1. Introduction
The avian infectious bronchitis virus (IBV) was first described in the USA in the 1930s and has since caused major economic losses to the global poultry industry [
1,
2]. Estimations of total losses in an infected flock vary but have been approximated to be up to USD 65,000 per week on a farm with around 1 million broilers [
3]. IBV is a coronavirus predominantly infecting chickens but has also been found to infect other avian species such as turkeys and pheasants [
4]. Infected birds mainly suffer from respiratory issues, but disease of the kidneys and gastrointestinal tract, as well as oviducts, is also reported for certain strains. Viral infection often leads to mortality of the birds due to secondary bacterial infections [
1,
3]. The infection of egg-laying chickens also has great economic consequences, as it causes a significant, and usually persistent, decline in egg production of the affected flock [
5]. Poultry continues to be a major protein source for humans, but the available vaccines against IBV are poorly cross-reactive. There are currently no effective therapeutics against infection and disease, and it remains a threat to the global food supply and the health of this avian species.
IBV is a single-stranded positive-sense RNA virus in the genus
Gammacoronavirus, order
Nidovirales. Like other coronaviruses, it has a large genome of around 28 kb in size. Besides its structural and accessory genes coded for in its 3′ third, IBV encodes two large polyproteins in the 5′ two-thirds of its genome. These polyproteins are cleaved by viral proteases, encoded in the polyprotein sequence, to release mature non-structural proteins or nsps [
6]. The papain-like protease encoded within nsp3, PLpro, releases the three N-terminal nsps, whilst the 3C-like or main protease (nsp5) cleaves the remainder and the majority of the cleavage sites in the polyproteins. This results in a total of 15 different non-structural protein subunits named nsp2-nsp16 [
7]. Besides its ability to cleave the polyproteins, IBV PLpro also has deubiquitinating (DUB) activity, which is dependent on the same catalytic site and is thought to help the virus evade antiviral innate immune responses [
8,
9]. This DUB activity, first confirmed to be present among the coronaviruses for SARS-CoV PLpro, describes the protease’s ability to cleave ubiquitin chains from cellular substrates and subsequently affect downstream signaling of these substrates in innate immune pathways [
10,
11]. It was shown that expression of IBV PLpro has a negative effect on the production of interferon β (IFN-β) and that PLpro is able to deubiquitinate factors in this innate immune pathway, such as melanoma differentiation-associated protein 5 (MDA5) and TANK-binding kinase 1 (TBK1) [
12,
13].
This basic principle of the DUB activity of coronavirus PLpro enzymes can be exploited to produce inhibitor molecules, as PLpro is known to bind ubiquitin. Previously, our labs have identified variants of ubiquitin (UbVs) from a phage-display library of sequence variants that were effective at inhibiting the PLpros of MERS-CoV and SARS-CoV-2, as well as the turnip yellow mosaic virus protease, due to their strong binding to the proteases [
14,
15,
16,
17]. By this tight binding to PLpro, these UbVs were not only able to inhibit the DUB activity of the proteases but also directly inhibit PLpro’s proteolytic activity and thereby significantly reduce viral titers in infected cells. As IBV PLpro was found to have similar proteolytic activity to that of other coronaviruses [
8], we aimed to develop UbVs that bind to IBV PLpro with high affinity and are able to inhibit the protease. We have utilized a phage-displayed library containing billions of ubiquitin sequence variants and performed repeated selections of these UbVs using a recombinant version of the papain-like proteases of the IBV laboratory-adapted Beaudette strain, as well as the M41 field strain of IBV.
These strains were selected because most research into IBV is based on the lab-adapted Beaudette strain, as it is one of the few that is able to successfully replicate in immortalized cell lines. However, the Beaudette strain is non-pathogenic to chickens and may not provide an accurate basis for the development of novel inhibitors. For this reason, the same strategy was applied to the IBV prototype “Mass” serotype strain M41, as this is a pathogenic strain of the virus that could provide a better opportunity to develop inhibitors that would be valuable to target real-world IBV infections. Recombinant PLpros derived from both strains were expressed in E. coli, affinity purified and used for UbV-selection. These UbVs were subsequently tested in cell-based assays against both viruses and their respective PLpro enzymes.
We were able to rapidly select UbVs with high affinity to IBV Beaudette PLpro, IBV M41 PLpro or both. Their tight binding to PLpro facilitated obstruction of the protease sites responsible for deubiquitination and immune evasion, as well as significantly inhibiting viral replication, showing the promise of such a protein product as a viral inhibitor for IBV. Here, we show that the UbV approach that was previously successful at targeting the human coronaviruses MERS-CoV and SARS-CoV-2 [
14,
15] is also a feasible approach for targeting relevant veterinary coronaviruses, such as IBV.
3. Discussion
To this day, the coronavirus avian infectious bronchitis virus (IBV) has a major detrimental impact on the global poultry industry [
1]. The virus not only causes respiratory disease but also causes decreases in egg quality and production, as it affects the oviducts of chickens. Even though vaccines against this virus are available, they are not adequate at halting infection and/or spread for multiple reasons, as they are poorly cross-protective. Furthermore, the vaccines used are often live attenuated vaccines which have been reported to recombine with field strains, as well as exert immunological pressure, consequently stimulating the emergence of new variant viruses [
24,
25]. This in turn promotes the genetic diversity of IBV, making it even more difficult to produce effective vaccines.
Some progress has been made regarding antiviral agents. Recently, a host defense peptide, called chicken cathelicidins 2, was identified as having anti-IBV activity [
26]. However, clear differences in effectivity between study models and IBV strains were found, calling into question the usefulness of such a peptide as a broadly effective antiviral treatment for multiple strains. Other antivirals that have previously been suggested to work against coronaviruses like SARS-CoV-2, such as ivermectin, were unfortunately ineffective at inhibiting IBV replication [
27]. Besides these options, many researchers have assessed the usefulness of traditional Chinese medicine or other plant-based substances against IBV infection. Some of these, such as a combination of Shegandilong with doxycycline, or the use of plant extracts, were indeed found to inhibit IBV infection to a certain extent in vivo [
28,
29]. Furthermore, the natural product myricetin was found to have antiviral activity against IBV PLpro [
30]. These have, however, not been approved for treatment or prophylaxis. New strategies for fighting and/or preventing IBV are therefore sorely needed.
