Antimicrobial, Quorum Sensing Inhibition, and Anti-Cancer Activities of Silver Nanoparticles Synthesized from Kenyan Bacterial Endophytes of Teclea nobilis
Abstract
1. Introduction
2. Results
2.1. Molecular Identification of Bacterial Isolates
2.2. Chemical Characterization of Endophytic Bacteria Crude Extracts
2.3. Silver Nanoparticle Biosynthesis
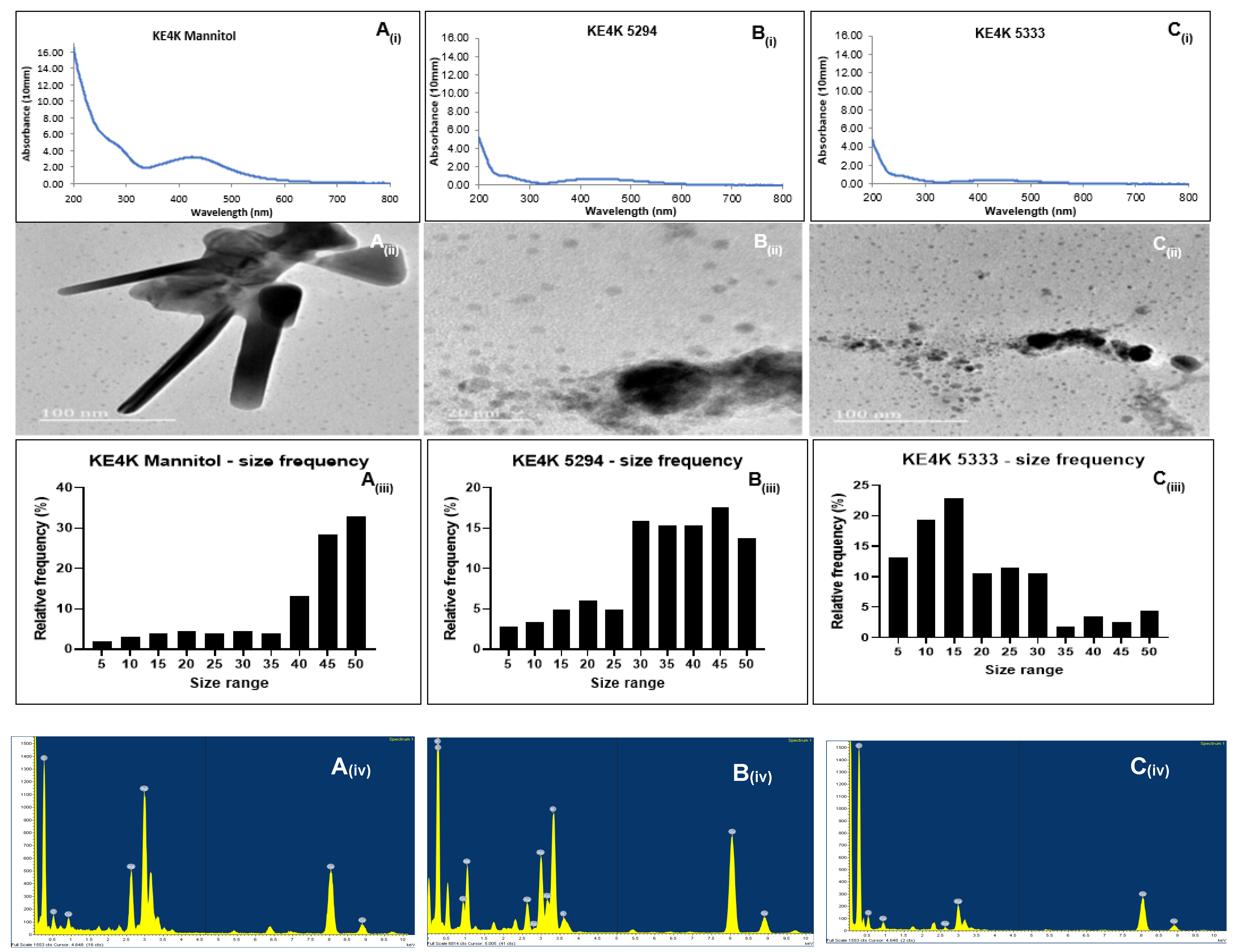
2.4. Characterization of Nanoparticles
2.4.1. UV-Visible Spectroscopy Confirmation of Nanoparticle Synthesis
2.4.2. Fourier-Transform Infrared Spectroscopy Analysis
2.4.3. High-Resolution Transmission Electron Microscopy Characterization
2.4.4. Zeta Potential Determination
2.5. Antimicrobial Activity Testing
2.6. Quorum Sensing Inhibitory Potential
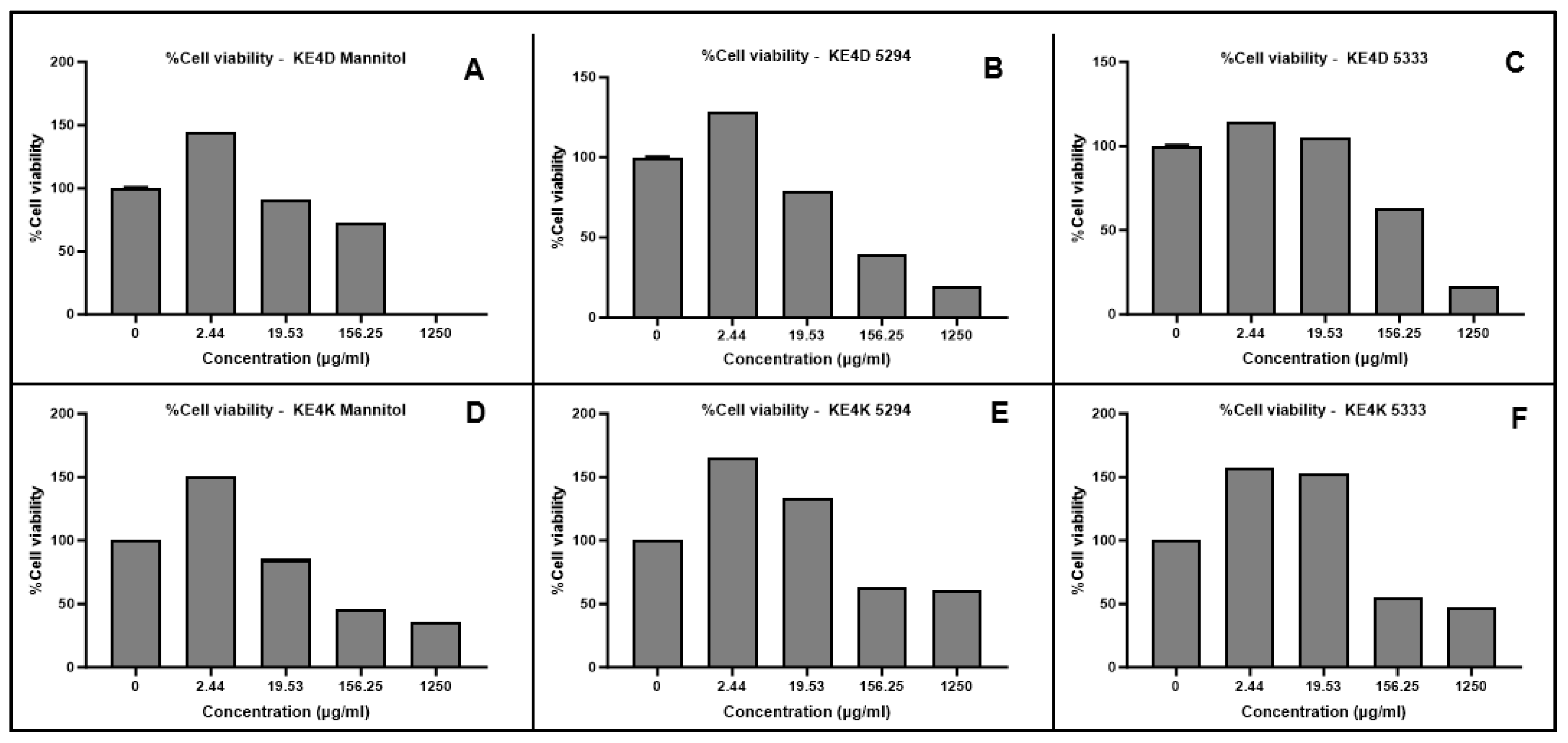
2.7. Cytotoxicity Assessment Using MTT Assay
3. Discussion
4. Materials and Methods
4.1. Isolation of Endophytic Bacteria
4.2. 16S rRNA Gene Amplification and Sequencing
4.3. Bacterial Fermentations and Extract Characterization
4.4. Ethyl Acetate Extraction, Fourier-Transform Infrared Spectroscopy and Gas Chromatography–Mass Spectrometry of Crude Extracts
4.5. Bacterial Silver Nanoparticle Synthesis
4.6. Characterization of Silver Nanoparticles
4.6.1. UV-Visible Spectroscopy
4.6.2. Fourier-Transform Infrared Spectroscopy
4.6.3. High-Resolution Transmission Electron Microscopy (HR-TEM)
4.6.4. Energy-Dispersive X-Ray Analysis (EDX)
4.6.5. Zeta Potential Assessment
4.7. Biological Activity Assessment of Endophytic Bacteria AgNPs
4.7.1. Antimicrobial Activity Assessment
4.7.2. Anti-Quorum Sensing Activity
4.7.3. Anti-Cancer Assays
Cell Culture
Cytotoxicity Test—MTT Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eshboev, F.; Mamadalieva, N.; Nazarov, P.A.; Hussain, H.; Katanaev, V.; Egamberdieva, D.; Azimova, S. Antimicrobial action mechanisms of natural compounds isolated from endophytic microorganisms. Antibiotics 2024, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Agarwal, S.; Verma, K.; Bhardwaj, R.; Mathur, V. Therapeutic compounds from medicinal plant endophytes: Molecular and metabolic adaptations. J. Appl. Microbiol. 2023, 134, lxad074. [Google Scholar] [CrossRef]
