Oral Treatment with EGCG, Folic Acid, Vitamin B12, and Hyaluronic Acid Improves HPV Clearance and Counteracts Its Persistence: A Clinical Study
Abstract
1. Introduction
2. Results
2.1. Clearance of HPV After 6 Months of Treatment
2.2. Resolution of HPV-Induced Cervical Lesions After 6 Months of Treatment
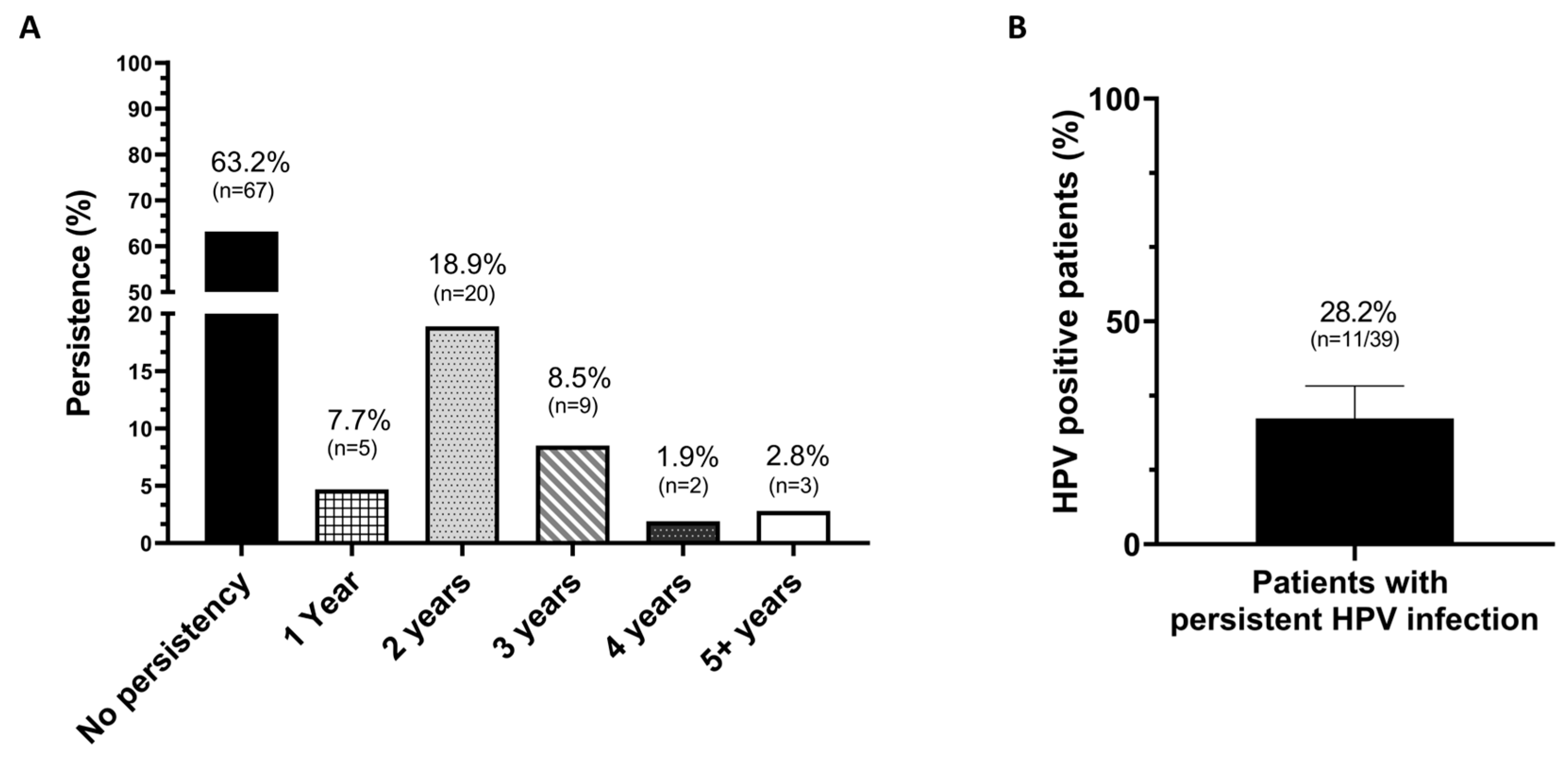
2.3. HPV Persistence After 6 Months of Treatment
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Treatment Regimen
4.3. Cervical Cytology
4.4. HPV DNA Test
4.5. Statistical Analysis
4.6. Safety Evaluation of EGCG: Insights from EFSA Scientific Opinion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers 2016, 2, 16086. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk-Bonikowska, B.; Rudnicka, L. HPV Infections-Classification, Pathogenesis, and Potential New Therapies. Int. J. Mol. Sci. 2024, 25, 7616. [Google Scholar] [CrossRef]
- Shanmugasundaram, S.; You, J. Targeting Persistent Human Papillomavirus Infection. Viruses 2017, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Szymonowicz, K.A.; Chen, J. Biological and clinical aspects of HPV-related cancers. Cancer Biol. Med. 2020, 17, 864–878. [Google Scholar] [CrossRef]
- Koshiol, J.; Lindsay, L.; Pimenta, J.M.; Poole, C.; Jenkins, D.; Smith, J.S. Persistent human papillomavirus infection and cervical neoplasia: A systematic review and meta-analysis. Am. J. Epidemiol. 2008, 168, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, G.; Herzum, A.; Pastorino, A.; Dezzana, M.; Casazza, S.; Mavilia, M.G.; Copello, F.; Parodi, A.; Drago, F. Prevalence of genital HPV infection in STI and healthy populations and risk factors for viral persistence. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 885–888. [Google Scholar] [CrossRef]
- de Sanjosé, S.; Brotons, M.; Pavón, M.A. The natural history of human papillomavirus infection. Best. Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Travé, G.; Zanier, K. HPV-mediated inactivation of tumor suppressor p53. Cell Cycle 2016, 15, 2231–2232. [Google Scholar] [CrossRef]
- Friedman, M.J.; Lee, H.; Kwon, Y.C.; Oh, S. Dynamics of Viral and Host 3D Genome Structure upon Infection. J. Microbiol. Biotechnol. 2022, 32, 1515–1526. [Google Scholar] [CrossRef]
- Yap, J.K.W.; Kehoe, S.T.; Woodman, C.B.J.; Dawson, C.W. The Major Constituent of Green Tea, Epigallocatechin-3-Gallate (EGCG), Inhibits the Growth of HPV18-Infected Keratinocytes by Stimulating Proteasomal Turnover of the E6 and E7 Oncoproteins. Pathogens 2021, 10, 459. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Lu, J.L.; Liang, Y.R.; Li, Q.S. Suppressive Effects of EGCG on Cervical Cancer. Molecules 2018, 23, 2334. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Han, J.H.; Song, Y.; Lee, J.H.; Choi, S.Y.; Park, Y.M. Epigallocatechin-3-gallate Can Prevent Type 2 Human Papillomavirus E7 from Suppressing Interferon-Stimulated Genes. Int. J. Mol. Sci. 2021, 22, 2418. [Google Scholar] [CrossRef] [PubMed]
