Withania somnifera and Chrysanthemum zawadskii Herbich var. latilobum (Maxim.) Kitamura Complex Attenuates Obesity in High-Fat-Diet-Induced Obese Mice
Abstract
1. Introduction
2. Results
2.1. Effects of ASC on Body Weight, Food Intake, FER, and Organ and Adipose Tissue Weight in HFD-Induced Obese Mice
2.2. Effects of ASC on Biochemical Parameters in HFD-Induced Obese Mice
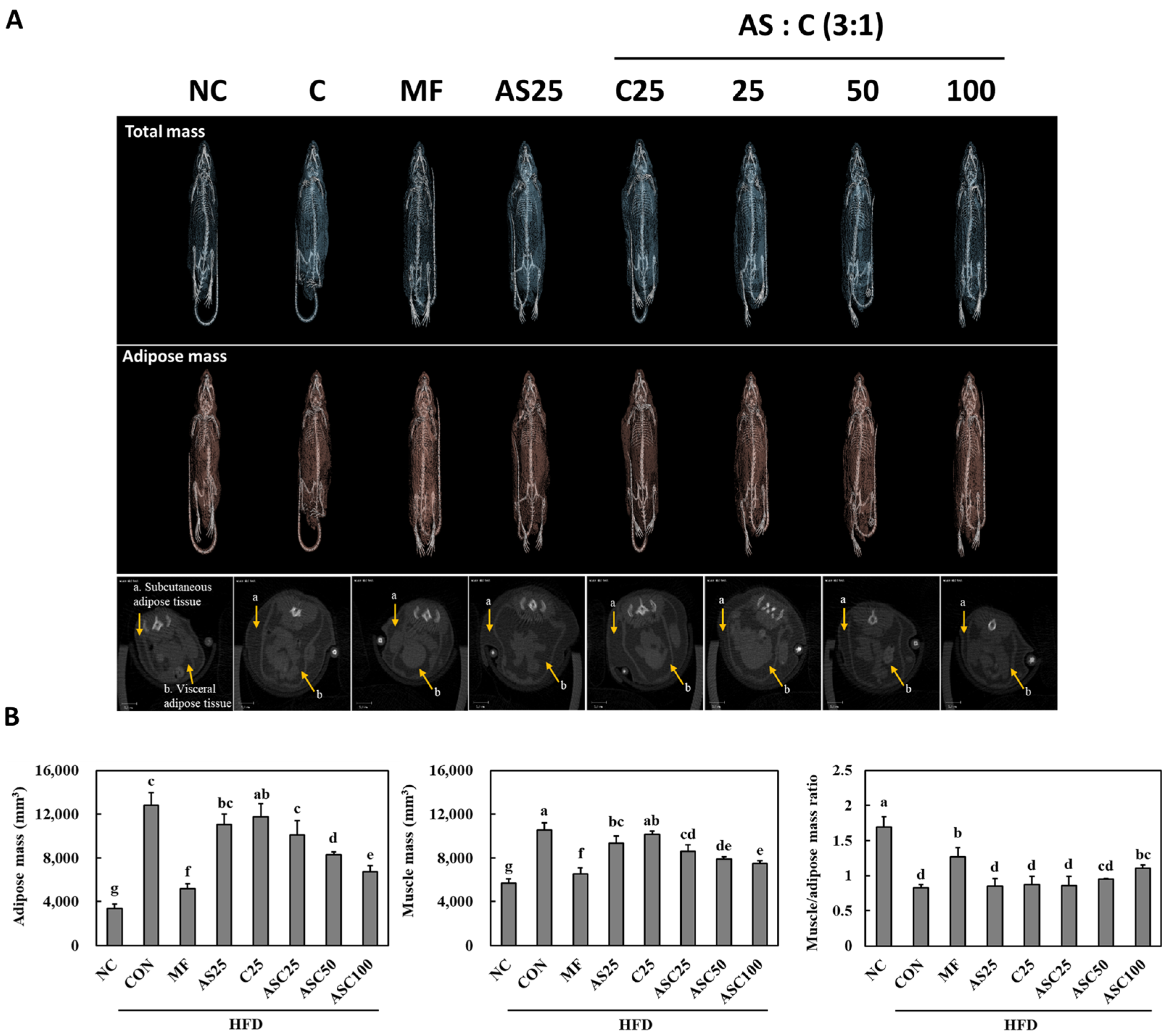
2.3. Effects of AS, C, and ASC on Adipose and Muscle Mass in HFD-Induced Obese Mice
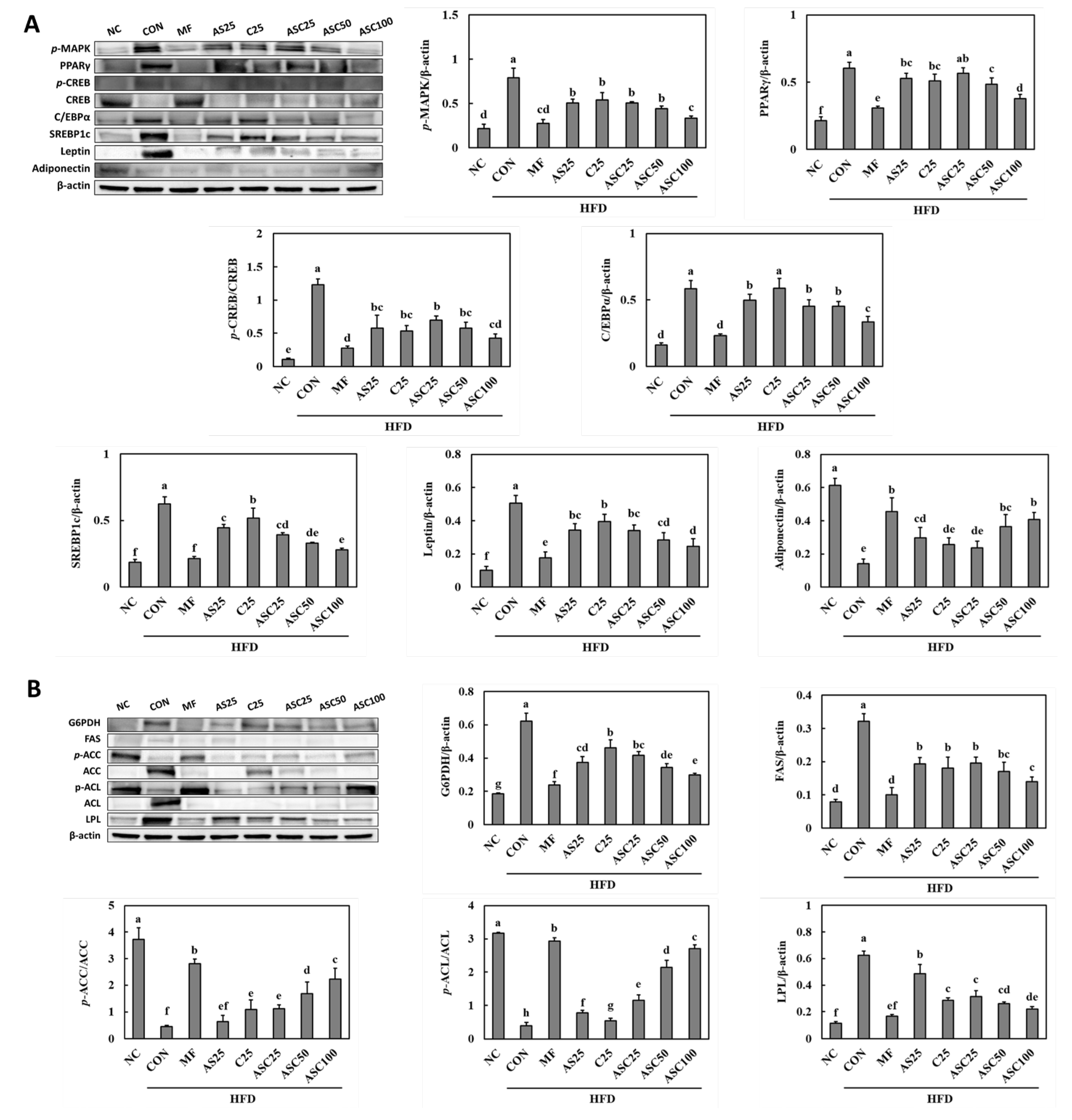
2.4. Effects of AS, C, and ASC on Adipogenesis and Lipogenesis Pathways in HFD-Induced Obese Mice
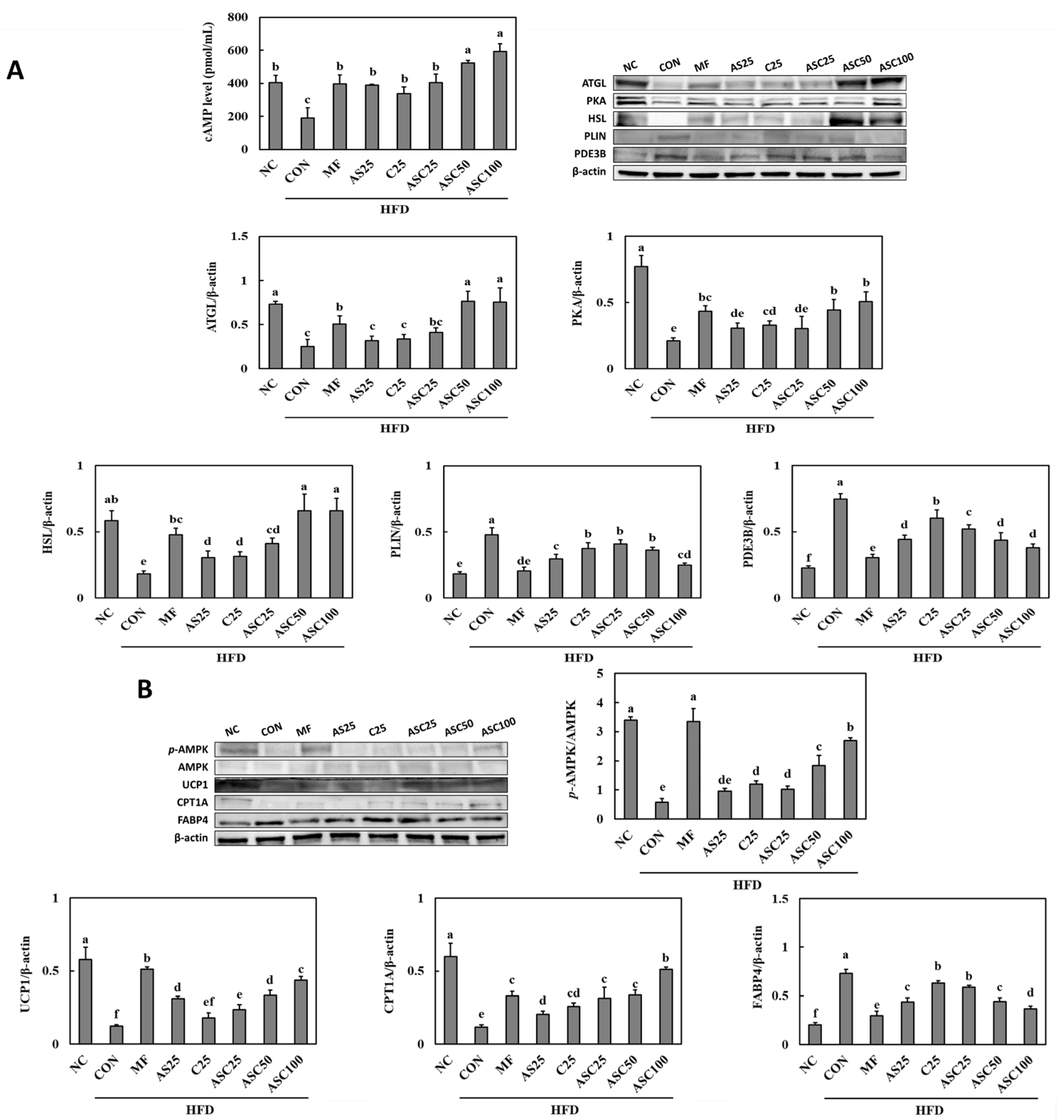
2.5. Effects of AS, C, and ASC on Lipolysis Pathway and Energy Metabolism in HFD-Induced Obese Mice
3. Discussion
4. Materials and Methods
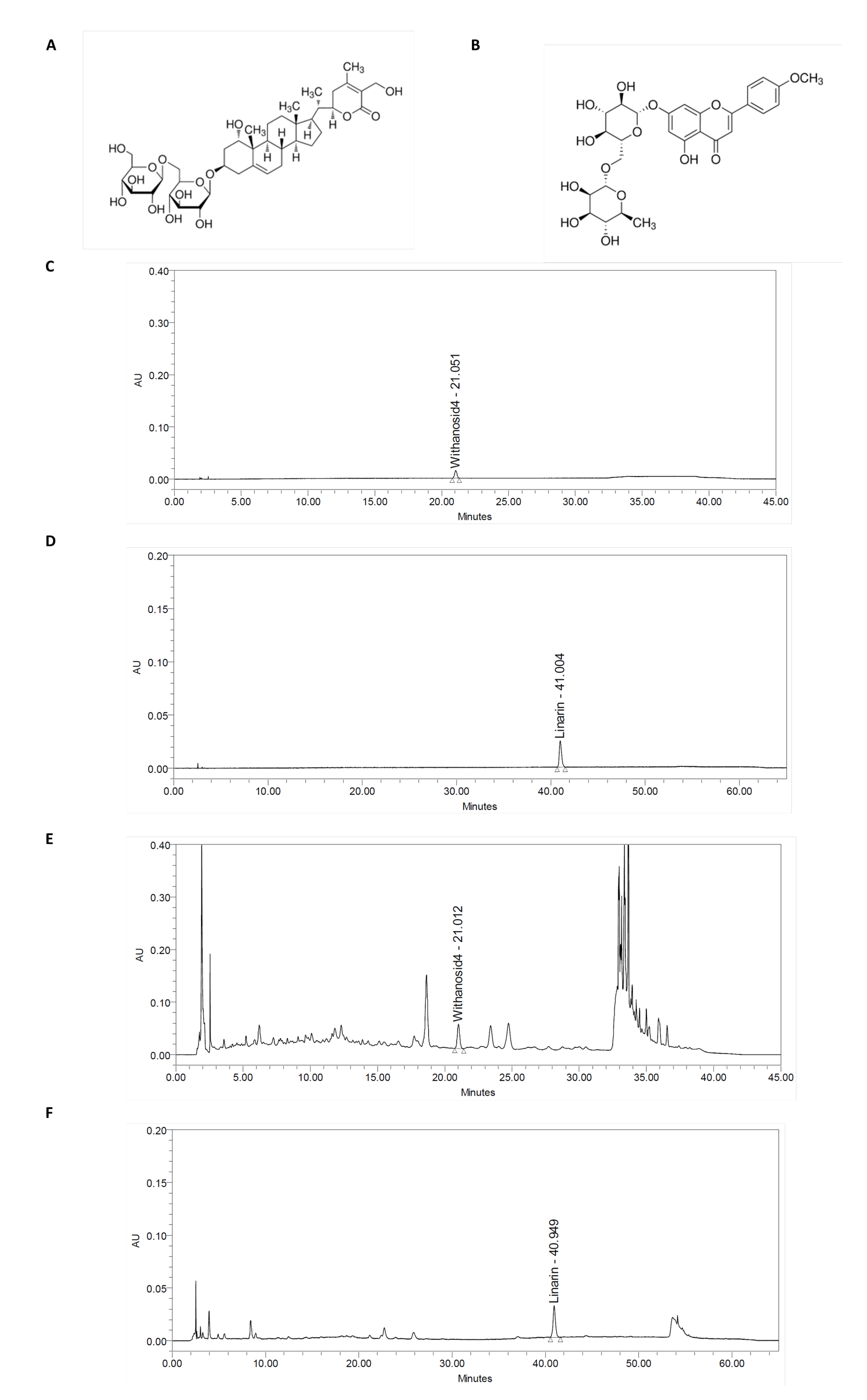
4.1. Preparation of Withania somnifera and Chrysanthemum zawadskii Herbich Var. Latilobum (Maxim.) Kitamura Complex (ASC) and Quantitative of Withanoside IV and Linarin
4.2. Animal
4.3. Biochemical Factors in Serum and Feces
4.4. Micro-CT
4.5. Western Blot
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2016, 378, 804–814. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Haslam, D.W.; James, W.P.T. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Yun, J.W. Possible anti-obesity therapeutics from nature—A review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kapoor, J.; Anishetty, S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of Ashwagandha root. Indian J. Psychol. Med. 2012, 34, 255–262. [Google Scholar] [CrossRef]
