Evaluation of Antibacterial, Antifungal, Antiviral, and Anticancer Potential of Extract from the Fern Dryopteris erythrosora
Abstract
1. Introduction
2. Results and Discussion
2.1. Bioactive Compounds and Antioxidant Potential of DEE
2.2. Antimicrobial Potential of DEE
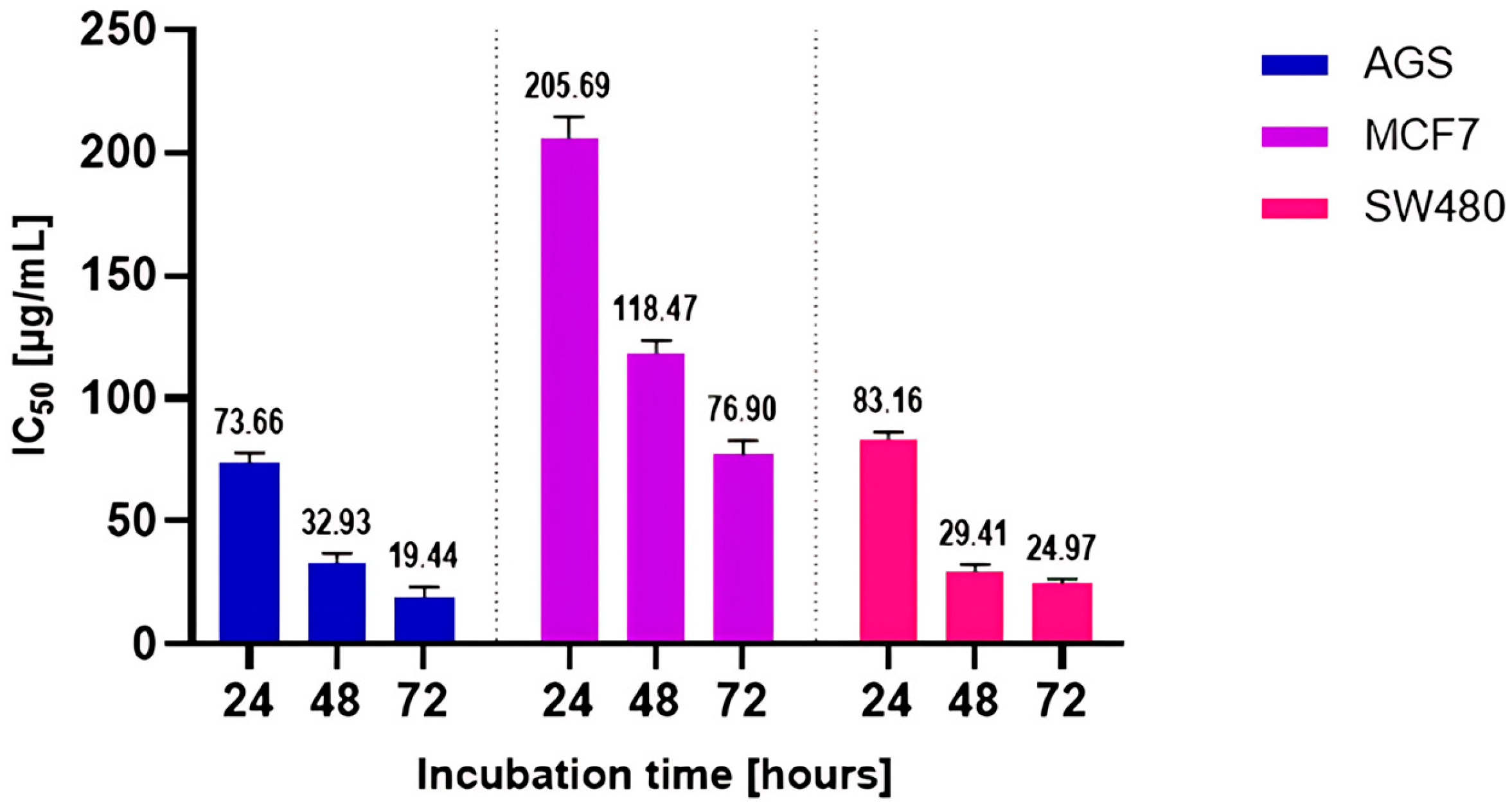
2.3. Effect of DEE Against Human Cancer Cell Lines
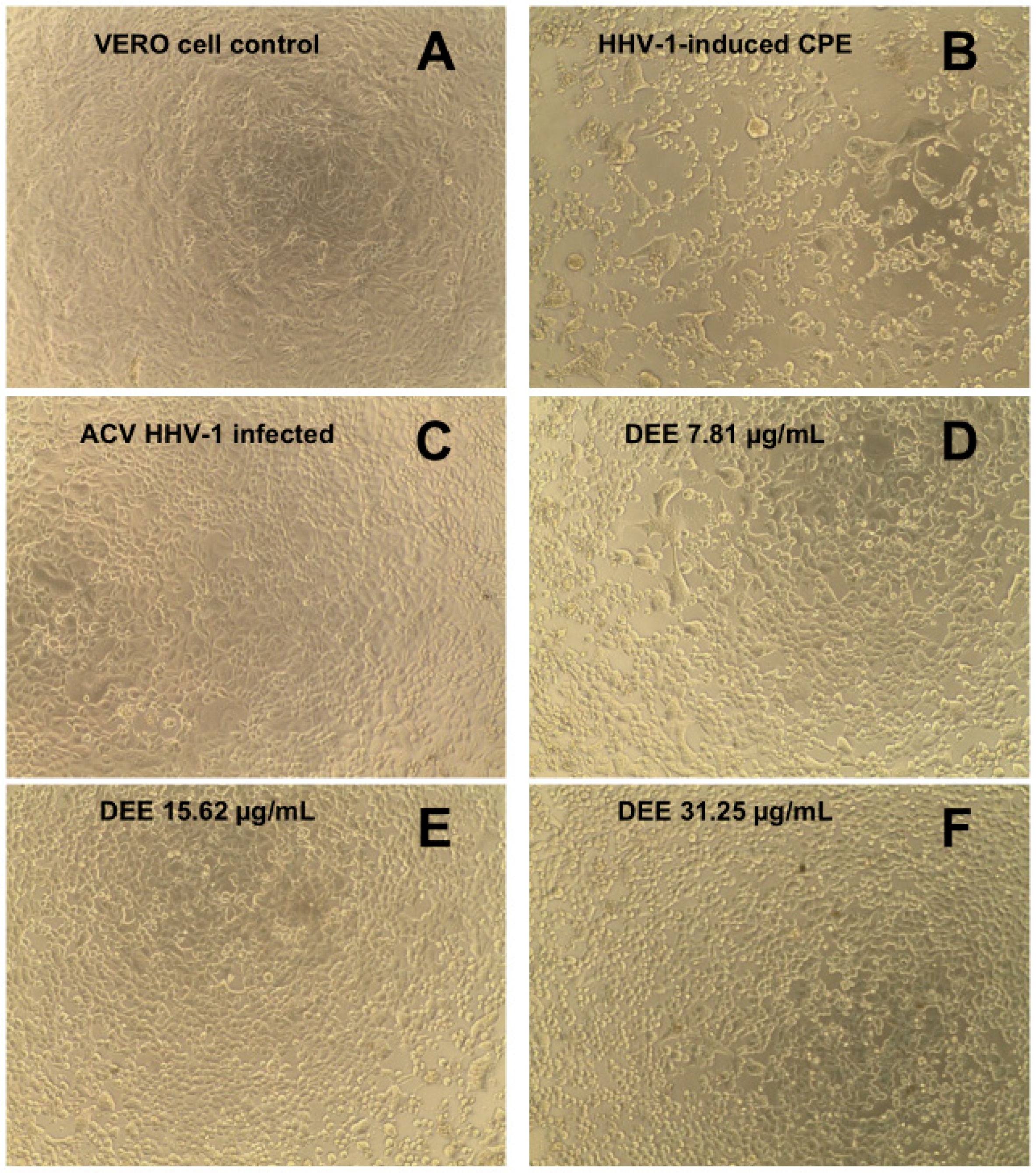
2.4. Cytotoxicity and Antiviral Activity of DEE
3. Materials and Methods
3.1. Preparation of Dryopteris erythrosora Extract (DEE)
3.2. Total Phenolic Content (TPC)
3.3. Total Flavonoid Content (TFC)
3.4. Ferric Reducing Antioxidant Power (FRAP) Assay
3.5. Antibacterial and Antifungal Activity
3.6. Anticancer Activity
3.7. Hemolytic Activity
3.8. Cytotoxicity and Antiviral Properties
3.8.1. Cytotoxicity Testing
3.8.2. Antiviral Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dekebo, A. Introductory Chapter: Plant Extracts. In Plant Extracts; IntechOpen: London, UK, 2019. [Google Scholar]
- Shu, Y.-Z. Recent Natural Products Based Drug Development: A Pharmaceutical Industry Perspective. J. Nat. Prod. 1998, 61, 1053–1071. [Google Scholar] [CrossRef] [PubMed]
- Rates, S.M.K. Plants as Source of Drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul Maqsummiya, Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-Derived Bioactive Compounds in Colon Cancer Treatment: An Updated Review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef]
- El Menyiy, N.; Guaouguaou, F.-E.; El Baaboua, A.; El Omari, N.; Taha, D.; Salhi, N.; Shariati, M.A.; Aanniz, T.; Benali, T.; Zengin, G.; et al. Phytochemical Properties, Biological Activities and Medicinal Use of Centaurium Erythraea Rafn. J. Ethnopharmacol. 2021, 276, 114171. [Google Scholar] [CrossRef]
- Gupta, A.; Atkinson, A.N.; Pandey, A.K.; Bishayee, A. Health-promoting and Disease-mitigating Potential of Verbascum thapsus L. (Common Mullein): A Review. Phytother. Res. 2022, 36, 1507–1522. [Google Scholar] [CrossRef]
