Automated Behavioral Analysis of Schizophrenia-like Phenotypes in Repeated MK-801-Treated Mice Using IntelliCage
Abstract
1. Introduction
2. Results
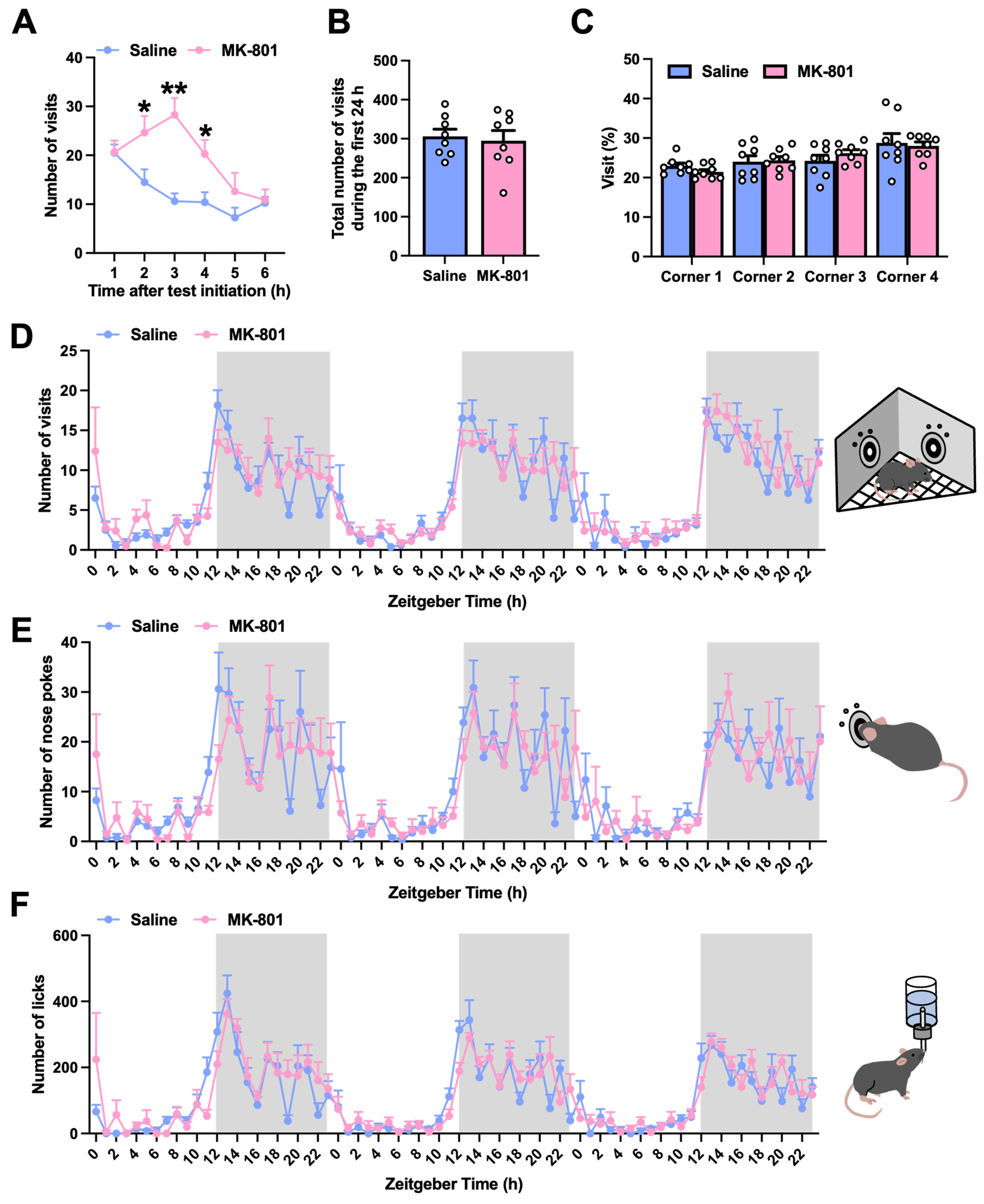
2.1. Exploratory Behavior and Circadian Activity in Repeated MK-801-Treated Mice
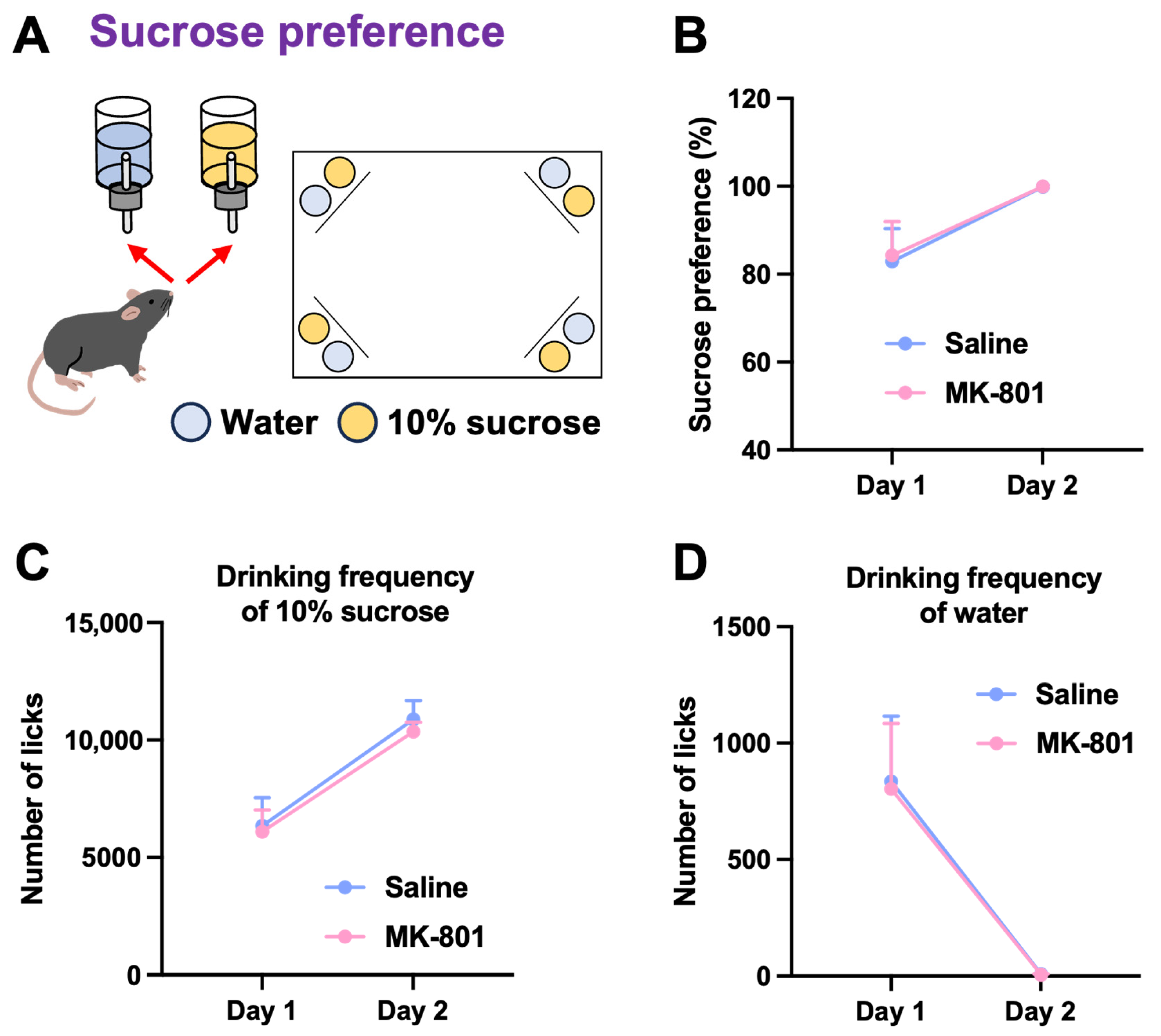
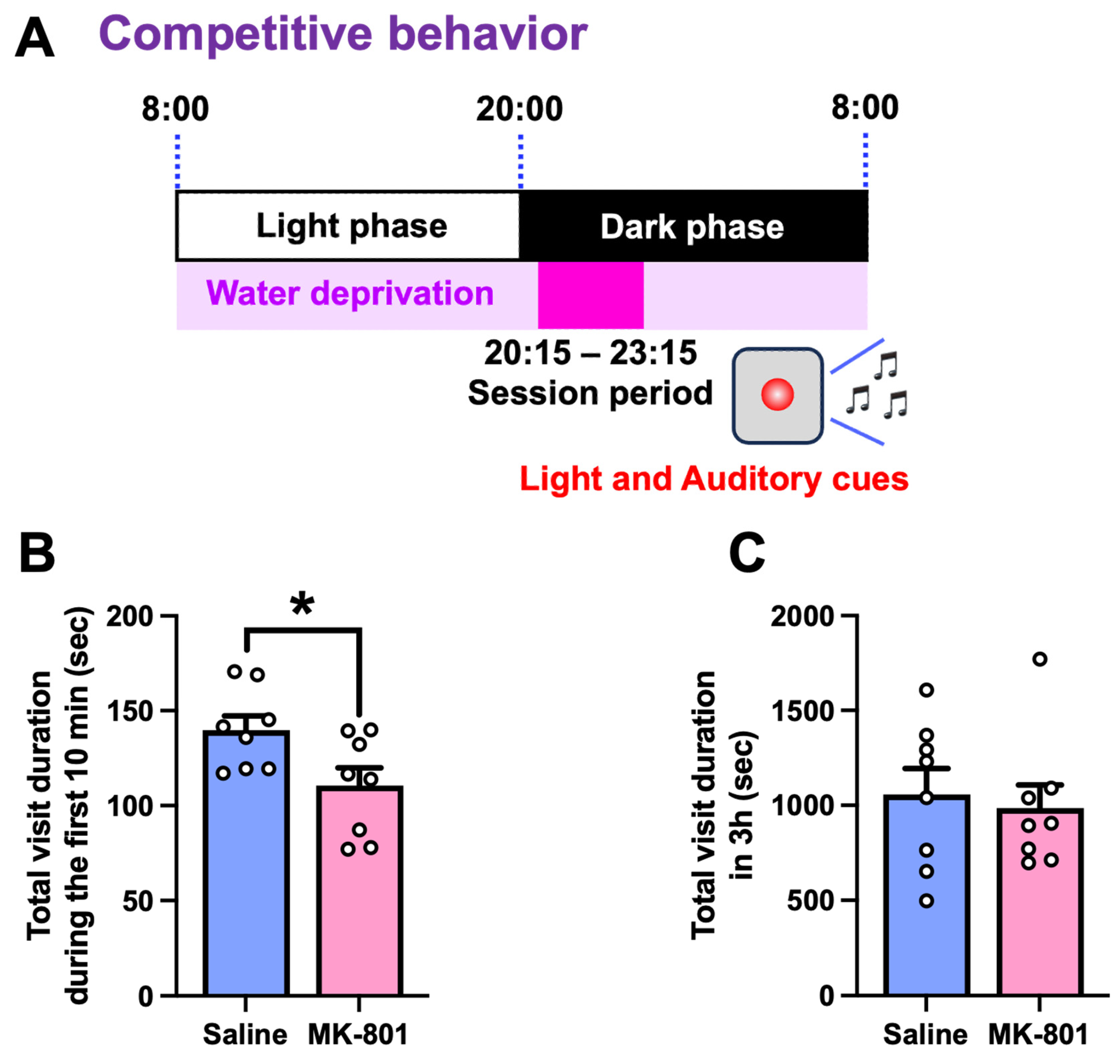
2.2. Sucrose Preference and Competitive Behavior in Repeated MK-801-Treated Mice
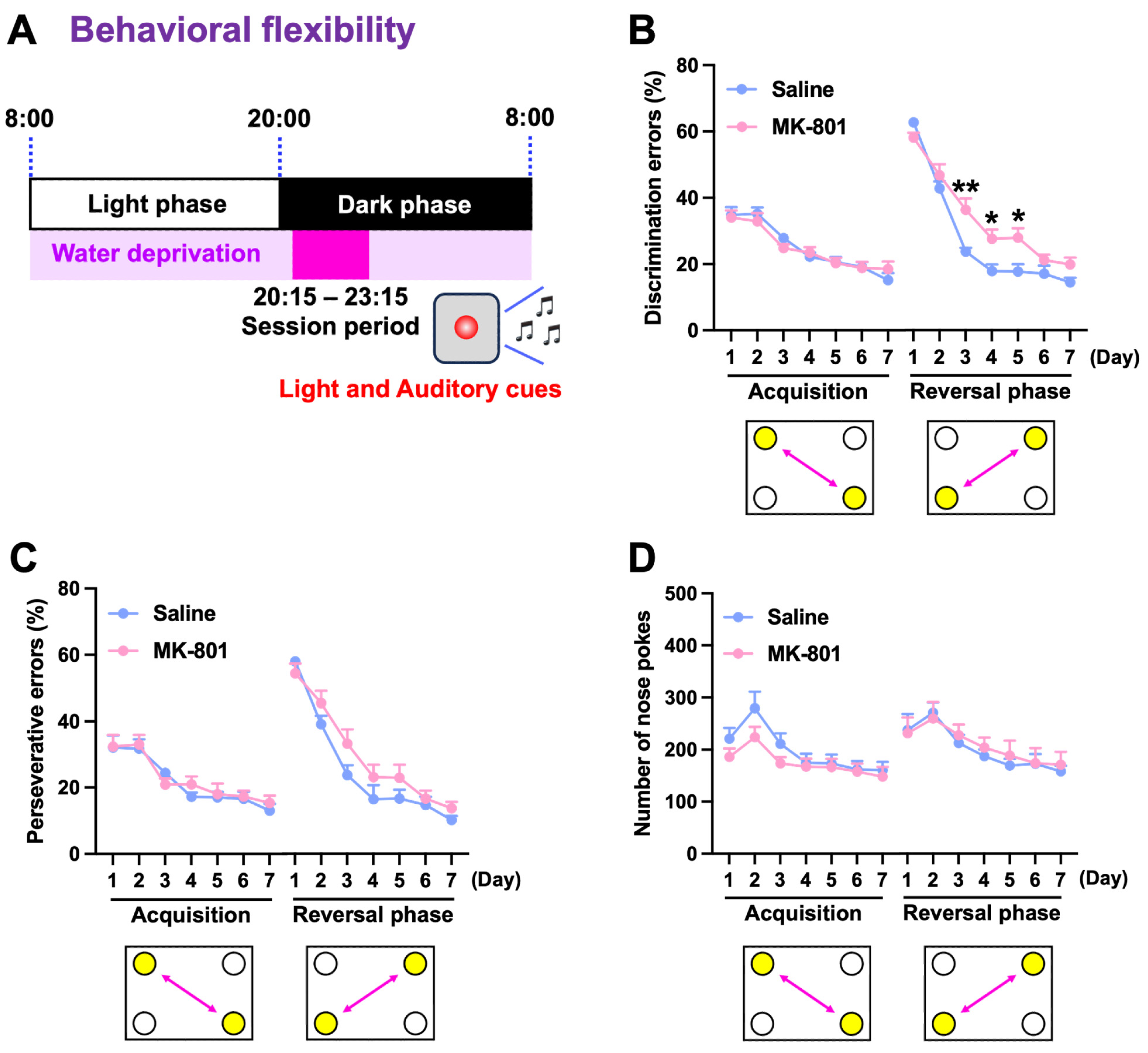
2.3. Cognitive Flexibility in Repeated MK-801-Treated Mice
3. Discussion
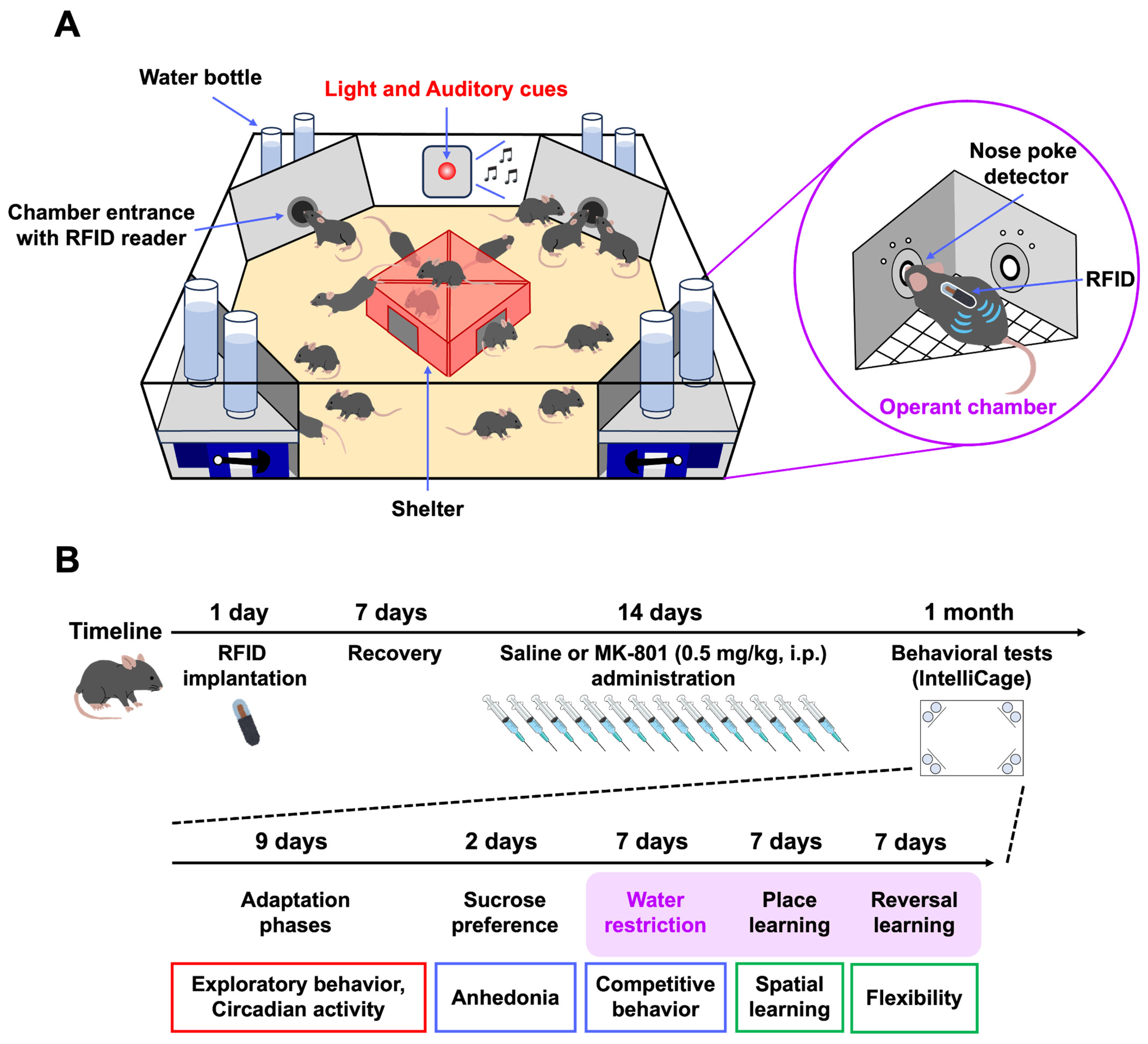
4. Materials and Methods
4.1. Mice
4.2. Drug Administration
4.3. IntelliCage
4.4. Behavioral Tests
4.4.1. Adaptation Phase
4.4.2. Sucrose Preference Test
4.4.3. Competition Test
4.4.4. Behavioral Flexibility Test
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NMDA | N-methyl-D-aspartate |
| RFID | radio frequency identification |
References
