Omega-3 Fatty Acid Consumption Alters Uterine Contraction: A Comparative Study on Different Breeds of Rats
Abstract
1. Introduction
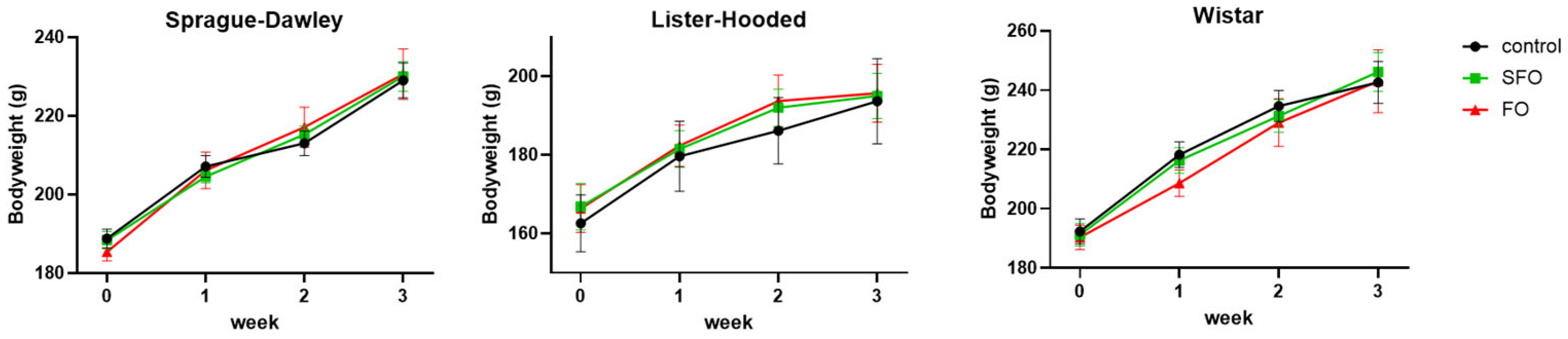
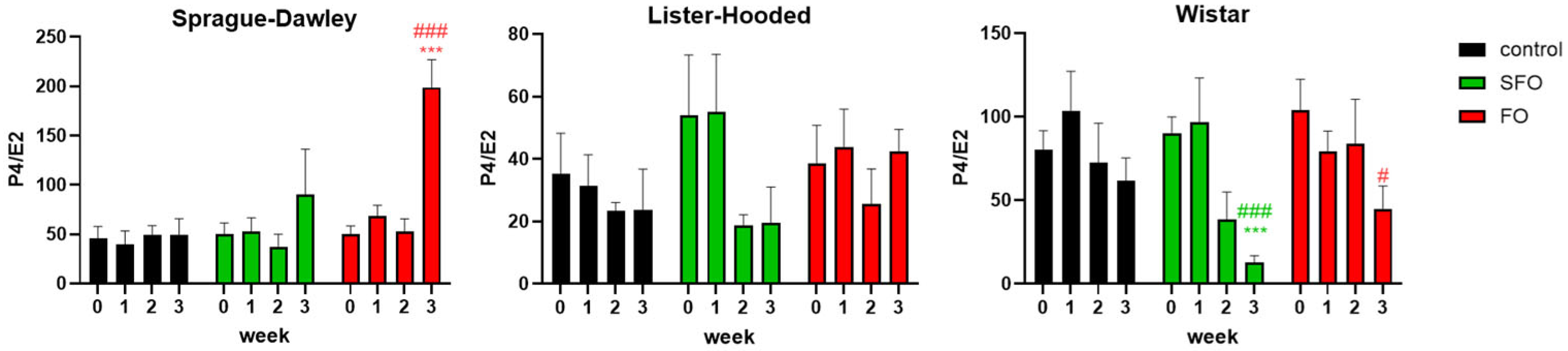
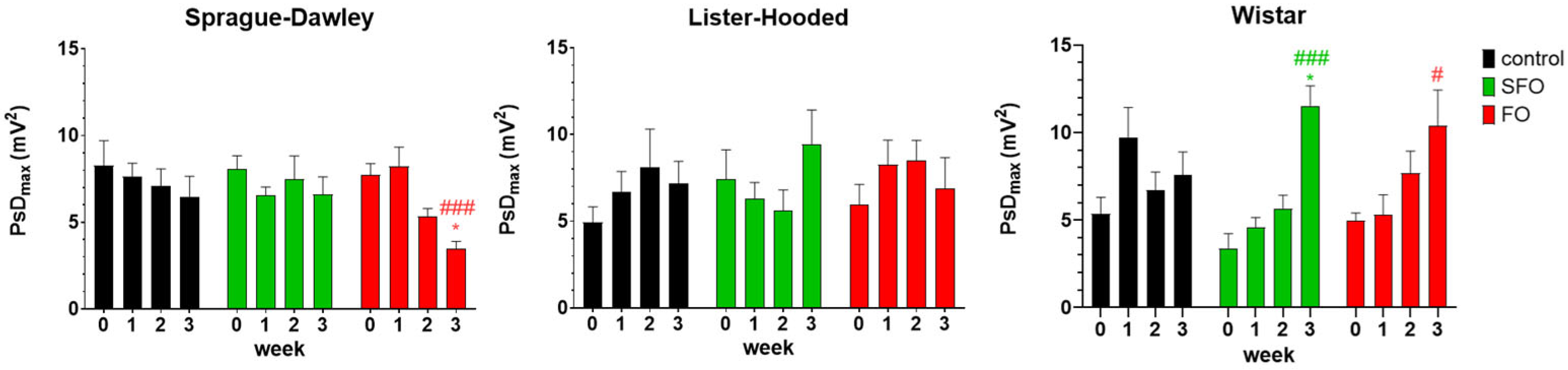
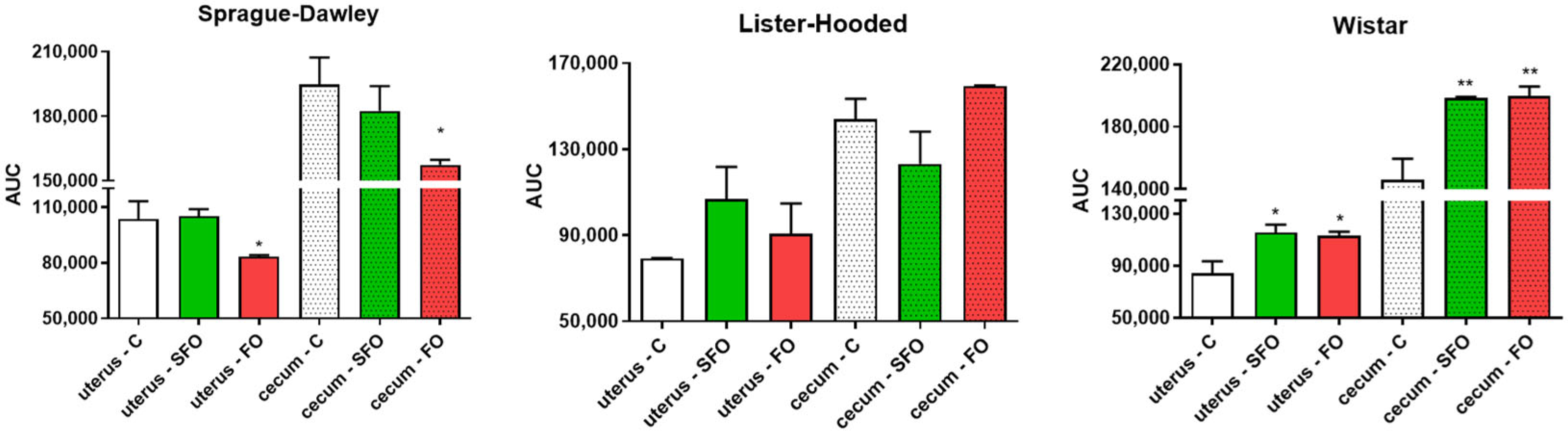
2. Results
2.1. Gonadotropic and Sex Hormone Levels
2.2. In Vivo Contractility
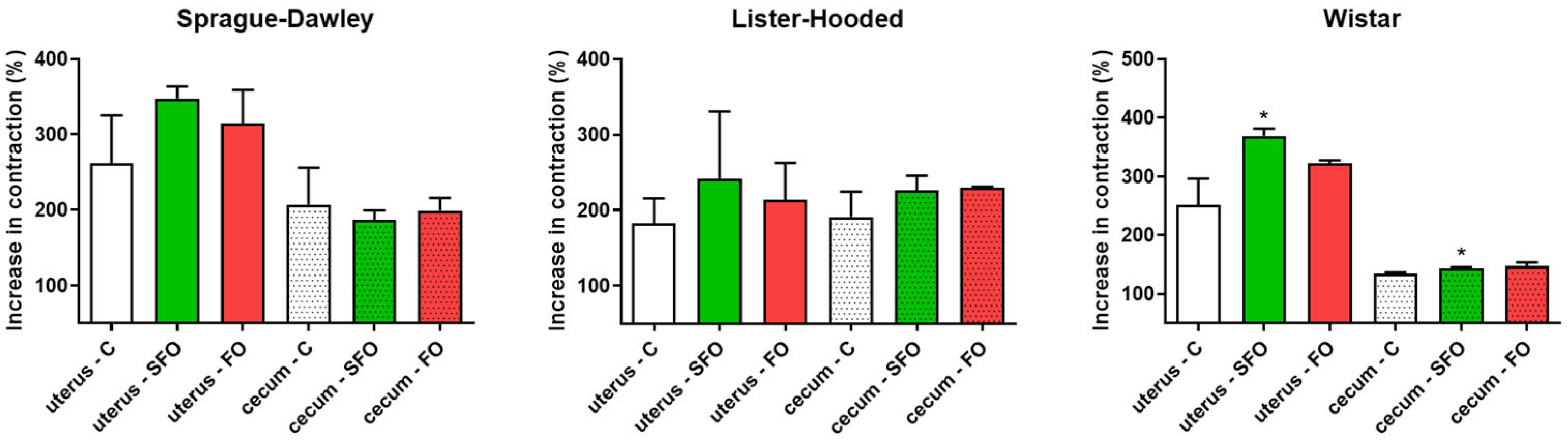
2.3. In Vitro Contractility
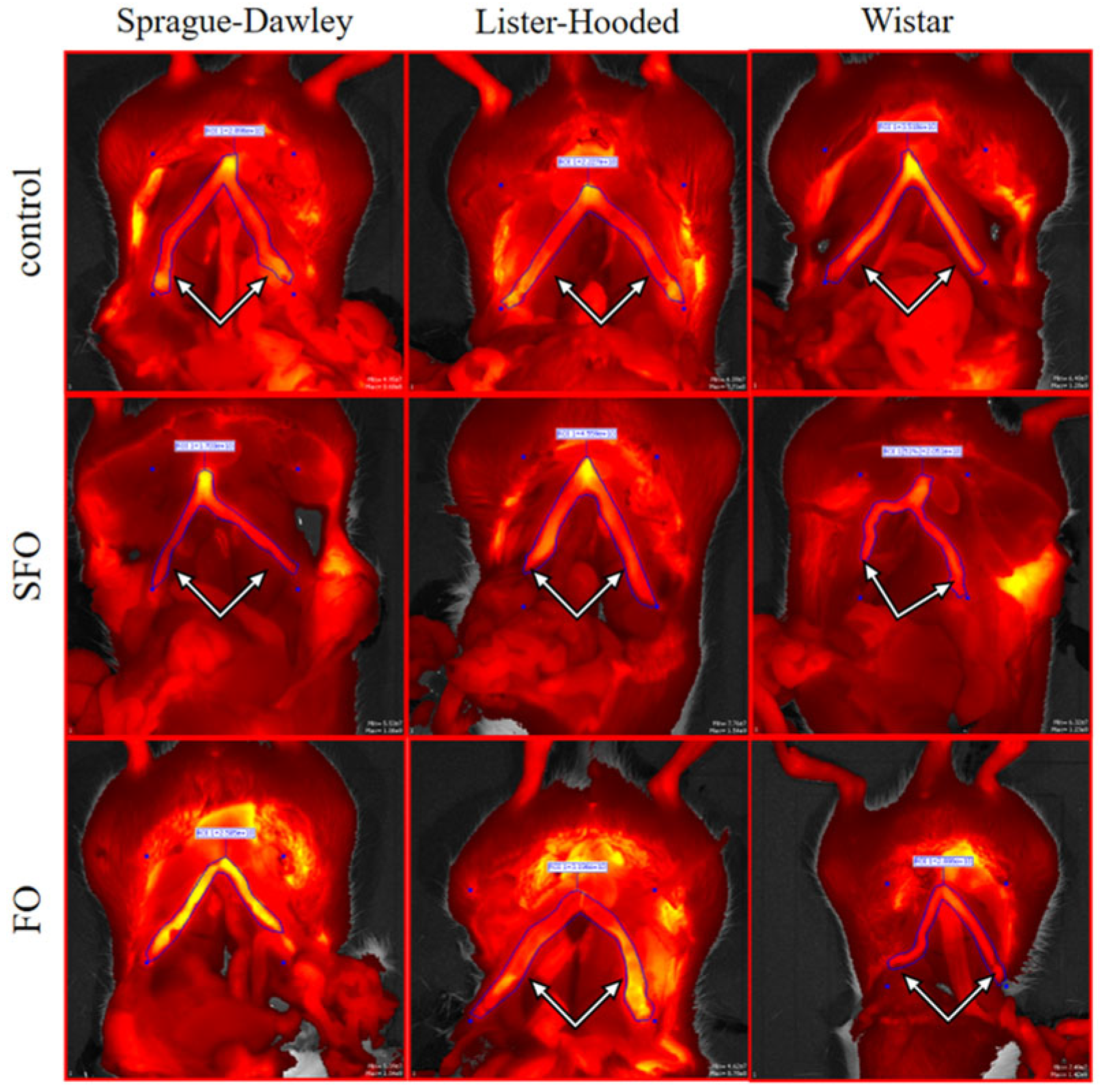
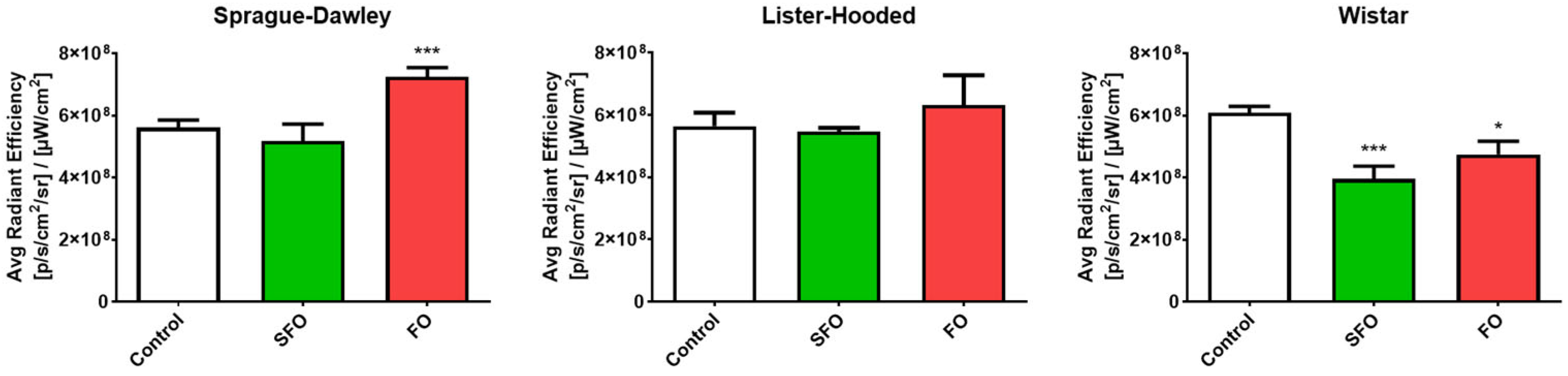
2.4. Uterine Activity of αvβ3 Integrin
3. Discussion
4. Materials and Methods
4.1. Housing and Handling of the Animals
4.2. Plasma Sample Collection and Hormone Analysis
4.3. Smooth Muscle Electromyographic Measurements
4.4. Contractility Studies in Isolated Organ Baths
4.5. In Vivo Imaging Protocol
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| ALA | α-linolenic acid |
| AUC | area under the curve |
| COX | cyclooxygenase |
| cpm | cycles per minute |
| DHA | docosahexaenoic acid |
| ELISA | enzyme-linked immunosorbent assay |
| EPA | eicosapentaenoic acid |
| FO | fish oil |
| MUFA | monounsaturated fatty acid |
| PCOS | polycystic ovary syndrome |
| PG | prostaglandin |
| PsDmax | maximum power spectrum density |
| PUFAs | polyunsaturated fatty acids |
| ROI | regions of interest |
| SFA | saturated fatty acid |
| SFO | sunflower oil |
| SMEMG | smooth muscle electromyography |
References
