Targeting of Epithelial Cell Adhesion Molecule-Expressing Malignant Tumors Using an Albumin-Binding Domain-Fused Designed Ankyrin Repeat Protein: Effect of the Molecular Architecture
Abstract
1. Introduction
2. Results and Discussion
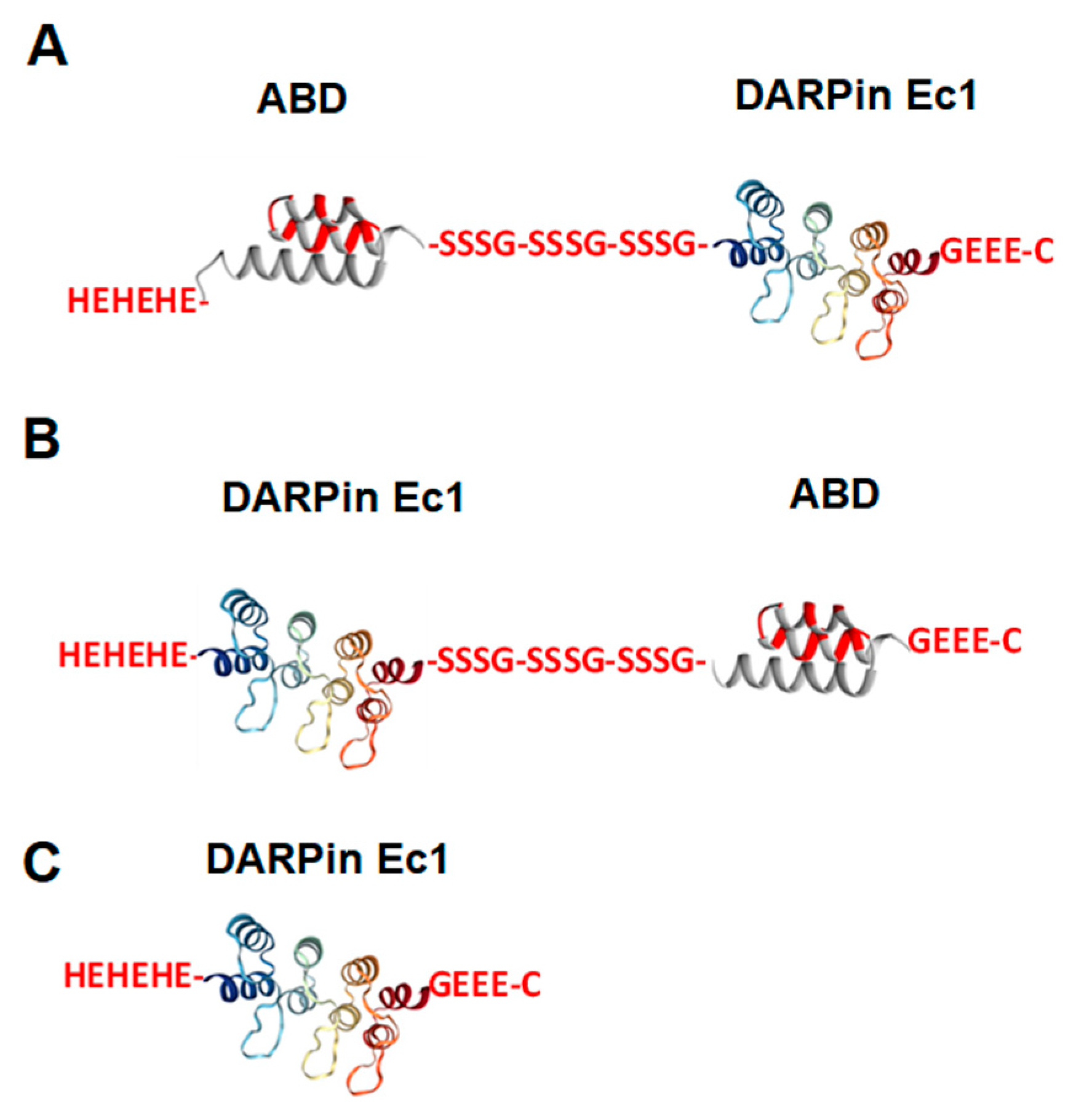
2.1. Production, Purification, and Characterization of ABD-Fused DARPin Ec1 Variants and Ec1 and Conjugation of DOTA Chelator
2.2. Radiolabeling of Ec1 Variants with 111In and In Vitro Stability
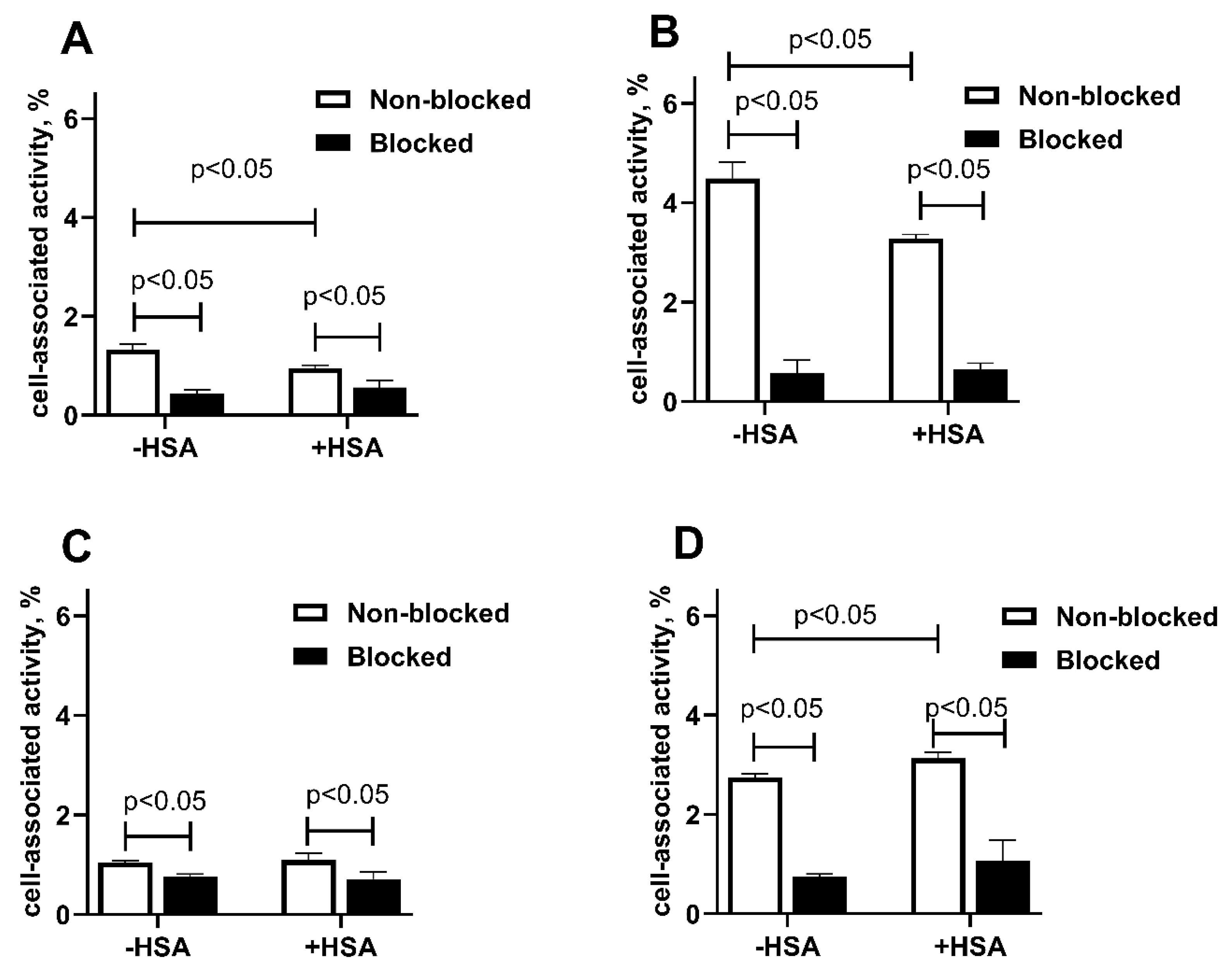
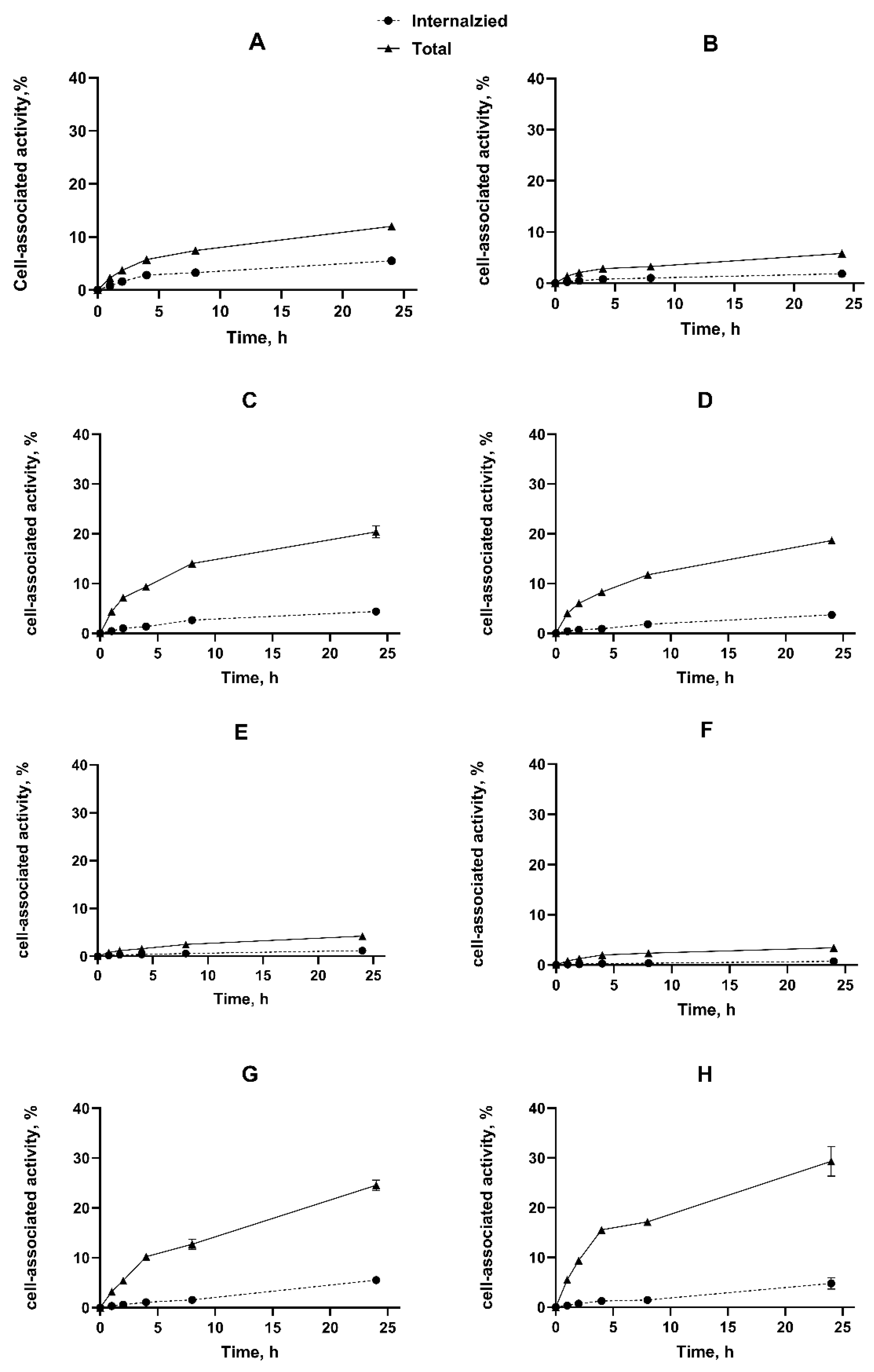
2.3. In Vitro Studies
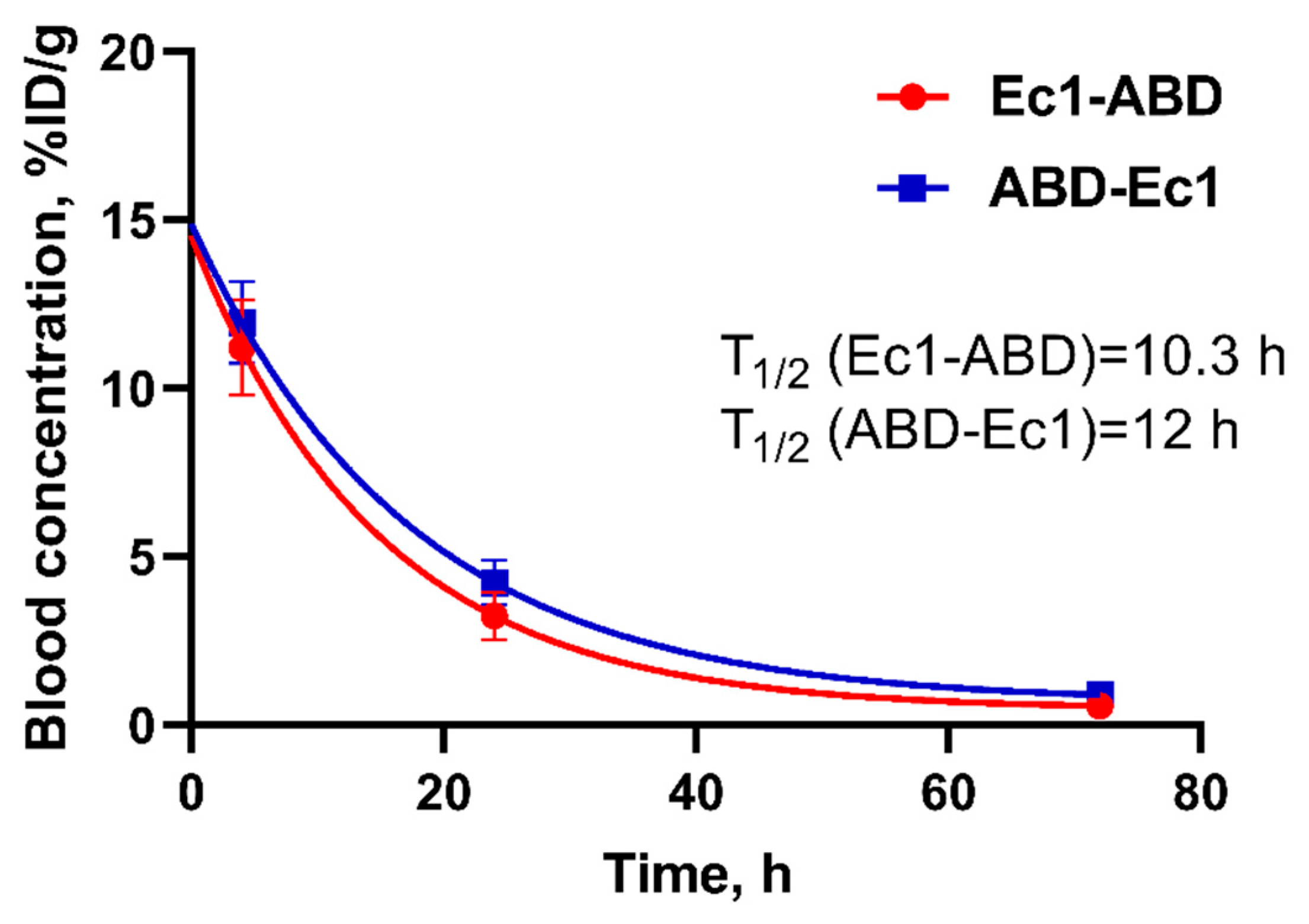
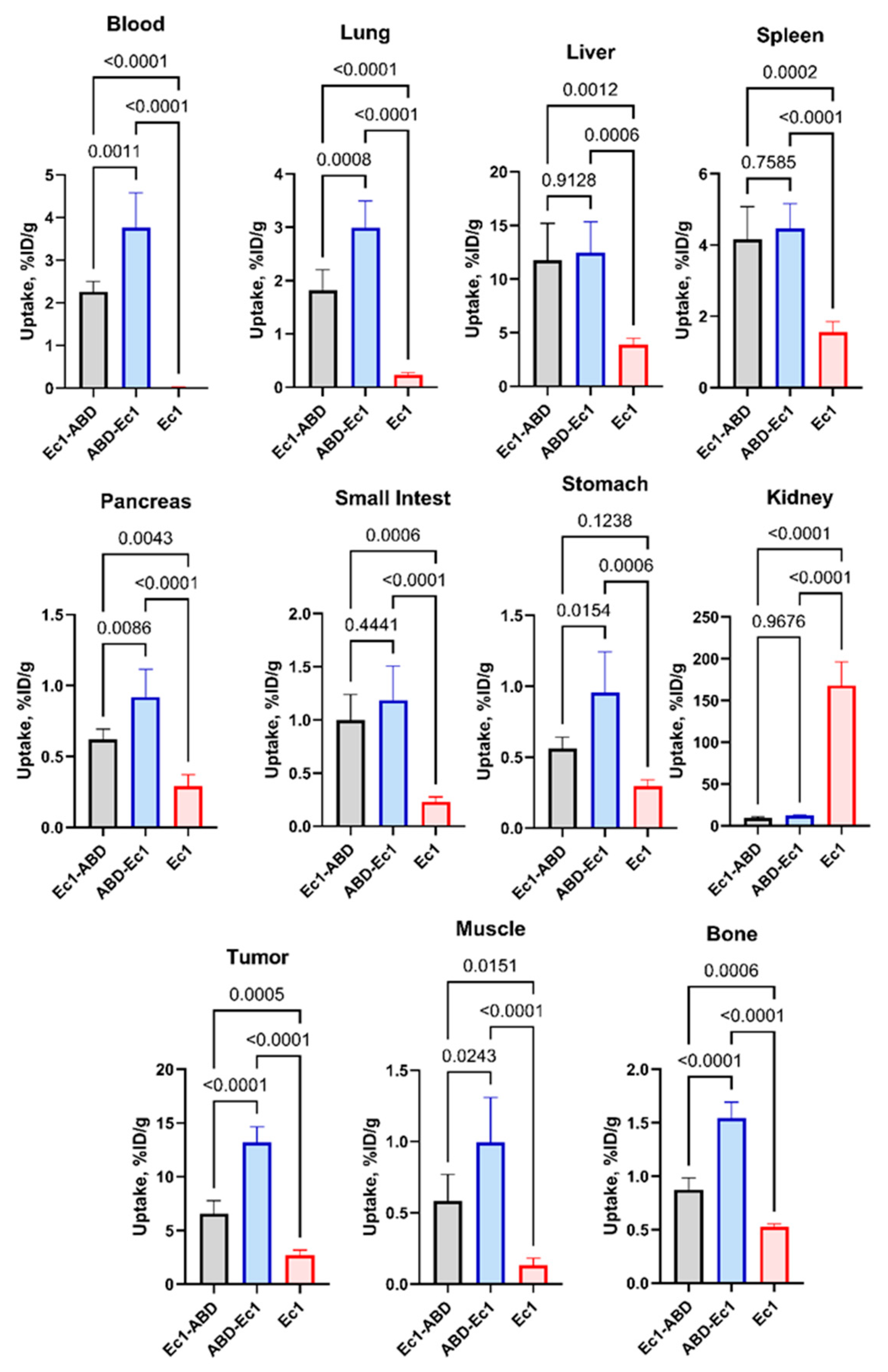
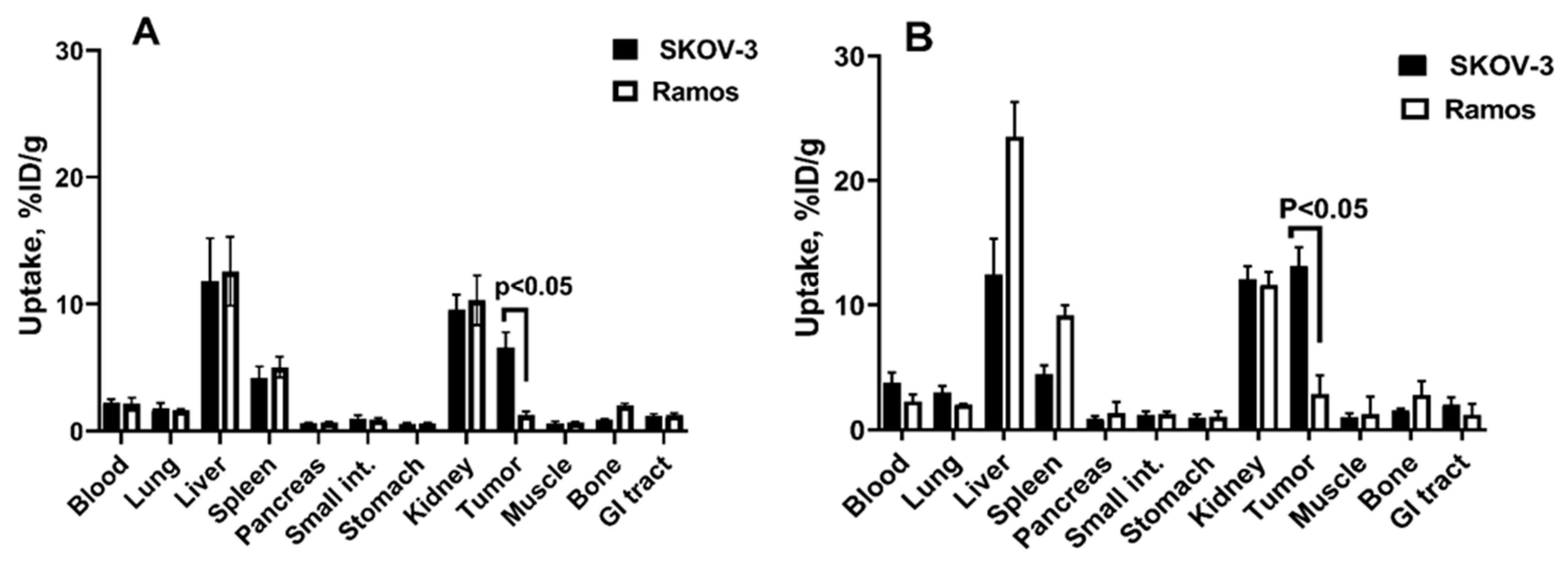
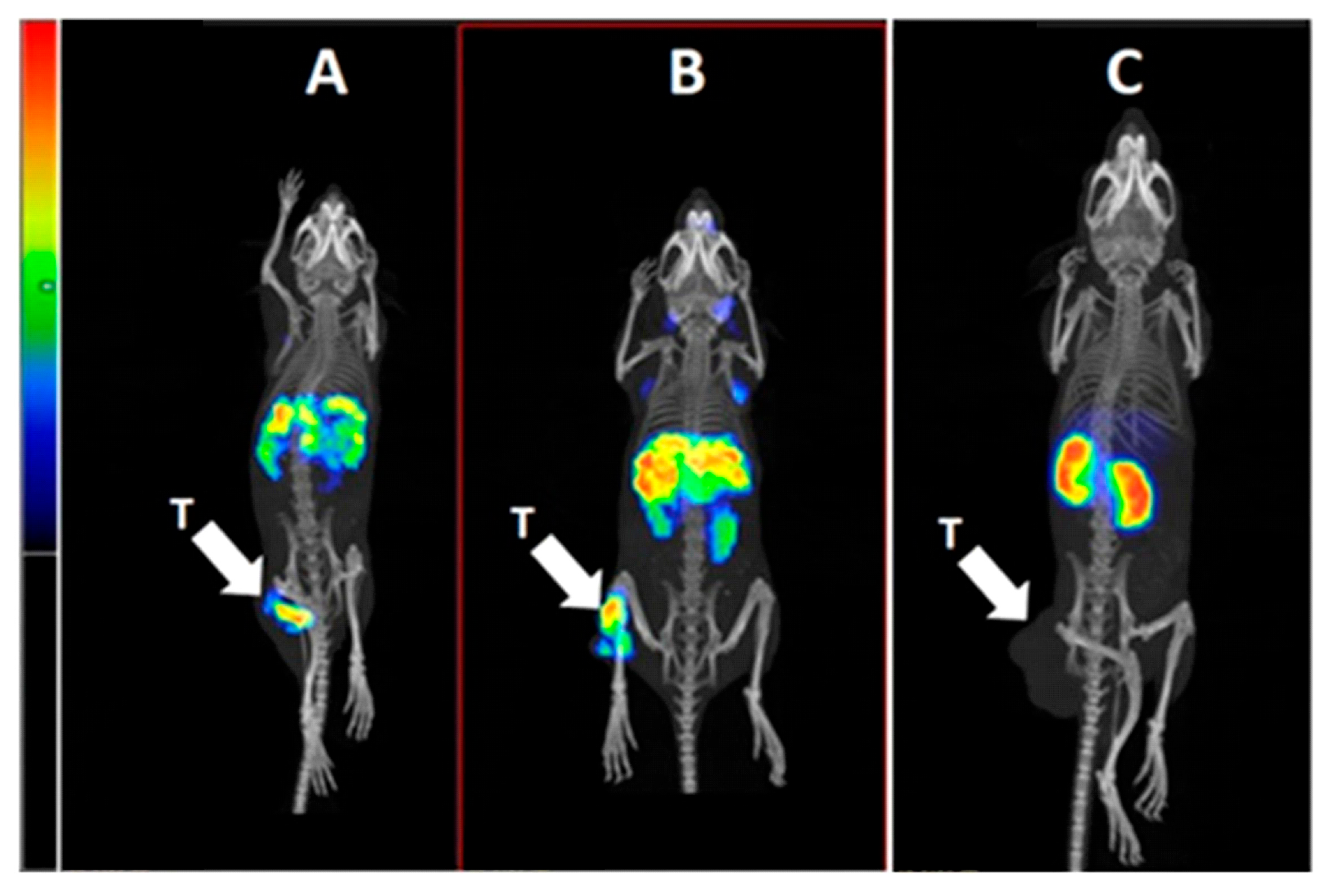
2.4. In Vivo Studies
3. Conclusions
4. Materials and Methods
4.1. General
4.2. Statistical Analysis
4.3. Production, Purification, and Characterization of ABD-Fused DARPin Ec1 Variants and Ec1 and Conjugation of DOTA Chelator
4.4. Radiolabeling of DARPin Ec1 with 111In and In Vitro Stability
4.5. In Vitro Studies
4.6. In Vivo Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
References
- Dumontet, C.; Reichert, J.M.; Senter, P.D.; Lambert, J.M.; Beck, A. Antibody-drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 2023, 22, 641–661. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Tarantino, P.; Rich, J.R.; LoRusso, P.M.; de Vries, E.G.E. The Journey of Antibody-Drug Conjugates: Lessons Learned from 40 Years of Development. Cancer Discov. 2024, 14, 2089–2108. [Google Scholar] [CrossRef]
- Gebauer, M.; Skerra, A. Engineered Protein Scaffolds as Next-Generation Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 391–415. [Google Scholar] [CrossRef]
- Vazquez-Lombardi, R.; Phan, T.G.; Zimmermann, C.; Lowe, D.; Jermutus, L.; Christ, D. Challenges and opportunities for non-antibody scaffold drugs. Drug Discov. Today 2015, 20, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Owens, B. Faster, deeper, smaller-the rise of antibody-like scaffolds. Nat. Biotechnol. 2017, 35, 602–603. [Google Scholar] [CrossRef]
- Ståhl, S.; Gräslund, T.; Eriksson Karlström, A.; Frejd, F.Y.; Nygren, P.Å.; Löfblom, J. Affibody Molecules in Biotechnological and Medical Applications. Trends Biotechnol. 2017, 35, 691–712. [Google Scholar] [CrossRef] [PubMed]
- Binz, H.K.; Stumpp, M.T.; Forrer, P.; Amstutz, P.; Plückthun, A. Designing repeat proteins: Well-expressed, soluble and stable proteins from combinatorial libraries of consensus ankyrin repeat proteins. J. Mol. Biol. 2003, 332, 489–503. [Google Scholar] [CrossRef]
- Boersma, Y.L. Advances in the Application of Designed Ankyrin Repeat Proteins (DARPins) as Research Tools and Protein Therapeutics. Methods Mol. Biol. 2018, 1798, 307–327. [Google Scholar] [CrossRef]