- Wankhede, S.; Langade, D.; Joshi, K.; Sinha, S.R.; Bhattacharyya, S. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2015, 12, 43. [Google Scholar] [CrossRef]
- Singh, N.; Bhalla, M.; de Jager, P.; Gilca, M. An overview on Ashwagandha: A Rasayana (rejuvenator) of Ayurveda. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 208–213. [Google Scholar] [CrossRef]
- Sikandan, A.; Shinomiya, T.; Nagahara, Y. Ashwagandha root extract exerts anti-inflammatory effects in HaCaT cells by inhibiting the MAPK/NF-kappaB pathways and by regulating cytokines. Int. J. Mol. Med. 2018, 42, 425–434. [Google Scholar]
- Mohmmed Hegab, A.M.; Hassanin, S.O.; Mekky, R.H.; Abuzahrah, S.S.; Hamza, A.A.; Talaat, I.M.; Amin, A. Withania somnifera Ameliorates Doxorubicin-Induced Nephrotoxicity and Potentiates Its Therapeutic Efficacy Targeting SIRT1/Nrf2, Oxidative Stress, Inflammation, and Apoptosis. Pharmaceuticals 2025, 18, 248. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.O.; Shin, J.Y.; Kang, H.J.; Park, J.H.; Hao, S.; Wang, F.; Jang, S.I. Anti-inflammatory effect of Chrysanthemum zawadskii, peppermint, Glycyrrhiza glabra herbal mixture in lipopolysaccharide-stimulated RAW264.7 macrophages. Mol. Med. Rep. 2021, 24, 532. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, H.K.; Lee, H.S. Hypoglycemic effect of standardized Chrysanthemum zawadskii ethanol extract in high-fat diet/streptozotocin-induced diabetic mice and rats. Food Sci. Biotechnol. 2018, 27, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Bódis, K.; Roden, M. Energy metabolism of white adipose tissue and insulin resistance in humans. Eur. J. Clin. Investig. 2018, 48, e13017. [Google Scholar]