- Kubica, P.; Szopa, A.; Dominiak, J.; Luczkiewicz, M.; Ekiert, H. Verbena officinalis (Common Vervain)—A Review on the Investigations of This Medicinally Important Plant Species. Planta Med. 2020, 86, 1241–1257. [Google Scholar] [CrossRef]
- Cao, H.; Chai, T.-T.; Wang, X.; Morais-Braga, M.F.B.; Yang, J.-H.; Wong, F.-C.; Wang, R.; Yao, H.; Cao, J.; Cornara, L.; et al. Phytochemicals from Fern Species: Potential for Medicine Applications. Phytochem. Rev. 2017, 16, 379–440. [Google Scholar] [CrossRef]
- Muhammad, M.; Ismail, Z.S.; Schneider, H.; Hawkins, J.A. Medicinal Use of Ferns: An Ethnobotanical Review. Sains Malays. 2020, 49, 1003–1014. [Google Scholar] [CrossRef]
- Cao, J.; Xia, X.; Chen, X.; Xiao, J.; Wang, Q. Characterization of Flavonoids from Dryopteris Erythrosora and Evaluation of Their Antioxidant, Anticancer and Acetylcholinesterase Inhibition Activities. Food Chem. Toxicol. 2013, 51, 242–250. [Google Scholar] [CrossRef]
- Yun, S.; Bai, J. Synergistic Antimicrobial Effects of Dryopteris erythrosora Extract and Mild Heat Treatment against Staphylococcus aureus. LWT 2023, 173, 114260. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, S.; Zabihi, E.; Abedian, Z.; Pourbagher, R.; Motevalizadeh Ardekani, A.; Mostafazadeh, A.; Akhavan-Niaki, H. Total Phenolic and Flavonoid Contents of Aqueous Extract of Stinging Nettle and In Vitro Antiproliferative Effect on Hela and BT-474 Cell Lines. Int. J. Mol. Cell. Med. 2014, 3, 102–107. [Google Scholar] [PubMed]
- Mohammed, F.S.; Kına, E.; Uysal, İ.; Sevindik, M. Total Phenolic, Flavonoid Contents, Antioxidant and Antimicrobial Activities of Hesperis pendula. Prospect. Pharm. Sci. 2023, 21, 57–61. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant Activity, Total Phenolic and Total Flavonoid Contents of Whole Plant Extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef]
- Sulaiman, C.T.; Balachandran, I. Total Phenolics and Total Flavonoids in Selected Indian Medicinal Plants. Indian J. Pharm. Sci. 2012, 74, 258–260. [Google Scholar] [CrossRef]