- Binz, H.K.; Bakker, T.R.; Phillips, D.J.; Cornelius, A.; Zitt, C.; Göttler, T.; Sigrist, G.; Fiedler, U.; Ekawardhani, S.I.; Saliba, J.A.; et al. Design and characterization of MP0250, a tri-specific anti-HGF/anti-VEGF DARPin® drug candidate. MAbs 2017, 8, 1262–1269. [Google Scholar] [CrossRef]
- Stefan, N.; Martin-Killias, P.; Wyss-Stoeckle, S.; Honegger, A.; Zangemeister-Wittke, U.; Plückthun, A. DARPins recognizing the tumor-associated antigen EpCAM selected by phage and ribosome display and engineered for multivalency. J. Mol. Biol. 2011, 413, 826–843. [Google Scholar] [CrossRef]
- Zahnd, C.; Pecorari, F.; Straumann, N.; Wyler, E.; Plückthun, A. Selection and characterization of Her2 binding-designed ankyrin repeat proteins. J. Biol. Chem. 2006, 281, 35167–35175. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Forrer, P.; Plückthun, A. Efficient selection of DARPins with sub-nanomolar affinities using SRP phage display. J. Mol. Biol. 2008, 382, 1211–1227. [Google Scholar] [CrossRef] [PubMed]
- Bragina, O.; Chernov, V.; Schulga, A.; Konovalova, E.; Garbukov, E.; Vorobyeva, A.; Orlova, A.; Tashireva, L.; Sörensen, J.; Zelchan, R.; et al. Phase I Trial of 99mTc-(HE)3-G3, a DARPin-Based Probe for Imaging of HER2 Expression in Breast Cancer. J. Nucl. Med. 2022, 4, 528–535. [Google Scholar] [CrossRef]
- Zelchan, R.; Chernov, V.; Medvedeva, A.; Rybina, A.; Bragina, O.; Mishina, E.; Larkina, M.; Varvashenya, R.; Fominykh, A.; Schulga, A.; et al. Phase I Clinical Evaluation of Designed Ankyrin Repeat Protein [99mTc]Tc(CO)3-(HE)3-Ec1 for Visualization of EpCAM-Expressing Lung Cancer. Cancers 2024, 16, 2815. [Google Scholar] [CrossRef]
- Maetzel, D.; Denzel, S.; Mack, B.; Canis, M.; Went, P.; Benk, M.; Kieu, C.; Papior, P.; Baeuerle, P.A.; Munz, M.; et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat. Cell Biol. 2009, 2, 162–171. [Google Scholar] [CrossRef]
- Armstrong, A.; Eck, S.L. EpCAM: A new therapeutic target for an old cancer antigen. Cancer Biol. Ther. 2003, 4, 320–326. [Google Scholar] [CrossRef]
- Patriarca, C.; Macchi, R.M.; Marschner, A.K.; Mellstedt, H. Epithelial cell adhesion molecule expression (CD326) in cancer: A short review. Cancer Treat. Rev. 2012, 38, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Amoury, M.; Kolberg, K.; Pham, A.T.; Hristodorov, D.; Mladenov, R.; Di Fiore, S.; Helfrich, W.; Kiessling, F.; Fischer, R.; Pardo, A.; et al. Granzyme B-based cytolytic fusion protein targeting EpCAM specifically kills triple negative breast cancer cells in vitro and inhibits tumor growth in a subcutaneous mouse tumor model. Cancer Lett. 2016, 372, 201–209. [Google Scholar] [CrossRef]
