The Mechanical Properties of Breast Cancer Cells and Their Surrounding Microenvironment
Abstract
1. Breast Cancer Cells and Their Mechanical Properties
2. The Mechanical Properties of the ECM and the Cytoskeleton
2.1. Integrin and Collagen
2.2. Basement Membrane
2.3. Fibroblasts and the ECM
3. Changes Induced in BC Cells Following Exposure to Forces
3.1. Forces Inducing Epithelial-to-Mesenchymal Transition (EMT), Dormancy, and Stemness
3.2. Changes Induced by Passing Through Narrow Pores
4. Genes Linking BC Phenotype and Mechanical Forces, and the Implications for Treatment
5. Conclusions
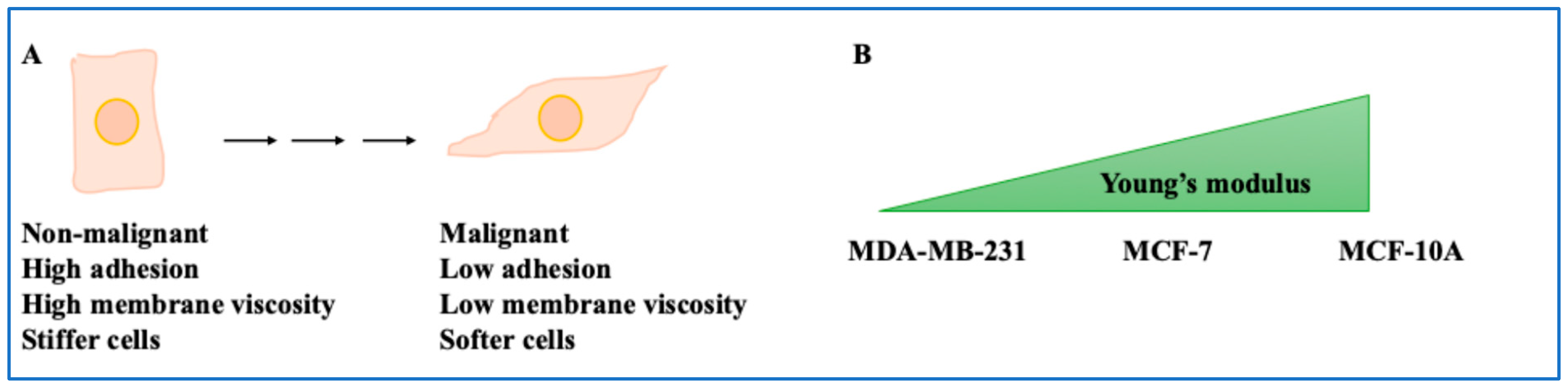
- Non-invasive BC cells showed viscous membranes but stiffer cells, while metastatic cells showed lower viscous membranes but softer cells. The higher the cell’s malignant potential, the lower the adhesion and the higher the cell deformability [8]. For example, MCF-7 demonstrated greater adhesion and intracellular forces, and stiff phenotypes compared to invasive cells [10,11,12,13]. Overall, this study shed light on the mechanical properties of the BC cells.
- The ECM also played a role in force dynamics. In the presence of HA, β1-integrin and CD44 were required for traction force generation [15]. This was important since the interplay between transmembrane proteins, GAGs and mechanical forces can inform future studies. Integrin-related mechanical tensions and BC cells during migration were also significant [16]. Collagen pore size and number increase influenced the mechanical properties of MDA-MB-231 cells by reducing the forces exerted by the cells on the ECM [17]. This finding applies to the composition of various hydrogels and scaffoldings used in BC studies. A stronger invasion capacity was obtained for a higher elastic modulus for thick fibril networks [18]. This directly impacted invasive phenotypes and therefore is significant. BC cells also used force and proteases to invade the basement membrane, as shown by others [19,44]. If the cancer cell was in contact with the ECM rather than a fibroblast, it experienced higher stress levels. In addition, TGFβ-activated fibroblasts could form a ring around the BC cells, modulating their movement [20,21]. Both aspects can be considered when designing systems to emulate the BC tumour microenvironment.