- Decandia, D.; Landolfo, E.; Sacchetti, S.; Gelfo, F.; Petrosini, L.; Cutuli, D. N-3 PUFA Improve Emotion and Cognition during Menopause: A Systematic Review. Nutrients 2022, 14, 1982. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 Long-Chain Polyunsaturated Fatty Acids, EPA and DHA: Bridging the Gap between Supply and Demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef]
- Liang, D.; Minikes, A.M.; Jiang, X. Ferroptosis at the Intersection of Lipid Metabolism and Cellular Signaling. Mol. Cell 2022, 82, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Taylor, D.S.; Yu-Poth, S.; Huth, P.; Moriarty, K.; Fishell, V.; Hargrove, R.L.; Zhao, G.; Etherton, T.D. Polyunsaturated Fatty Acids in the Food Chain in the United States. Am. J. Clin. Nutr. 2000, 71, 179S–188S. [Google Scholar] [CrossRef]
- Wathes, D.C.; Abayasekara, D.R.E.; Aitken, R.J. Polyunsaturated Fatty Acids in Male and Female Reproduction. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Covington, M.B. Omega-3 Fatty Acids. Am. Fam. Physician 2004, 70, 133–140. [Google Scholar]
- Qiu, B.; Zandkarimi, F.; Bezjian, C.T.; Reznik, E.; Soni, R.K.; Gu, W.; Jiang, X.; Stockwell, B.R. Phospholipids with Two Polyunsaturated Fatty Acyl Tails Promote Ferroptosis. Cell 2024, 187, 1177–1190.e18. [Google Scholar] [CrossRef]
- Benatti, P.; Nicolai, R.; Calvani, M.; Peluso, G. Polyunsaturated Fatty Acids: Biochemical, Nutritional and Epigenetic Properties. J. Am. Coll. Nutr. 2004, 23, 281–302. [Google Scholar] [CrossRef]
- Salek, M.; Clark, C.C.T.; Taghizadeh, M.; Jafarnejad, S. N-3 Fatty Acids as Preventive and Therapeutic Agents in Attenuating PCOS Complications. EXCLI J. 2019, 18, 558–575. [Google Scholar] [CrossRef]