- Jenkins, S.V.; Nima, Z.A.; Vang, K.B.; Kannarpady, G.; Nedosekin, D.A.; Zharov, V.P.; Griffin, R.J.; Biris, A.S.; Dings, R.P.M. Triple-negative breast cancer targeting and killing by EpCAM-directed plasmonically active nanodrug systems. NPJ Precis. Oncol. 2017, 1, 27. [Google Scholar] [CrossRef]
- Andersson, Y.; Inderberg, E.M.; Kvalheim, G.; Herud, T.M.; Engebraaten, O.; Flatmark, K.; Dueland, S.; Fodstad, Ø. Immune stimulatory effect of anti-EpCAM immunotoxin-improved overall survival of metastatic colorectal cancer patients. Acta Oncol. 2020, 59, 404–409. [Google Scholar] [CrossRef]
- Chaudry, M.A.; Sales, K.; Ruf, P.; Lindhofer, H.; Winslet, M.C. EpCAM an immunotherapeutic target for gastrointestinal malignancy: Current experience and future challenges. Br. J. Cancer. 2007, 96, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Tolcher, A.W.; Forero, A.; Vanhove, G.F.; Takimoto, C.; Bauer, R.J.; Hammond, L.A.; Patnaik, A.; White, M.L.; Shen, S.; et al. ING-1, a monoclonal antibody targeting Ep-CAM in patients with advanced adenocarcinomas. Clin. Cancer Res. 2004, 10, 7555–7565. [Google Scholar] [CrossRef] [PubMed]
- Deyev, S.M.; Vorobyeva, A.; Schulga, A.; Abouzayed, A.; Günther, T.; Garousi, J.; Konovalova, E.; Ding, H.; Gräslund, T.; Orlova, A.; et al. Effect of a radiolabel biochemical nature on tumor-targeting properties of EpCAM-binding engineered scaffold protein DARPin Ec1. Int. J. Biol. Macromol. 2020, 145, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Deyev, S.M.; Xu, T.; Liu, Y.; Schulga, A.; Konovalova, E.; Garousi, J.; Rinne, S.S.; Larkina, M.; Ding, H.; Gräslund, T.; et al. Influence of the Position and Composition of Radiometals and Radioiodine Labels on Imaging of Epcam Expression in Prostate Cancer Model Using the DARPin Ec1. Cancers 2021, 13, 3589. [Google Scholar] [CrossRef]
- Vorobyeva, A.; Konovalova, E.; Xu, T.; Schulga, A.; Altai, M.; Garousi, J.; Rinne, S.S.; Orlova, A.; Tolmachev, V.; Deyev, S. Feasibility of Imaging EpCAM Expression in Ovarian Cancer Using Radiolabeled DARPin Ec1. Int. J. Mol. Sci. 2020, 21, 3310. [Google Scholar] [CrossRef]
- Vorobyeva, A.; Bezverkhniaia, E.; Konovalova, E.; Schulga, A.; Garousi, J.; Vorontsova, O.; Abouzayed, A.; Orlova, A.; Deyev, S.; Tolmachev, V. Radionuclide Molecular Imaging of EpCAM Expression in Triple-Negative Breast Cancer Using the Scaffold Protein DARPin Ec1. Molecules 2020, 25, 4719. [Google Scholar] [CrossRef]
- Kontermann, R.E. Strategies for extended serum half-life of protein therapeutics. Curr. Opin. Biotechnol. 2011, 22, 868–876. [Google Scholar] [CrossRef]