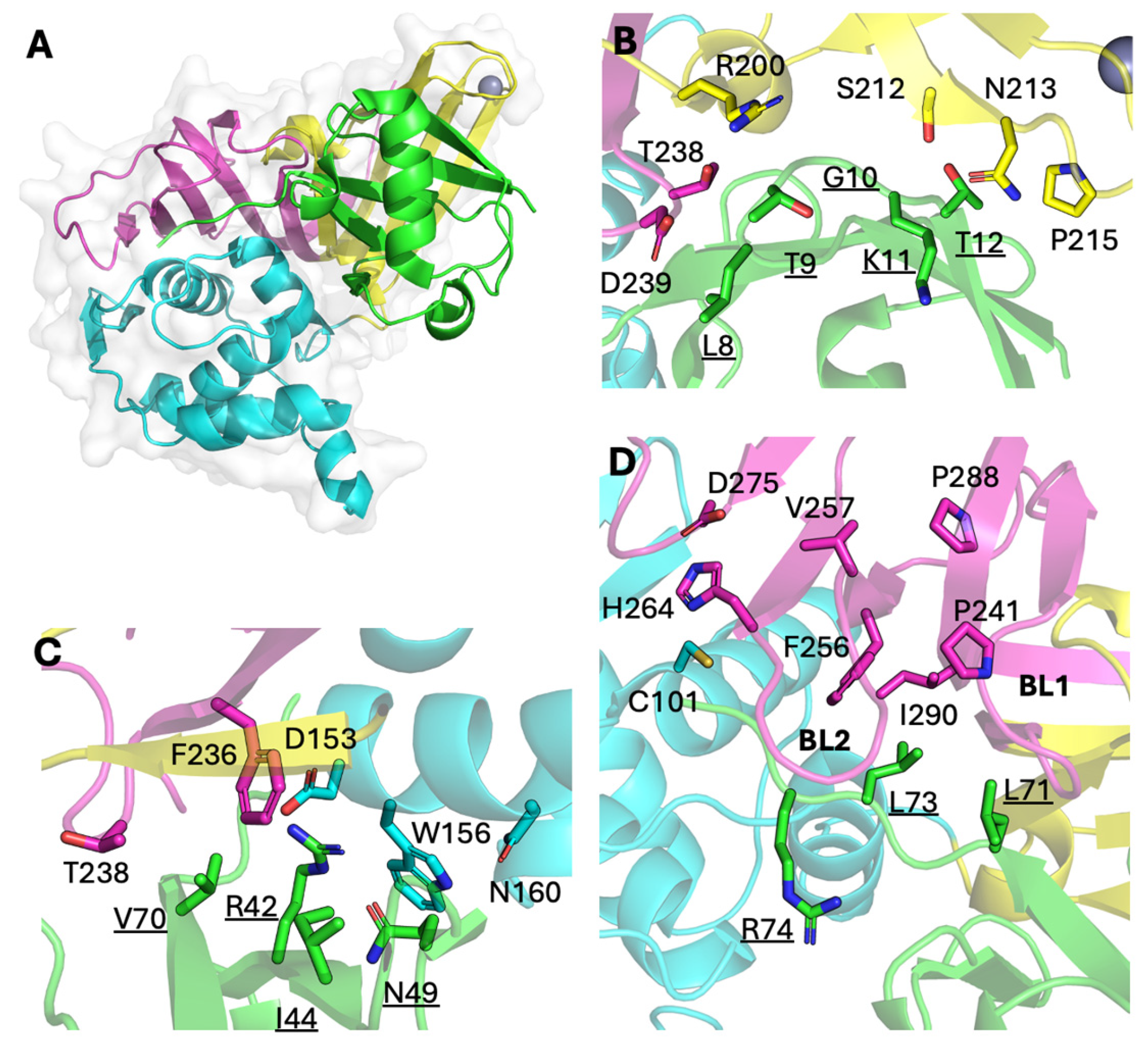
The DUB activity of IBV PLpro has been previously established; however, the genome sequence of an IBV QX-strain was used, which is largely restricted to southern China and is quite divergent from the Massachusetts-like strains found globally [
9,
31]. The authors also claimed that PLpro domain itself had no DUB activity and that only when the nsp3 transmembrane domain downstream of the protease domain was included in their construct did they detect such activity. Accordingly, we investigated whether a similar condition applied to the PLpro domain of the Beaudette and M41 strains. As our strategy was highly dependent on the binding of PLpro to ubiquitin, and thus DUB activity, we deemed it imperative to confirm PLpro activity before UbV selection.
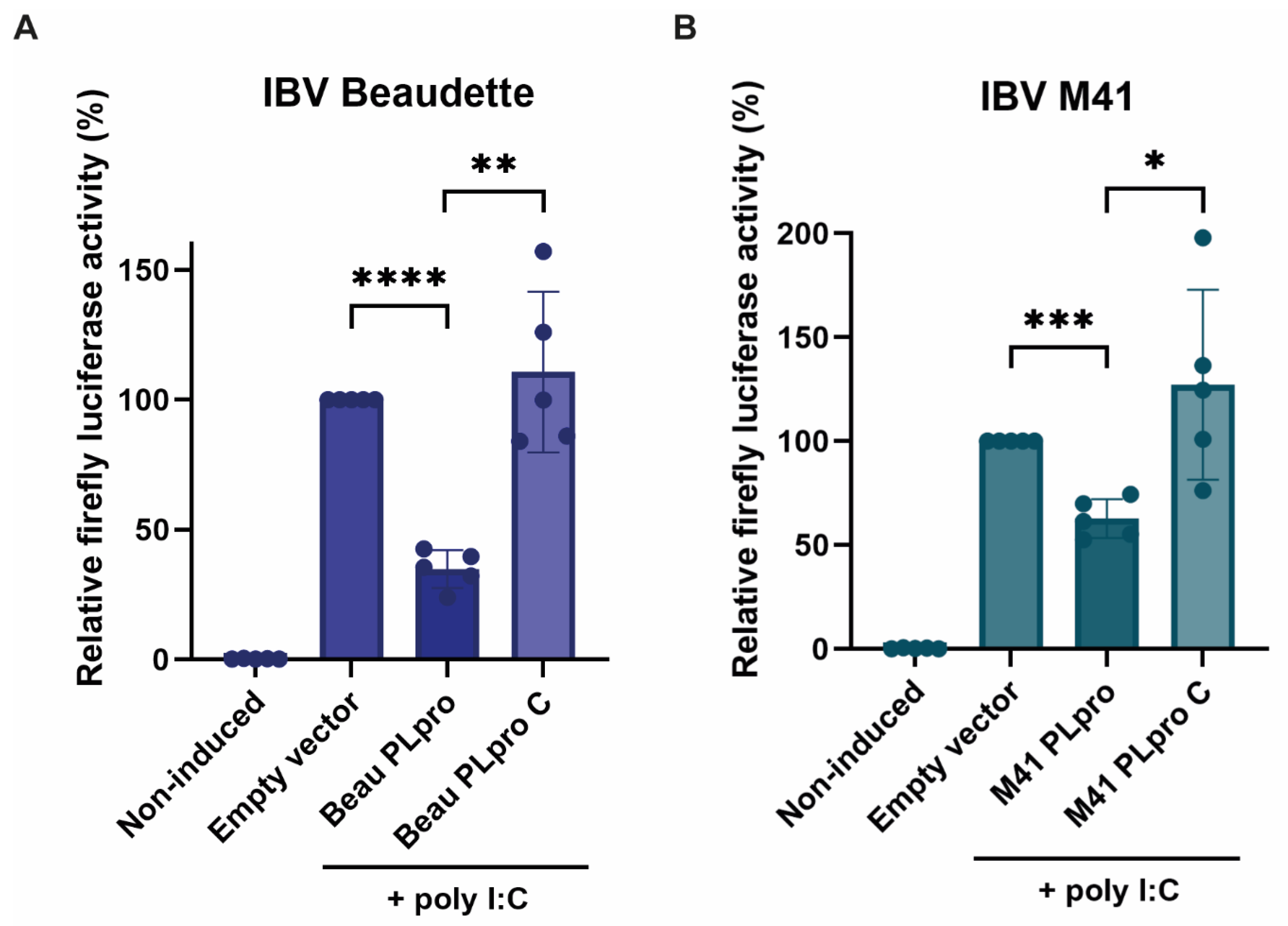
We found that the IBV PLpro domains from both the Beaudette and M41 strains each exhibit a clear DUB activity, which is not present when the catalytic mutant of the proteases is expressed instead. This suggests an important role for the catalytic cysteine in this activity, which is expected, as deubiquitination entails a proteolytic cleavage of the covalent ubiquitin–substrate isopeptide bond. Furthermore, we found that both PLpro enzymes have a detrimental effect on the innate immune response in the cell, as measured by a reduction in activity of the chicken IFN-β promoter in our assay. Even though similar results have been published before, they were obtained with the use of the human instead of the chicken IFN-β promoter, possibly yielding skewed results [
32]. Others that did use the chicken IFN-β promoter only included IBV PLpro from the M41 strain in their experiments [
12].