- Jung, J.; Lee, M.; Park, S.H.; Cho, W.; Kim, J.; Eun, S.; Lee, J. Rose Petal Extract Ameliorates Obesity in High Fat Diet-Induced Obese Mice. Prev. Nutr. Food Sci. 2024, 29, 125–134. [Google Scholar] [CrossRef]
- Tsuru, H.; Osaka, M.; Hiraoka, Y.; Yoshida, M. HFD-induced hepatic lipid accumulation and inflammation are decreased in Factor D deficient mouse. Sci. Rep. 2020, 10, 17593. [Google Scholar] [CrossRef]
- VanSaun, M.N.; Lee, I.K.; Washington, M.K.; Matrisian, L.; Gorden, D.L. High fat diet induced hepatic steatosis establishes a permissive microenvironment for colorectal metastases and promotes primary dysplasia in a murine model. Am. J. Pathol. 2009, 175, 355–364. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- El-Eshmawy, M.M. Impact of obesity on liver function tests: Is nonalcoholic fatty liver disease the only player? A review article. Porto. Biomed. J. 2023, 8, e228. [Google Scholar] [CrossRef]
- Jalili, V.; Poorahmadi, Z.; Hasanpour Ardekanizadeh, N.; Gholamalizadeh, M.; Ajami, M.; Houshiarrad, A.; Hajipour, A.; Shafie, F.; Alizadeh, A.; Mokhtari, Z.; et al. The association between obesity with serum levels of liver enzymes, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and gamma-glutamyl transferase in adult women. Endocrinol. Diabetes Metab. 2022, 5, e367. [Google Scholar] [CrossRef]
- Huang, G.; Xu, J.; Zhang, Z.; Cai, L.; Liu, H.; Yu, X. Total cholesterol and high density lipoprotein cholesterol ratio is associated with metabolic syndrome in a very elderly Chinese population. Sci. Rep. 2022, 12, 15212. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, M.B.; Dzaye, O.; Bøtker, H.E.; Jensen, J.M.; Maeng, M.; Bentzon, J.F.; Kanstrup, H.; Sørensen, H.T.; Leipsic, J.; Blankstein, R.; et al. Low-Density Lipoprotein Cholesterol Is Predominantly Associated with Atherosclerotic Cardiovascular Disease Events in Patients with Evidence of Coronary Atherosclerosis: The Western Denmark Heart Registry. Circulation 2023, 147, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.W.; Kamili, A.; Tandy, S.; Weir, J.M.; Gaire, R.; Wong, G.; Meikle, P.J.; Cohn, J.S.; Rye, K.A. Dietary sphingomyelin lowers hepatic lipid levels and inhibits intestinal cholesterol absorption in high-fat-fed mice. PLoS ONE 2013, 8, e55949. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity. Molecules 2016, 21, 1404. [Google Scholar] [CrossRef]
- Boden, G. Obesity and free fatty acids. Endocrinol. Metab. Clin. North Am. 2008, 37, 635–646. [Google Scholar] [CrossRef]
- Deng, L.; Jia, L.; Wu, X.L.; Cheng, M. Association Between Body Mass Index and Glycemic Control in Type 2 Diabetes Mellitus: A Cross-Sectional Study. Diabetes Metab. Syndr. Obes. 2025, 18, 555–563. [Google Scholar] [CrossRef]
- Nie, Y.; Luo, F. Dietary Fiber: An Opportunity for a Global Control of Hyperlipidemia. Oxidative Med. Cell. Longev. 2021, 2021, 5542342. [Google Scholar] [CrossRef]
- Kamili, A.; Wat, E.; Chung, R.W.; Tandy, S.; Weir, J.M.; Meikle, P.J.; Cohn, J.S. Hepatic accumulation of intestinal cholesterol is decreased and fecal cholesterol excretion is increased in mice fed a high-fat diet supplemented with milk phospholipids. Nutr. Metab. 2010, 7, 90. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef]
- Crewe, C.; Zhu, Y.; Paschoal, V.A.; Joffin, N.; Ghaben, A.L.; Gordillo, R.; Oh, D.Y.; Liang, G.; Horton, J.D.; Scherer, P.E. SREBP-regulated adipocyte lipogenesis is dependent on substrate availability and redox modulation of mTORC1. JCI Insight 2019, 5, e129397. [Google Scholar] [CrossRef]