- Zahnd, C.; Kawe, M.; Stumpp, M.T.; de Pasquale, C.; Tamaskovic, R.; Nagy-Davidescu, G.; Dreier, B.; Schibli, R.; Binz, H.K.; Waibel, R.; et al. Efficient tumor targeting with high-affinity designed ankyrin repeat proteins: Effects of affinity and molecular size. Cancer Res. 2010, 70, 1595–1605. [Google Scholar] [CrossRef]
- Brandl, F.; Merten, H.; Zimmermann, M.; Béhé, M.; Zangemeister-Wittke, U.; Plückthun, A. Influence of size and charge of unstructured polypeptides on pharmacokinetics and biodistribution of targeted fusion proteins. J. Control. Release 2019, 307, 379–392. [Google Scholar] [CrossRef]
- Steiner, D.; Merz, F.W.; Sonderegger, I.; Gulotti-Georgieva, M.; Villemagne, D.; Phillips, D.J.; Forrer, P.; Stumpp, M.T.; Zitt, C.; Binz, H.K. Half-life extension using serum albumin-binding DARPin® domains. Protein Eng. Des. Sel. 2017, 30, 583–591. [Google Scholar] [CrossRef]
- Deyev, S.M.; Oroujeni, M.; Garousi, J.; Gräslund, T.; Li, R.; Rosly, A.H.B.; Orlova, A.; Konovalova, E.; Schulga, A.; Vorobyeva, A.; et al. Preclinical Evaluation of HER2-Targeting DARPin G3: Impact of Albumin-Binding Domain (ABD) Fusion. Int. J. Mol. Sci. 2024, 25, 4246. [Google Scholar] [CrossRef]
- Zhang, J.; Bodenko, V.; Larkina, M.; Bezverkhniaia, E.; Xu, T.; Liao, Y.; Abouzayed, A.; Plotnikov, E.; Tretyakova, M.; Yuldasheva, F.; et al. Half-life extension via ABD-fusion leads to higher tumor uptake of an affibody-drug conjugate compared to PAS- and XTENylation. J. Control. Release 2024, 370, 468–478. [Google Scholar] [CrossRef]
- Hartimath, S.V.; Alizadeh, E.; Solomon, V.R.; Chekol, R.; Bernhard, W.; Hill, W.; Parada, A.C.; Barreto, K.; Geyer, C.R.; Fonge, H. Preclinical Evaluation of 111In-Labeled PEGylated Maytansine Nimotuzumab Drug Conjugates in EGFR-Positive Cancer Models. J. Nucl. Med. 2019, 60, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Chomet, M.; Schreurs, M.; Nguyen, M.; Howng, B.; Villanueva, R.; Krimm, M.; Vasiljeva, O.; van Dongen, G.A.M.S.; Vugts, D.J. The tumor targeting performance of anti-CD166 Probody drug conjugate CX-2009 and its parental derivatives as monitored by 89Zr-immuno-PET in xenograft bearing mice. Theranostics 2020, 10, 5815–5828. [Google Scholar] [CrossRef]
- Jauw, Y.W.S.; Menke-van der Houven van Oordt, C.W.; Hoekstra, O.S.; Hendrikse, N.H.; Vugts, D.J.; Zijlstra, J.M.; Huisman, M.C.; van Dongen, G.A.M.S. Immuno-Positron Emission Tomography with Zirconium-89-Labeled Monoclonal Antibodies in Oncology: What Can We Learn from Initial Clinical Trials? Front. Pharmacol. 