholar] [CrossRef]
- Motelică, L.; Voicu, G.; Chircov, C.; Surdu, V.-A.; Truşcă, R.; Vasile, B.Ș.; Ficai, D.; Oprea, O.; Marta, D.; Peteu, V.E.; et al. Aspartic Acid Functionalized Magnetic Nanoparticles for Enhanced Internalization in Tumoral Cell. J. Aust. Ceram. Soc. 2025, 61, 265–283. [Google Scholar] [CrossRef]
- Farooq, M.A.; Aquib, M.; Farooq, A.; Haleem Khan, D.; Joelle Maviah, M.B.; Sied Filli, M.; Kesse, S.; Boakye-Yiadom, K.O.; Mavlyanova, R.; Parveen, A.; et al. Recent Progress in Nanotechnology-Based Novel Drug Delivery Systems in Designing of Cisplatin for Cancer Therapy: An Overview. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1674–1692. [Google Scholar] [CrossRef]
- Yu, H.; Tang, Z.; Zhang, D.; Song, W.; Zhang, Y.; Yang, Y.; Ahmad, Z.; Chen, X. Pharmacokinetics, Biodistribution and in Vivo Efficacy of Cisplatin Loaded Poly(l-Glutamic Acid)-g-Methoxy Poly(Ethylene Glycol) Complex Nanoparticles for Tumor Therapy. J. Control. Release 2015, 205, 89–97. [Google Scholar] [CrossRef]
- Liang, X.-J.; Meng, H.; Wang, Y.; He, H.; Meng, J.; Lu, J.; Wang, P.C.; Zhao, Y.; Gao, X.; Sun, B.; et al. Metallofullerene Nanoparticles Circumvent Tumor Resistance to Cisplatin by Reactivating Endocytosis. Proc. Natl. Acad. Sci. USA 2010, 107, 7449–7454. [Google Scholar] [CrossRef]
- Alvarez-Berríos, M.P.; Vivero-Escoto, J.L. In Vitro Evaluation of Folic Acid-Conjugated Redox-Responsive Mesoporous Silica Nanoparticles for the Delivery of Cisplatin. Int. J. Nanomed. 2016, 11, 6251–6265. [Google Scholar] [CrossRef]
- Shah, A.S.; Surnar, B.; Kolishetti, N.; Dhar, S. Intersection of Inorganic Chemistry and Nanotechnology for the Creation of New Cancer Therapies. Acc. Mater. Res. 2022, 3, 283–296. [Google Scholar] [CrossRef]
- Mensah, L.B.; Morton, S.W.; Li, J.; Xiao, H.; Quadir, M.A.; Elias, K.M.; Penn, E.; Richson, A.K.; Ghoroghchian, P.P.; Liu, J.; et al. Layer-by-layer Nanoparticles for Novel Delivery of Cisplatin and PARP Inhibitors for Platinum-based Drug Resistance Therapy in Ovarian Cancer. Bioeng Transl. Med. 2019, 4, e10131. [Google Scholar] [CrossRef]
- Hernández-Gil, J.; Cobaleda-Siles, M.; Zabaleta, A.; Salassa, L.; Calvo, J.; Mareque-Rivas, J.C. An Iron Oxide Nanocarrier Loaded With a Pt(IV) Prodrug and Immunostimulatory dsRNA for Combining Complementary Cancer Killing Effects. Adv. Healthc. Mater. 2015, 4, 1034–1042. [Google Scholar] [CrossRef]
- Bozeman, E.N.; Gao, N.; Qian, W.; Wang, A.Z.; Yang, L. Abstract 3635: Enhanced Intra-Tumoral Immune Cell Infiltration Following Tumor Targeted Delivery of Chemotherapy Using Theranostic Nanoparticles in an Orthotopic Mouse Pancreatic Model. Cancer Res. 2014, 74, 3635. [Google Scholar] [CrossRef]
- Hoffmann, C.; Shen, C.; Tourneau, C.L. Nanoparticle Therapy for Head and Neck Cancers. Curr. Opin. Oncol. 2022, 34, 177–184. [Google Scholar] [CrossRef]