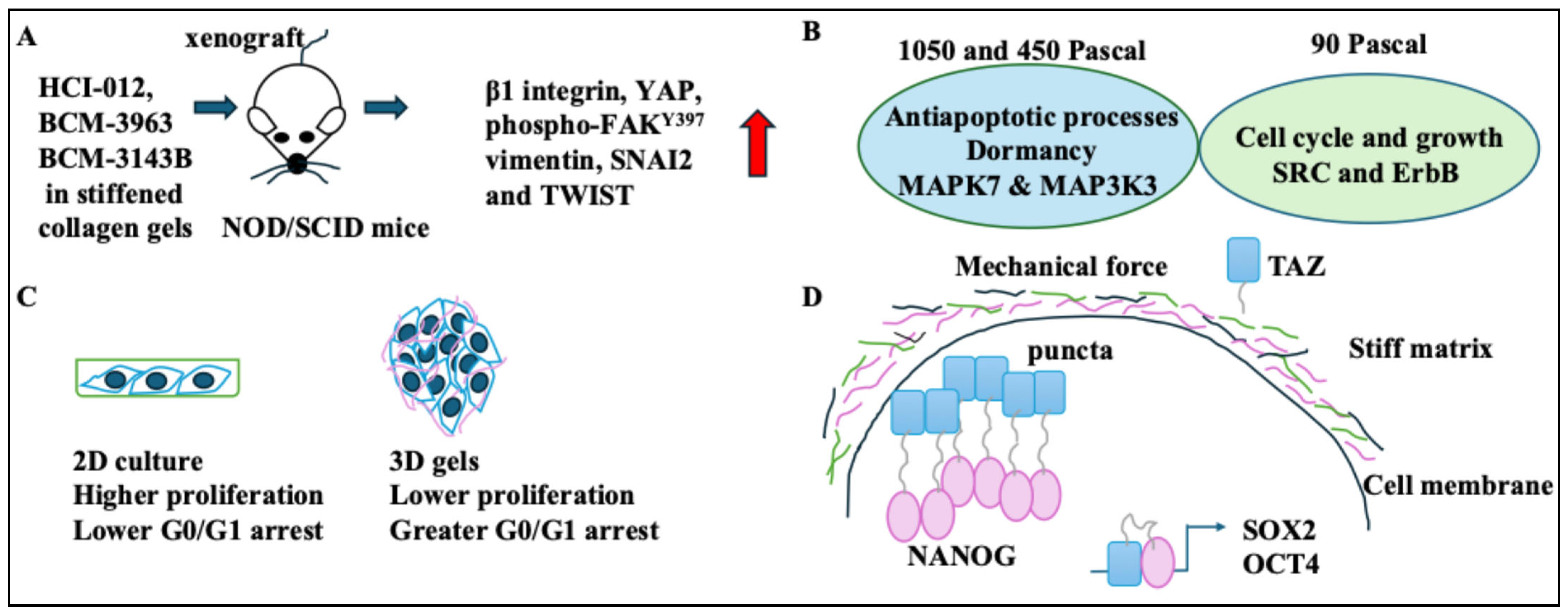
- Changes in BC cells following force exposure were discussed. HER2+ BC xenograft tumours in stiffened collagen gels expressed markers of EMT and β1 integrin [22]. This indicates a direct link between stiffened matrices and increased cell motility. The MCF-7 cells grown in higher Pascal force matrices became dormant. As such, the greater the stiffness of the gel, the greater the G0/G1 arrest and the potential for dormancy [24,25]. These aspects were particularly interesting when considering in vivo changes that induce dormancy following “mechanosensing” [45]. BC cells in stiff matrices showed high NANOG and TAZ levels and stemness [26]. In other words, the niche stiffness could impact cancer stemness in BC cells, and this was orchestrated by a host of key stemness transcriptional regulators. On a side note, changes to BC cells following transit through narrow capillaries were discussed. As a result, nuclear rupture of BC cells occurred. These constriction forces triggered inflammatory pathways and changed proliferation and nuclear stiffness. Cells also became more deformable [27,43].