- Kim, E.-J.; Choi, J.-Y.; Yu, M.-R.; Kim, M.-Y.; Lee, S.-H.; Lee, B.-H. Total Polyphenols, Total Flavonoid Contents, and Antioxidant Activity of Korean Natural and Medicinal Plants. Korean J. Food Sci. Technol. 2012, 44, 337–342. [Google Scholar] [CrossRef]
- Rani, A.; Uzair, M.; Ali, S.; Qamar, M.; Ahmad, N.; Abbas, M.W.; Esatbeyoglu, T. Dryopteris Juxtapostia Root and Shoot: Determination of Phytochemicals; Antioxidant, Anti-Inflammatory, and Hepatoprotective Effects; and Toxicity Assessment. Antioxidants 2022, 11, 1670. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Alam, F.; Khan, S.H.A.; Asad, M.H.H. Bin Phytochemical, Antimicrobial, Antioxidant and Enzyme Inhibitory Potential of Medicinal Plant Dryopteris ramosa (Hope) C. Chr. BMC Complement. Med. Ther. 2021, 21, 197. [Google Scholar] [CrossRef]
- Baloch, R.; Uzair, M.; Chauhdary, B.A.; Hayat, m.m. Phytochemical Analysis, Antioxidant and Cytotoxic Activities of Dryopteris ramosa. Biomed. Res. 2019, 30, 764–769. [Google Scholar]
- Wang, Y.; Liu, B.; Wang, X.; Fan, Y. Comparison of Constituents and Antioxidant Activity of Above-Ground and Underground Parts of Dryopteris Crassirhizoma Nakai Based on HS-SPME-GC-MS and UPLC/Q-TOF-MS. Molecules 2022, 27, 4991. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, H.; Sonboli, A.; Mahmoodi Kordi, F.; Dehghan, H.; Bahadori, M.B. Cytotoxicity, Antioxidant Activity and Phenolic Content of Eight Fern Species, from North of Iran. Pharm. Sci. 2015, 21, 18–24. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, J.; Dai, X.; Chen, X.; Wang, Q. Flavonoid Contents and Free Radical Scavenging Activity of Extracts from Leaves, Stems, Rachis and Roots of Dryopteris erythrosora. Iran. J. Pharm. 2011, 11, 991–997. [Google Scholar]
- Chang, Y.; Hu, J.L.; Jiang, S.J.; Qiang, S. Study on the Distribution and the Total Flavonoids Content of Medicial Pteridophytes in Nanjing Zijin Mountain. J. Northeast Agric. Univ. 2005, 36, 320–323. [Google Scholar]
