Immune Modulation with Nanodiscs: Surface Charge Dictates Cellular Interactions and Activation of Macrophages and Dendritic-like Cells
Abstract
1. Introduction
2. Results
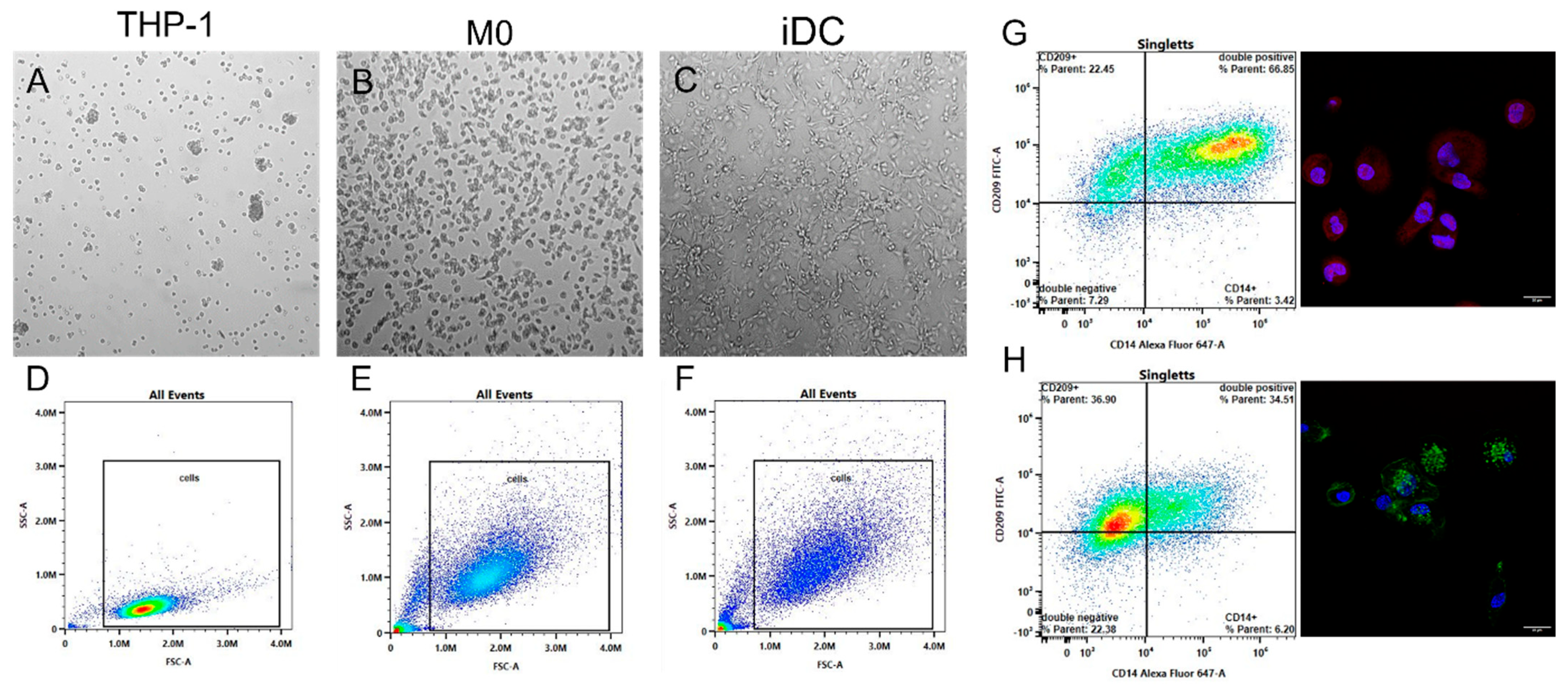
2.1. Differentiation and Characterization of Monocytes
2.2. Nanoparticle–Immune Cell Interaction
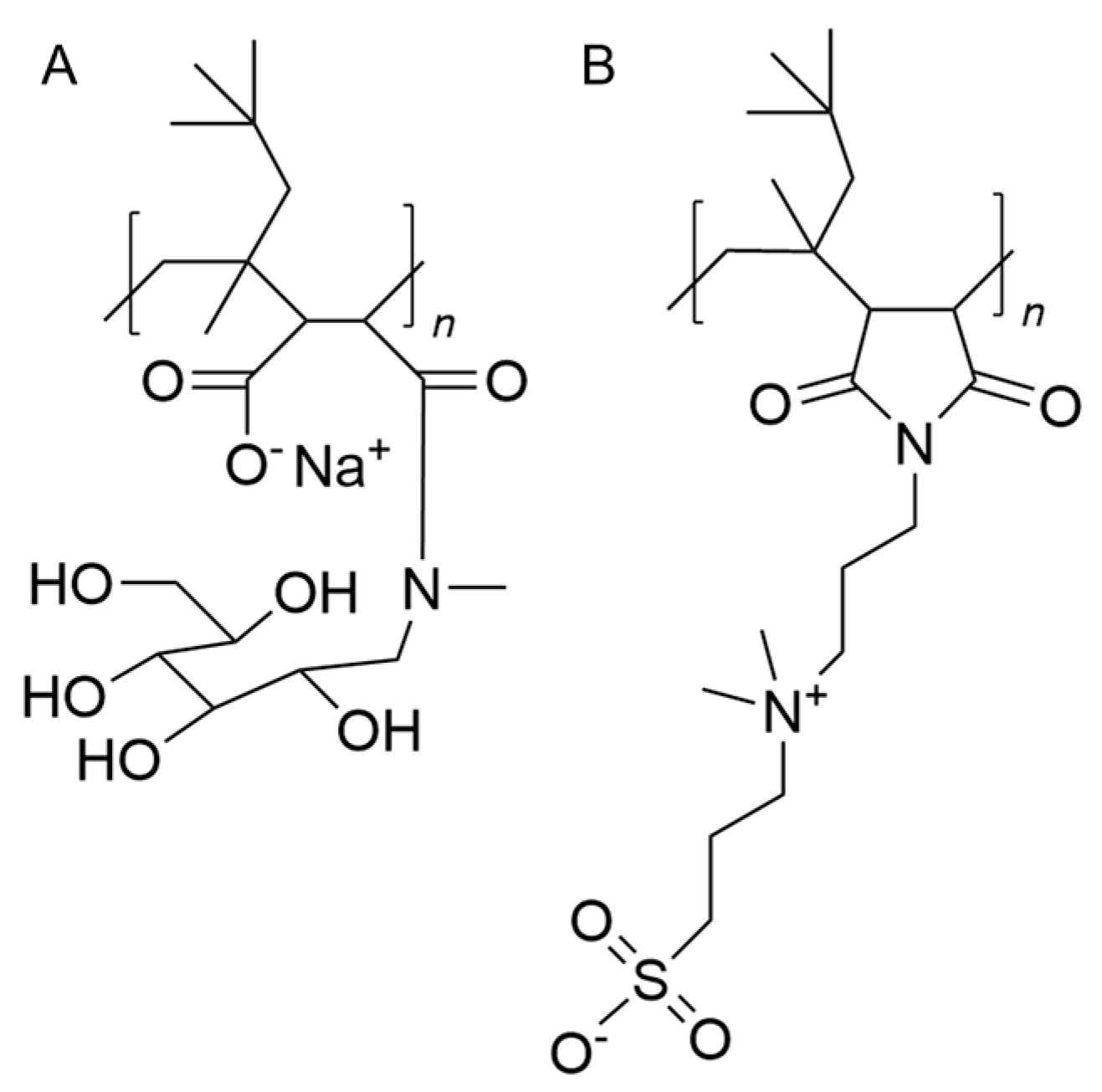
2.2.1. Nanoparticle Characterization
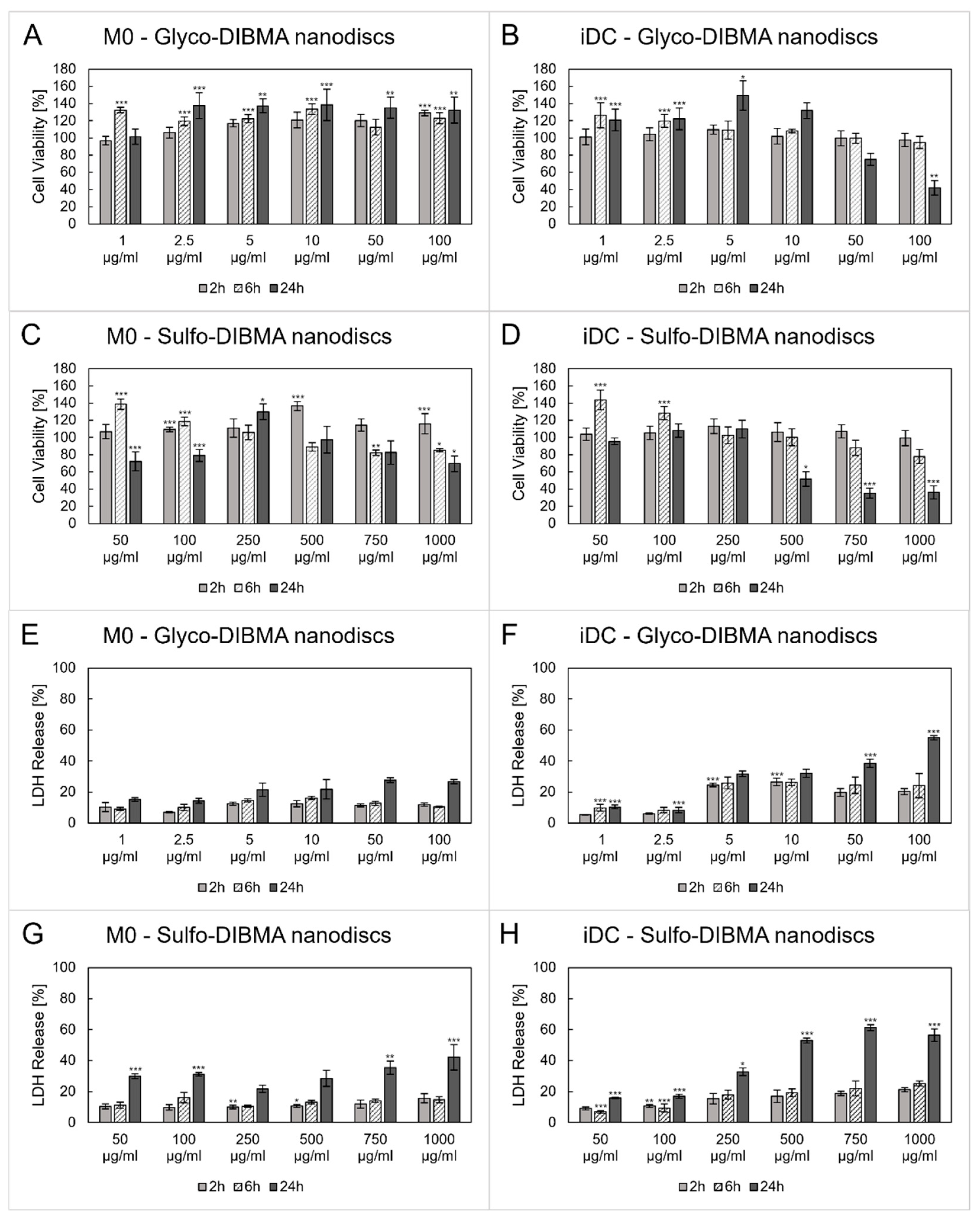
2.2.2. Cytotoxicity
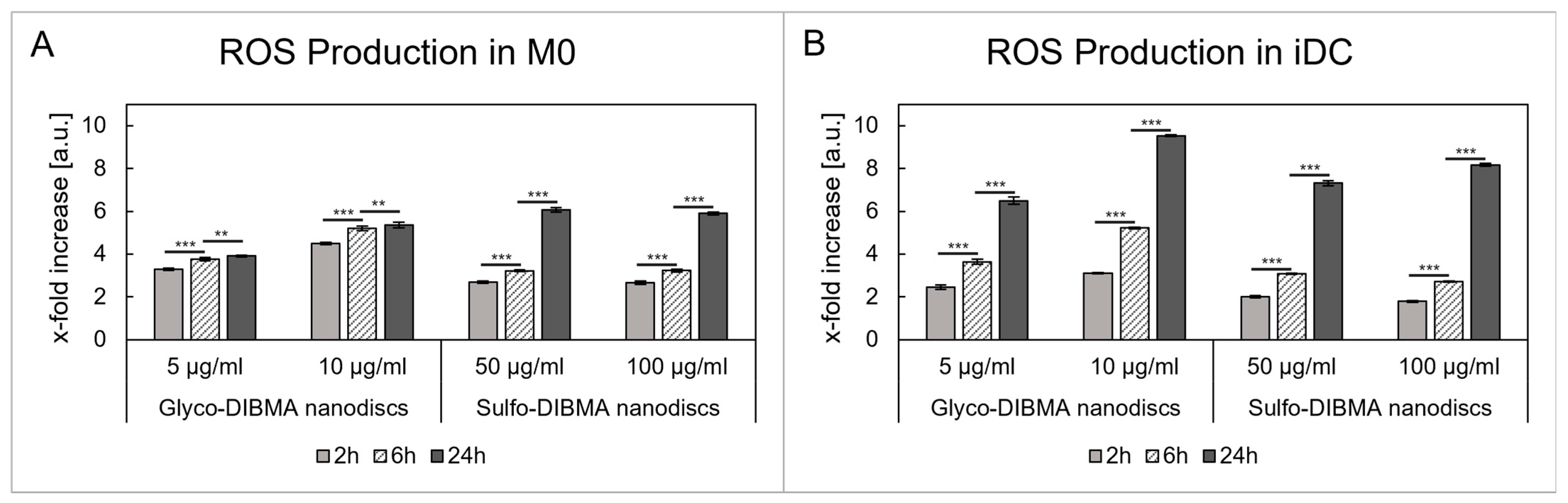
2.2.3. ROS Production
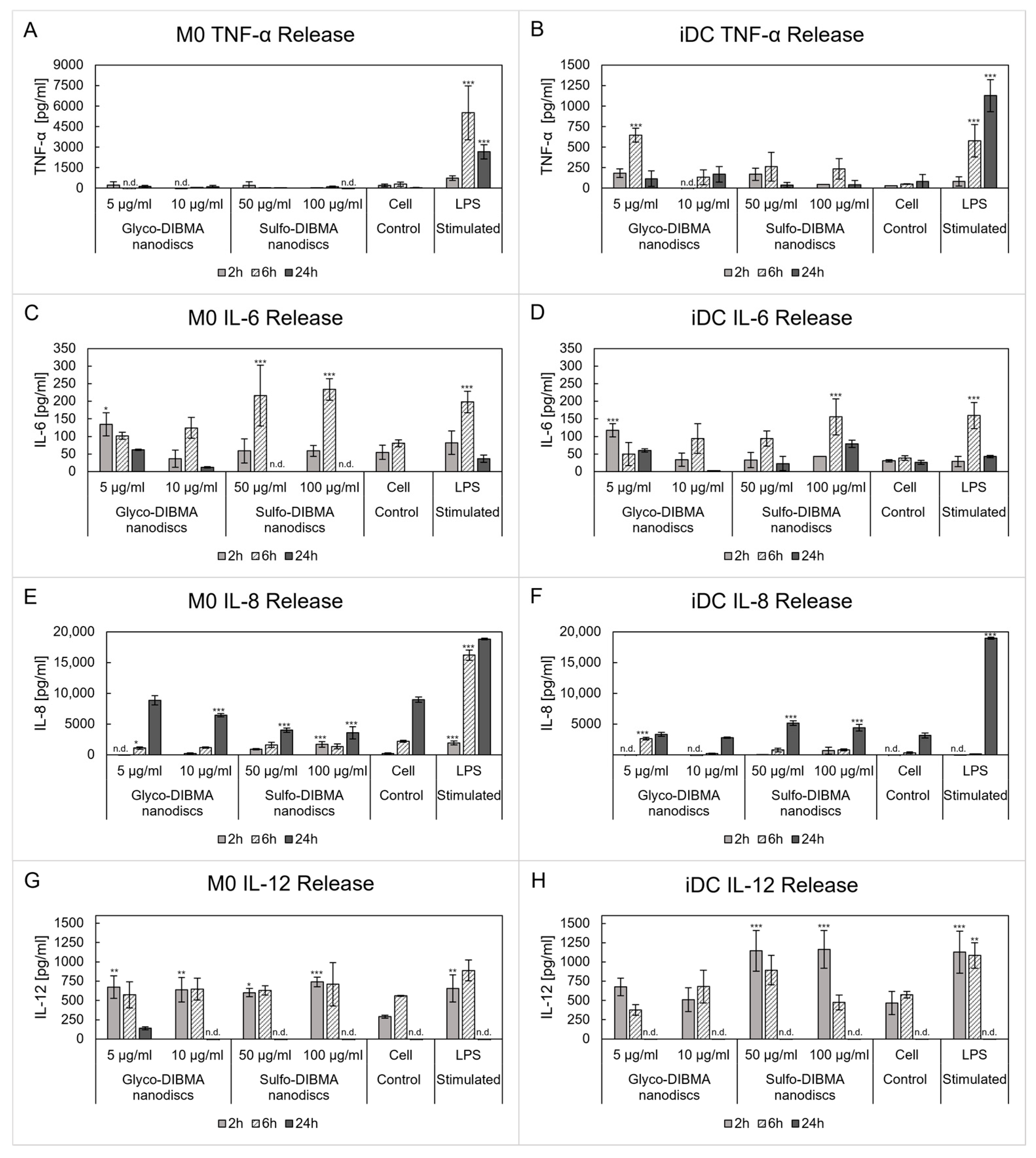
2.2.4. Cytokine Release
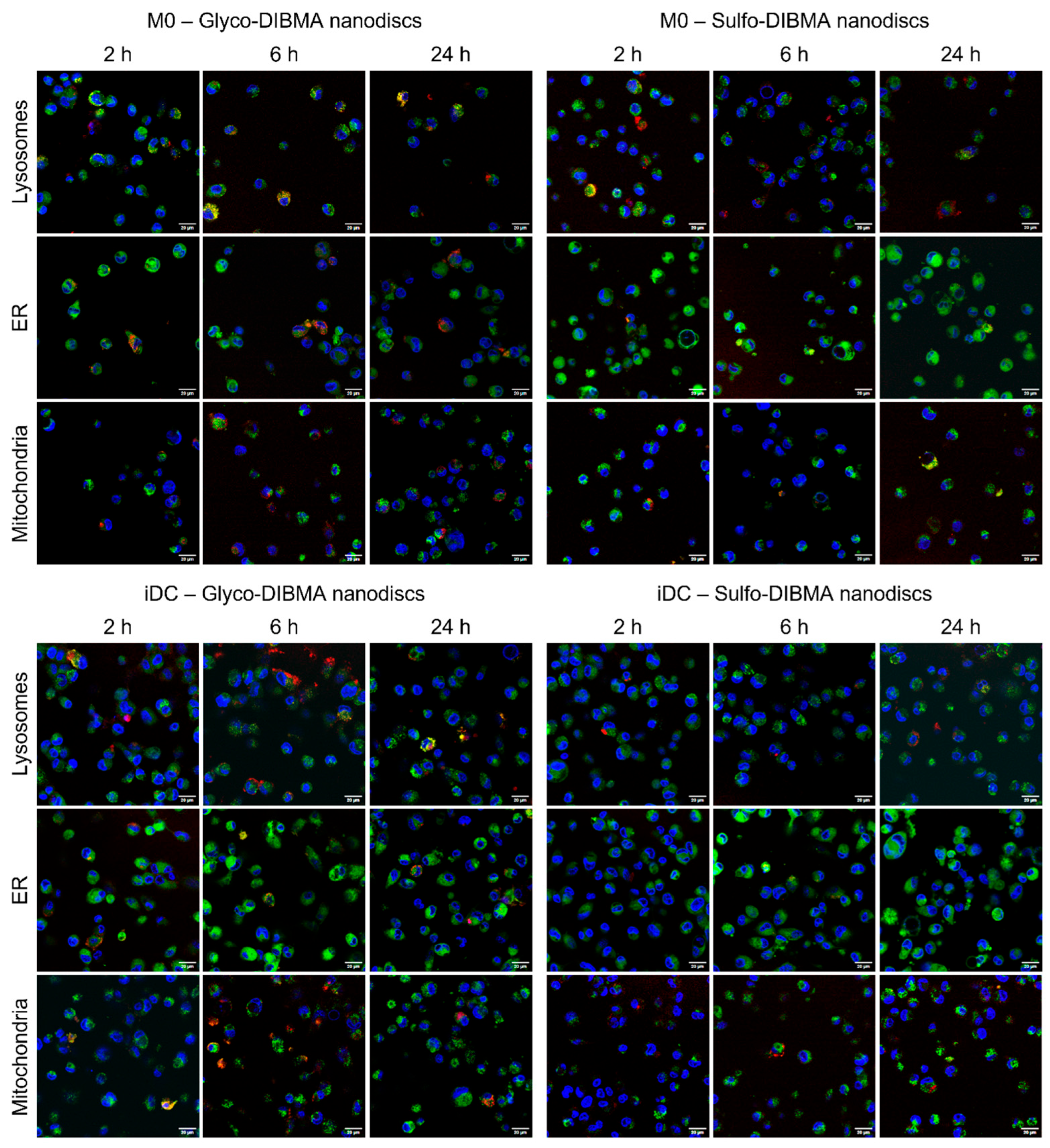
2.2.5. Uptake and Co-Localization Studies
2.2.6. Immune Cell Activation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Differentiation
4.3. Cell Characterization
4.3.1. Fluorescence Microscopy
4.3.2. Spectral Flow Cytometry
4.3.3. Atomic Force Microscopy
4.3.4. Scanning and Transmission Electron Microscopy
4.4. Nanodisc Interaction Studies
4.4.1. Nanodisc Preparation
4.4.2. Cytotoxicity
4.4.3. Generation of ROS
4.4.4. Cytokine Production—ELISA
4.4.5. Uptake and Co-Localization Studies
4.4.6. Activation of M0 and iDCs
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carton, F.; Malatesta, M. In Vitro Models of Biological Barriers for Nanomedical Research. Int. J. Mol. Sci. 2022, 23, 8910. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy. Asthma Clin. Immunol. 2018, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Groell, F.; Jordan, O.; Borchard, G. In vitro models for immunogenicity prediction of therapeutic proteins. Eur. J. Pharm. Biopharm. 2018, 130, 128–142. [Google Scholar] [CrossRef]
- den Haan, J.M.M.; Arens, R.; van Zelm, M.C. The activation of the adaptive immune system: Cross-talk between antigen-presenting cells, T cells and B cells. Immunol. Lett. 2014, 162, 103–112. [Google Scholar] [CrossRef]