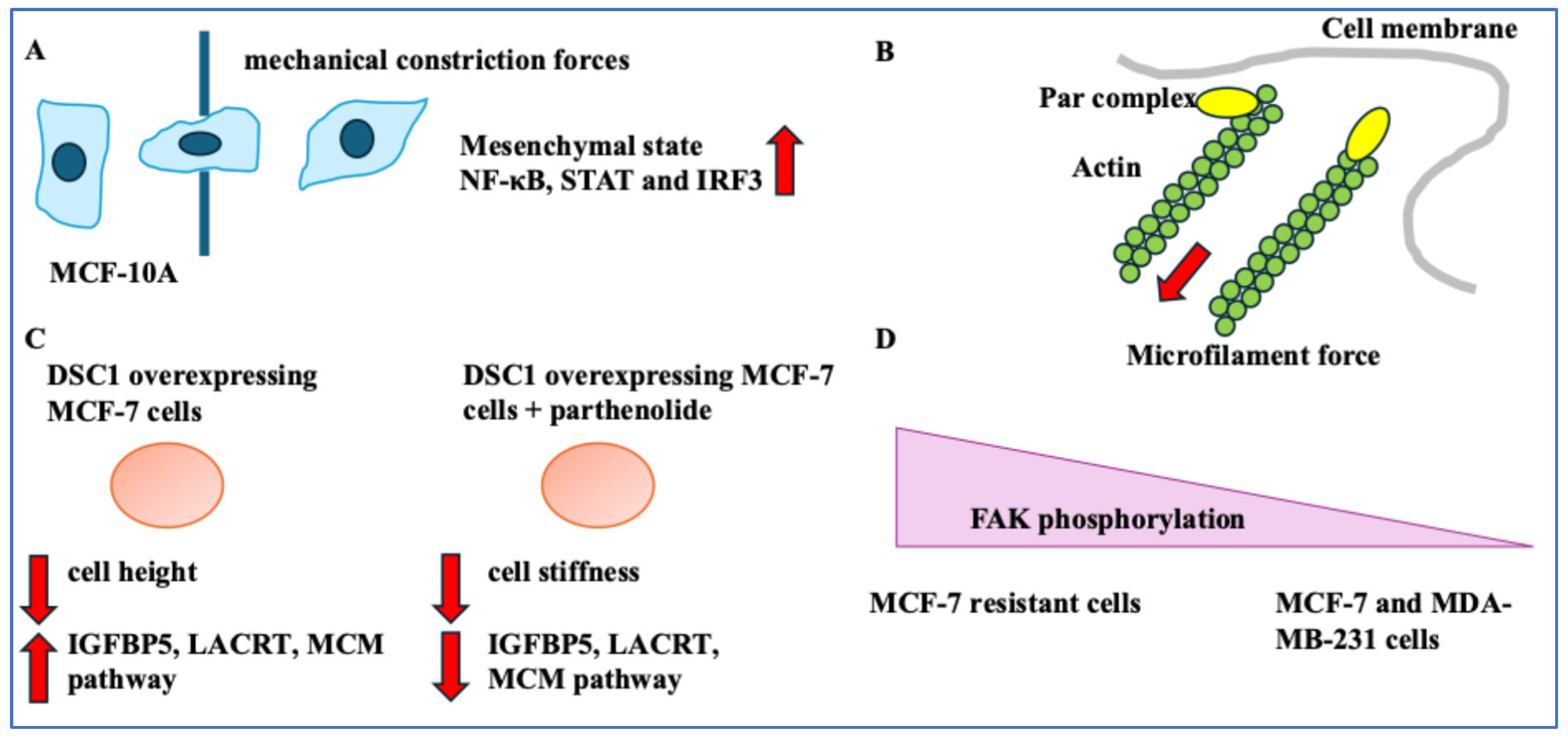
- Genes linked BC phenotypes with mechanical forces. Inhibiting the interaction between Par3 and aPKC increased BC cell migration. Inhibiting NF-κB suppressed DSC1 levels and proliferation and affected cell stiffness [28,29]. This suggested gene expression, the cytoskeleton, and important BC cell activities, including migration and proliferation, were linked to the mechanical properties of BC cells. Finally, implications for diagnostics and treatment were assessed. Image processing and intricate algorithms could be diagnostic tools to detect cells with unusually high metabolic needs [30]. Also, vinculin and FAK activity could be BC treatment resistance indicators. The implications of cell–ECM junction tightness and high vinculin were limited migration post-treatment [31,32]. The dual role of vinculin in these two scenarios indicates the complexity of its function in the cell. Mechanical methods can suppress metastasis and alter the disease progression route [33,34]. Overall, understanding the mechanics of BC cells and the ECM will improve treatment.