- Xu, C.; Yu, Y.; Sun, Y.; Kong, L.; Yang, C.; Hu, M.; Yang, T.; Zhang, J.; Hu, Q.; Zhang, Z. Transformable Nanoparticle-Enabled Synergistic Elicitation and Promotion of Immunogenic Cell Death for Triple-Negative Breast Cancer Immunotherapy. Adv. Funct. Mater. 2019, 29, 1905213. [Google Scholar] [CrossRef]
- Fu, G.; Wu, Y.; Zhao, G.; Chen, X.; Xu, Z.; Sun, J.; Tian, J.; Cheng, Z.; Shi, Y.; Jin, B. Activation of cGAS-STING Signal to Inhibit the Proliferation of Bladder Cancer: The Immune Effect of Cisplatin. Cells 2022, 11, 3011. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Chen, X.; Xu, Z.; Su, J.; Tian, J.; Shi, Y.; Xu, C.; Pan, H.; Jin, B. Cisplatin Inhibits Bladder Cancer Proliferation Through cGAS-STING Pathway. 2020. Available online: https://www.researchgate.net/publication/347859388_Cisplatin_Inhibits_Bladder_Cancer_Proliferation_Through_cGAS-STING_Pathway/fulltext/5fed0030a6fdccdcb81ae3e7/Cisplatin-Inhibits-Bladder-Cancer-Proliferation-Through-cGAS-STING-Pathway.pdf (accessed on 27 May 2025).
- Wang, W.; Yang, F.; Zhang, L.; Wang, M.; Yin, L.; Dong, X.; Xiao, H.; Xing, N. Targeting DNA Damage and Repair Machinery via Delivering WEE1 Inhibitor and Platinum (IV) Prodrugs to Stimulate STING Pathway for Maximizing Chemo-Immunotherapy in Bladder Cancer. Adv. Mater. 2024, 36, 2308762. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Wen, R.; Kolishetti, N.; Dhar, S. A Prodrug of Two Approved Drugs, Cisplatin and Chlorambucil, for Chemo War Against Cancer. Mol. Cancer Ther. 2017, 16, 625–636. [Google Scholar] [CrossRef]
- Zhao, Q.; Liang, G.; Guo, B.; Wang, W.; Yang, C.; Chen, D.; Yang, F.; Xiao, H.; Xing, N. Polyphotosensitizer-Based Nanoparticles With Michael Addition Acceptors Inhibiting GST Activity and Cisplatin Deactivation for Enhanced Chemotherapy and Photodynamic Immunotherapy. Adv. Sci. 2023, 10, 2300175. [Google Scholar] [CrossRef]
- Afrin, H.; Geetha Bai, R.; Kumar, R.; Ahmad, S.S.; Agarwal, S.K.; Nurunnabi, M. Oral Delivery of RNAi for Cancer Therapy. Cancer Metastasis Rev. 2023, 42, 699–724. [Google Scholar] [CrossRef]
- Tolue Ghasaban, F.; Maharati, A.; Zangouei, A.S.; Zangooie, A.; Moghbeli, M. MicroRNAs as the Pivotal Regulators of Cisplatin Resistance in Head and Neck Cancers. Cancer Cell Int. 2023, 23, 170. [Google Scholar] [CrossRef]
- Kumar, K.; Rani, V.; Mishra, M.; Chawla, R. New Paradigm in Combination Therapy of siRNA with Chemotherapeutic Drugs for Effective Cancer Therapy. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100103. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Elnagheeb, M. Mesoporous Silica Nanoparticles Loaded with Cisplatin and Phthalocyanine for Combination Chemotherapy and Photodynamic Therapy in Vitro. Nanomaterials 2015, 5, 2302–2316. [Google Scholar] [CrossRef]
- Duan, X.; He, C.; Kron, S.J.; Lin, W. Nanoparticle Formulations of Cisplatin for Cancer Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 776–791. [Google Scholar] [CrossRef]