- van Helden, S.F.G.; van Leeuwen, F.N.; Figdor, C.G. Human and murine model cell lines for dendritic cell biology evaluated. Immunol. Lett. 2008, 117, 191–197. [Google Scholar] [CrossRef]
- Nascimento, C.R.; Fernandes, N.A.R.; Maldonado, L.A.G.; Junior, C.R. Comparison of monocytic cell lines U937 and THP-1 as macrophage models for in vitro studies. Biochem. Biophys. Rep. 2022, 32, 101383. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Hölken, J.M.; Teusch, N. The Monocytic Cell Line THP-1 as a Validated and Robust Surrogate Model for Human Dendritic Cells. Int. J. Mol. Sci. 2023, 24, 1452. [Google Scholar] [CrossRef]
- Ray, P.; Haideri, N.; Haque, I.; Mohammed, O.; Chakraborty, S.; Banerjee, S.; Quadir, M.; Brinker, A.; Banerjee, S. The Impact of Nanoparticles on the Immune System: A Gray Zone of Nanomedicine. J. Immunol. Sci. 2021, 5, 19–33. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef]
- Mu, Q.; Deng, H.; An, X.; Liu, G.; Liu, C. Designing nanodiscs as versatile platforms for on-demand therapy. Nanoscale 2024, 16, 2220–2234. [Google Scholar] [CrossRef] [PubMed]
- Noh, I.; Guo, Z.; Zhou, J.; Gao, W.; Fang, R.H.; Zhang, L. Cellular Nanodiscs Made from Bacterial Outer Membrane as a Platform for Antibacterial Vaccination. ACS Nano 2023, 17, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Grimme, S.; Ganesh, B.; Gopisetty, A.; Sheng, J.R.; Martinez, O.; Jayarama, S.; Artinger, M.; Meriggioli, M.; Prabhakar, B.S. Nanodisc-Incorporated Hemagglutinin Provides Protective Immunity against Influenza Virus Infection. J. Virol. 2010, 84, 361–371. [Google Scholar] [CrossRef]
- Kuai, R.; Ochyl, L.J.; Bahjat, K.S.; Schwendeman, A.; Moon, J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017, 16, 489–496. [Google Scholar] [CrossRef]
- Kuai, R.; Sun, X.; Yuan, W.; Ochyl, L.J.; Xu, Y.; Najafabadi, A.H.; Scheetz, L.; Yu, M.Z.; Balwani, I.; Schwendeman, A.; et al. Dual TLR agonist nanodiscs as a strong adjuvant system for vaccines and immunotherapy. J. Control. Release 2018, 282, 131–139. [Google Scholar] [CrossRef]