- Leaver, H.A.; Howie, A.; Wilson, N.H. The Biosynthesis of the 3-Series Prostaglandins in Rat Uterus after Alpha-Linolenic Acid Feeding: Mass Spectroscopy of Prostaglandins E and F Produced by Rat Uteri in Tissue Culture. Prostaglandins Leukot. Essent. Fat. Acids 1991, 42, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Naraoka, Y.; Hosokawa, M.; Minato-Inokawa, S.; Sato, Y. Severity of Menstrual Pain Is Associated with Nutritional Intake and Lifestyle Habits. Healthcare 2023, 11, 1289. [Google Scholar] [CrossRef]
- Harel, Z. Dysmenorrhea in Adolescents. Ann. N. Y. Acad. Sci. 2008, 1135, 185–195. [Google Scholar] [CrossRef]
- Snipe, R.M.J.; Brelis, B.; Kappas, C.; Young, J.K.; Eishold, L.; Chui, J.M.; Vatvani, M.D.; Nigro, G.M.D.; Hamilton, D.L.; Convit, L.; et al. Omega-3 Long Chain Polyunsaturated Fatty Acids as a Potential Treatment for Reducing Dysmenorrhoea Pain: Systematic Literature Review and Meta-Analysis. Nutr. Diet. 2024, 81, 94–106. [Google Scholar] [CrossRef]
- Hashimoto, M.; Makino, N.; Inazumi, T.; Yoshida, R.; Sugimoto, T.; Tsuchiya, S.; Sugimoto, Y. Effects of an Ω3 Fatty Acid-Biased Diet on Luteolysis, Parturition, and Uterine Prostanoid Synthesis in Pregnant Mice. Biochem. Biophys. Res. Commun. 2022, 589, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Paulesu, L.; Bhattacharjee, J.; Bechi, N.; Romagnoli, R.; Jantra, S.; Ietta, F. Pro-Inflammatory Cytokines in Animal and Human Gestation. Curr. Pharm. Des. 2010, 16, 3601–3615. [Google Scholar] [CrossRef]