- McCutcheon, R.A.; Reis Marques, T.; Howes, O.D. Schizophrenia-An Overview. JAMA Psychiatry 2020, 77, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Uno, Y.; Coyle, J.T. Glutamate hypothesis in schizophrenia. Psychiatry Clin. Neurosci. 2019, 73, 204–215. [Google Scholar] [CrossRef]
- Janus, A.; Lustyk, K.; Pytka, K. MK-801 and cognitive functions: Investigating the behavioral effects of a non-competitive NMDA receptor antagonist. Psychopharmacology 2023, 240, 2435–2457. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Tian, B.; Liu, H.; Wu, S.; Wang, W. The effects and mechanism of environmental enrichment on MK-801 induced cognitive impairment in rodents with schizophrenia. Front. Cell Neurosci. 2022, 16, 1024649. [Google Scholar] [CrossRef]
- Liang, J.Q.; Chen, X.; Cheng, Y. Paeoniflorin Rescued MK-801-Induced Schizophrenia-Like Behaviors in Mice via Oxidative Stress Pathway. Front. Nutr. 2022, 9, 870032. [Google Scholar] [CrossRef]
- Kubota, H.; Kunisawa, K.; Niijima, M.; Hirakawa, M.; Mori, Y.; Hasegawa, M.; Fujigaki, S.; Fujigaki, H.; Yamamoto, Y.; Saito, K.; et al. Deficiency of kynurenine 3-monooxygenase exacerbates impairment of prepulse inhibition induced by phencyclidine. Biochem. Biophys. Res. Commun. 2022, 629, 142–151. [Google Scholar] [CrossRef]