- Choudhury, S.; Baksi, S.; Bandyopadhyay, B.; Roy, D. Assessment of phytochemicals, antioxidant activity and enzyme production of endophytic fungi isolated from medicinal plant sources. J. Adv. Zool. 2023, 44, 1611–1620. [Google Scholar] [CrossRef]
- Jha, P.; Kaur, T.; Chhabra, I.; Panja, A.; Paul, S.; Kumar, V.; Malik, T. Endophytic fungi: Hidden treasure chest of antimicrobial metabolites interrelationship of endophytes and metabolites. Front. Microbiol. 2023, 14, 37497538. [Google Scholar] [CrossRef]
- Kashyap, N.; Singh, S.K.; Yadav, N.; Singh, V.K.; Kumari, M.; Kumar, D.; Kumar, A. Biocontrol screening of endophytes: Applications and limitations. Plants 2023, 12, 2480. [Google Scholar] [CrossRef]
- Allan, J.; Belz, S.; Hoeveler, A.; Hugas, M.; Okuda, H.; Patri, A.; Anklam, E. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul. Toxicol. Pharm. 2021, 122, 104885. [Google Scholar] [CrossRef]
- Dey, A.; Pandey, G.; Rawtani, D. Functionalized nanomaterials driven antimicrobial food packaging: A technological advancement in food science. Food Control 2022, 131, 108469. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahmad, R.; Zeyaullah, M.; Khare, S.K. Microbial nano-factories: Synthesis and biomedical applications. Front. Chem. 2021, 9, 194. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Sheikh, H.I.; Sarkar, T.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbiologically-synthesized nanoparticles and their role in silencing the biofilm signaling cascade. Front. Microbiol. 2021, 12, 180. [Google Scholar] [CrossRef]
- Kamnev, A.A.; Dyatlova, Y.A.; Kenzhegulov, O.A.; Vladimirova, A.A.; Mamchenkova, P.V.; Tugarova, A.V. Fourier transform infrared (FTIR) spectroscopic analyses of microbiological samples and biogenic selenium nanoparticles of microbial origin: Sample preparation effects. Molecules 2021, 26, 1146. [Google Scholar] [CrossRef]
- Ng, H.S.; Wan, P.K.; Kondo, A.; Chang, J.S.; Lan, J.C.W. Production and recovery of ectoine: A review of current state and future prospects. Processes 2023, 11, 339. [Google Scholar] [CrossRef]
- Sabzevar, A.H.; Hashemitabar, G.R.; Rad, M.; Vatandoost, J. Synthesis and biological properties of silver chloride nanoparticles using cell-free extracts of Aeromonas hydrophila and antibacterial activity against drug-resistant bacteria. Braz. Arch. Biol. Technol. 2021, 64, e21210010. [Google Scholar] [CrossRef]