Having established both the DUB activity of the protease as well as its negative effect on innate immunity, it was deemed appropriate to screen for UbV binding. UbV selection using both Beaudette and M41 PLpros provided us with eight promising original UbVs (
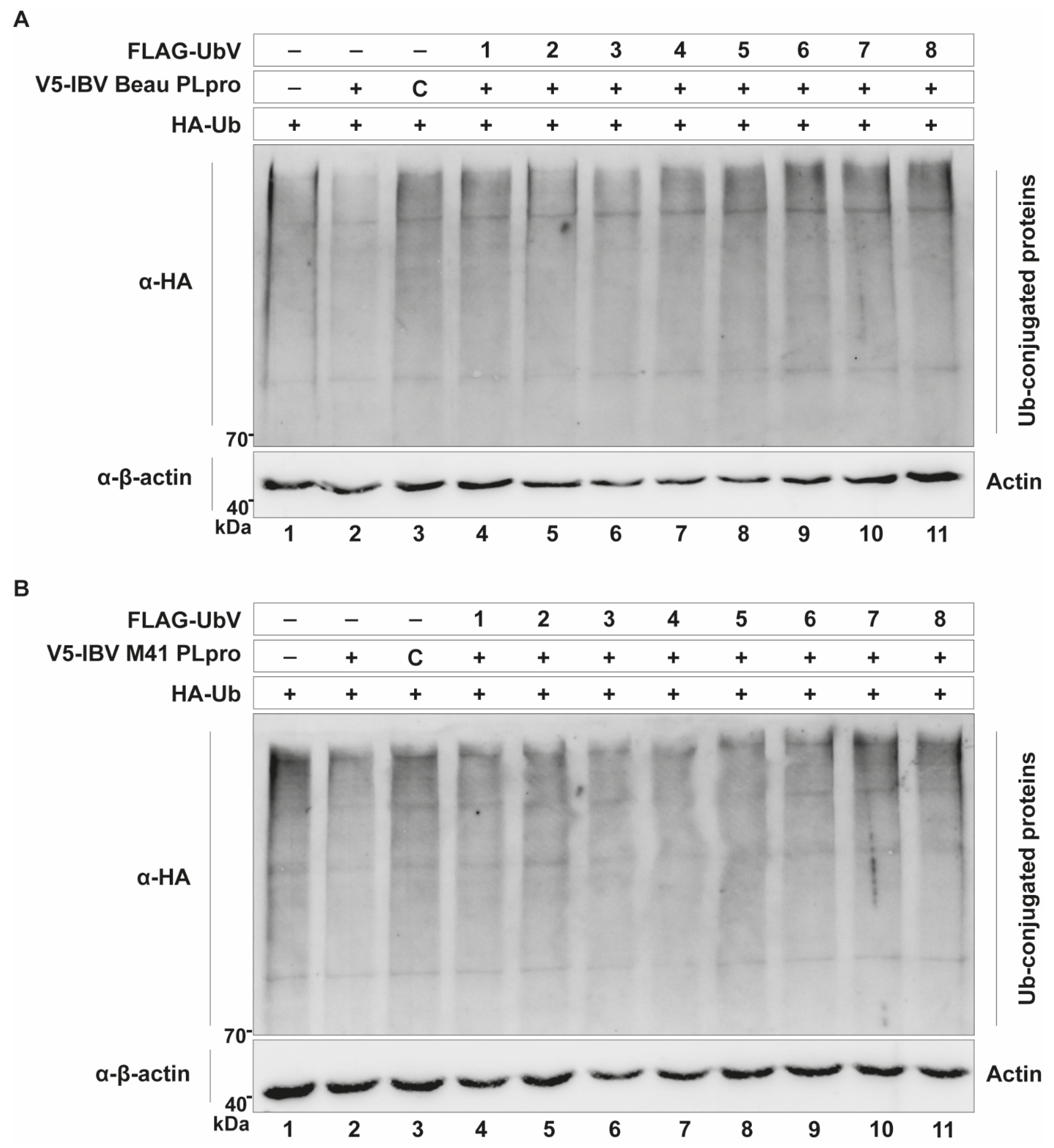
Table 1). When tested in a cell-based DUB assay, UbVs 1, 2, 5 and 8 were found to inhibit the DUB activity of either Beaudette PLpro, M41 PLpro or both (
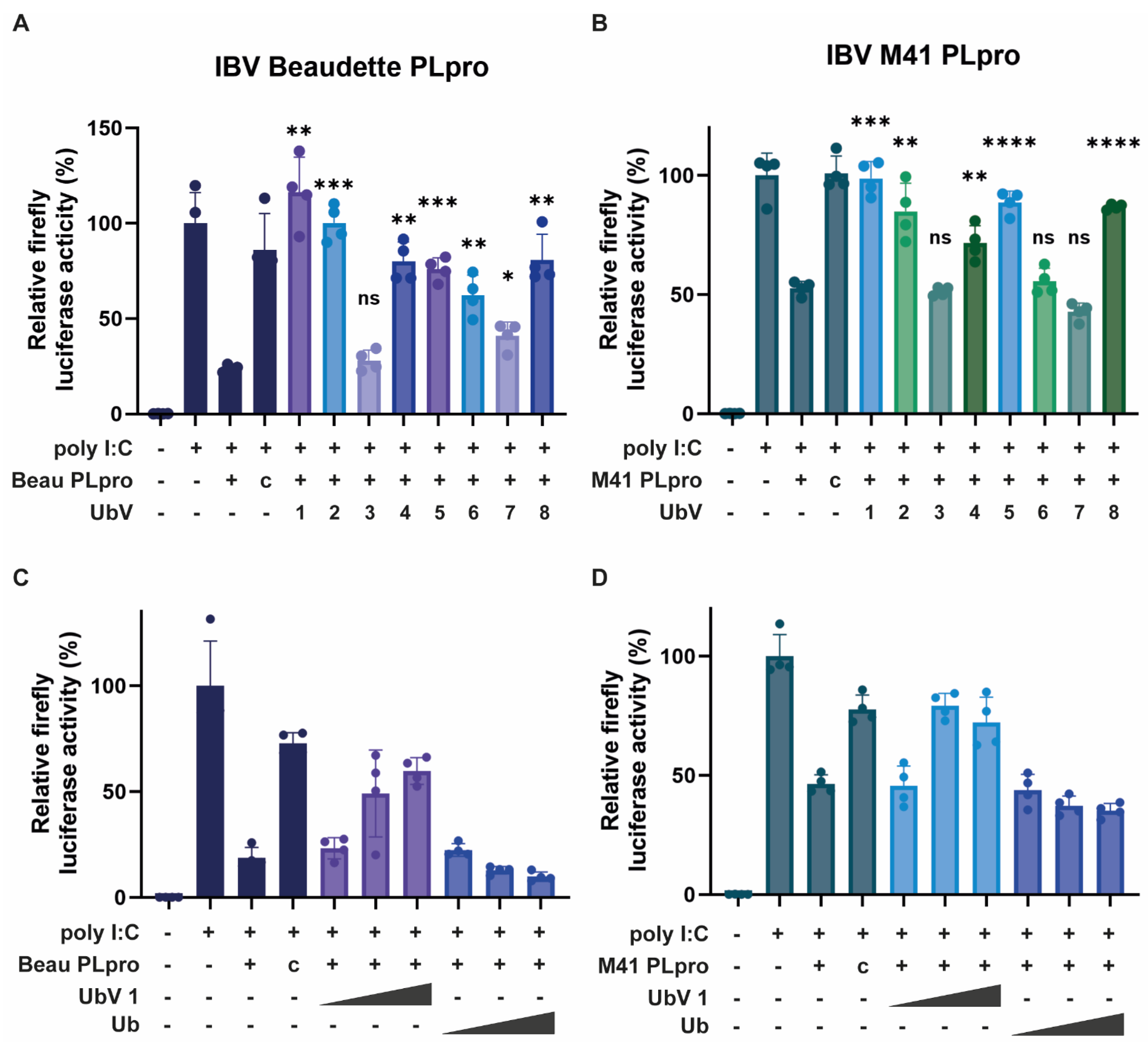
Figure 3). Assessing their effectiveness in the luciferase assay showed that expression of these same UbVs inhibited the detrimental effect of the PLpro enzymes on the innate immune response in the cell significantly (
Figure 4). UbVs 1, 2, 5 and 8 were subsequently assessed in biochemical in vitro studies.