- Tung, K.T.S.; Wong, R.S.; Mak, R.T.W. Maternal N-3 PUFA Intake During Pregnancy and Perinatal Mental Health Problems: A Systematic Review of Recent Evidence. Curr. Nutr. Rep. 2023, 12, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Newberry, S.J.; Chung, M.; Booth, M.; Maglione, M.A.; Tang, A.M.; O’Hanlon, C.E.; Wang, D.D.; Okunogbe, A.; Huang, C.; Motala, A.; et al. Omega-3 Fatty Acids and Maternal and Child Health: An Updated Systematic Review. Evid. Rep./Technol. Assess. 2016, 224, 1–826. [Google Scholar] [CrossRef]
- Kim, P.Y.; Zhong, M.; Kim, Y.S.; Sanborn, B.M.; Allen, K.G.D. Long Chain Polyunsaturated Fatty Acids Alter Oxytocin Signaling and Receptor Density in Cultured Pregnant Human Myometrial Smooth Muscle Cells. PLoS ONE 2012, 7, e41708. [Google Scholar] [CrossRef]
- Baker, H.J.; Lindsey, J.R.; Wesibroth, S.H. The Laboratory Rat: Biology and Diseases; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1. [Google Scholar]
- Gáspár, R.; Földesi, I.; Havass, J.; Márki, A.; Falkay, G. Characterization of Late-Pregnant Rat Uterine Contraction via the Contractility Ratio in Vitro Significance of A1-Adrenoceptors. Life Sci. 2001, 68, 1119–1129. [Google Scholar] [CrossRef]
- Szucs, K.F.; Vigh, D.; Mirdamadi, M.; Samavati, R.; Barna, T.; Schaffer, A.; Alasaad, K.; Gaspar, R. Smooth Muscle Electromyography for Detecting Major Alterations in the Estrus Cycle in Rats. PLoS ONE 2024, 19, e0307932. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.R.; Blesson, C.S.; Fatima, I.; Kitchlu, S.; Jain, S.K.; Mehrotra, P.K.; Dwivedi, A. Expression of AVβ3 Integrin in Rat Endometrial Epithelial Cells and Its Functional Role during Implantation. Gen. Comp. Endocrinol. 