- Piyathilake, C.J.; Henao, O.L.; Macaluso, M.; Cornwell, P.E.; Meleth, S.; Heimburger, D.C.; Partridge, E.E. Folate is associated with the natural history of high-risk human papillomaviruses. Cancer Res. 2004, 64, 8788–8793. [Google Scholar] [CrossRef]
- Piyathilake, C.J.; Badiga, S.; Paul, P.; Vijayaraghavan, K.; Vedantham, H.; Sudula, M.; Sowjanya, P.; Ramakrishna, G.; Shah, K.V.; Partridge, E.E.; et al. Indian women with higher serum concentrations of folate and vitamin B12 are significantly less likely to be infected with carcinogenic or high-risk (HR) types of human papillomaviruses (HPVs). Int. J. Womens Health 2010, 2, 7–12. [Google Scholar] [CrossRef]
- Jin, S.; Lin, F.; Yang, L.; Zhang, Q. Association between dietary folate intake and HPV infection: NHANES 2005-2016. PLoS ONE 2024, 19, e0306636. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Ding, L.; Meng, D.; Liu, H.; Wang, J.; Song, L.; Lyu, Y.J.; Jia, H.X.; Hao, M.; Tian, Z.Q.; et al. [Relationship between serum folate and CIN1 prognosis and its interaction with HR-HPV infection]. Zhonghua Zhong Liu Za Zhi 2021, 43, 866–871. [Google Scholar] [CrossRef]
- Petca, A.; Borislavschi, A.; Zvanca, M.E.; Petca, R.C.; Sandru, F.; Dumitrascu, M.C. Non-sexual HPV transmission and role of vaccination for a better future (Review). Exp. Ther. Med. 2020, 20, 186. [Google Scholar] [CrossRef]
- Woodman, C.B.; Collins, S.I.; Young, L.S. The natural history of cervical HPV infection: Unresolved issues. Nat. Rev. Cancer 2007, 7, 11–22. [Google Scholar] [CrossRef]
- Nyman, E.; Henricson, J.; Ghafouri, B.; Anderson, C.D.; Kratz, G. Hyaluronic Acid Accelerates Re-epithelialization and Alters Protein Expression in a Human Wound Model. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2221. [Google Scholar] [CrossRef]
- Waggett, S.; Lyles, E.; Schlesinger, T. Update on Low-Molecular Weight Hyaluronic Acid in Dermatology: A Scoping Review. EMJ Dermatol. 2024, 134–146. [Google Scholar] [CrossRef]
- Yang, H.; Song, L.; Zou, Y.; Sun, D.; Wang, L.; Yu, Z.; Guo, J. Role of Hyaluronic Acids and Potential as Regenerative Biomaterials in Wound Healing. ACS Appl. Bio Mater. 2021, 4, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Chiantera, V.; Gerli, S.; Proietti, S.; Lepore, E.; Unfer, V.; Carugno, J.; Favilli, A. Preventing Persistence of HPV Infection with Natural Molecules. Pathogens 2023, 12, 416. [Google Scholar] [CrossRef]