- Su, T.; Zhang, Y.; Valerie, K.; Wang, X.Y.; Lin, S.; Zhu, G. STING activation in cancer immunotherapy. Theranostics 2019, 9, 7759–7771. [Google Scholar] [CrossRef]
- Dane, E.L.; Belessiotis-Richards, A.; Backlund, C.; Wang, J.; Hidaka, K.; Milling, L.E.; Bhagchandani, S.; Melo, M.B.; Wu, S.; Li, N.; et al. STING agonist delivery by tumour-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. Nat. Mater. 2022, 21, 710–720. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Danielczak, B.; Rasche, M.; Lenz, J.; Patallo, E.P.; Weyrauch, S.; Mahler, F.; Agbadaola, M.T.; Meister, A.; Babalola, J.O.; Vargas, C.; et al. A bioinspired glycopolymer for capturing membrane proteins in native-like lipid-bilayer nanodiscs. Nanoscale 2022, 14, 1855–1867. [Google Scholar] [CrossRef]
- Lenz, J.; Larsen, A.H.; Keller, S.; Luchini, A. Effect of Cholesterol on the Structure and Composition of Glyco-DIBMA Lipid Particles. Langmuir 2023, 39, 3569–3579. [Google Scholar] [CrossRef] [PubMed]
- Glueck, D.; Grethen, A.; Das, M.; Mmeka, O.P.; Patallo, E.P.; Meister, A.; Rajender, R.; Kins, S.; Räschle, M.; Victor, J.; et al. Electroneutral Polymer Nanodiscs Enable Interference-Free Probing of Membrane Proteins in a Lipid-Bilayer Environment. Small 2022, 18, 2202492. [Google Scholar] [CrossRef] [PubMed]
- Eggenreich, L.; Vargas, C.; Kolar, C.; Keller, S. Lipid exchange among electroneutral Sulfo-DIBMA nanodiscs is independent of ion concentration. Biol. Chem. 2023, 404, 703–713. [Google Scholar] [CrossRef]
- Jeitler, R.; Glader, C.; Tetyczka, C.; Zeiringer, S.; Absenger-Novak, M.; Selmani, A.; Fröhlich, E.; Roblegg, E. Investigation of Cellular Interactions of Lipid-Structured Nanoparticles with Oral Mucosal Epithelial Cells. Front. Mol. Biosci. 2022, 9, 917921. [Google Scholar] [CrossRef]