Funding
Conflicts of Interest
Abbreviations
| Cx43 | connexin 43 |
| DSC1 | desmocolin-1 |
| ECM | extracellular matrix |
| EMT | epithelial-to-mesenchymal transition |
| ER+ | oestrogen receptor-positive |
| FAK | focal adhesion kinase |
| GAG | glycosaminoglycan |
| HA | hyaluronic acid |
| HER2+ | human epidermal growth factor receptor-positive |
| IGFBP5 | insulin growth factor binding receptor 5 |
| IPN | Interpenetrating network |
| nN | Nanonewton |
| NLS | nuclear localisation signal |
| NOD/SCID | non-obese diabetic/severe combined immunodeficiency |
| PR+ | progesterone receptor-positive |
| QPI | quantitative phase imaging |
| TGT | tension gauge tethering |
| TN− | triple-negative |
References
- Maughan, K.L.; Lutterbie, M.A.; Ham, P.S. Treatment of breast cancer. Am. Fam. Physician 2010, 81, 1339–1346. [Google Scholar] [PubMed]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E., Jr.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.K.; Guan, J.L. Breast Cancer: Multiple Subtypes within a Tumor? Trends Cancer 2017, 3, 753–760. [Google Scholar] [CrossRef]
- Prat, A.; Pineda, E.; Adamo, B.; Galván, P.; Fernández, A.; Gaba, L.; Díez, M.; Viladot, M.; Arance, A.; Muñoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015, 24 (Suppl. 2), S26–S35. [Google Scholar] [CrossRef]
- Koh, J.; Kim, M.J. Introduction of a New Staging System of Breast Cancer for Radiologists: An Emphasis on the Prognostic Stage. Korean J. Radiol. 2019, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Alibert, C.; Goud, B.; Manneville, J. Are cancer cells really softer than normal cells? Biol. Cell 2017, 109, 167–189. [Google Scholar] [CrossRef]
- Fostok, S.F.; El-Sibai, M.; El-Sabban, M.; Talhouk, R.S. Gap Junctions and Wnt Signaling in the Mammary Gland: A Cross-Talk? J. Mammary Gland. Biol. Neoplasia 2019, 24, 17–38. [Google Scholar] [CrossRef]
- Habli, Z.; Zantout, A.; Al-Haj, N.; Saab, R.; El-Sabban, M.; Khraiche, M.L. Single-Cell Fluidic Force Spectroscopy Reveals Dynamic Mechanical Fingerprints of Malignancy in Breast Cancer. ACS Appl. Mater. Interfaces 2024, 16, 50147–50159. [Google Scholar] [CrossRef]
- Dessard, M.; Manneville, J.B.; Berret, J.F. Cytoplasmic viscosity is a potential biomarker for metastatic breast cancer cells. Nanoscale Adv. 2024, 6, 1727–1738. [Google Scholar] [CrossRef]
- Habli, Z.; Zantout, A.; El-Sabban, M.; Khraiche, M.L. Investigating malignancy-dependent mechanical properties of breast cancer cells. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 1–4. [Google Scholar]
- Zbiral, B.; Weber, A.; Vivanco, M.D.M.; Toca-Herrera, J.L. Characterization of Breast Cancer Aggressiveness by Cell Mechanics. Int. J. Mol. Sci. 2023, 24, 12208. [Google Scholar] [CrossRef]
- Starodubtseva, M.N.; Shkliarava, N.M.; Chelnokova, I.A.; Villalba, M.I.; Krylov, A.Y.; Nadyrov, E.A.; Kasas, S. Mechanical Properties and Nanomotion of BT-20 and ZR-75 Breast Cancer Cells Studied by Atomic Force Microscopy and Optical Nanomotion Detection Method. Cells 2023, 12, 2362. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Vivanco, M.D.M.; Toca-Herrera, J.L. Application of self-organizing maps to AFM-based viscoelastic characterization of breast cancer cell mechanics. Sci. Rep. 2023, 13, 3087. [Google Scholar] [CrossRef] [PubMed]