- Kwon, D.-Y.; Kang, O.H.; Choi, J.-G.; Lee, Y.-S.; Oh, Y.-C.; Chae, H.-S.; Lee, G.-H.; Park, P.-S.; Kim, Y.-C.; Sohn, D.H.; et al. Antibacterial Effect of Dryopteris crassirhizoma against Methicillin-Resistant Staphylococcus aureus. Fitoterapia 2007, 78, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Takuli, P.; Khulbe, K.; Kumar, P.; Parki, A.; Syed, A.; Elgorban, A.M. Phytochemical Profiling, Antioxidant and Antibacterial Efficacy of a Native Himalayan Fern: Woodwardia unigemmata (Makino) Nakai. Saudi J. Biol. Sci. 2020, 27, 1961–1967. [Google Scholar] [CrossRef]
- Kuete, V.; Efferth, T. Cameroonian Medicinal Plants: Pharmacology and Derived Natural Products. Front. Pharmacol. 2010, 1, 123. [Google Scholar] [CrossRef]
- Silva, A.C.O.; Santana, E.F.; Saraiva, A.M.; Coutinho, F.N.; Castro, R.H.A.; Pisciottano, M.N.C.; Amorim, E.L.C.; Albuquerque, U.P. Which Approach Is More Effective in the Selection of Plants with Antimicrobial Activity? Evid. -Based Complement. Altern. Med. 2013, 2013, 308980. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Malca-García, G.; Glenn, A.; Sharon, D.; Chait, G.; Díaz, D.; Pourmand, K.; Jonat, B.; Somogy, S.; Guardado, G.; et al. Minimum Inhibitory Concentrations of Medicinal Plants Used in Northern Peru as Antibacterial Remedies. J. Ethnopharmacol. 2010, 132, 101–108. [Google Scholar] [CrossRef]
- Kang, C.-G.; Hah, D.-S.; Kim, C.-H.; Kim, Y.-H.; Kim, E.-K.; Kim, J.-S. Evaluation of Antimicrobial Activity of the Methanol Extracts from 8 Traditional Medicinal Plants. Toxicol. Res. 2011, 27, 31–36. [Google Scholar] [CrossRef]
- McMurray, R.L.; Ball, M.E.E.; Tunney, M.M.; Corcionivoschi, N.; Situ, C. Antibacterial Activity of Four Plant Extracts Extracted from Traditional Chinese Medicinal Plants against Listeria monocytogenes, Escherichia coli, and Salmonella enterica Subsp. Enterica Serovar Enteritidis. Microorganisms 2020, 8, 962. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Malca, G.; Glenn, A.; Sharon, D.; Nilsen, B.; Parris, B.; Dubose, D.; Ruiz, D.; Saleda, J.; Martinez, M.; et al. Toxicity of Medicinal Plants Used in Traditional Medicine in Northern Peru. J. Ethnopharmacol. 