2009, 160, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Carolina, V.; Mariangeles, C.; Delia, W.; Monica, G.; Mirta, K.; Claudio, B. Are Integrins and Ligands Correlated at Pig Placental Interface during Pregnancy? Reprod. Fertil. 2023, 4, e220079. [Google Scholar] [CrossRef] [PubMed]
- Surman, M.; Wilczak, M.; Bzowska, M.; Tylko, G.; Przybyło, M. The Proangiogenic Effects of Melanoma-Derived Ectosomes Are Mediated by Avβ5 Integrin Rather than Avβ3 Integrin. Cells 2024, 13, 1336. [Google Scholar] [CrossRef]
- Mohd Mokhtar, H.; Giribabu, N.; Salleh, N. Testosterone Down-Regulates Expression of Avβ3-Integrin, E-Cadherin and Mucin-1 during Uterine Receptivity Period in Rats (Testosteron Menindas Ekspresi Avβ3-Integrin, E-Cadherin Dan Mucin-1 Semasa Tempoh Reseptif Rahim Dalam Tikus). Sains Malays. 2018, 47, 2509–2517. [Google Scholar] [CrossRef]
- Lu, L.; Li, X.; Lv, L.; Xu, Y.; Wu, B.; Huang, C. Dietary and Serum N-3 PUFA and Polycystic Ovary Syndrome: A Matched Case–Control Study. Br. J. Nutr. 2022, 128, 114–123. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, L.; Bao, T.; Ge, J. Effectiveness of Omega-3 Fatty Acid for Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Reprod. Biol. Endocrinol. 2018, 16, 27. [Google Scholar] [CrossRef]
- Choi, J.E.; Park, Y. EPA and DHA, but Not ALA, Have Antidepressant Effects with 17β-Estradiol Injection via Regulation of a Neurobiological System in Ovariectomized Rats. J. Nutr. Biochem. 2017, 49, 101–109. [Google Scholar] [CrossRef]