- Ezenwafor, T.; Anye, V.; Madukwe, J.; Amin, S.; Obayemi, J.; Odusanya, O.; Soboyejo, W. Nanoindentation study of the viscoelastic properties of human triple negative breast cancer tissues: Implications for mechanical biomarkers. Acta Biomater. 2023, 158, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Cheung, B.C.H.; Chen, X.; Davis, H.J.; Nordmann, C.S.; Toth, J.; Hodgson, L.; Segall, J.E.; Shenoy, V.B.; Wu, M. Identification of CD44 as a key engager to hyaluronic acid-rich extracellular matrices for cell traction force generation and tumor invasion in 3D. Matrix Biol. 2025, 135, 1–11. [Google Scholar] [CrossRef]
- Kim, Y.; Tram, L.T.H.; Kim, K.A.; Kim, B.C. Defining Integrin Tension Required for Chemotaxis of Metastatic Breast Cancer Cells in Confinement. Adv. Healthc. Mater. 2023, 12, e2202747. [Google Scholar] [CrossRef]
- Blázquez-Carmona, P.; Ruiz-Mateos, R.; Barrasa-Fano, J.; Shapeti, A.; Martín-Alfonso, J.E.; Domínguez, J.; Van Oosterwyck, H.; Reina-Romo, E.; Sanz-Herrera, J.A. Quantitative atlas of collagen hydrogels reveals mesenchymal cancer cell traction adaptation to the matrix nanoarchitecture. Acta Biomater. 2024, 185, 281–295. [Google Scholar] [CrossRef]
- Sapudom, J.; Riedl, P.; Schricker, M.; Kroy, K.; Pompe, T. Physical network regimes of 3D fibrillar collagen networks trigger invasive phenotypes of breast cancer cells. Biomater. Adv. 2024, 163, 213961. [Google Scholar] [CrossRef]
- Chang, J.; Saraswathibhatla, A.; Song, Z.; Varma, S.; Sanchez, C.; Alyafei, N.H.K.; Indana, D.; Slyman, R.; Srivastava, S.; Liu, K.; et al. Cell volume expansion and local contractility drive collective invasion of the basement membrane in breast cancer. Nat. Mater. 2024, 23, 711–722. [Google Scholar] [CrossRef]
- Connaughton, M.; Dabagh, M. Modeling Physical Forces Experienced by Cancer and Stromal Cells Within Different Organ-Specific Tumor Tissue. IEEE J. Transl. Eng. Health Med. 2024, 12, 413–434. [Google Scholar] [CrossRef]
- Santos, A.R.M.P.; Kirkpatrick, B.E.; Kim, M.; Anseth, K.S.; Park, Y. 2D co-culture model reveals a biophysical interplay between activated fibroblasts and cancer cells. Acta Biomater. 2024, 190, 264–272. [Google Scholar] [CrossRef]
- Stashko, C.; Hayward, M.K.; Northey, J.J.; Pearson, N.; Ironside, A.J.; Lakins, J.N.; Oria, R.; Goyette, M.-A.; Mayo, L.; Russnes, H.G.; et al. A convolutional neural network STIFMap reveals associations between stromal stiffness and EMT in breast cancer. Nat. Commun. 2023, 14, 3561. [Google Scholar] [CrossRef]
- Jahangiri, L. Epithelial to Mesenchymal Transition in Neuroblastoma: Mechanisms and Therapeutic Considerations. Curr. Tissue Microenviron. Rep. 2024, 5, 91–108. [Google Scholar] [CrossRef]