2011, 137, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Zia-ur-Rehman; Rasheed, H.M.; Bashir, K.; Gurgul, A.; Wahid, F.; Che, C.-T.; Shahzadi, I.; Khan, T. UHPLC-MS/MS-GNPS Based Phytochemical Investigation of Dryopteris ramosa (Hope) C. Chr. and Evaluation of Cytotoxicity against Liver and Prostate Cancer Cell Lines. Heliyon 2022, 8, e11286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J.; Xu, W.; Xia, Y.; Zhu, H.; Wang, J.; Li, Y.; Wang, G.; Zhang, Y.; Chen, N. Undescribed Phloroglucinol Derivatives with Antiviral Activities from Dryopteris atrata (Wall. Ex Kunze) Ching. Phytochemistry 2023, 208, 113585. [Google Scholar] [CrossRef]
- Maryam, M.; Te, K.K.; Wong, F.C.; Chai, T.T.; Low, G.K.K.; Gan, S.C.; Chee, H.Y. Antiviral Activity of Traditional Chinese Medicinal Plants Dryopteris crassirhizoma and Morus alba against Dengue virus. J. Integr. Agric. 2020, 19, 1085–1096. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, M.; He, M.; Gu, M.; Lin, M.; Zhang, G. Selection of Solvent for Extraction of Antioxidant Components from Cynanchum auriculatum, Cynanchum bungei, and Cynanchum wilfordii Roots. Food Sci. Nutr. 2019, 7, 1337–1343. [Google Scholar] [CrossRef]
- Olech, M.; Łyko, L.; Nowak, R. Influence of Accelerated Solvent Extraction Conditions on the LC-ESI-MS/MS Polyphenolic Profile, Triterpenoid Content, and Antioxidant and Anti-Lipoxygenase Activity of Rhododendron luteum Sweet Leaves. Antioxidants 2020, 9, 822. [Google Scholar] [CrossRef]
- Wayne, P.A. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 28. [Google Scholar]
- Khabnadideh, S.; Rezaei, Z.; Pakshir, K.; Zomorodian, K.; Ghafari, N. Synthesis and Antifungal Activity of Benzimidazole, Benzotriazole and Aminothiazole Derivatives. Res. Pharm. Sci. 2012, 7, 65–72. [Google Scholar]