- Rahman, S.; Rahman, L.; Khalil, A.T.; Ali, N.; Zia, D.; Ali, M.; Shinwari, Z.K. Endophyte-mediated synthesis of silver nanoparticles and their biological applications. Appl. Microbiol. Biotechnol. 2019, 103, 2551–2569. [Google Scholar] [CrossRef] [PubMed]
- Saeki, E.K.; Yamada, A.Y.; De Araujo, L.A.; Anversa, L.; de Oliveira Garcia, D.; Barros De Souza, R.L.; Nakazato, G. Subinhibitory concentrations of biogenic silver nanoparticles affect motility and biofilm formation in Pseudomonas aeruginosa. Front. Cell Infect. Microbiol. 2021, 11, 253. [Google Scholar] [CrossRef]
- Awadelkareem, A.M.; Siddiqui, A.J.; Noumi, E.; Ashraf, S.A.; Hadi, S.; Snoussi, M.; Adnan, M. Biosynthesized silver nanoparticles derived from probiotic Lactobacillus rhamnosus (AgNPs-LR) targeting biofilm formation and quorum sensing-mediated virulence factors. Antibiotics 2023, 12, 986. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Patel, M.; Jahan, S.; Abdelgadir, A.; Alam, M.J.; Alshahrani, M.M.; Adnan, M. Silver nanoparticles derived from probiotic Lactobacillus casei—A novel approach for combating bacterial infections and cancer. Probiotics Antimicrob. Proteins 2023, 1–18. [Google Scholar] [CrossRef]
- Mookherjee, A.; Singh, S.; Maiti, M.K. Quorum sensing inhibitors: Can endophytes be prospective sources? Arch. Microbiol. 2018, 200, 355–369. [Google Scholar] [CrossRef]
- Jabeen, S.; Qureshi, R.; Munazir, M.; Maqsood, M.; Munir, M.; Shah, S.S.H.; Rahim, B.Z. Application of green synthesized silver nanoparticles in cancer treatment—A critical review. Mater. Res. Express 2021, 8, 092001. [Google Scholar] [CrossRef]
- Miranda, R.R.; Sampaio, I.; Zucolotto, V. Exploring silver nanoparticles for cancer therapy and diagnosis. Colloid. Surface B Biointerfaces 2022, 210, 112254. [Google Scholar] [CrossRef]
- Chadive, D.K.; Gurrala, P.; Dowlathabad, M.R. Biogenic synthesis of silver nanoparticles from the leaf extract of Erythroxylum Monogynum Roxb: Evaluation of antibacterial and anticancer effects. Nano-Struct. Nano-Objects 2024, 39, 101222. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Jawad, K.H.; Çevik, S.; Sulaiman, G.M.; Albukhaty, S.; Sasikumar, P. Investigating the antimicrobial, antioxidant, and anticancer effects of Elettaria cardamomum seed extract conjugated to green synthesized silver nanoparticles by laser ablation. Plasmonics 2024, 19, 1187–1200. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Mohamed, B.S.; Zayed, M.; El-Sabbagh, S.M. Antibacterial, antibiofilm, and anticancer activity of silver-nanoparticles synthesized from the cell-filtrate of Streptomyces enissocaesilis. BMC Biotechnol. 2024, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Kiani, Z.; Aramjoo, H.; Chamani, E.; Siami-Aliabad, M.; Mortazavi-Derazkola, S. In vitro cytotoxicity against K562 tumour cell line, antibacterial, antioxidant, antifungal and catalytic activities of biosynthesized silver nanoparticles using Sophora pachycarpa extract. Arab. J. Chem. 2022, 15, 103677. [Google Scholar] [CrossRef]
- Mobaraki, F.; Momeni, M.; Jahromi, M.; Kasmaie, F.M.; Barghbani, M.; Yazdi, M.E.T.; Hosseini, S.M. Apoptotic, antioxidant and cytotoxic properties of synthesized AgNPs using green tea against human testicular embryonic cancer stem cells. Process Biochem. 2022, 119, 106–118. [Google Scholar] [CrossRef]
- Takáč, P.; Michalková, R.; Čižmáriková, M.; Bedlovičová, Z.; Balážová, L.; Takáčová, G. The role of silver nanoparticles in the diagnosis and treatment of cancer: Are there any perspectives for the future? Life 2023, 13, 466. [Google Scholar] [CrossRef]
- Zaib, S.; Shah, H.S.; Khan, I.; Jawad, Z.; Sarfraz, M.; Riaz, H.; Ahmed, D.A.E.M. Fabrication and evaluation of anti-cancer potential of diosgenin incorporated chitosan-silver nanoparticles; in vitro, in silico and in vivo studies. Int. J. Biol. Macromol. 2024, 254, 127975. [Google Scholar] [CrossRef]
- Meena, M.; Zehra, A.; Swapnil, P.; Marwal, A.; Yadav, G.; Sonigra, P. Endophytic nanotechnology: An approach to study scope and potential applications. Front. Chem. 2021, 9, 47. [Google Scholar] [CrossRef]
- Sarhan, A.; Fahmy, T.; Habib, A. Optical, AC conductivity and antibacterial activity enhancement of chitosan-silver nanocomposites for optoelectronic and biomedical applications. Phys. Scr. 2024, 99, 105905. [Google Scholar] [CrossRef]
- Kumar, C.M.K.; Yugandhar, P.; Savithramma, N. Biological synthesis of silver nanoparticles from Adansonia digitata L. fruit pulp extract, characterization, and its antimicrobial properties. J. Intercult. Ethnopharmacol. 2016, 5, 79. [Google Scholar] [CrossRef]