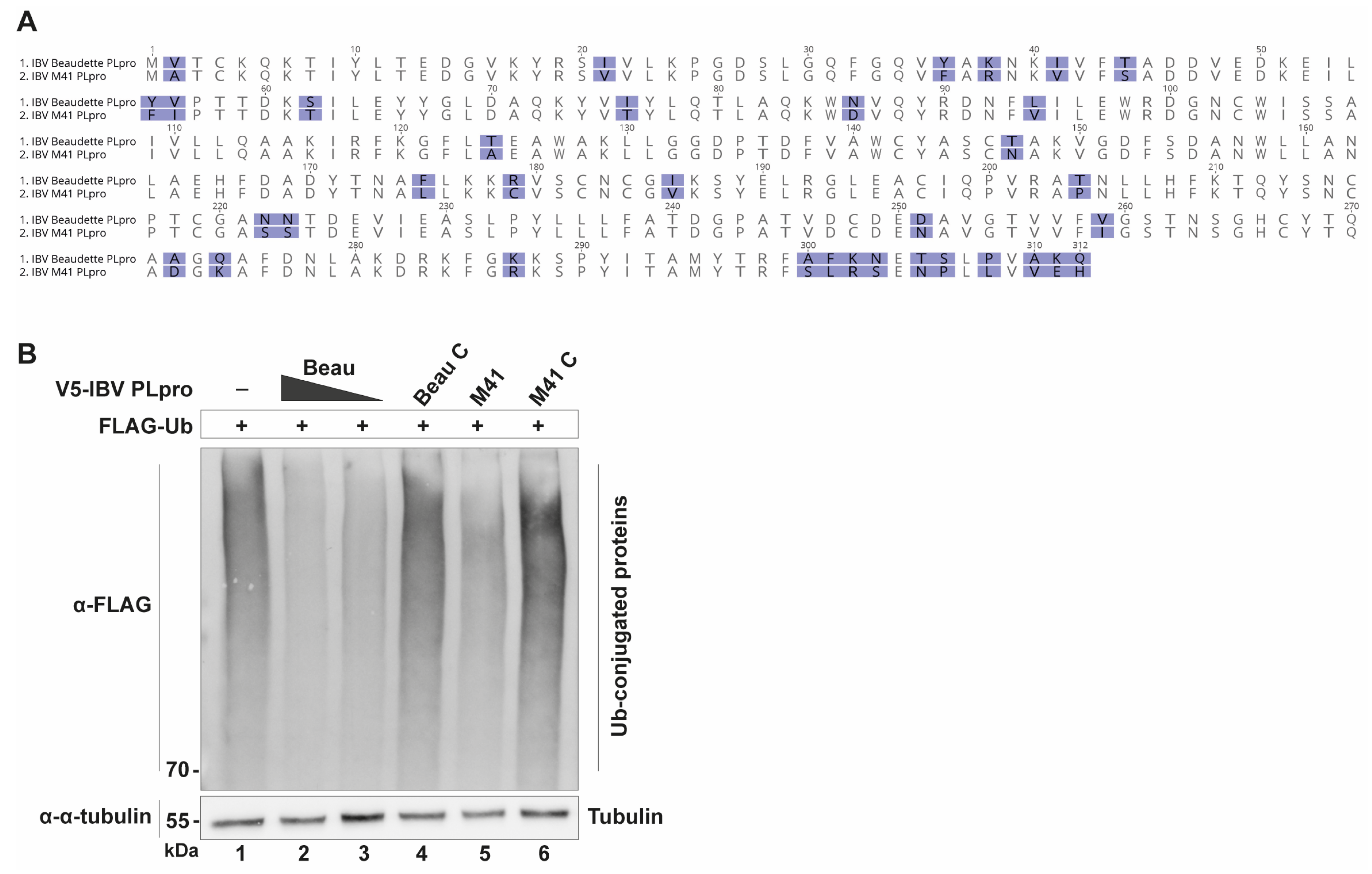
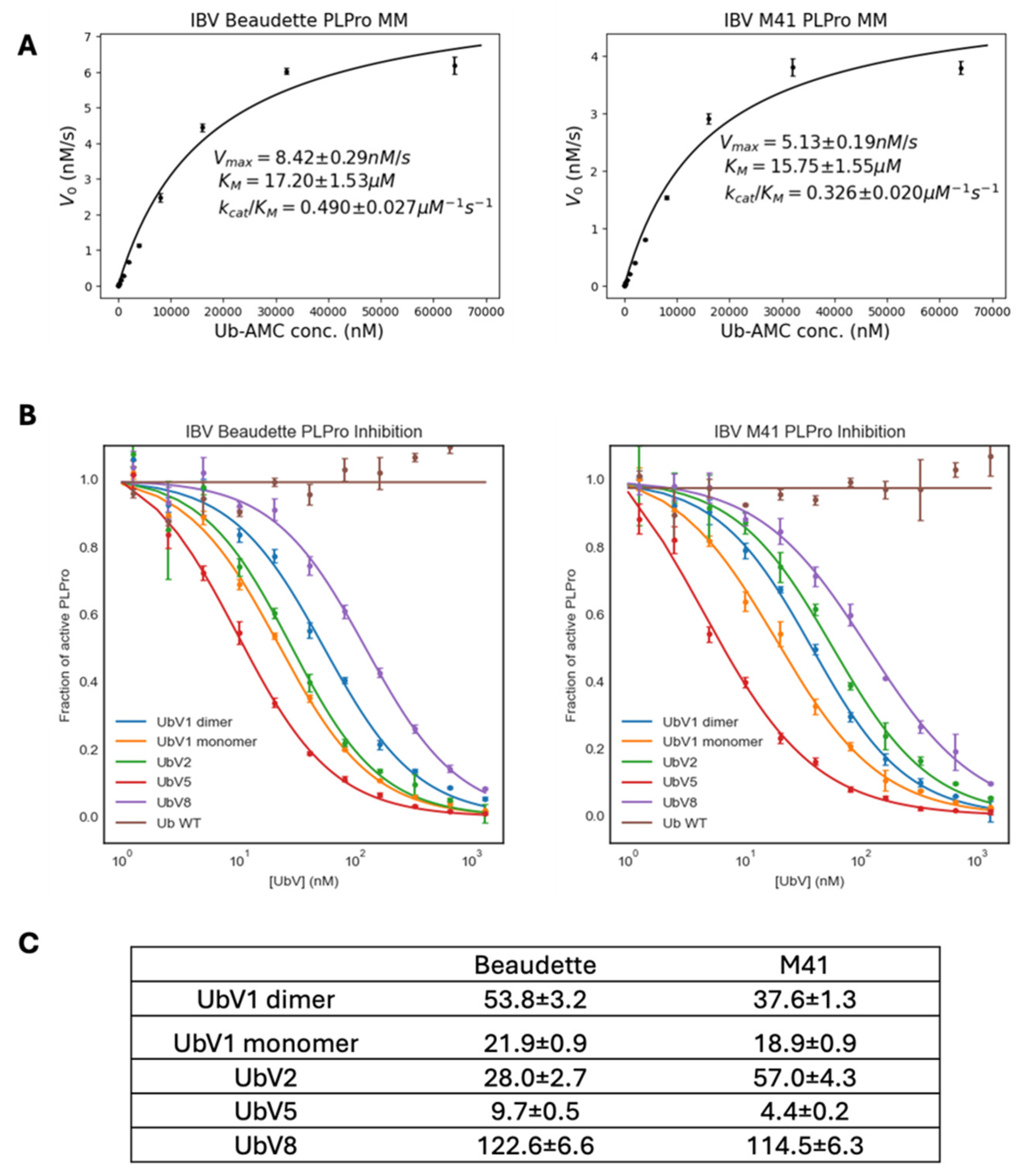
The recombinant PLpro enzymes from Beaudette and M41 showed similar Michaelis–Menten kinetics (
Figure 5A), which was not unexpected given that their sequence differences do not map to active site or Ub binding interfaces (
Figure 1). The k
cat/K
M value of Beaudette PLpro (0.49 ± 0.027 µM
−1s
−1) was different from previous reports using an LRGG-AMC substrate (0.67 ± 0.11 mM
−1s
−1) [
8], due to the significant differences between substrates. Ub-AMC is a full-length Ub conjugated to a fluorophore on its C-terminus, while the short peptide substrate used by Kong and co-workers only mimics the four C-terminal amino acids of Ub. Ub-AMC is arguably a better mimic of the natural interaction between PLpro and Ub. Furthermore, in our biochemical assay, all UbVs tested showed IC
50 values in the nanomolar range (
Figure 5C) compared to wildtype Ub, which did not show appreciable inhibition of PLpro DUB activity. For both Beaudette and M41 PLpros, UbV 5 showed the strongest inhibition (IC
50 ~9.7 nM and ~4.4 nM, respectively). These low IC
50 values of UbVs agree with our hypothesis that the UbVs selected by phage display inhibit PLpro DUB activity efficiently.
In contrast with the other tested UbVs, UbV 1 has a G10A substitution compared to ubiquitin, which leads to a conformational change that promotes dimerization, as well as a possibly increasing affinity for the target [
16,
18,
33]. In previous studies, this substitution led to the exchange of a β1 strand between two UbV chains. Therefore, UbV 1 likely exists in both monomeric and dimeric conformations. Both UbV 1 conformations were purified, and their IC