- Deng, Y.; Govers, C.; Beest, E.T.; van Dijk, A.J.; Hettinga, K.; Wichers, H.J. A THP-1 Cell Line-Based Exploration of Immune Responses Toward Heat-Treated BLG. Front. Nutr. 2021, 7, 612397. [Google Scholar] [CrossRef]
- Bosshart, H.; Heinzelmann, M. THP-1 cells as a model for human monocytes. Ann. Transl. Med. 2016, 4, 438. [Google Scholar] [CrossRef]
- Xing, F.; Wang, J.; Hu, M.; Yu, Y.; Chen, G.; Liu, J. Comparison of immature and mature bone marrow-derived dendritic cells by atomic force microscopy. Nanoscale Res. Lett. 2011, 6, 1. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Phagocytosis: Our Current Understanding of a Universal Biological Process. Front. Immunol. 2020, 11, 531655. [Google Scholar] [CrossRef]
- Labernadie, A.; Thibault, C.; Vieu, C.; Maridonneau-Parini, I.; Charrière, G.M. Dynamics of podosome stiffness revealed by atomic force microscopy. Proc. Natl. Acad. Sci. USA 2010, 107, 21016–21021. [Google Scholar] [CrossRef]
- Schimpel, C.; Werzer, O.; Fröhlich, E.; Leitinger, G.; Absenger-Novak, M.; Teubl, B.; Zimmer, A.; Roblegg, E. Atomic force microscopy as analytical tool to study physico-mechanical properties of intestinal cells. Beilstein J. Nanotechnol. 2015, 6, 1457–1466. [Google Scholar] [CrossRef]
- Linder, S.; Wiesner, C. Tools of the trade: Podosomes as multipurpose organelles of monocytic cells. Cell. Mol. Life Sci. 2015, 72, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Forrester, M.A.; Wassall, H.J.; Hall, L.S.; Cao, H.; Wilson, H.M.; Barker, R.N.; Vickers, M.A. Similarities and differences in surface receptor expression by THP-1 monocytes and differentiated macrophages polarized using seven different conditioning regimens. Cell. Immunol. 2018, 332, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, I.; Ostuni, R.; Marek, L.R.; Barresi, S.; Barbalat, R.; Barton, G.M.; Granucci, F.; Kagan, J.C. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 2011, 147, 868–880. [Google Scholar] [CrossRef]