- Ibrahim, E.H.; Kilany, M.; Ghramh, H.A.; Khan, K.A.; Islam, S. Cellular proliferation/cytotoxicity and antimicrobial potentials of green synthesized silver nanoparticles (AgNPs) using Juniperus procera. Saudi J. Biol. Sci. 2019, 26, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Kralova, K.; Jampilek, J. Responses of medicinal and aromatic plants to engineered nanoparticles. Appl. Sci. 2021, 11, 1813. [Google Scholar] [CrossRef]
- Math, H.H.; Shashiraj, K.N.; Kumar, R.S.; Rudrappa, M.; Bhat, M.P.; Basavarajappa, D.S.; Nayaka, S. Investigation of in vitro anticancer and apoptotic potential of biofabricated silver nanoparticles from Cardamine hirsuta (L.) leaf extract against Caco-2 cell line. Inorganics 2023, 11, 322. [Google Scholar] [CrossRef]
- Damavandi, M.S.; Shojaei, H.; Esfahani, B.N. The anti-cancer and antibacterial potential of bioactive secondary metabolites derived from bacterial endophytes in association with Artemisia absinthium. Sci. Rep. 2023, 13, 18473. [Google Scholar] [CrossRef]
- Duan, C.; Xiao, X.; Yu, Y.; Xu, M.; Zhang, Y.; Liu, X.; Wang, J. In situ Raman characterization of the stability of blueberry anthocyanins in aqueous solutions under perturbations in temperature, UV, pH. Food Chem. 2024, 431, 137155. [Google Scholar] [CrossRef]
- Samuel, M.S.; Jose, S.; Selvarajan, E.; Mathimani, T.; Pugazhendhi, A. Biosynthesized silver nanoparticles using Bacillus amyloliquefaciens; Application for cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol. Photoch. Photobio. B 2020, 202, 111642. [Google Scholar] [CrossRef]
- Jain, A.S.; Pawar, P.S.; Sarkar, A.; Junnuthula, V.; Dyawanapelly, S. Bionanofactories for green synthesis of silver nanoparticles: Toward antimicrobial applications. Int. J. Mol. Sci. 2021, 22, 11993. [Google Scholar] [CrossRef]
- Das, I.; Bharali, P.; Gogoi, P.; Borah, A.; Borah, D. Green synthesis of silver nanoparticles using microbial biosurfactant produced by a newly isolated Klebsiella sp. strain RGUDBI03 through a single step method and exploring its role in enhancing the chickpea and rice seed germination. BioNanoSci 2024, 14, 4693–4709. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Aires, A.; Falco, V.; Valentão, P.; Poeta, P. Phenolic compounds classification and their distribution in winemaking by-products. Eur. Food Res. Technol. 2023, 249, 207–239. [Google Scholar] [CrossRef]