- Han, R.; Sun, X.; Wu, Y.; Yang, Y.H.; Wang, Q.C.; Zhang, X.T.; Ding, T.; Yang, J.-T. Proteomic and Phosphoproteomic Profiling of Matrix Stiffness-Induced Stemness-Dormancy State Transition in Breast Cancer Cells. J. Proteome Res. 2024, 23, 4658–4673. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qiu, S.; Liu, X.; Guo, F.; Zhai, J.; Li, Z.; Deng, L.; Ge, L.; Qian, H.; Yang, L.; et al. Extracellular matrix-derived mechanical force governs breast cancer cell stemness and quiescence transition through integrin-DDR signaling. Signal Transduct. Target. Ther. 2023, 8, 247. [Google Scholar] [CrossRef]
- Liu, X.; Ye, Y.; Zhu, L.; Xiao, X.; Zhou, B.; Gu, Y.; Si, H.; Liang, H.; Liu, M.; Li, J.; et al. Niche stiffness sustains cancer stemness via TAZ and NANOG phase separation. Nat. Commun. 2023, 14, 238. [Google Scholar] [CrossRef]
- Perea Paizal, J.; Au, S.H.; Bakal, C. Nuclear rupture induced by capillary constriction forces promotes differential effects on metastatic and normal breast cells. Sci. Rep. 2024, 14, 14793. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, Q.; Chen, S.; Zhao, W.; Zhao, X.; Ruan, Q.; Zheng, Z.; Zhao, H.; Ma, T.; Guo, J.; et al. Par3 promotes breast cancer invasion and migration through pull tension and protein nanoparticle-induced osmotic pressure. Biomed. Pharmacother. 2022, 155, 113739. [Google Scholar] [CrossRef] [PubMed]
- Lapcik, P.; Sulc, P.; Janacova, L.; Jilkova, K.; Potesil, D.; Bouchalova, P.; Müller, P.; Bouchal, P. Desmocollin-1 is associated with pro-metastatic phenotype of luminal A breast cancer cells and is modulated by parthenolide. Cell. Mol. Biol. Lett. 2023, 28, 68. [Google Scholar] [CrossRef]
- Cano, Á.; Yubero, M.L.; Millá, C.; Puerto-Belda, V.; Ruz, J.J.; Kosaka, P.M.; Calleja, M.; Malumbres, M.; Tamayo, J. Rapid mechanical phenotyping of breast cancer cells based on stochastic intracellular fluctuations. iScience 2024, 27, 110960. [Google Scholar] [CrossRef]
- Choi, J.; Park, S. A nanomechanical strategy involving focal adhesion kinase for overcoming drug resistance in breast cancer. Nanomedicine 2022, 43, 102559. [Google Scholar] [CrossRef]
- Metsiou, D.N.; Deligianni, D.; Giannopoulou, E.; Kalofonos, H.; Koutras, A.; Athanassiou, G. Adhesion strength and anti-tumor agents regulate vinculin of breast cancer cells. Front. Oncol. 2022, 12, 811508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yu, T.; Chai, X.; Zhang, S.; Liu, J.; Zhou, Y.; Yin, D.; Zhang, C. Gradient Rotating Magnetic Fields Impairing F-Actin-Related Gene CCDC150 to Inhibit Triple-Negative Breast Cancer Metastasis by Inactivating TGF-β1/SMAD3 Signaling Pathway. Research 2024, 7, 0320. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, F.; Yao, C.; Lv, W.; Peng, H.; Stanciu, S.G.; Stenmark, H.A.; Song, Y.M.; Jiang, B.; Wu, A. “Double-punch” strategy against triple-negative breast cancer via a synergistic therapy of magneto-mechanical force enhancing NIR-II hypothermal ablation. Biomaterials 2022, 291, 121868. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Mäntylä, V.M.; Lehtonen, A.J.; Korhonen, V.; Srbova, L.; Pokki, J. Quantifying the Influence of X-ray Irradiation on Cell-Size-Scale Viscoelasticity of Collagen Type 1. J. Biomech. Eng. 2024, 146, 044501. [Google Scholar] [CrossRef]