50 values were measured, which were comparable. This suggests that the conformational change caused by dimerization may not affect its binding mode to PLpro.
Previously, we established that UbVs are promising antiviral agents against MERS-CoV and SARS-CoV-2 PLpro [
14,
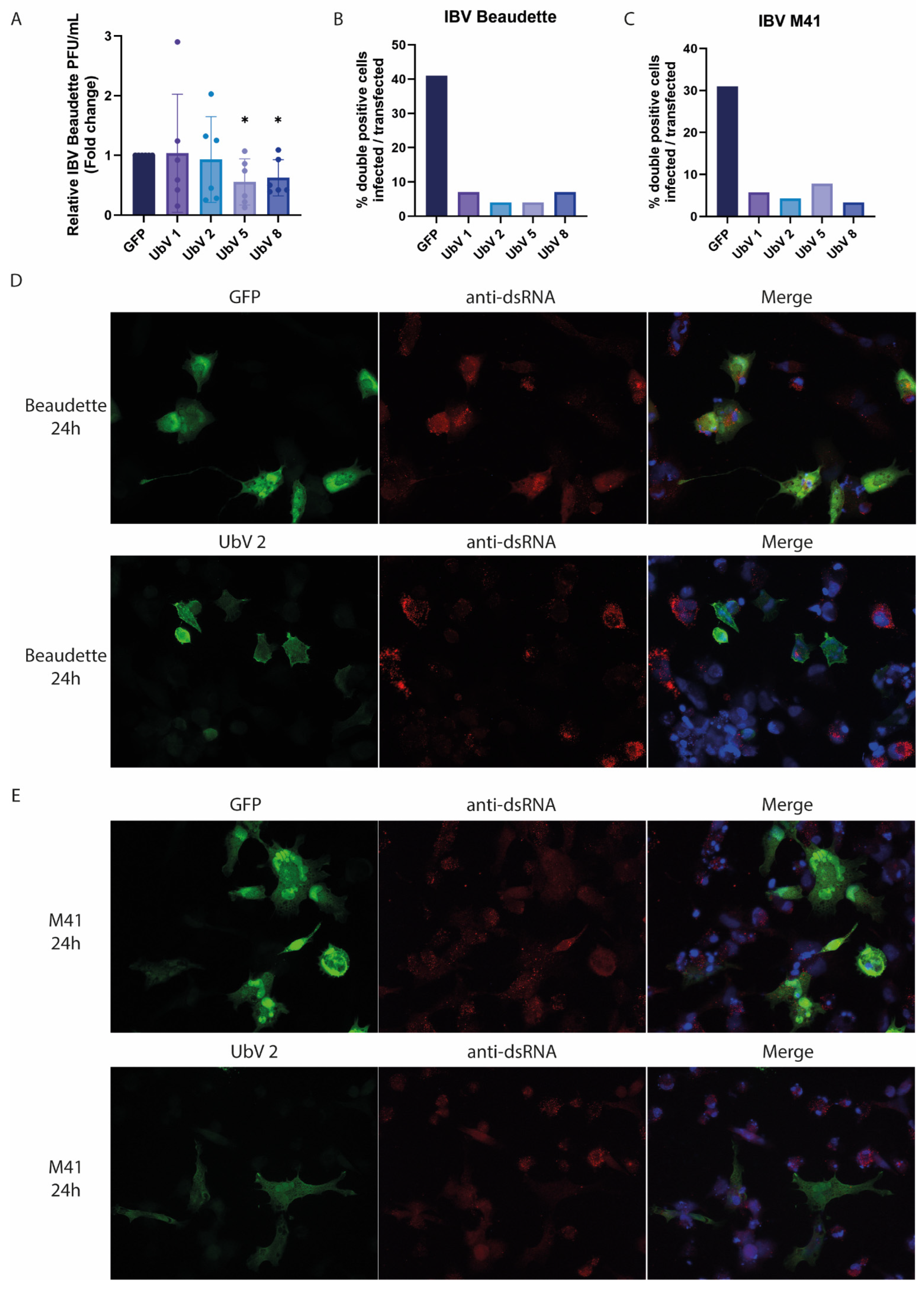
15]. In the current study, we extended this strategy to a veterinary coronavirus, IBV. The tested UbVs show promising inhibition of the various functionalities of IBV PLpro from two strains, as well as seemingly inhibiting viral infection, as assessed by immunofluorescence assay. The limited effect on infectious viral progeny titers that we observed in our assays was likely due to the transfection efficiency in the DF-1 cells being relatively low. This leaves many cells susceptible to infection and available to produce normal virus titers, rendering the difference with GFP-transfected control cells too small to be measured. For future research, another set-up of this experiment, for example using cell lines that stably express the UbVs, would likely yield more convincing results. Nonetheless, in vitro, the selected UbVs seemed similarly effective at inhibiting the PLpro enzymes from both IBV Beaudette and M41. Structural modeling revealed that the amino acid differences between Beaudette and M41 do not overlap with the binding interfaces of Ub and are thus not likely to interfere with UbV binding either. Thus, this UbV approach may be an efficient way to target a variety of IBV strains, holding promise for broad-acting anti-IBV effects.