- Frega, A.; Gentili, C.; Proietti, S.; Lepore, E.; Unfer, V.; Fuso, A. Epigallocatechin gallate, folic acid, vitamin B12, and hyaluronic acid significantly increase apoptosis and p53 expression in HeLa cells. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5240–5245. [Google Scholar] [CrossRef]
- Aragona, C.; Bezerra Espinola, M.S.; Bilotta, G.; Porcaro, G.; Calcagno, M. Evaluating the Efficacy of Pervistop((R)), a New Combination Based on EGCG, Folic Acid, Vitamin B12 and Hyaluronic Acid on Patients with Human Papilloma Virus (HPV) Persistent Infections and Cervical Lesions: A Pilot Study. J. Clin. Med. 2023, 12, 2171. [Google Scholar] [CrossRef]
- Tinelli, A.; Gustapane, S.; Licchelli, M.; Coluccia, A.C.; Panese, G.; Proietti, S.; Gambioli, R. Treatment with Epigallocatechin Gallate, Folic Acid, Vitamin B12, and Hyaluronic Acid Decreases HPV Positivity in Women Attending Regional Screening in Puglia. Microorganisms 2024, 12, 1897. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, M.; Incocciati, B.; Di Fraia, L.; Unfer, V. Counteracting HPV Cervical and Anal Infection through Dietary Supplementation of EGCG, Folic Acid, Vitamin B12 and Hyaluronic Acid: Clinical Case Reports. J. Clin. Med. 2024, 13, 3597. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Liu, H.; Feugang, J.M.; Hao, Z.; Chow, H.H.; Garcia, F. Green tea compound in chemoprevention of cervical cancer. Int. J. Gynecol. Cancer 2010, 20, 617–624. [Google Scholar] [CrossRef]
- Kciuk, M.; Alam, M.; Ali, N.; Rashid, S.; Glowacka, P.; Sundaraj, R.; Celik, I.; Yahya, E.B.; Dubey, A.; Zerroug, E.; et al. Epigallocatechin-3-Gallate Therapeutic Potential in Cancer: Mechanism of Action and Clinical Implications. Molecules 2023, 28, 5246. [Google Scholar] [CrossRef]
- Xiao, S.; Tang, Y.S.; Kusumanchi, P.; Stabler, S.P.; Zhang, Y.; Antony, A.C. Folate Deficiency Facilitates Genomic Integration of Human Papillomavirus Type 16 DNA In Vivo in a Novel Mouse Model for Rapid Oncogenic Transformation of Human Keratinocytes. J. Nutr. 2018, 148, 389–400. [Google Scholar] [CrossRef]
- Gao, F.; Yang, C.X.; Mo, W.; Liu, Y.W.; He, Y.Q. Hyaluronan oligosaccharides are potential stimulators to angiogenesis via RHAMM mediated signal pathway in wound healing. Clin. Investig. Med. 2008, 31, E106–E116. [Google Scholar] [CrossRef]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract. Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef]