- Hasegawa, M.; Niijima, M.; Kunisawa, K.; Teshigawara, T.; Kubota, H.; Fujigaki, S.; Fujigaki, H.; Yamamoto, Y.; Kim, H.C.; Saito, K.; et al. Maternal immune activation induces neurodevelopmental impairments of adult offspring through alterations in tryptophane-kynurenine pathway in the placenta. Biochem. Biophys. Res. Commun. 2024, 737, 150922. [Google Scholar] [CrossRef]
- Lipp, H.P.; Krackow, S.; Turkes, E.; Benner, S.; Endo, T.; Russig, H. IntelliCage: The development and perspectives of a mouse- and user-friendly automated behavioral test system. Front. Behav. Neurosci. 2023, 17, 1270538. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, C.; Rigoli, F.; Averbeck, B.; Shergill, S.S. The value of novelty in schizophrenia. Schizophr. Res. 2018, 192, 287–293. [Google Scholar] [CrossRef]
- Martin, E.A.; Li, L.Y.; Castro, M.K. Electrophysiological responses to images ranging in motivational salience: Attentional abnormalities associated with schizophrenia-spectrum disorder risk. Sci. Rep. 2020, 10, 4578. [Google Scholar] [CrossRef]
- Kapur, S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 2003, 160, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.H.V. The Temporality of Aberrant Salience and Schizophrenia. Front. Integr. Neurosci. 2022, 16, 925716. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; Schriever, S.C.; Kullmann, S.; Zimprich, A.; Peter, A.; Gailus-Durner, V.; Fuchs, H.; Hrabe de Angelis, M.; Wurst, W.; Tschop, M.H.; et al. Diabetes type 2 risk gene Dusp8 is associated with altered sucrose reward behavior in mice and humans. Brain Behav. 2021, 11, e01928. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, B.J., 3rd; Jones, B.J.; McFalls, J.A., Jr.; Pisa, A.M. Social class and type of schizophrenia. Eur. Psychiatry 2006, 21, 233–237. [Google Scholar] [CrossRef]
- McLeod, H.J.; Coertze, L.; Moore, E. The relationship between insight and social rank appraisals in people with schizophrenia. Br. J. Clin. Psychol. 2009, 48 Pt 3, 329–334. [Google Scholar] [CrossRef]
