Folate Receptor Alpha in Advanced Epithelial Ovarian Cancer: Diagnostic Role and Therapeutic Implications of a Clinically Validated Biomarker
Abstract
1. Introduction
2. Mirvetuximab Soravtansine-Gynx Clinical Trials
3. Ventana FOLR1 (FOLR1 2.1) RxDx Assay
3.1. Pre-Analytical Considerations for FOLR1 Immunohistochemistry
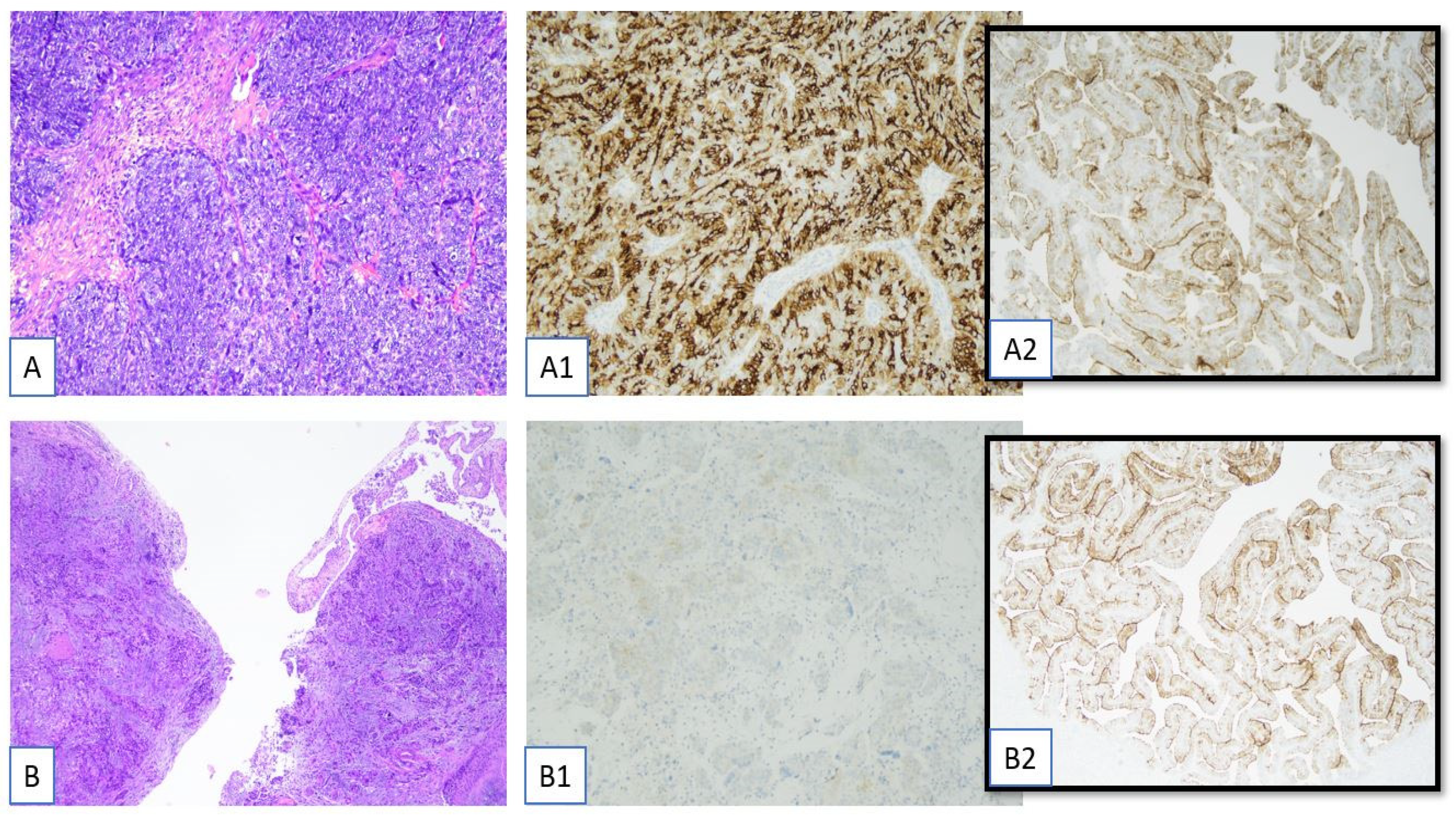
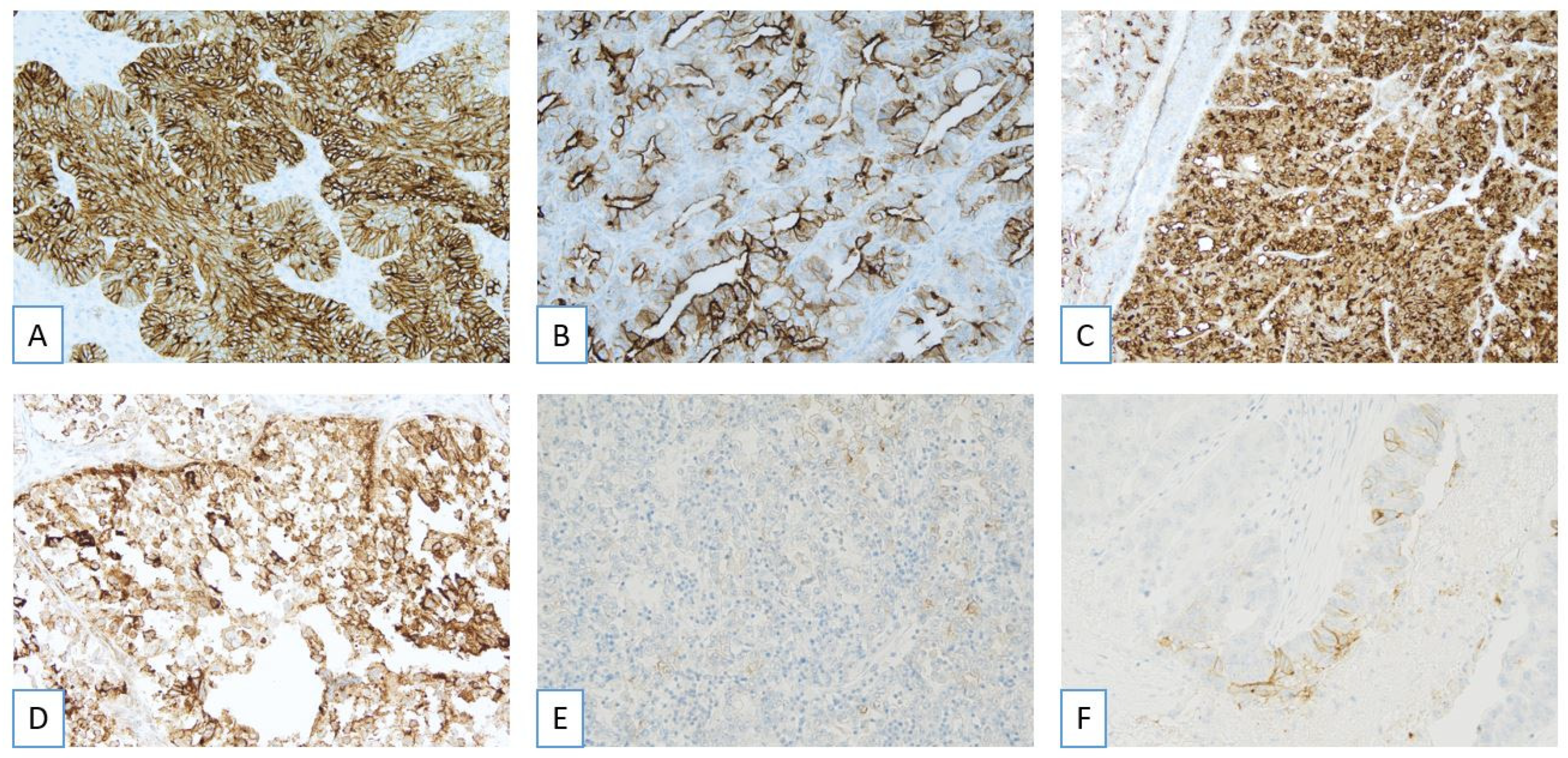
3.2. Pathological Evaluation of FRα: Scoring Criteria
3.3. Pathological Evaluation of FRα: Complex Staining Patterns and Borderline Cases
3.4. Immunohistochemical Report of the Ventana FOLR1 RxDx Assay
- Assay methodology:The report should state that the evaluation was performed using the Ventana FOLR1 RxDx Assay, which employs the FOLR1-2.1 monoclonal antibody on the Benchmark Ultra IHC platform. This ensures standardization and clinical validation of the assay for determining FOLR1 status in ovarian cancer.