- Chang, J.; Chaudhuri, O. Beyond proteases: Basement membrane mechanics and cancer invasion. J. Cell Biol. 2019, 218, 2456–2469. [Google Scholar] [CrossRef]
- Tang, D.; Gao, J.; Wang, S.; Ye, N.; Chong, Y.; Huang, Y.; Wang, J.; Li, B.; Yin, W.; Wang, D. Cancer-associated fibroblasts promote angiogenesis in gastric cancer through galectin-1 expression. Tumour Biol. 2016, 37, 1889–1899. [Google Scholar] [CrossRef]
- Sosa, M.S.; Bragado, P.; Aguirre-Ghiso, J.A. Mechanisms of disseminated cancer cell dormancy: An awakening field. Nat. Rev. Cancer 2014, 14, 611–622. [Google Scholar] [CrossRef]
- Jahangiri, L. Cancer Stem Cell Markers and Properties Across Gastrointestinal Cancers. Curr. Tissue Microenviron. Rep. 2023, 4, 77–89. [Google Scholar] [CrossRef]
- Guan, L.Y.; Lin, S.Z.; Chen, P.C.; Lv, J.Q.; Li, B.; Feng, X.Q. Interfacial Organization and Forces Arising from Epithelial–Cancerous Monolayer Interactions. ACS Nano 2023, 17, 24668–24684. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz Motamed, P.; Abouali, H.; Poudineh, M.; Maftoon, N. Experimental measurement and numerical modeling of deformation behavior of breast cancer cells passing through constricted microfluidic channels. Microsyst. Nanoeng. 2024, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Gangoda, L.; Keerthikumar, S.; Fonseka, P.; Edgington, L.E.; Ang, C.S.; Ozcitti, C.; Bogyo, M.; Parker, B.S.; Mathivanan, S. Inhibition of cathepsin proteases attenuates migration and sensitizes aggressive N-Myc amplified human neuroblastoma cells to doxorubicin. Oncotarget 2015, 6, 11175–11190. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Bravo-Cordero, J.J. Regulation of dormancy during tumor dissemination: The role of the ECM. Cancer Metastasis Rev. 2023, 42, 99–112. [Google Scholar] [CrossRef]

| Players | Mechanism | References |
|---|---|---|
| Non-invasive and metastatic BC cells | Non-invasive cells had viscous membranes but were stiff, and metastatic cells had less viscous membranes but were soft. | [8,9] |
| MCF-10A compared to MCF-7 and MDA-MB-231 cells | Highest Young’s modulus (stiffness) in non-malignant cells. | [11] |
| HA, traction force, β1-integrin in HA gels, and CD44 | Blocking CD44 or β1-integrin using antibodies in H2 conditions (collagen I and higher HA gels) reduced traction forces. | [15] |
| Integrin, adhesion, and EGF | With EGF, cells became more polarised, and it affected integrin-mediated adhesion and cell migration. | [16] |
| Collagen properties in hydrogels | Collagen pore size and number affected the mechanical properties of MDA-MB-231 cells by reducing the forces these cells exerted on the ECM. | [17] |
| MDA-MB-231 cells and aggressiveness | The higher elastic modulus for thick fibril networks induced a stronger invasion capacity in MDA-MB-231 cells. | [18] |
| Basement membrane breach, FAK, forces, and proteases | Basement membrane openings had high FAK activity; BC cells used force and proteases to stretch and invade the basement membrane. | [19] |