- Park, E.K.; Jung, H.S.; Yang, H.I.; Yoo, M.C.; Kim, C.; Kim, K.S. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm. Res. 2007, 56, 45–50. [Google Scholar] [CrossRef]
- Longo, V.; Aloi, N.; Presti, E.L.; Fiannaca, A.; Longo, A.; Adamo, G.; Urso, A.; Meraviglia, S.; Bongiovanni, A.; Cibella, F.; et al. Impact of the flame retardant 2,2’4,4’-tetrabromodiphenyl ether (PBDE-47) in THP-1 macrophage-like cell function via small extracellular vesicles. Front. Immunol. 2023, 13, 1069207. [Google Scholar] [CrossRef]
- Kim, C.-H. DC-SIGNs. Glycobiol. Innate. Immunol. 2022, 585–606. [Google Scholar] [CrossRef]
- Bullwinkel, J.; Lüdemann, A.; Debarry, J.; Singh, P.B. Epigenotype switching at the CD14 and CD209 genes during differentiation of human monocytes to dendritic cells. Epigenetics 2011, 6, 45–51. [Google Scholar] [CrossRef]
- Dong, Y.; Tang, H.; Dai, H.; Zhao, H.; Wang, J. The application of nanodiscs in membrane protein drug discovery & development and drug delivery. Front. Chem. 2024, 12, 1444801. [Google Scholar]
- Gao, Y.; Huang, D.; Huang, S.; Li, H.; Xia, B. Rational design of ROS generation nanosystems to regulate innate immunity of macrophages, dendrtical and natural killing cells for immunotherapy. Int. Immunopharmacol. 2024, 139, 112695. [Google Scholar] [CrossRef]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. “Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Zhang, Y.; Choksi, S.; Chen, K.; Pobezinskaya, Y.; Linnoila, I.; Liu, Z.G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013, 23, 898–914. [Google Scholar] [CrossRef] [PubMed]