- Świątek, Ł.; Sieniawska, E.; Sinan, K.I.; Maciejewska-Turska, M.; Boguszewska, A.; Polz-Dacewicz, M.; Senkardes, I.; Guler, G.O.; Bibi Sadeer, N.; Mahomoodally, M.F.; et al. LC-ESI-QTOF-MS/MS Analysis, Cytotoxic, Antiviral, Antioxidant, and Enzyme Inhibitory Properties of Four Extracts of Geranium pyrenaicum Burm. f.: A Good Gift from the Natural Treasure. Int. J. Mol. Sci. 2021, 22, 7621. [Google Scholar] [CrossRef]

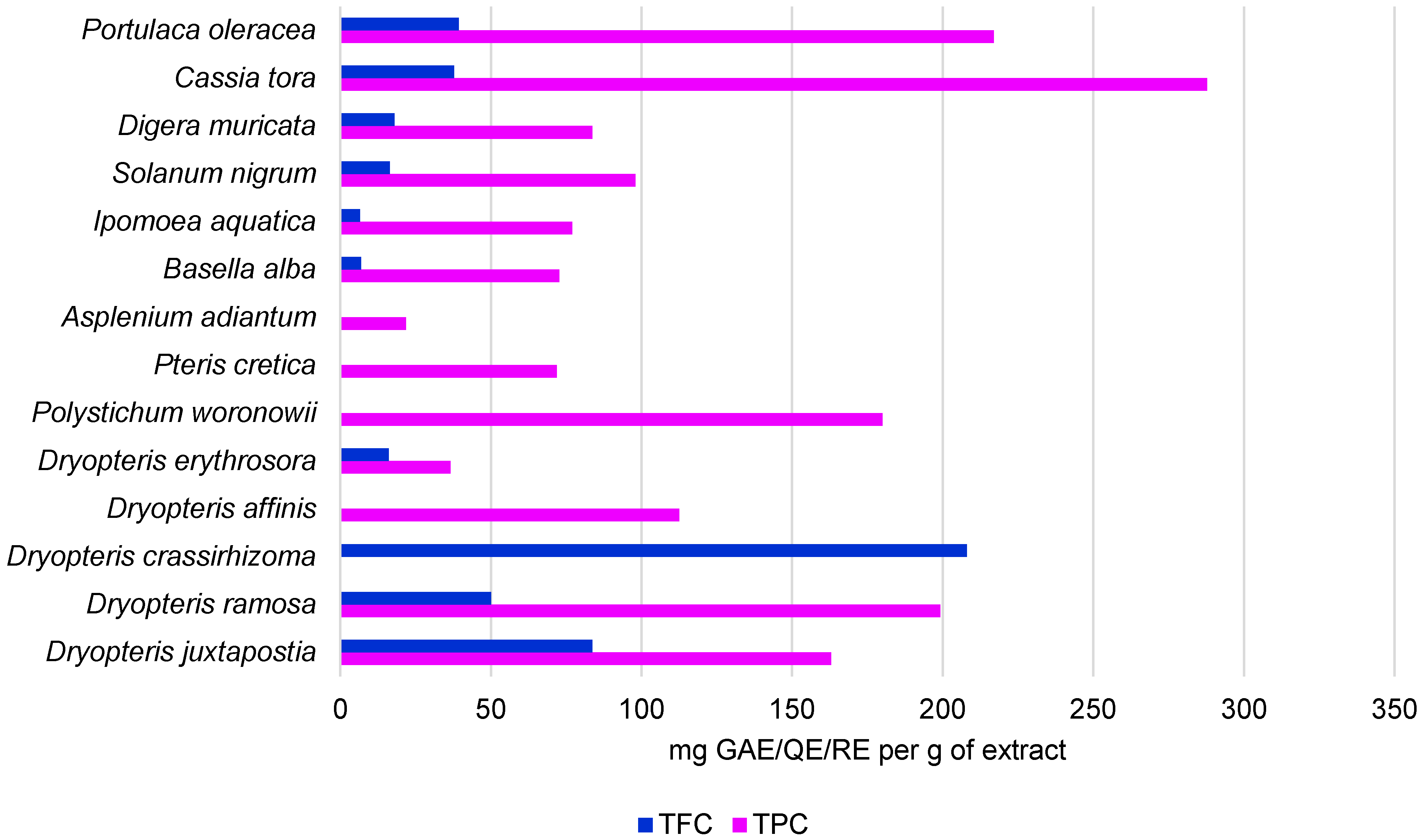





| Species | Solvent Used for Extraction | TPC | TFC | Reference |
|---|---|---|---|---|
| Dryopteris juxtapostia | methanol; dichloromethan | 163.00 mg GAE/g; 222.00 mg GAE/g; | 83.7 mg QE/g; 51 mg QE/g | [18] |
| Dryopteris ramosa | ethyl acetate; methanol; water | - | 45.28 μg QE/mg; 36.94 μg QE/mg; 25.69 μgQE/mg | [20] |
| Dryopteris ramosa | methanol; dichloromethan | 199.20 ± 4.50 mg GAE/g; 184.20 ± 4.04 mg GAE/g | 50.13 ± 3.51 mg RE/g; 73.02 ± 1.00 mg RE/g; | [21] |
| Dryopteris crassirhizoma | ethanol | - | 208.09 ± 5.89 mg RE/g | [22] |
| Dryopteris affinis | methanol | 112.54 ± 8.09 mg GAE/g | - | [23] |
| Polystichum woronowii | methanol | 180.00 ± 12.5 mg GAE/g | - | [23] |
| Pteris cretica | methanol | 71.86 ± 5.34 mg GAE/g | - | [23] |
| Asplenium adiantum | methanol | 21.85 ± 3.12 mg GAE/g | - | [23] |
| Basella alba | methanol | 72.66 ± 0.46 mg GAE/g | 6.97 ± 0.62 mg QE/g | [18] |
| Ipomoea aquatica | methanol | 77.06 ± 0.70 mg GAE/g | 6.61 ± 0.42 mg QE/g | [18] |
| Solanum nigrum | methanol | 97.96 ± 0.62 mg GAE/g | 16.42 ± 0.39 mg QE/g | [18] |
| Digera muricata | methanol | 83.69 ± 0.46 mg GAE/g | 18.00 ± 0.68 mg QE/g | [18] |
| Cassia tora | methanol | 287.73 ± 0.16 mg GAE/g | 37.86 ± 0.53 mg QE/g | [18] |
| Portulaca oleracea | methanol | 216.96 ± 0.87 mg GAE/g | 39.38 ± 0.57 mg QE/g | [18] |
| Microorganisms | MIC (mg/mL) |
|---|---|
| Escherichia coli | 1.25 |
| Klebsiella pneumoniae | 0.375 |
| Staphylococcus aureus | 0.375 |
| Staphylococcus epidermidis | 0.75 |
| Candida albicans | >10 |
| MIC Range (µg/mL) | Kuete and Efferth (2010) Classification [28] | Silva et al. (2013) Classification [29] |
|---|---|---|
| <100 | Significant activity | Highly active |
| 100–500 | Moderate activity | Active |