2016, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Menke-van der Houven van Oordt, C.W.; McGeoch, A.; Bergstrom, M.; McSherry, I.; Smith, D.A.; Cleveland, M.; Al-Azzam, W.; Chen, L.; Verheul, H.; Hoekstra, O.S.; et al. Immuno-PET Imaging to Assess Target Engagement: Experience from 89Zr-Anti-HER3 mAb (GSK2849330) in Patients with Solid Tumors. J. Nucl. Med. 2019, 60, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Garousi, J.; Lindbo, S.; Borin, J.; von Witting, E.; Vorobyeva, A.; Oroujeni, M.; Mitran, B.; Orlova, A.; Buijs, J.; Tolmachev, V.; et al. Comparative evaluation of dimeric and monomeric forms of ADAPT scaffold protein for targeting of HER2-expressing tumours. Eur. J. Pharm. Biopharm. 2019, 134, 37–48. [Google Scholar] [CrossRef]
- Garousi, J.; von Witting, E.; Borin, J.; Vorobyeva, A.; Altai, M.; Vorontsova, O.; Konijnenberg, M.W.; Oroujeni, M.; Orlova, A.; Tolmachev, V.; et al. Radionuclide therapy using ABD-fused ADAPT scaffold protein: Proof of Principle. Biomaterials 2021, 266, 120381. [Google Scholar] [CrossRef]
- Hofstrom, C.; Orlova, A.; Altai, M.; Wangsell, F.; Gräslund, T.; Tolmachev, V. Use of a HEHEHE purification tag instead of a hexahistidine tag improves biodistribution of affibody molecules site-specifically labeled with (99m)Tc, (111)In, and (125)I. J. Med. Chem. 2011, 54, 3817–3826. [Google Scholar] [CrossRef]
- Xu, T.; Vorobyeva, A.; Schulga, A.; Konovalova, E.; Vorontsova, O.; Ding, H.; Gräslund, T.; Tashireva, L.A.; Orlova, A.; Tolmachev, V.; et al. Imaging-Guided Therapy Simultaneously Targeting HER2 and EpCAM with Trastuzumab and EpCAM-Directed Toxin Provides Additive Effect in Ovarian Cancer Model. Cancers 2021, 13, 3939. [Google Scholar] [CrossRef]
- Xu, T.; Liu, Y.; Schulga, A.; Konovalova, E.; Deyev, S.M.; Tolmachev, V.; Vorobyeva, A. Epithelial cell adhesion molecule-targeting designed ankyrin repeat protein-toxin fusion Ec1-LoPE exhibits potent cytotoxic action in prostate cancer cells. Oncol. Rep. 2022, 47, 94. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Schliemann, C.; Palumbo, A.; Zuberbühler, K.; Villa, A.; Kaspar, M.; Trachsel, E.; Klapper, W.; Menssen, H.D.; Neri, D. Complete eradication of human B-cell lymphoma xenografts using rituximab in combination with the immunocytokine L19-IL2. Blood 2009, 113, 2275–2283. [Google Scholar] [CrossRef]
- Wu, C.; Li, H.; Zhao, H.; Zhang, W.; Chen, Y.; Yue, Z.; Lu, Q.; Wan, Y.; Tian, X.; Deng, A. Potentiating antilymphoma efficacy of chemotherapy using a liposome for in-tegration of CD20 targeting, ultra-violet irradiation polymerizing, and controlled drug delivery. Nanoscale Res. Let. 2014, 9, 447. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Konshina, A.G.; Bocharov, E.V.; Konovalova, E.V.; Schulga, A.A.; Tolmachev, V.; Deyev, S.M.; Efremov, R.G. Structural Basis of Activity of HER2-Targeting Construct Composed of DARPin G3 and Albumin-Binding Domains. Int. J. Mol. Sci. 2024, 25, 11370. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Wittrup, K.D. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol. Cancer Ther. 2009, 8, 2861–2871. [Google Scholar] [CrossRef]