- Muir, R.; Liu, G.; Khan, R.; Shmygol, A.; Quenby, S.; Gibson, R.A.; Muhlhausler, B.; Elmes, M. Maternal Obesity-Induced Decreases in Plasma, Hepatic and Uterine Polyunsaturated Fatty Acids during Labour Is Reversed through Improved Nutrition at Conception. Sci. Rep. 2018, 8, 3389. [Google Scholar] [CrossRef]
- Kothencz, A.; Hajagos-Tóth, J.; Szucs, K.F.; Schaffer, A.; Gáspár, R. α-Tocopherol Potentiates the Cervical Resistance Decreasing Effects of COX Inhibitors in Pregnant Rats: The Putative Role of Cyclooxygenase-2 Inhibition. J. Pharmacol. Exp. Ther. 2019, 368, 292–298. [Google Scholar] [CrossRef]
- Sims, S.M.; Daniel, E.E.; Garfield, R.E. Improved Electrical Coupling in Uterine Smooth Muscle Is Associated with Increased Numbers of Gap Junctions at Parturition. J. Gen. Physiol. 1982, 80, 353–375. [Google Scholar] [CrossRef]
- Pribék, I.K.; Szűcs, K.F.; Süle, M.; Grosz, G.; Ducza, E.; Vigh, D.; Tóth, E.; Janka, Z.; Kálmán, J.; Datki, Z.L.; et al. Detection of Acute Stress by Smooth Muscle Electromyography: A Translational Study on Rat and Human. Life Sci. 2021, 277, 119492. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Teresa, M.; Cai, J.; Zhang, C.; Wong, S.; Yan, Z.; Khojasteh, S.C.; Zhang, D. Comparative Assessment for Rat Strain Differences in Metabolic Profiles of 14 Drugs in Wistar Han and Sprague Dawley Hepatocytes. Xenobiotica 2021, 51, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.K.G.; Hulan, H.W.; Trenholm, H.L.; Corner, A.H. Growth, Lipid Metabolism and Pathology of Two Strains of Rats Fed High Fat Diets. J. Nutr. 1979, 109, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Sakai, N.; Kim, H.