- Tumor histotype:The histopathological classification of the tumor must be clearly specified. Most commonly, the assay is applied to high-grade serous carcinoma (HGSC), but other histological subtypes should be explicitly mentioned when applicable.
- Anatomic location of the tissue sample:The report should identify whether the analyzed tissue is from a primary site (ovary, salpinx, or peritoneum) or a metastatic lesion, as this may influence the interpretation and clinical implications.
- Internal control tissue evaluation:The presence of normal fallopian tube epithelium on the same slide is essential for internal quality control. The report must document whether control tissue is present and morphologically adequate (i.e., well preserved and with an expected staining pattern), which confirms the technical validity of the assay.
- Assessment of tumor cell adequacy:It is critical to confirm that the tissue section contains a minimum of 100 viable tumor cells. If this threshold is not met, the report should specify the reason for inadequacy (e.g., artifacts from freezing or fixation, necrosis, or absence of invasive tumor tissue), and the case may be considered non-evaluable.
- Scoring procedure and interobserver agreement:The report should state whether shared scoring was performed among multiple observers (e.g., consensus reading by two or three pathologists) or if a single observer evaluated the case. Shared scoring improves reproducibility and reduces interobserver variability in borderline or complex cases.
- Quantitative scoring results:For each participating observer (e.g., Operators I, II, III), the report should indicate the percentage of tumor cells showing membranous staining with moderate (2+) or strong (3+) intensity. This data supports both the final interpretation and the transparency of evaluation.
- Use of additional slides for reevaluation:If the initial slide was suboptimal or yielded an equivocal result, the report should document whether reevaluation on other included tissue sections was performed and whether it affected the final interpretation.
- Final interpretive classification:Based on established criteria, the tumor should be classified as FOLR1-positive, FOLR1-negative, or indeterminate. This classification must integrate staining intensity, percentage of positive tumor cells, and internal control status.
- Therapeutic implications:The report should conclude with a clinical interpretation indicating whether the patient is eligible, not eligible, or indeterminate for treatment with mirvetuximab soravtansine, an antibody–drug conjugate approved for FOLR1-positive ovarian cancer.