- Mead, J.R.; Irvine, S.A.; Ramji, D.P. Lipoprotein lipase: Structure, function, regulation, and role in disease. J. Mol. Med. 2002, 80, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.S.; Liu, W.; Van de Velde, S.; Matsumura, S.; Wiater, E.; Huang, L.; Montminy, M. Activation of the adipocyte CREB/CRTC pathway in obesity. Commun. Biol. 2021, 4, 1214. [Google Scholar] [CrossRef] [PubMed]
- Bolsoni-Lopes, A.; Alonso-Vale, M.I. Lipolysis and lipases in white adipose tissue—An update. Arch. Endocrinol. Metab. 2015, 59, 335–342. [Google Scholar] [CrossRef]
- Degerman, E.; Ahmad, F.; Chung, Y.W.; Guirguis, E.; Omar, B.; Stenson, L.; Manganiello, V. From PDE3B to the regulation of energy homeostasis. Curr. Opin. Pharmacol. 2011, 11, 676–682. [Google Scholar] [CrossRef]
- O’Neill, H.M.; Holloway, G.P.; Steinberg, G.R. AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: Implications for obesity. Mol. Cell. Endocrinol. 2013, 366, 135–151. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamada, T. UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes. Front. Endocrinol. 2020, 11, 498. [Google Scholar] [CrossRef]
- Park, S.H.; Park, J.; Lee, M.; Kim, J.; Eun, S.; Jun, W.; Kim, O.K.; Lee, J. Antiobesity effect of Kaempferia parviflora accompanied by inhibition of lipogenesis and stimulation of lipolysis. Food Nutr Res. 2023, 67, 10–29219. [Google Scholar] [CrossRef]
| NC | CON | MF | AS25 | C25 | ASC25 | ASC50 | ASC100 | |
|---|---|---|---|---|---|---|---|---|
| Initial body weight (g) | 20.97 ± 0.75 ns | 21.16 ± 1.09 | 20.59 ± 0.64 | 20.71 ± 0.92 | 21.03 ± 1.37 | 20.81 ± 0.87 | 20.81 ± 1.09 | 20.66 ± 0.84 |