- Griess, B.; Mir, S.; Datta, K.; Teoh-Fitzgerald, M. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic. Biol. Med. 2020, 147, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Kotsias, F.; Hoffmann, E.; Amigorena, S.; Savina, A. Reactive oxygen species production in the phagosome: Impact on antigen presentation in dendritic cells. Antioxid. Redox Signal. 2013, 18, 714–729. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Baranov, M.V.; Kumar, M.; Sacanna, S.; Thutupalli, S.; van den Bogaart, G. Modulation of Immune Responses by Particle Size and Shape. Front. Immunol. 2021, 11, 607945. [Google Scholar] [CrossRef]
- Li, K.; Underhill, D.M. C-Type Lectin Receptors in Phagocytosis. Curr. Top. Microbiol. Immunol. 2020, 429, 1–18. [Google Scholar]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Zhang, Y.; Tekobo, S.; Tu, Y.; Zhou, Q.; Jin, X.; Dergunov, S.A.; Pinkhassik, E.; Yan, B. Permission to enter cell by shape: Nanodisk vs Nanosphere. ACS Appl. Mater. Interfaces 2012, 4, 4099–4105. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Cytokines as biomarkers of nanoparticle immunotoxicity. Chem. Soc. Rev. 2013, 42, 5552–5576. [Google Scholar] [CrossRef]
- Ma, X.; Yan, W.; Zheng, H.; Du, Q.; Zhang, L.; Ban, Y.; Li, N.; Wei, F. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Research 2015, 4, F1000 Faculty Rev-1465. [Google Scholar] [CrossRef]
- Brzicova, T.; Javorkova, E.; Vrbova, K.; Zajicova, A.; Holan, V.; Pinkas, D.; Philimonenko, V.; Sikorova, J.; Klema, J.; Topinka, J.; et al. Molecular Responses in THP-1 Macrophage-Like Cells Exposed to Diverse Nanoparticles. Nanomaterials 2019, 9, 687. [Google Scholar] [CrossRef] [PubMed]
- Ebner, S.; Ratzinger, G.; Krösbacher, B.; Schmuth, M.; Weiss, A.; Reider, D.; Kroczek, R.A.; Herold, M.; Heufler, C.; Fritsch, P.; et al. Production of IL-12 by human monocyte-derived dendritic cells is optimal when the stimulus is given at the onset of maturation, and is further enhanced by IL-4. J. Immunol. 2001, 166, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Han, T.H.; Jin, P.; Ren, J.; Slezak, S.; Marincola, F.M.; Stroncek, D.F. Evaluation of 3 clinical dendritic cell maturation protocols containing lipopolysaccharide and interferon-gamma. J. Immunother. 