3.5. Comparison with Other FRα IHC Methods
4. Tissue Selection and FRα Expression Heterogeneity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webb, P.M.; Ibiebele, T.I.; Hughes, M.C.; Beesley, J.; van der Pols, J.C.; Chen, X.; Nagle, C.M.; Bain, C.J.; Chenevix-Trench, G.; the Australian Cancer Study (Ovarian Cancer); et al. Folate and related micronutrients, folate-metabolising genes and risk of ovarian cancer. Eur. J. Clin. Nutr. 2011, 65, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, T.; Muminovic, M.; Nano, O.; Vulfovich, M. Folate Receptor Alpha—A Novel Approach to Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 1046. [Google Scholar] [CrossRef]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the folate receptor α in oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Goldman, I.D. Folate and thiamine transporters mediated by facilitative carriers (SLC19A1-3 and SLC46A1) and folate receptors. Mol. Asp. Med. 2013, 34, 373–385. [Google Scholar] [CrossRef]
- Kelemen, L.E. The role of folate receptor α in cancer development, progression and treatment: Cause, consequence or innocent bystander? Int. J. Cancer 2006, 119, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Bax, H.J.; Chauhan, J.; Stavraka, C.; Santaolalla, A.; Osborn, G.; Khiabany, A.; Grandits, M.; López-Abente, J.; Palhares, L.C.G.F.; Hak, C.C.W.; et al. Folate receptor alpha in ovarian cancer tissue and patient serum is associated with disease burden and treatment outcomes. Br. J. Cancer 2023, 128, 342–353. [Google Scholar] [CrossRef]
- Moore, K.N.; Angelergues, A.; Konecny, G.E.; García, Y.; Banerjee, S.; Lorusso, D.; Lee, J.-Y.; Moroney, J.W.; Colombo, N.; Roszak, A.; et al. Mirvetuximab Soravtansine in FRα-Positive, Platinum-Resistant Ovarian Cancer. N. Engl. J. Med. 2023, 389, 2162–2174. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Zheng, W. Mirvetuximab soravtansine: Current and future applications. J. Hematol. Oncol. 2025, 18, 33. [Google Scholar] [CrossRef]
- Gilbert, L.; Oaknin, A.; Matulonis, U.A.; Mantia-Smaldone, G.M.; Lim, P.C.; Castro, C.M.; Provencher, D.; Memarzadeh, S.; Method, M.; Wang, J.; et al. Safety and efficacy of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol. Oncol. 2023, 170, 241–247. [Google Scholar] [CrossRef]
- Richardson, D.L.; Moore, K.N.; Vergote, I.; Gilbert, L.; Martin, L.P.; Mantia-Smaldone, G.M.; Castro, C.M.; Provencher, D.; Matulonis, U.A.; Stec, J.; et al. Phase 1b study of mirvetuximab soravtansine, a folate receptor alpha (FRα)–targeting antibody-drug conjugate, in combination with carboplatin and bevacizumab in patients with platinum-sensitive ovarian cancer. Gynecol. Oncol. 2024, 185, 186–193. [Google Scholar] [CrossRef]
- Coleman, R.L.; Lorusso, D.; Oaknin, A.; Cecere, S.C.; Denys, H.; Colombo, N.; van Gorp, T.; Konner, J.A.; Marin, M.R.; Harter, P.; et al. Mirvetuximab soravtansine in folate receptor alpha (FRα)–high platinum-resistant ovarian cancer: Final overall survival and post hoc sequence of therapy subgroup results from the SORAYA trial. Int. J. Gynecol. Cancer 2024, 34, 1119–1125. [Google Scholar] [CrossRef]
- Secord, A.A.; Lewin, S.; Murphy, C.; Cecere, S.; Barquín, A.; Gálvez-Montosa, F.; Mathews, C.; Konecny, G.; Ray-Coquard, I.; Oaknin, A.; et al. The efficacy and safety of mirvetuximab soravtansine in FRα-positive, third-line and later, recurrent platinum-sensitive ovarian cancer: The single-arm phase II PICCOLO trial. Ann. Oncol. 2025, 36, 321–330. [Google Scholar] [CrossRef]