-S.; Ishizuka, M.; Kazusaka, A.; Fujita, S. Strain differences in diazepam metabolism at its three metabolic sites in sprague-dawley, brown norway, dark agouti, and wistar strain rats. Drug Metab. Dispos. 2004, 32, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Szucs, K.F.; Grosz, G.; Süle, M.; Nagy, A.; Tiszai, Z.; Samavati, R.; Gáspár, R. Identification of Myoelectric Signals of Pregnant Rat Uterus: New Method to Detect Myometrial Contraction. Croat. Med. J. 2017, 58, 141–148. [Google Scholar] [CrossRef]
- Szucs, K.F.; Nagy, A.; Grosz, G.; Tiszai, Z.; Gaspar, R. Correlation between Slow-Wave Myoelectric Signals and Mechanical Contractions in the Gastrointestinal Tract: Advanced Electromyographic Method in Rats. J. Pharmacol. Toxicol. Methods 2016, 82, 37–44. [Google Scholar] [CrossRef]
- Schaffer, A.; Ducza, E.; Bódi, N.; Bagyánszki, M.; Szalai, Z.; Mirdamadi, M.; Barna, T.; Szűcs, K.F.; Gáspár, R. The Ontogenies of Endometrial and Myometrial Leptin and Adiponectin Receptors in Pregnant Rats: Their Putative Impact on Uterine Contractility. Life Sci. 2022, 297, 120465. [Google Scholar] [CrossRef]


| SFO | FO | |||
|---|---|---|---|---|
| Daily Dose (mg) | Total Dose (g) | Daily Dose (mg) | Total Dose (g) | |
| SFA | 94.90 | 1.90 | 268.40 | 5.37 |
| MUFA | 302.50 | 6.05 | 351.80 | 7.04 |
| n-6 PUFAs | 521.70 | 10.43 | 61.40 | 1.23 |