2009, 32, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Yasin, Z.N.M.; Idrus, F.N.M.; Hoe, C.H.; Yvonne-Tee, G.B. Macrophage polarization in THP-1 cell line and primary monocytes: A systematic review. Differentiation 2022, 128, 67–82. [Google Scholar] [CrossRef]
- Dzopalic, T.; Rajkovic, I.; Dragicevic, A.; Colic, M. The response of human dendritic cells to co-ligation of pattern-recognition receptors. Immunol. Res. 2012, 52, 20–33. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, J. Properties of immature and mature dendritic cells: Phenotype, morphology, phagocytosis, and migration. RSC Adv. 2019, 9, 11230–11238. [Google Scholar] [CrossRef]
- Agadjanyan, M.G.; Kim, J.J.; Trivedi, N.; Wilson, D.M.; Monzavi-Karbassi, B.; Morrison, L.D.; Nottingham, L.K.; Dentchev, T.; Tsai, A.; Dang, K.; et al. CD86 (B7-2) Can Function to Drive MHC-Restricted Antigen-Specific CTL Responses In Vivo. J. Immunol. 1999, 162, 3417–3427. [Google Scholar] [CrossRef]
- Stern, L.J.; Calvo-Calle, J.M. HLA-DR: Molecular insights and vaccine design. Curr. Pharm. Des. 2009, 15, 3249–3261. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, J. CD4 T Helper Cell Subsets and Related Human Immunological Disorders. Int. J. Mol. Sci. 2020, 21, 8011. [Google Scholar] [CrossRef]
- Hinke, D.M.; Andersen, T.K.; Gopalakrishnan, R.P.; Fossum, E.; Braathen, R.; Bogen, B. Antigen bivalency of antigen-presenting cell-targeted vaccines increases B cell responses. CellReports 2022, 39, 110901. [Google Scholar] [CrossRef]
- Sokolov, I.; Dokukin, M.E.; Guz, N.V. Method for quantitative measurements of the elastic modulus of biological cells in AFM indentation experiments. Methods 2013, 60, 202–213. [Google Scholar] [CrossRef]



| Glyco-DIBMA Nanodiscs | Sulfo-DIBMA Nanodiscs | |||||
|---|---|---|---|---|---|---|
| Size [nm] | PDI | Zeta potential [mV] | Size [nm] | PDI | Zeta potential [mV] | |