In the future, it would be interesting to explore the possibility of producing genetically modified chickens that express one or more IBV-inhibiting UbVs, aiming to render the animals resistant to IBV infection. Indeed, others have been able to genetically edit chicken primordial germ cells to contain a mutated version of the protein ANP32A, which in turn prevents the interaction of the avian influenza viral polymerase with this host protein [
34]. These germ cells were then used to produce a genetically modified chicken resistant to low-dose influenza infection. Further research is needed to determine whether UbVs are sufficiently efficacious as antiviral agents in whole organisms to treat and/or prevent coronavirus infections.
4. Materials and Methods
4.1. Cell and Virus Culture
Chicken embryonic fibroblasts (DF-1 cells) were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Lonza, Basel, Switzerland), supplemented with 10% fetal bovine serum (FBS, Capricorn Scientific, Ebsdorfergrund, Germany), 1× non-essential amino acids (NEAA, Merck, Darmstadt, Germany), and 1× glutamine (Ala-Gln, Merck). The cell line tested negative for mycoplasma.
IBV strains Beaudette and M41 were propagated in fertilized chicken eggs or DF-1 cells and used for infection experiments inside BSL-2 facilities at the Roslin Institute and the Leiden University Medical Center. IBV M41 was a kind gift from Sjaak de Wit at Royal GD (Deventer, The Netherlands). The complete genome reference sequence of these strains is available under GenBank accession numbers NC_001451.1 (IBV Beaudette) and DQ834384.1 (IBV M41).
4.2. Antibodies
For Western blot analysis, the following antibodies and corresponding dilutions were used: mouse anti-FLAG (clone M2, #F1804, Sigma-Aldrich, Saint Louis, MO, USA, diluted 1:2000), mouse anti-HA (HA.C5, Genetex, Irvine, CA, USA, 1:1000) and mouse anti-α-Tubulin (clone B-5-1-2, #T5168, Sigma-Aldrich, diluted 1:2000). These primary antibodies were detected with biotin-conjugated goat anti-mouse IgG (#31802, Invitrogen, Waltham, MA, USA, diluted 1:2000) and tertiary antibody Cy3-conjugated mouse anti-biotin (#200-162-211, Jackson, Avon, CT, USA, diluted 1:2500).
For immunofluorescence assays, the following antibodies and dilutions were used: rabbit anti-FLAG (#F7425, Merck, diluted 1:500), mouse anti-dsRNA (clone J2, Scicons, Springville, CA, USA, diluted 1:1000) Alexa488-conjugated goat-anti-rabbit IgG (Invitrogen, diluted 1:300) and Cy3-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA, diluted 1:1000). Nuclei were stained with Hoechst 33258 (Thermo Fisher Scientific, Waltham, MA, USA, diluted 1:100).
4.3. Plasmid Construction
Plasmids pcDNA3-V5-IBV-PLpro-Beaudette, pcDNA3-V5-IBV-PLpro-Beaudette-C, pcDNA3-V5-IBV-PLpro-M41 and pcDNA3-V5-IBV-PLpro-M41-C were constructed using synthetic gBlocks (IDT), cloned in-frame with a V5 tag on the N-terminal side into a pcDNA3 vector using the XhoI and KpnI restriction enzymes. The GenBank sequences NC_001451.1 (IBV Beaudette) and DQ834384.1 (IBV M41) were used to determine the sequences of PLpro at genome positions aa1348-1659 (Beau) and aa1350-1661 (M41). Catalytic mutants were produced by site-directed mutagenesis to create C101A mutations in both constructs. The pcDNA3-FLAG-UbV plasmids were cloned into the same vector using the NheI and BamHI restriction enzymes. Constructs were verified by Sanger sequencing. Other plasmids were described elsewhere or provided by others: pLenti6.3-FLAG-Ub.WT [
14], pRK5-HA-Ub was a gift from Ted Dawson (Addgene plasmid #17608;
http://n2t.net/addgene:17608, URL accessed on 13 April 2022; RRID:Addgene_17608 [
35]) and pcDNA3.1-empty (Invitrogen). Sequence alignments were produced using Geneious version 10.2 created by Biomatters, New York, NY, USA.
For the luciferase assay, the pGL3-Basic Vector was used to clone the chicken IFN-β promoter region. This insert was ordered as gBlock (IDT), rehydrated and digested using NheI and HindIII restriction enzymes, as was the vector backbone. These were ligated using T4 DNA ligase (NEB, Ipswich, MA, USA).
For expression and purification, the sequences encoding the IBV PLpro protease domains of IBV Beaudette and IBV M41 were identified and ordered as gBlocks (IDT), as mentioned above, and ligated into the bacterial expression vector pETM11, which provides the inserted sequence with a His6-tag.
4.4. Expression and Purification of IBV PLpro for UbV Screening
His
6-IBV PLpro enzymes were expressed from the pETM11-based vector in
E. coli BL21 (DE3) cells (Stratagene). Transformed bacterial cells were grown in 2×YT medium containing kanamycin, overnight. The next day, the prewarmed Luria–Bertani (LB) medium was spiked with the overnight culture and supplemented with kanamycin. The cultures were incubated at 37 °C with shaking until the OD
600 reached 0.7. Cultures were then induced with IPTG (final concentration 0.1 mM) and incubated overnight at 16 °C, 180 rpm. The overnight cultures were spun down, and cell pellets were lysed using bacterial lysis buffer (50 mM NaH
2PO
4, 300 mM NaCl, 30 mM Imidazole, 10% glycerol and cOmplete
TM Protease Inhibitor Cocktail (Roche, Basel, Switzerland)), supplemented with lysozyme, Triton X-100 and Benzonase. Lysates were incubated on a rotator for a few hours at 4 °C. HisPur Ni-NTA Resin slurry (Thermo Fisher Scientific) was then used for protein purification by batch method using the corresponding user guide. Finally, a buffer exchange was performed using a 10 kDa cut-off centrifugal filter tube to remove excess salts. Eluted protein was analyzed by SDS-PAGE (containing 2,2,2-trichloroethanol) and subsequently analyzed upon UV exposure to verify proper size and purity of the sample (
Figure S5).