| Total n-3 PUFAs | 0.83 | 0.02 | 238.50 | 4.71 |
| » ALA | 0.83 | 0.02 | 20.7 | 0.41 |
| » EPA | - | - | 85.38 | 1.71 |
| » DHA | - | - | 115.18 | 2.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szucs, K.F.; Vigh, D.; Mirdamadi, S.; Samavati, R.; Schaffer, A.; Barna, T.; Tóth, T.; Bázár, G.; Baranyay, H.; Gaspar, R. Omega-3 Fatty Acid Consumption Alters Uterine Contraction: A Comparative Study on Different Breeds of Rats. Int. J. Mol. Sci. 2025, 26, 5221. https://doi.org/10.3390/ijms26115221
Szucs KF, Vigh D, Mirdamadi S, Samavati R, Schaffer A, Barna T, Tóth T, Bázár G, Baranyay H, Gaspar R. Omega-3 Fatty Acid Consumption Alters Uterine Contraction: A Comparative Study on Different Breeds of Rats. International Journal of Molecular Sciences. 2025; 26(11):5221. https://doi.org/10.3390/ijms26115221
Chicago/Turabian StyleSzucs, Kalman F., Dora Vigh, Seyedmohsen Mirdamadi, Reza Samavati, Annamaria Schaffer, Tamara Barna, Tamás Tóth, György Bázár, Henrik Baranyay, and Robert Gaspar. 2025. "Omega-3 Fatty Acid Consumption Alters Uterine Contraction: A Comparative Study on Different Breeds of Rats" International Journal of Molecular Sciences 26, no. 11: 5221. https://doi.org/10.3390/ijms26115221
APA StyleSzucs, K. F., Vigh, D., Mirdamadi, S., Samavati, R., Schaffer, A., Barna, T., Tóth, T., Bázár, G., Baranyay, H., & Gaspar, R. (2025). Omega-3 Fatty Acid Consumption Alters Uterine Contraction: A Comparative Study on Different Breeds of Rats. International Journal of Molecular Sciences, 26(11), 5221. https://doi.org/10.3390/ijms26115221