- Van Gorp, T.; Moore, K.N.; Konecny, G.E.; Leary, A.; García-García, Y.; Banerjee, S.; Lorusso, D.; Lee, J.-Y.; Moroney, J.W.; Caruso, G.; et al. Patient-reported outcomes from the MIRASOL trial evaluating mirvetuximab soravtansine versus chemotherapy in patients with folate receptor α-positive, platinum-resistant ovarian cancer: A randomised, open-label, phase 3 trial. Lancet Oncol. 2025, 26, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Previs, R.A.; Strickland, K.C.; Wallen, Z.; Ko, H.; Green, M.; Cooper, M.; Lyon, E.; Biorn, M.; Armetta, J.; Quarles, R.; et al. Analysis of real world FRα testing in ovarian, fallopian tube, and primary peritoneal cancers. Gynecol. Oncol. 2025, 192, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Oza, A.; Colombo, N.; Oaknin, A.; Scambia, G.; Lorusso, D.; Konecny, G.; Banerjee, S.; Murphy, C.; Tanyi, J.; et al. Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: Primary analysis of FORWARD I. Ann. Oncol. 2021, 32, 757–765. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Lorusso, D.; Oaknin, A.; Pignata, S.; Dean, A.; Denys, H.; Colombo, N.; Van Gorp, T.; Konner, J.A.; Marin, M.R.; et al. Efficacy and Safety of Mirvetuximab Soravtansine in Patients With Platinum-Resistant Ovarian Cancer With High Folate Receptor Alpha Expression: Results From the SORAYA Study. J. Clin. Oncol. 2023, 41, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A.; et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef]
- O’Malley, D.M.; Matulonis, U.A.; Birrer, M.J.; Castro, C.M.; Gilbert, L.; Vergote, I.; Martin, L.P.; Mantia-Smaldone, G.M.; Martin, A.G.; Bratos, R.; et al. Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol. Oncol. 2020, 157, 379–385. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Myers, T.; Wimberger, P.; Van Gorp, T.; Redondo, A.; Cibula, D.; Nicum, S.; Rodrigues, M.; Backes, F.J.; Barlin, J.N.; et al. Maintenance with mirvetuximab soravtansine plus bevacizumab vs bevacizumab in FRα-high platinum-sensitive ovarian cancer. Futur. Oncol. 2024, 20, 2423–2436. [Google Scholar] [CrossRef]
- James, R.L.; Sisserson, T.; Cai, Z.; Dumas, M.E.; Inge, L.J.; Ranger-Moore, J.; Mason, A.; Sloss, C.M.; McArthur, K. Development of an FRα Companion Diagnostic Immunohistochemical Assay for Mirvetuximab Soravtansine. Arch. Pathol. Lab. Med. 2024, 148, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Deutschman, E.; Fulton, R.; Sloss, C.M. Evaluation of Laboratory-Derived Immunohistochemical Assays for Folate Receptor α Expression in Epithelial Ovarian Cancer and Comparison with a Companion Diagnostic. Arch. Pathol. Lab. Med. 2025. [Google Scholar] [CrossRef] [PubMed]
- Lawson, B.C.; Marques-Piubelli, M.L.; Westin, S.N.; Malpica, A. Folate Receptor Immunohistochemical Staining and Gynecologic Tumors: Initial Experience with 216 Cases. Int. J. Gynecol. Pathol. 2025, 44, 167–173. [Google Scholar] [CrossRef]