- Martin-Killias, P.; Stefan, N.; Rothschild, S.; Plückthun, A.; Zangemeister-Wittke, U. A novel fusion toxin derived from an EpCAM-specific designed ankyrin repeat protein has potent antitumor activity. Clin. Cancer Res. 2011, 17, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Frey, R.; Zangemeister-Wittke, U.; Plückthun, A. Orthogonal assembly of a designed ankyrin repeat protein-cytotoxin conjugate with a clickable serum albumin module for half-life extension. Bioconjugate Chem. 2013, 24, 1955–1966. [Google Scholar] [CrossRef]
- Simon, M.; Stefan, N.; Borsig, L.; Plückthun, A.; Zangemeister-Wittke, U. Increasing the antitumor effect of an EpCAM-targeting fusion toxin by facile click PEGylation. Mol Cancer Ther. 2014, 13, 375–385. [Google Scholar] [CrossRef]
- Xiao, Y.; Mei, C.; Xu, D.; Yang, F.; Yang, M.; Bi, L.; Mao, J.; Pang, P.; Li, D. Identifi-cation of a CEACAM5 targeted nanobody for positron emission tomography imaging and near-infrared fluorescence imaging of colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2305–2318. [Google Scholar] [CrossRef] [PubMed]
- Dinh-Fricke, A.V.; Hantschel, O. Improving the pharmacokinetics, biodistribution and plasma stability of monobodies. Front Pharmacol. 2024, 15, 1393112. [Google Scholar] [CrossRef]
- Pastan, I.; Hassan, R.; Fitzgerald, D.J.; Kreitman, R.J. Immunotoxin therapy of cancer. Nat. Rev. Cancer. 2006, 6, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.; Proshkina, G.; Kutova, O.; Shilova, O.; Ryabova, A.; Schulga, A.; Stremovskiy, O.; Zdobnova, T.; Balalaeva, I.; Deyev, S. Recombinant targeted toxin based on HER2-specific DARPin possesses a strong selective cytotoxic effect in vitro and a potent antitumor activity in vivo. J. Control. Release 2016, 233, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Mazor, R.; Pastan, I. Immunogenicity of Immunotoxins Containing Pseudomonas Exotoxin A: Causes, Consequences, and Mitigation. Front. Immunol. 2020, 11, 1261. [Google Scholar] [CrossRef]
- Frøysnes, I.S.; Andersson, Y.; Larsen, S.G.; Davidson, B.; Øien, J.T.; Olsen, K.H.; Giercksky, K.E.; Julsrud, L.; Fodstad, Ø.; Dueland, S.; et al. Novel Treatment with Intraperitoneal MOC31PE Immunotoxin in Colorectal Peritoneal Metastasis: Results From the ImmunoPeCa Phase 1 Trial. Ann. Surg. Oncol. 2017, 24, 1916–1922. [Google Scholar] [CrossRef]
- Mazor, R.; Onda, M.; Pastan, I. Immunogenicity of therapeutic recombinant immunotoxins. Immunol. Rev. 2016, 270, 152–164. [Google Scholar] [CrossRef]