| 501–625 | Moderate activity | Moderately active |
| 626–1000 | Weak activity | Moderately active |
| 1001–2000 | – | Weakly active |
| >2000 | – | Inactive |
| Tested Plant | MIC of the Ethanol Extract Against E. coli [mg/mL] | MIC of the Ethanol Extract Against S. aureus [mg/mL] |
|---|---|---|
| Iresine herbstii Hook. | 256 | - |
| Apium graveolens L. | 32 | 256 |
| Vallesia glabra (Cav.) Link | 64 | 16 |
| Baccharis sp | 2 | 4 |
| Senecio sp. | 8 | 2 |
| Ochroma pyramidale (Cav. ex Lam.) Urb. | 1 | - |
| Senna bicapsularis (L.) Roxb. | 0.016 | 256 |
| Banisteriopsis caapi (Spruce ex Grieseb.) Morton | 0.0625 | 1 |
| Compounds | Concentration (µg/mL) | (CCID50/mL) a |
|---|---|---|
| DEE | 15.62 | 4.09 ± 0.51 |
| 31.25 | 4.25 ± 0.30 | |
| Acyclovir | 60 | 0 |
| Virus control | − | 5.85 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górka, K.; Koleśnik, M.; Salwa, K.; Kwaśnik, M.; Kubiński, K. Evaluation of Antibacterial, Antifungal, Antiviral, and Anticancer Potential of Extract from the Fern Dryopteris erythrosora. Int. J. Mol. Sci. 2025, 26, 5182. https://doi.org/10.3390/ijms26115182
Górka K, Koleśnik M, Salwa K, Kwaśnik M, Kubiński K. Evaluation of Antibacterial, Antifungal, Antiviral, and Anticancer Potential of Extract from the Fern Dryopteris erythrosora. International Journal of Molecular Sciences. 2025; 26(11):5182. https://doi.org/10.3390/ijms26115182
Chicago/Turabian StyleGórka, Kamila, Marcin Koleśnik, Kinga Salwa, Mateusz Kwaśnik, and Konrad Kubiński. 2025. "Evaluation of Antibacterial, Antifungal, Antiviral, and Anticancer Potential of Extract from the Fern Dryopteris erythrosora" International Journal of Molecular Sciences 26, no. 11: 5182. https://doi.org/10.3390/ijms26115182
APA StyleGórka, K., Koleśnik, M., Salwa, K., Kwaśnik, M., & Kubiński, K. (2025). Evaluation of Antibacterial, Antifungal, Antiviral, and Anticancer Potential of Extract from the Fern Dryopteris erythrosora. International Journal of Molecular Sciences, 26(11), 5182. https://doi.org/10.3390/ijms26115182