| Final body weight (g) | 32.23 ± 1.78 f | 48.11 ± 2.86 a | 35.47 ± 0.79 e | 45.27 ± 2.76 ab | 46.19 ± 4.26 ab | 43.83 ± 3.21 bc | 40.83 ± 2.28 cd | 38.57 ± 2.38 d |
| Weight gain * (g) | 11.26 ± 1.51 f | 26.94 ± 2.71 a | 14.88 ± 1.33 e | 24.56 ± 2.58 ab | 25.16 ± 5.88 ab | 23.02 ± 3.48 bc | 20.02 ± 2.28 cd | 17.91 ± 2.48 de |
| FER ** | 5.02 ± 0.87 b | 8.69 ± 0.87 a | 7.59 ± 0.68 a | 8.61 ± 0.90 a | 8.73 ± 1.80 a | 8.93 ± 1.35 a | 8.62 ± 0.98 a | 7.92 ± 1.09 a |
| Organ weight (g) | ||||||||
| Liver | 1.28 ± 0.10 f | 2.43 ± 0.16 a | 1.49 ± 0.12 e | 1.97 ± 0.24 bc | 2.13 ± 0.24 b | 1.82 ± 0.20 cd | 1.79 ± 0.15 de | 1.62 ± 0.15 de |
| Kidney | 0.40 ± 0.03 ns | 0.42 ± 0.05 | 0.41 ± 0.06 | 0.41 ± 0.04 | 0.42 ± 0.03 | 0.41 ± 0.02 | 0.42 ± 0.03 | 0.40 ± 0.01 |
| Spleen | 0.12 ± 0.03 ns | 0.13 ± 0.01 | 0.13 ± 0.04 | 0.11 ± 0.02 | 0.12 ± 0.03 | 0.11 ± 0.02 | 0.11 ± 0.03 | 0.11 ± 0.02 |
| Adipose tissue weight (g) | ||||||||
| Total WAT *** | 1.10 ± 0.19 g | 6.26 ± 0.39 a | 2.50 ± 0.29 f | 4.93 ± 0.34 bc | 5.15 ± 0.37 b | 4.62 ± 0.35 c | 3.95 ± 0.45 d | 3.28 ± 0.30 e |
| Subcutaneous WAT | 0.48 ± 0.11 f | 2.90 ± 0.20 a | 0.93 ± 0.24 e | 2.15 ± 0.19 b | 2.17 ± 0.29 b | 1.95 ± 0.21 bc | 1.73 ± 0.14 c | 1.40 ± 0.23 d |
| Visceral WAT | 0.63 ± 0.12 g | 3.36 ± 0.38 a | 1.57 ± 0.18 f | 2.78 ± 0.24 bc | 2.98 ± 0.23 b | 2.67 ± 0.25 c | 2.23 ± 0.36 d | 1.87 ± 0.22 e |
| NC | CON | MF | AS25 | C25 | ASC25 | ASC50 | ASC100 | |
|---|---|---|---|---|---|---|---|---|
| Serum | ||||||||
| ALT (mU/mL) | 51.04 ± 5.98 c | 99.90 ± 11.16 a | 62.79 ± 9.37 bc | 75.85 ± 15.25 b | 77.84 ± 12.01 b | 69.63 ± 8.38 b | 61.98 ± 8.14 bc | 63.36 ± 10.60 bc |