- Wang, R.; Hu, B.; Pan, Z.; Mo, C.; Zhao, X.; Liu, G.; Hou, P.; Cui, Q.; Xu, Z.; Wang, W.; et al. Antibody-Drug Conjugates (ADCs): Current and future biopharmaceuticals. J. Hematol. Oncol. 2025, 18, 51. [Google Scholar] [CrossRef]
- Gerdes, S.; Staubach, P.; Dirschka, T.; Wetzel, D.; Weirich, O.; Niesmann, J.; da Mota, R.; Rothhaar, A.; Ardabili, M.; Vlasitz, G.; et al. Izokibep for the treatment of moderate-to-severe plaque psoriasis: A phase II, randomized, placebo-controlled, double-blind, dose-finding multicentre study including long-term treatment. Br. J. Dermatol. 2023, 189, 381–391. [Google Scholar] [CrossRef]
- Xu, T.; Ding, H.; Vorobyeva, A.; Oroujeni, M.; Orlova, A.; Tolmachev, V.; Gräslund, T. Drug Conjugates Based on a Monovalent Affibody Targeting Vector Can Efficiently Eradicate HER2 Positive Human Tumors in an Experimental Mouse Model. Cancers 2020, 13, 85. [Google Scholar] [CrossRef]
- Garousi, J.; Ding, H.; von Witting, E.; Xu, T.; Vorobyeva, A.; Oroujeni, M.; Orlova, A.; Hober, S.; Gräslund, T.; Tolmachev, V. Targeting HER2 Expressing Tumors with a Potent Drug Conjugate Based on an Albumin Binding Domain-Derived Affinity Protein. Pharmaceutics 2021, 13, 1847. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Xu, T.; Ding, H.; Zhang, J.; Bodenko, V.; Tretyakova, M.S.; Belousov, M.V.; Liu, Y.; Oroujeni, M.; Orlova, A.; et al. Comparison of HER2-targeted affibody conjugates loaded with auristatin- and maytansine-derived drugs. J. Control. Release 2023, 355, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.M.; Iegre, J.; O’ Donovan, D.H.; Ölwegård Halvarsson, M.; Spring, D.R. Peptides as a platform for targeted therapeutics for cancer: Peptide-drug conjugates (PDCs). Chem. Soc. Rev. 2021, 50, 1480–1494. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Y.; Yin, H.; Chen, X.T.; Yao, J.F.; Ma, Y.N.; Song, M.; Xu, H.; Yu, Q.Y.; Du, S.S.; Qi, Y.K.; et al. Three Rounds of Stability-Guided Optimization and Systematical Evaluation of Oncolytic Peptide LTX-315. J. Med. Chem. 2024, 67, 3885–3908. [Google Scholar] [CrossRef]
- Nagy, Á.; Abouzayed, A.; Kanellopoulos, P.; Landmark, F.; Bezverkhniaia, E.; Tolmachev, V.; Orlova, A.; Eriksson, K.A. Evaluation of ABD-Linked RM26 Conjugates for GRPR-Targeted Drug Delivery. ACS Omega 2024, 9, 36122–36133. [Google Scholar] [CrossRef]
- Jonsson, A.; Dogan, J.; Herne, N.; Abrahmsén, L.; Nygren, P.A. Engineering of a femtomolar affinity binding protein to human serum albumin. Protein Eng. Des. Sel. 2008, 21, 515–527. [Google Scholar] [CrossRef]
- Tolmachev, V.; Orlova, A.; Andersson, K. Methods for radiolabelling of monoclonal antibodies. Methods Mol. Biol. 2014, 1060, 309–330. [Google Scholar] [CrossRef]