- Aggarwal, S.; Agarwal, P.; Gupta, N. A comprehensive narrative review of challenges and facilitators in the implementation of various HPV vaccination program worldwide. Cancer Med. 2024, 13, e6862. [Google Scholar] [CrossRef] [PubMed]
- Skinner, S.R.; Wheeler, C.M.; Romanowski, B.; Castellsague, X.; Lazcano-Ponce, E.; Del Rosario-Raymundo, M.R.; Vallejos, C.; Minkina, G.; Pereira Da Silva, D.; McNeil, S.; et al. Progression of HPV infection to detectable cervical lesions or clearance in adult women: Analysis of the control arm of the VIVIANE study. Int. J. Cancer 2016, 138, 2428–2438. [Google Scholar] [CrossRef]
- Grandi, G.; Botticelli, L.; Fraia, P.D.; Babalini, C.; Masini, M.; Unfer, V. The Association of Four Natural Molecules-EGCG, Folic Acid, Vitamin B12, and HA-To Counteract HPV Cervical Lesions: A Case Report. J. Pers. Med. 2023, 13, 567. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.L.; Villa, L.L.; Sobrinho, J.P.; Prado, J.M.; Rousseau, M.C.; Desy, M.; Rohan, T.E. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J. Infect. Dis. 1999, 180, 1415–1423. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Harris, R.; Sedjo, R.L.; Baldwin, S.; Roe, D.; Papenfuss, M.R.; Abrahamsen, M.; Inserra, P.; Olvera, S.; Hatch, K. Incidence, prevalence, and clearance of type-specific human papillomavirus infections: The Young Women’s Health Study. J. Infect. Dis. 2002, 186, 462–469. [Google Scholar] [CrossRef]
- Brown, D.R.; Shew, M.L.; Qadadri, B.; Neptune, N.; Vargas, M.; Tu, W.; Juliar, B.E.; Breen, T.E.; Fortenberry, J.D. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J. Infect. Dis. 2005, 191, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Moscicki, A.B.; Shiboski, S.; Broering, J.; Powell, K.; Clayton, L.; Jay, N.; Darragh, T.M.; Brescia, R.; Kanowitz, S.; Miller, S.B.; et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J. Pediatr. 1998, 132, 277–284. [Google Scholar] [CrossRef]
- Guo, P.; Wu, L.; Wang, H.; Wang, L.; Li, H.; Wang, H.; Wang, Y.; Shao, S.; Chen, S. The Relationship between Systemic Expression Levels of Immune Cells and Tumor Markers and High-Risk HPV Infection in Patients with Cervical Cancer, Cervical Intraepithelial Neoplasia, and Chronic Cervicitis, and its Clinical Significance. Int. J. Womens Health 2025, 17, 1263–1270. [Google Scholar] [CrossRef]
- Solomon, D.; Davey, D.; Kurman, R.; Moriarty, A.; O’Connor, D.; Prey, M.; Raab, S.; Sherman, M.; Wilbur, D.; Wright, T., Jr.; et al. The 2001 Bethesda System: Terminology for reporting results of cervical cytology. Jama 2002, 287, 2114–2119. [Google Scholar] [CrossRef]