- Floresco, S.B.; Zhang, Y.; Enomoto, T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav. Brain Res. 2009, 204, 396–409. [Google Scholar] [CrossRef]
- Izquierdo, A.; Brigman, J.L.; Radke, A.K.; Rudebeck, P.H.; Holmes, A. The neural basis of reversal learning: An updated perspective. Neuroscience 2017, 345, 12–26. [Google Scholar] [CrossRef]
- Waltz, J.A.; Gold, J.M. Probabilistic reversal learning impairments in schizophrenia: Further evidence of orbitofrontal dysfunction. Schizophr. Res. 2007, 93, 296–303. [Google Scholar] [CrossRef]
- Reddy, L.F.; Waltz, J.A.; Green, M.F.; Wynn, J.K.; Horan, W.P. Probabilistic Reversal Learning in Schizophrenia: Stability of Deficits and Potential Causal Mechanisms. Schizophr. Bull. 2016, 42, 942–951. [Google Scholar] [CrossRef]
- Hao, K.; Chen, F.; Xu, S.; Xiong, Y.; Xu, R.; Huang, H.; Shu, C.; Lv, Y.; Wang, G.; Wang, H. Cognitive impairment following maternal separation in rats mediated by the NAD+/SIRT3 axis via modulation of hippocampal synaptic