4.5. Selection of UbVs
UbV selection was performed as described by Zhang and Roscow [
36]. In brief, purified IBV Beaudette and M41 PLpro were diluted in PBS to 1 µM and coated on hydrophobic microplates overnight at 4 °C. In preparation for the first round of phage display, a seed culture of
E. coli OmniMAX was prepared by inoculating 2YT broth supplemented with tetracycline with an isolated colony and incubated overnight at 37 °C (assume all incubation steps are performed with shaking, unless stated otherwise). The next day, the seed culture was used to seed a mid-log phase OmniMAX culture. Unabsorbed protein was poured off, and the plate was blocked with 1%
w/
v bovine serum albumin (BSA)-supplemented PBS (PB buffer) by rinsing once, refilling and incubating at 4 °C for one hour. During this time, the phage library was thawed on ice and diluted 10-fold in PBS. One-fifth the volume of 20% polyethylene glycol/2.5 M NaCl (PEG-NaCl) was added and mixed before incubating on ice for 30 min. The solution was centrifuged at 11,000×
g for 30 min at 4 °C, after which supernatant was discarded, and the phage pellet was gently resuspended in 1 mL of 0.05% Tween 20- and 1%
w/
v BSA-supplemented PBS (PBT buffer). Once the blocked plate was finished incubating, the blocking solution was poured off, the phage library was added, and the plate was incubated at room temperature for one hour to allow phage binding to recombinant PLpro. Unbound phages were then poured off, and wells containing bound phage were washed four times with 0.05% Tween 20-supplemented PBS (PT buffer). Phages were eluted by incubation with 0.1 M HCl at room temperature for 5 min. The pH was neutralized by adding 12.5%
v/
v 1 M Tris-HCl (pH 11) and pooled eluted phages were supplemented with 1% BSA and stored at 4 °C. This solution is termed the phage output. Half of the round one output volume was used to inoculate the mid-log phase cells prepared earlier, which were then incubated at 37 °C for 30 min. M13K07 helper phages were cultured to a final concentration of 10
10 PFU/mL, and the culture was incubated at 37 °C for another hour. The culture was seeded into 2YT broth supplemented with carbenicillin and kanamycin and grown overnight at 37 °C. The next day, the culture was centrifuged at 11,000×
g for 10 min at 4 °C, and the supernatant was decanted and mixed with PEG/NaCl. After incubating on ice for ten minutes, the solution was centrifuged at 11,000×
g for ten minutes at 4 °C. The supernatant was discarded, and the phage pellet was resuspended in PBT, centrifuged at 16,200×
g for four minutes at 4 °C to pellet debris and stored at 4 °C. This enriched library solution was used as input for the next round of selection. Five subsequent rounds of selection were performed in a similar manner, with each round receiving the enriched input from its respective previous round. Only the post-selection washing step becomes more stringent with each round of selection, to increase the likelihood of enriching a binder with significantly increased affinity for IBV PLpro. A mid-log phase
E. coli OmniMAX culture was inoculated with ten-fold PBS dilutions of rounds four and five’s phage outputs and plated onto multiple LB plates supplemented with carbenicillin. Colonies were picked, inoculated into 2YT broth supplemented with carbenicillin and M13K07 helper phage and grown overnight at 37 °C. Cultures were centrifuged at 1200×
g for ten minutes at 4 °C, and supernatant was transferred to a new container and stored at 4 °C. These solutions, containing eluted and amplified phage DNA, were then analyzed via Sanger sequencing. Sequencing data were cleaned and analyzed using an in-house program (PDAS:
https://github.com/oroscow/phage_display_analysis_suite_pdas, URL accessed on 9 September 2023).
4.6. Luciferase Assays
DF-1 cells were seeded into 24-well plates and transfected when cell confluency was around 70%. Transfections were performed using pGL3-chIFNβ-luc, pRL-TK (Renilla) and different combinations of pcDNA3-V5-IBV-PLpro-Beaudette, pcDNA3-V5-IBV-PLpro-Beaudette-C, pcDNA3-V5-IBV-PLpro-M41, pcDNA3-V5-IBV-PLpro-M41-C and pcDNA3-FLAG-UbVs. pcDNA3.1-empty vector was used to normalize the total amount of transfected DNA per well. Poly I:C (HMW, Invivogen, San Diego, CA, USA) was used as an inducer of the chIFN-β promoter activity in this assay. Linear polyethylenimine (PEI 25K, Polysciences, Warrington, PA, USA) was used as a transfection method. Briefly, per 1 μg of plasmid DNA, 3 μL of PEI (1 μg/μL) was diluted in Opti-MEM medium (Lonza). The plasmid DNA was also diluted in Opti-MEM. Diluted PEI was then added to the diluted DNA, vortexed and incubated for 20 min. The mixture was then added to the cells (24-well plate, 5 × 104 cells/well) in a dropwise manner, and the cells were incubated at 37 °C, 5% CO2. Cells transfected with the luciferase assay were lysed 18 h post-transfection in 1×PLB (Promega, Madison, WI, USA). Read-out was performed using the Dual-Luciferase Reporter Assay System (Promega), and the CLARIOstar Plus Microplate Reader (BMG Labtech, Ortenberg, Germany) or EnVision Multilabel Plate Reader (PerkinElmer, Waltham, MA, USA). Corresponding graphs were created using Graphpad Prism (version 9). All luciferase assay experiments were performed at least three times independently, with four technical replicates each. Unpaired two-tailed Student’s t-tests were performed to determine statistical significance.
4.7. Deubiquitination (DUB) Assays
Deubiquitination assays were performed as described previously [
14]. For the current study, DF-1 cells were seeded into 12-well plates and transfected when cell confluency was around 70%. Combinations of tagged ubiquitin, PLpro and UbVs were expressed to assess the ability of PLpro to deubiquitinate (poly-) ubiquitinated proteins in the cells and whether the UbVs can inhibit this effect. Transfections were performed using pRK5-HA-Ub.WT and different combinations of pcDNA3-V5-IBV-PLpro Beaudette, pcDNA3-V5-IBV-PLpro-Beaudette-C, pcDNA3-V5-IBV-PLpro-M41, pcDNA3-V5-IBV-PLpro-M41-C and pcDNA3-FLAG-UbVs. pcDNA3.1-empty vector was used to normalize the total amount of transfected DNA per well. pcDNA3-GFP was co-transfected as a transfection efficiency control. PEI was used as a transfection method. Briefly, per 2 μg of combined plasmid DNA, 6 μL of PEI was diluted in Opti-MEM medium. The plasmid DNA was also diluted in Opti-MEM. Diluted PEI was then added to the diluted DNA, vortexed and incubated for 20 min. The mixture was then added to the cells (12-well plate, 1 × 10
5 cells/well) in a dropwise manner, and the cells were incubated at 37 °C, 5% CO
2, for 24 h. At 24 hpt, the cells were lysed in 2× Laemmli Sample Buffer (LSB, 250 mM Tris-base (pH 6.8), 4% SDS, 20% glycerol, 10 mM DTT, 0.01% Bromophenol blue) and boiled, before Western blotting. All DUB assays were performed independently at least three times.