| AST (mU/mL) | 94.15 ± 5.97 c | 138.38 ± 5.63 a | 113.66 ± 9.38 b | 113.15 ± 13.65 b | 120.13 ± 8.45 b | 109.71 ± 4.55 b | 110.97 ± 11.58 b | 109.02 ± 7.15 b |
| Triglyceride (mM) | 4.83 ± 0.77 e | 26.34 ± 2.06 a | 8.92 ± 0.65 e | 17.03 ± 3.97 c | 20.87 ± 1.29 b | 16.78 ± 2.52 c | 13.68 ± 2.16 d | 11.88 ± 0.76 de |
| Total chol (μg/μL) | 9.31 ± 6.60 e | 46.90 ± 5.77 a | 24.83 ± 5.63 d | 41.38 ± 6.17 ab | 43.22 ± 9.42 ab | 37.24 ± 7.55 bc | 36.32 ± 5.63 bcd | 28.97 ± 3.78 cd |
| LDL-chol (μg/μL) | 0.23 ± 0.01 e | 0.76 ± 0.08 a | 0.32 ± 0.05 d | 0.51 ± 0.07 b | 0.57 ± 0.07 b | 0.50 ± 0.05 bc | 0.49 ± 0.06 bc | 0.41 ± 0.01 c |
| HDL-chol (μg/μL) | 1.42 ± 0.07 c | 2.45 ± 0.19 a | 1.87 ± 0.10 b | 1.97 ± 0.12 b | 2.06 ± 0.02 b | 2.08 ± 0.13 b | 1.97 ± 0.36 b | 1.86 ± 0.18 b |
| LDL/HDL ratio | 0.16 ± 0.01 d | 0.31 ± 0.03 a | 0.17 ± 0.03 d | 0.26 ± 0.04 bc | 0.28 ± 0.03 ab | 0.24 ± 0.02 bc | 0.25 ± 0.03 bc | 0.22 ± 0.01 c |
| FFA (mM) | 0.29 ± 0.03 d | 0.59 ± 0.06 a | 0.35 ± 0.04 cd | 0.44 ± 0.04 b | 0.52 ± 0.06 a | 0.44 ± 0.07 b | 0.44 ± 0.08 b | 0.41 ± 0.05 bc |
| Glucose (nmol/μL) | 11.68 ± 0.86 g | 21.28 ± 1.51 a | 13.13 ± 0.87 f | 18.50 ± 0.85 bc | 19.75 ± 1.04 a | 17.81 ± 1.01 c | 16.13 ± 0.59 d | 14.85 ± 0.57 e |
| Insulin (ng/mL) | 0.25 ± 0.07 g | 0.91 ± 0.07 a | 0.40 ± 0.07 f | 0.65 ± 0.02 c | 0.75 ± 0.06 b | 0.60 ± 0.01 cd | 0.54 ± 0.02 de | 0.51 ± 0.02 e |
| HbA1c (%) | 4.57 ± 0.26 d | 11.14 ± 0.68 a | 5.58 ± 0.57 e | 8.51 ± 0.49 c | 10.03 ± 0.65 b | 8.38 ± 0.25 c | 7.50 ± 0.76 cd | 7.03 ± 0.66 d |
| Feces | ||||||||
| Triglyceride (mM) | 1.34 ± 0.33 f | 2.17 ± 0.78 e | 4.97 ± 0.41 b | 3.37 ± 0.46 cd | 3.07 ± 0.33 d | 3.77 ± 0.39 c | 5.14 ± 0.47 b | 6.44 ± 0.42 a |