| Radiochemical Yield, % | Radiochemical Yield After EDTA Treatment, % | Isolated Yield, % | Radiochemical Purity, % | % Stability in PBS (1 h at 37 °C) | % Stability (×1000 EDTA, 1 h at 37 °C) | |
|---|---|---|---|---|---|---|
| Ec1-ABD | 92 ± 7 | 79 ± 18 | 46 ± 16 | 99 ± 2 | 90.7 ± 0.6 | 91.7 ± 1.5 |
| ABD-Ec1 | 61 ± 24 | 52 ± 23 | 38 ± 18 | 100 ± 1 | 96.9 ± 2.6 | 94.8 ± 1.8 |
| Ec1 | 60 | 52 | 20 | 96 | - | - |
| Compound | ka (1/(M*s)) (103) | kd (1/s) (10−6) | KD (nM) |
|---|---|---|---|
| [111In]In-Ec1-ABD (−HSA) | 7.4 ± 2.7 | 3.5 ± 0.6 | 0.523 ± 0.212 |
| [111In]In-Ec1-ABD (+HSA) | 10.2 ± 5.8 | 21.2 ± 4.0 | 2.6 ± 1.9 |
| [111In]In-ABD-Ec1(−HSA) | 8.0 ± 3.4 | 2.8 ± 0.5 | 0.368 ± 0.086 |
| [111In]In-ABD-Ec1(+HSA) | 8.0 ± 6.6 | 7.3 ± 6.5 | 0.863 ± 0.103 |
| [111In]In-Ec1 * | - | - | 0.21 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolmachev, V.; Vorobyeva, A.; Rosly, A.H.B.; Garousi, J.; Liu, Y.; Gräslund, T.; Papalanis, E.; Schulga, A.; Konovalova, E.; Orlova, A.; et al. Targeting of Epithelial Cell Adhesion Molecule-Expressing Malignant Tumors Using an Albumin-Binding Domain-Fused Designed Ankyrin Repeat Protein: Effect of the Molecular Architecture. Int. J. Mol. Sci. 2025, 26, 5236. https://doi.org/10.3390/ijms26115236
Tolmachev V, Vorobyeva A, Rosly AHB, Garousi J, Liu Y, Gräslund T, Papalanis E, Schulga A, Konovalova E, Orlova A, et al. Targeting of Epithelial Cell Adhesion Molecule-Expressing Malignant Tumors Using an Albumin-Binding Domain-Fused Designed Ankyrin Repeat Protein: Effect of the Molecular Architecture. International Journal of Molecular Sciences. 2025; 26(11):5236. https://doi.org/10.3390/ijms26115236
Chicago/Turabian StyleTolmachev, Vladimir, Anzhelika Vorobyeva, Alia Hani Binti Rosly, Javad Garousi, Yongsheng Liu, Torbjörn Gräslund, Eleftherios Papalanis, Alexey Schulga, Elena Konovalova, Anna Orlova, and et al. 2025. "Targeting of Epithelial Cell Adhesion Molecule-Expressing Malignant Tumors Using an Albumin-Binding Domain-Fused Designed Ankyrin Repeat Protein: Effect of the Molecular Architecture" International Journal of Molecular Sciences 26, no. 11: 5236. https://doi.org/10.3390/ijms26115236
APA StyleTolmachev, V., Vorobyeva, A., Rosly, A. H. B., Garousi, J., Liu, Y., Gräslund, T., Papalanis, E., Schulga, A., Konovalova, E., Orlova, A., Deyev, S. M., & Oroujeni, M. (2025). Targeting of Epithelial Cell Adhesion Molecule-Expressing Malignant Tumors Using an Albumin-Binding Domain-Fused Designed Ankyrin Repeat Protein: Effect of the Molecular Architecture. International Journal of Molecular Sciences, 26(11), 5236. https://doi.org/10.3390/ijms26115236