4.8. Western Blot Analysis
To visualize the deubiquitination assays, proteins were separated on an 8% SDS-PAGE gel, after which the gel was blotted onto a 0.2 µm Amersham Hybond P PVDF Western blotting membrane (Cytivia/Merck, Marlborough, MA, USA) and blocked in 1× casein (#C5890, Sigma-Aldrich) for 1 h. The membrane was stained overnight with anti-HA antibody to visualize the ubiquitin smears. The membranes were then washed 3 times with PBS containing 0.05% Tween-20 (PBST), followed by incubation for 1 h with the secondary antibody in 0.5× casein. After washing again with PBST, the membranes were incubated in the dark with the tertiary antibody in 0.5× casein for 1 h. The membranes were washed in PBST and PBS and visualized. The membrane was then re-stained with anti-α-Tubulin or anti-β-actin antibody overnight as a loading control. Similar steps were taken for secondary and tertiary antibody incubations.
4.9. Immunofluorescence Assay
DF-1 cells were seeded onto coverslips from sustained culture, transfected with UbVs, infected with IBV Beaudette or IBV M41 for 24 h and subsequently fixed in 3% paraformaldehyde. IFAs were performed as described previously [
15]. Briefly, after proper fixing, the coverslips were washed with PBS containing 10 mM glycine. The coverslips were then incubated with PBS containing 0.1% Triton X-100 for 10 min to permeabilize the cells. After this, the coverslips were washed in PBS before incubation with primary antibody in PBS with 5% FCS for 1 h. After incubation, the coverslips were washed again with PBS before incubation with secondary antibody in PBS/5% FCS for 1 h. The coverslips were mounted onto microscope slides with ProLong Glass antifade mounting fluid (Thermo Fisher Scientific/Invitrogen). Results were assessed using Zeiss Axioskop 2 and the AxioVision software (version 4.7) (Carl Zeiss Microscopy, LLC, White Plains, NY, USA).
4.10. IBV Infection Experiments
To determine the reduction in viral progeny upon UbV expression, DF-1 cells were seeded into 12-well plates and transfected with the different pcDNA3-FLAG-UbV plasmids when the cell confluency was around 70%, before being infected with IBV. Briefly, per 2 μg of plasmid DNA, 6 μL of PEI was diluted in Opti-MEM medium (Lonza). The plasmid DNA was also diluted in Opti-MEM. Diluted PEI was then added to the diluted DNA, vortexed and incubated for 20 min. The mixture was then added to the cells (12-well plate, 1 × 105 cells/well) in a dropwise manner, and the cells were incubated at 37 °C, 5% CO2. After 24 h of transfection, the cells were infected with either IBV Beaudette or IBV M41 at an MOI of 0.5. Supernatants were collected 24 h after infection for viral titration and immunofluorescence microscopy.
To determine IBV Beaudette viral titers, the supernatants were subjected to plaque assay on DF-1 cells. Briefly, 10−1 to 10−6 dilutions of supernatants were made in PBS/DEAE+2%FCS and added to the appropriate wells of 12-well plates. The plates were then incubated like this for an hour while shaking at 37 °C. Afterwards, the inoculum was removed and replaced with Avicel-containing semi-solid overlay, and the plates were incubated for 2–3 days at 37 °C. After 2–3 days, the cells were fixed with 4% formaldehyde and plaques visualized with crystal violet staining. Corresponding graphs were created using Graphpad Prism. Experiments were performed independently 3 times, and plaque assays were performed in duplicate for each sample with similar results. Work using live virus was performed inside biosafety cabinets at Biosafety Level 2 laboratories at either the Roslin Institute, University of Edinburgh, Scotland, or at the Leiden University Medical Center, The Netherlands.
4.11. Expression and Purification of Proteins
E. coli BL21 (DE3) cells were transformed with the pETM11 vectors mentioned above and grown in Kanamycin-supplemented LB media at 37 °C. Then, 1 mM of IPTG was added when OD600 reached 0.5, and cells were further incubated in a shaker at 18 °C for 16 h. Overnight culture was collected by centrifugation, and the cell pellet was lysed by Emulsiflex with lysis buffer (50 mM Tris-HCl pH 7.5, 200 mM NaCl, 10 mM β-mercaptoethanol). Cell lysates were centrifuged to remove cell debris, and the supernatant was mixed with Ni-NTA resin at 4 °C for 1 h, and then Ni-NTA slurry was loaded onto the open column. Resin was washed with the lysis buffer supplemented with 30 mM Imidazole. Proteins were eluted by buffer (50 mM Tris-HCl pH 7.5, 200 mM NaCl, 250 mM Imidazole, 13.3 mM β-mercaptoethanol). For PLpro, the eluate was mixed with TEV protease to cleave off the His6 tag and incubated at 4 °C for 16 h. After cleavage, the sample was loaded on a Ni-NTA column to capture TEV protease and the remaining His6 tag. Flowthrough was further purified by size exclusion chromatography (Superdex 75) with buffer (50 mM Tris-HCl pH 7.5, 50 mM NaCl, 1 mM DTT). Fractions with corresponding retention volumes were pooled together for biochemical assays. For UbV purification, the eluate from Ni-NTA column purification was directly purified by size exclusion chromatography (Superdex 75) without His-tag cleavage.
4.12. In Vitro DUB Activity Assay
PLpro enzymes were diluted in the assay buffer (20 mM Tris-HCl pH 8.0, 150 mM NaCl, 2 mM DTT, 0.05% BSA). DUB kinetics were measured for 1 nM PLpro with a range of Ub-AMC substrate concentration (0 to 64 µM). With 10 µL reaction volume in a 384 well plate, free AMC fluorescence was quantified by a plate reader (SpectraMax iD5, Molecular Devices, San Jose, CA, USA) with an excitation wavelength of 380 nm and an emission wavelength of 460 nm. The concentration of free AMC was estimated by the standard curve of AMC standards. Initial velocity of enzyme activity was estimated by the regression to the exponential equation Y = A (1 − exp(−B * t)) + C. Initial velocities of different substrate concentrations were plotted to estimate kcat and KM for the Michaelis–Menten kinetics (V = Vmax * [S]/(KM + [S]), where [S] is the initial substrate concentration). To determine the IC50 of UbVs, an assay was performed with 1 nM of PLpro and 500 nM of Ub-AMC. Using a range of concentrations (0 to 1.28 µM), UbVs were mixed with PLpro before the addition of Ub-AMC and incubated at 20 °C for 15 min. The enzyme reaction was started by adding Ub-AMC, and the fluorescence of free AMC was measured by SpectraMax iD5 with the same parameters as above.
4.13. Model Visualization
The X-ray crystal structure of IBV Beaudette PLpro and wildtype Ub complex (PDB: 5BZ0) was visualized by pymol2 (The PyMOL Molecular Graphics System, Version 2.5.4 Schrödinger, LLC, New York, NY, USA).