- Mohammadinezhad, F.; Talebi, A.; Allahyartorkaman, M.; Nahavandi, R.; Vesal, M.; Akbarzadeh, A. Preparation, Characterization and Cytotoxic Studies of Cisplatin-Containing Nanoliposomes on Breast Cancer Cell Lines. Asian Pac. J. Cancer Biol. 2023, 8, 155–159. [Google Scholar] [CrossRef]
- Zhao, C.; Cheng, R.; Yang, Z.; Tian, Z. Nanotechnology for Cancer Therapy Based on Chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef] [PubMed]
- Amreddy, N.; Babu, A.; Panneerselvam, J.; Srivastava, A.; Muralidharan, R.; Chen, A.; Zhao, Y.D.; Munshi, A.; Ramesh, R. Chemo-Biologic Combinatorial Drug Delivery Using Folate Receptor-Targeted Dendrimer Nanoparticles for Lung Cancer Treatment. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Ramani, S.M.; Penchalaneni, J.; Padma, K.R. Emerging Updates on Tracking New Landscapes in Nanotechnology for the Diagnosis and Ovarian Cancer Therapy. J. Assoc. Med. Sci. 2024, 57, 124–134. [Google Scholar] [CrossRef]
- Qiu, S.; Sun, L.; Zhang, Y.; Han, S. Downregulation of BAG3 Attenuates Cisplatin Resistance by Inhibiting Autophagy in Human Epithelial Ovarian Cancer Cells. Oncol. Lett. 2019, 18, 1969–1978. [Google Scholar] [CrossRef]
- Liu, H.-T.; Wang, T.; Hsu, Y.; Chou, C.; Huang, K.; Hsu, C.; Liang, H.; Chang, H.; Lee, T.; Tsai, P. Nanoparticulated Honokiol Mitigates Cisplatin-Induced Chronic Kidney Injury by Maintaining Mitochondria Antioxidant Capacity and Reducing Caspase 3-Associated Cellular Apoptosis. Antioxidants 2019, 8, 466. [Google Scholar] [CrossRef]
- Bei, M.F.; Domocoș, D.; Szilagyi, G.; Varga, D.M.; Pogan, M.D. Influence of Vitamins and Antioxidants in Oral Carcinogenesis – A Review. Pharmacophore 2023, 14, 39–45. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, T.; Cui, M.; Li, N.; Li, Q.; Zhu, L.; Duan, S.; Wang, Y.; Guo, Y. A Novel Targeted Co-Delivery Nanosystem for Enhanced Ovarian Cancer Treatment via Multidrug Resistance Reversion and mTOR-Mediated Signaling Pathway. J. Nanobiotechnol. 2021, 19, 444. [Google Scholar] [CrossRef]
- Duan, Y.; Shen, C.; Zhang, Y.; Luo, Y. Advanced Diagnostic and Therapeutic Strategies in Nanotechnology for Lung Cancer. Front. Oncol. 2022, 12, 1031000. [Google Scholar] [CrossRef]
- Aftab, Z.; Bukhari, S.M.; Abubakar, M.; Sultan, H.M.; Zubair, M.; Abou Niaaj, M.A. Innovative Nanoparticle Synthesis and Multifaceted Applications in Medicine and Cancer Therapy. J. Clin. Nurs. Res. 2024, 8, 21–35. [Google Scholar] [CrossRef]
- Bortot, B.; Mongiat, M.; Valencic, E.; Dal Monego, S.; Licastro, D.; Crosera, M.; Adami, G.; Rampazzo, E.; Ricci, G.; Romano, F.; et al. Nanotechnology-Based Cisplatin Intracellular Delivery to Enhance Chemo-Sensitivity of Ovarian Cancer. Int. J. Nanomed. 2020, 15, 4793–4810. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, D.; Nicolosi, S.; Cocetta, V.; Salvalaio, M.; Pagetta, A.; Ragazzi, E.; Montopoli, M.; Pasut, G. Cisplatin Liposome and 6-Amino Nicotinamide Combination to Overcome Drug Resistance in Ovarian Cancer Cells. Oncotarget 2018, 9, 16847–16860. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Du, X.; Kleitz, F.; Qiao, S.Z. Cancer-Cell-Specific Nuclear-Targeted Drug Delivery by Dual-Ligand-Modified Mesoporous Silica Nanoparticles. Small 2015, 11, 5919–5926. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Madni, A.; Tahir, N.; Parveen, F.; Khan, S.; Jan, N.; Ali, A.; Abdurrahim, M.; Farooq, U.; Khan, M.I. Co-Delivery of Curcumin and Cisplatin to Enhance Cytotoxicity of Cisplatin Using Lipid-Chitosan Hybrid Nanoparticles. Int. J. Nanomed. 2020, 15, 2207–2217. [Google Scholar] [CrossRef]
- Tsvetkova, D.; Ivanova, S. Application of Approved Cisplatin Derivatives in Combination Therapy against Different Cancer Diseases. Molecules 2022, 27, 2466. [Google Scholar] [CrossRef]
- Sun, J.; Li, L.; Cai, W.; Chen, A.; Zhang, R. Multifunctional Hybrid Nanoprobe for Photoacoustic/PET/MR Imaging-Guided Photothermal Therapy of Laryngeal Cancer. ACS Appl. Bio Mater. 2021, 4, 5312–5323. [Google Scholar] [CrossRef]
- Rahim, M.A.; Jan, N.; Khan, S.; Shah, H.; Madni, A.; Khan, A.; Jabar, A.; Khan, S.; Elhissi, A.; Hussain, Z.; et al. Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting. Cancers 2021, 13, 670. [Google Scholar] [CrossRef]
- Xu, B.; Zeng, M.; Zeng, J.; Feng, J.; Yu, L. Meta-Analysis of Clinical Trials Comparing the Efficacy and Safety of Liposomal Cisplatin Versus Conventional Nonliposomal Cisplatin in Nonsmall Cell Lung Cancer (NSCLC) and Squamous Cell Carcinoma of the Head and Neck (SCCHN). Medicine 2018, 97, e13169. [Google Scholar] [CrossRef]
- Browning, R.J.; Thomas Reardon, P.J.; Parhizkar, M.; Pedley, R.; Edirisinghe, M.; Knowles, J.C.; Stride, E. Drug Delivery Strategies for Platinum-Based Chemotherapy. Acs Nano 2017, 11, 8560–8578. [Google Scholar] [CrossRef]
- Poy, D.; Akbarzadeh, A.; Shahmabadi, H.E.; Ebrahimifar, M.; Farhangi, A.A.; Zarabi, M.F.; Akbari, A.; Saffari, Z.; Siami, F. Preparation, Characterization, and Cytotoxic Effects of Liposomal Nanoparticles Containing Cisplatin: An in Vitro Study. Chem. Biol. Drug Des. 2016, 88, 568–573. [Google Scholar] [CrossRef]
- Deshpande, N.; Jayakannan, M. Cisplatin-Stitched Polysaccharide Vesicles for Synergistic Cancer Therapy of Triple Antagonistic Drugs. Biomacromolecules 2016, 18, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Ji, X.; Luo, J. Rational Nanocarrier Design Towards Clinical Translation of Cancer Nanotherapy. Biomed. Mater. 2021, 16, 032005. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.J.; Ciura, K.; Ma, Y.; Mikołajczyk, A.; Jagiełło, K.; Wan, Y.; Gao, Y.; Zheng, J.; Zhong, S.; Puzyn, T.; et al. Toward the Integration of Machine Learning and Molecular Modeling for Designing Drug Delivery Nanocarriers. Adv. Mater. 2024, 36, e2407793. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).