plasticity. Transl. Psychiatry 2025, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Van den Buuse, M. Modeling the positive symptoms of schizophrenia in genetically modified mice: Pharmacology and methodology aspects. Schizophr. Bull. 2010, 36, 246–270. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, D.H.; Gardner, W.; Marchese, C.; Balzinger, M.; Ramanathan, C.; Schott, M.; Rozov, S.; Veleanu, M.; Vestring, S.; Normann, C.; et al. Prefrontal cortex molecular clock modulates development of depression-like phenotype and rapid antidepressant response in mice. Nat. Commun. 2024, 15, 7257. [Google Scholar] [CrossRef] [PubMed]
- Horigane, S.I.; Ozawa, Y.; Zhang, J.; Todoroki, H.; Miao, P.; Haijima, A.; Yanagawa, Y.; Ueda, S.; Nakamura, S.; Kakeyama, M.; et al. A mouse model of Timothy syndrome exhibits altered social competitive dominance and inhibitory neuron development. FEBS Open Bio 2020, 10, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubota, H.; Zhang, X.; Khalili, M.; Zhou, X.; Wen, Y.; Nagai, T. Automated Behavioral Analysis of Schizophrenia-like Phenotypes in Repeated MK-801-Treated Mice Using IntelliCage. Int. J. Mol. Sci. 2025, 26, 5184. https://doi.org/10.3390/ijms26115184
Kubota H, Zhang X, Khalili M, Zhou X, Wen Y, Nagai T. Automated Behavioral Analysis of Schizophrenia-like Phenotypes in Repeated MK-801-Treated Mice Using IntelliCage. International Journal of Molecular Sciences. 2025; 26(11):5184. https://doi.org/10.3390/ijms26115184
Chicago/Turabian StyleKubota, Hisayoshi, Xinjian Zhang, Masoumeh Khalili, Xinzhu Zhou, Yu Wen, and Taku Nagai. 2025. "Automated Behavioral Analysis of Schizophrenia-like Phenotypes in Repeated MK-801-Treated Mice Using IntelliCage" International Journal of Molecular Sciences 26, no. 11: 5184. https://doi.org/10.3390/ijms26115184
APA StyleKubota, H., Zhang, X., Khalili, M., Zhou, X., Wen, Y., & Nagai, T. (2025). Automated Behavioral Analysis of Schizophrenia-like Phenotypes in Repeated MK-801-Treated Mice Using IntelliCage. International Journal of Molecular Sciences, 26(11), 5184. https://doi.org/10.3390/ijms26115184