| Total chol (μg/mL) | 0.39 ± 0.19 e | 0.59 ± 0.22 d | 1.36 ± 0.12 b | 1.00 ± 0.16 c | 0.91 ± 0.19 c | 1.05 ± 0.05 c | 1.39 ± 0.19 b | 1.70 ± 0.14 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-H.; Park, J.; Yoo, E.; Jung, J.; Park, M.-R.; Kim, S.; Kim, J.-L.; Lee, J.W.; Kim, O.-k.; Lee, M. Withania somnifera and Chrysanthemum zawadskii Herbich var. latilobum (Maxim.) Kitamura Complex Attenuates Obesity in High-Fat-Diet-Induced Obese Mice. Int. J. Mol. Sci. 2025, 26, 5230. https://doi.org/10.3390/ijms26115230
Park S-H, Park J, Yoo E, Jung J, Park M-R, Kim S, Kim J-L, Lee JW, Kim O-k, Lee M. Withania somnifera and Chrysanthemum zawadskii Herbich var. latilobum (Maxim.) Kitamura Complex Attenuates Obesity in High-Fat-Diet-Induced Obese Mice. International Journal of Molecular Sciences. 2025; 26(11):5230. https://doi.org/10.3390/ijms26115230
Chicago/Turabian StylePark, Seong-Hoo, Jeongjin Park, Eunhee Yoo, Jaeeun Jung, Mi-Ryeong Park, Soyoung Kim, Jong-Lae Kim, Jong Wook Lee, Ok-kyung Kim, and Minhee Lee. 2025. "Withania somnifera and Chrysanthemum zawadskii Herbich var. latilobum (Maxim.) Kitamura Complex Attenuates Obesity in High-Fat-Diet-Induced Obese Mice" International Journal of Molecular Sciences 26, no. 11: 5230. https://doi.org/10.3390/ijms26115230
APA StylePark, S.-H., Park, J., Yoo, E., Jung, J., Park, M.-R., Kim, S., Kim, J.-L., Lee, J. W., Kim, O.-k., & Lee, M. (2025). Withania somnifera and Chrysanthemum zawadskii Herbich var. latilobum (Maxim.) Kitamura Complex Attenuates Obesity in High-Fat-Diet-Induced Obese Mice. International Journal of Molecular Sciences, 26(11), 5230. https://doi.org/10.3390/ijms26115230