- Martin, L.P.; Konner, J.A.; Moore, K.N.; Seward, S.M.; Matulonis, U.A.; Perez, R.P.; Su, Y.; Berkenblit, A.; Ruiz-Soto, R.; Birrer, M.J. Characterization of folate receptor alpha (FRα) expression in archival tumor and biopsy samples from relapsed epithelial ovarian cancer patients: A phase I expansion study of the FRα-targeting antibody-drug conjugate mirvetuximab soravtansine. Gynecol. Oncol. 2017, 147, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Lorusso, D.; Oaknin, A.; Oza, A.; Colombo, N.; Van Gorp, T.; O'Malley, D.M.; Banerjee, S.; Murphy, C.G.; Harter, P.; et al. Safety and tolerability of mirvetuximab soravtansine monotherapy for folate receptor alpha–expressing recurrent ovarian cancer: An integrated safety summary. Gynecol. Oncol. 2024, 191, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Rushton, T.; Krause, H.B.; Samec, T.; Elliott, A.; Karnezis, A.N.; Toboni, M.D.; Thaker, P.H.; Braxton, D.R.; Oberley, M.; Gershenson, D.M.; et al. Characterizing the genomic landscape through the lens of FOLR1 status in low and high grade serous ovarian carcinoma. Gynecol. Oncol. 2024, 191, 80–85. [Google Scholar] [CrossRef]
- Saito, A.; Nishikawa, T.; Yoshida, H.; Mizoguchi, C.; Kitadai, R.; Yamamoto, K.; Yazaki, S.; Kojima, Y.; Ishikawa, M.; Kato, T.; et al. Folate receptor alpha is widely expressed and a potential therapeutic target in uterine and ovarian carcinosarcoma. Gynecol. Oncol. 2023, 176, 115–121. [Google Scholar] [CrossRef]

| Feature | Description |
|---|---|
| Clone | Mouse monoclonal anti-FOLR1, clone FOLR1-2.1 |
| Platform | BenchMark IHC/ISH |
| Detection Kit | OptiView DAB IHC |
| Cut-off for positivity | ≥75% viable tumor cells with 2+/3+ membranous staining |
| Control tissue | Normal fallopian tube epithelium (moderate 2+ staining) |
| Validated specimens | FFPE primary/metastatic HGSOC tissue (resection, biopsy) |
| Non-validated specimens | Cytology samples, decalcified bone metastases |
| Score | Intensity Description | Included in Scoring |
|---|---|---|
| 0 | No signal | No |
| 1+ | Faint gold/light brown, partial/circumferential | No |
| 2+ | Chocolate brown, partial/circumferential | Yes (if ≥75% cells) |
| 3+ | Thick dark brown/black, partial/circumferential | Yes (if ≥75% cells) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zannoni, G.F.; Santoro, A.; d’Amati, A.; D’Alessandris, N.; Scaglione, G.; Padial Urtueta, B.; Valente, M.; Narducci, N.; Addante, F.; Spadola, S.; et al. Folate Receptor Alpha in Advanced Epithelial Ovarian Cancer: Diagnostic Role and Therapeutic Implications of a Clinically Validated Biomarker. Int. J. Mol. Sci. 2025, 26, 5222. https://doi.org/10.3390/ijms26115222
Zannoni GF, Santoro A, d’Amati A, D’Alessandris N, Scaglione G, Padial Urtueta B, Valente M, Narducci N, Addante F, Spadola S, et al. Folate Receptor Alpha in Advanced Epithelial Ovarian Cancer: Diagnostic Role and Therapeutic Implications of a Clinically Validated Biomarker. International Journal of Molecular Sciences. 2025; 26(11):5222. https://doi.org/10.3390/ijms26115222
Chicago/Turabian StyleZannoni, Gian Franco, Angela Santoro, Antonio d’Amati, Nicoletta D’Alessandris, Giulia Scaglione, Belen Padial Urtueta, Michele Valente, Nadine Narducci, Francesca Addante, Saveria Spadola, and et al. 2025. "Folate Receptor Alpha in Advanced Epithelial Ovarian Cancer: Diagnostic Role and Therapeutic Implications of a Clinically Validated Biomarker" International Journal of Molecular Sciences 26, no. 11: 5222. https://doi.org/10.3390/ijms26115222
APA StyleZannoni, G. F., Santoro, A., d’Amati, A., D’Alessandris, N., Scaglione, G., Padial Urtueta, B., Valente, M., Narducci, N., Addante, F., Spadola, S., Bragantini, E., & Angelico, G. (2025). Folate Receptor Alpha in Advanced Epithelial Ovarian Cancer: Diagnostic Role and Therapeutic Implications of a Clinically Validated Biomarker. International Journal of Molecular Sciences, 26(11), 5222. https://doi.org/10.3390/ijms26115222