| PBS | 14.9 ± 0.3 | 0.307 ± 0.026 | −15.5 ± 2.9 | 13.2 ± 1.2 | 0.262 ± 0.018 | −3.5 ± 1.6 |
| RPMI (0 h) | 13.0 ± 0.5 | 0.215 ± 0.011 | −12.6 ± 3.3 | 17.7 ± 1.0 | 0.325 ± 0.009 | −2.3 ± 0.7 |
| RPMI (24 h) | 13.1 ± 0.3 | 0.250 ± 0.007 | −12.7 ± 0.8 | 11.4 ± 0.3 | 0.191 ± 0.038 | −2.0 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeiringer, S.; Derler, M.; Mussbacher, M.; Kolesnik, T.; Fröhlich, E.; Leitinger, G.; Kolb, D.; Tutz, S.; Vargas, C.; Keller, S.; et al. Immune Modulation with Nanodiscs: Surface Charge Dictates Cellular Interactions and Activation of Macrophages and Dendritic-like Cells. Int. J. Mol. Sci. 2025, 26, 5154. https://doi.org/10.3390/ijms26115154
Zeiringer S, Derler M, Mussbacher M, Kolesnik T, Fröhlich E, Leitinger G, Kolb D, Tutz S, Vargas C, Keller S, et al. Immune Modulation with Nanodiscs: Surface Charge Dictates Cellular Interactions and Activation of Macrophages and Dendritic-like Cells. International Journal of Molecular Sciences. 2025; 26(11):5154. https://doi.org/10.3390/ijms26115154
Chicago/Turabian StyleZeiringer, Scarlett, Martina Derler, Marion Mussbacher, Tatjana Kolesnik, Eleonore Fröhlich, Gerd Leitinger, Dagmar Kolb, Sarah Tutz, Carolyn Vargas, Sandro Keller, and et al. 2025. "Immune Modulation with Nanodiscs: Surface Charge Dictates Cellular Interactions and Activation of Macrophages and Dendritic-like Cells" International Journal of Molecular Sciences 26, no. 11: 5154. https://doi.org/10.3390/ijms26115154
APA StyleZeiringer, S., Derler, M., Mussbacher, M., Kolesnik, T., Fröhlich, E., Leitinger, G., Kolb, D., Tutz, S., Vargas, C., Keller, S., & Roblegg, E. (2025). Immune Modulation with Nanodiscs: Surface Charge Dictates Cellular Interactions and Activation of Macrophages and Dendritic-like Cells. International Journal of Molecular Sciences, 26(11), 5154. https://doi.org/10.3390/ijms26115154