| Softer and stiffer ECMs, BC cells, and invasion | In tissues with softer ECMs, cancer cells experienced high stress compared to stiffer ECMs. | [20] |
| Coculture of fibroblasts with BC cells (COAFs) | Vinculin and inner negative traction forces were localised to activated fibroblasts, while COAFs had low migratory capacity. | [21] |
| Stiff ECMs, invasion, and EMT | Stiff ECMs promoted growth, invasiveness, and EMT. These tumours increased β1 integrin, YAP, FAKY397, vimentin, SNAI2, and TWIST. | [22,23] |
| Matrices with 450 and 1050 Pascal forces or 90 Pascal forces | 450 and 1050 Pascal groups showed low proliferation, and 90 Pascal groups showed high proliferation. | [24] |
| 3D Matrigel with 450 Pascal (high stiffness) | The greater the stiffness of the gel, the higher the G0/G1 arrest; 3D Matrigel with 450 Pascal forces drove cell cycle arrest and quiescence. | [25] |
| Stiff matrices and BC cells | The abundance of ALDH1+CK+ (stem cells) was greater in stiff matrices. | [26] |
| Constriction forces, proliferation, and BC cells | Constriction forces increased proliferation and Lamin A/C reorganisation in MCF-10A cells more than in BC cells. | [27] |
| Par3, the cytoskeleton, and invasion | The Par3-siRNA-mediated knockdown in MDA-MB-231 cells reduced cytoskeletal stability and induced cancer invasion. | [28] |
| NF-κB inhibitor (parthenolide) and DSC1 | Parthenolide decreased DSC1 levels in BC cells. DSC1 expression reduced cell height, and parthenolide decreased stiffness in these cells. | [29] |
| MDA-MB-231 metastatic cells and metabolism | MDA-MB-231 metastatic cells showed a greater increase in dry mass fluctuations and areas of high metabolic activity. | [30] |
| MDA-MB-231 cells, MCF-7 resistant cells, and MCF-7 cells | Actin stress fibres and vinculin were high in MDA-MB-231- and MCF-7-resistant cells compared to MCF-7 cells. | [31] |
| Post-treatment BC cells | In post-treatment BC cells, large adhesion forces were needed to separate cells from the ECM, migration was low, and vinculin was high. | [32] |
| Gradient RMF and magnetic photothermal converter | Gradient RMF affected the CCDC150 gene in BC cells and suppressed migration. TNBC was treated with a magnetic photothermal converter. | [33,34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahangiri, L. The Mechanical Properties of Breast Cancer Cells and Their Surrounding Microenvironment. Int. J. Mol. Sci. 2025, 26, 5183. https://doi.org/10.3390/ijms26115183
Jahangiri L. The Mechanical Properties of Breast Cancer Cells and Their Surrounding Microenvironment. International Journal of Molecular Sciences. 2025; 26(11):5183. https://doi.org/10.3390/ijms26115183
Chicago/Turabian StyleJahangiri, Leila. 2025. "The Mechanical Properties of Breast Cancer Cells and Their Surrounding Microenvironment" International Journal of Molecular Sciences 26, no. 11: 5183. https://doi.org/10.3390/ijms26115183
APA StyleJahangiri, L. (2025). The Mechanical Properties of Breast Cancer Cells and Their Surrounding Microenvironment. International Journal of Molecular Sciences, 26(11), 5183. https://doi.org/10.3390/ijms26115183