- Ejegod, D.M.; Junge, J.; Franzmann, M.; Kirschner, B.; Bottari, F.; Sideri, M.; Sandri, M.T.; Bonde, J. Clinical and analytical performance of the BD Onclarity™ HPV assay for detection of CIN2+ lesions on SurePath samples. Papillomavirus Res. 2016, 2, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, M.; Giubbi, C.; Sechi, I.; Bottari, F.; Iacobone, A.D.; Musumeci, R.; Perdoni, F.; Muresu, N.; Piana, A.; Fruscio, R.; et al. Evaluation of BD Onclarity™ HPV Assay on Self-Collected Vaginal and First-Void Urine Samples as Compared to Clinician-Collected Cervical Samples: A Pilot Study. Diagnostics 2022, 12, 3075. [Google Scholar] [CrossRef] [PubMed]
- Enders, C.; Lang, G.E.; Lang, G.K.; Werner, J.U. [ISO 9001:2015 Certification in Quality Management]. Klin. Monbl Augenheilkd. 2017, 234, 886–890. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipic, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Treatment Group |
|---|---|
| Number of patients (n) | 106 |
| Anatomical sites | Cervical |
| Age (years) | 44.5 ± 8.9 |
| Viral persistence | |
| 39 |
| 5 |
| 20 |
| 9 |
| 2 |
| 3 |
| HPV Genotype | |
| 106 |
| 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 |
| Cervical Lesions | |
| 39 |
| 46 |
| 67 |
| Cytology | |
| 36 |
| 5 |
| 5 |
| 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcaro, G.; Pavone-Cossut, M.R.; Moretti, S.; Bilotta, G.; Aragona, C.; Unfer, V. Oral Treatment with EGCG, Folic Acid, Vitamin B12, and Hyaluronic Acid Improves HPV Clearance and Counteracts Its Persistence: A Clinical Study. Int. J. Mol. Sci. 2025, 26, 5251. https://doi.org/10.3390/ijms26115251
Porcaro G, Pavone-Cossut MR, Moretti S, Bilotta G, Aragona C, Unfer V. Oral Treatment with EGCG, Folic Acid, Vitamin B12, and Hyaluronic Acid Improves HPV Clearance and Counteracts Its Persistence: A Clinical Study. International Journal of Molecular Sciences. 2025; 26(11):5251. https://doi.org/10.3390/ijms26115251
Chicago/Turabian StylePorcaro, Giuseppina, Maria Rosaria Pavone-Cossut, Sonia Moretti, Gabriele Bilotta, Cesare Aragona, and Vittorio Unfer. 2025. "Oral Treatment with EGCG, Folic Acid, Vitamin B12, and Hyaluronic Acid Improves HPV Clearance and Counteracts Its Persistence: A Clinical Study" International Journal of Molecular Sciences 26, no. 11: 5251. https://doi.org/10.3390/ijms26115251
APA StylePorcaro, G., Pavone-Cossut, M. R., Moretti, S., Bilotta, G., Aragona, C., & Unfer, V. (2025). Oral Treatment with EGCG, Folic Acid, Vitamin B12, and Hyaluronic Acid Improves HPV Clearance and Counteracts Its Persistence: A Clinical Study. International Journal of Molecular Sciences, 26(11), 5251. https://doi.org/10.3390/ijms26115251