- Polozsányi, Z.; Galádová, H.; Kaliňák, M.; Jopčík, M.; Kaliňáková, B.; Breier, A.; Šimkovič, M. The antimicrobial effects of myrosinase hydrolysis products derived from glucosinolates isolated from Lepidium draba. Plants 2024, 13, 995. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Rezaei, A.; Abdollahi, H.; Derikvand, Z.; Hemmati-Sarapardeh, A.; Mosavi, A.; Nabipour, N. Insights into the effects of pore size distribution on the flowing behavior of carbonate rocks: Linking a nano-based enhanced oil recovery method to rock typing. Nanomaterials 2020, 10, 972. [Google Scholar] [CrossRef] [PubMed]
- Héger, M.; Noiset, P.; Nkoba, K.; Vereecken, N.J. Traditional ecological knowledge and non-food uses of stingless bee honey in Kenya’s last pocket of tropical rainforest. J. Ethnobiol. Ethnomed. 2023, 19, 42. [Google Scholar] [CrossRef]
- Mbuvi, M.T.E.; Kungu, J.B.; Eshitera, A. the impact of governance regime on land cover and use change and forest structure: Insights from Kakamega and Loita forests, Kenya. Open J. For. 2022, 12, 185–215. [Google Scholar] [CrossRef]
- Tsipinana, S.; Husseiny, S.; Alayande, K.A.; Raslan, M.; Amoo, S.; Adeleke, R. Contribution of endophytes towards improving plant bioactive metabolites: A rescue option against red-taping of medicinal plants. Front. Plant Sci. 2023, 14, 1248319. [Google Scholar] [CrossRef]
- Tiwari, P.; Srivastava, Y.; Bae, H. Trends of pharmaceutical design of endophytes as anti-infectives. Curr. Top. Med. Chem. 2021, 21, 1572–1586. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumar, A. Secondary metabolites from endophytic fungi: Production, methods of analysis, and diverse pharmaceutical potential. Symbiosis 2023, 90, 111–125. [Google Scholar] [CrossRef]
- Fareed, N.; Nisa, S.; Bibi, Y.; Fareed, A.; Ahmed, W.; Sabir, M.; Qayyum, A. Green synthesized silver nanoparticles using carrot extract exhibited strong antibacterial activity against multidrug-resistant bacteria. J. King Saud Univ.-Sci. 2023, 35, 102477. [Google Scholar] [CrossRef]
- Sivakumar, S.; Subban, M.; Chinnasamy, R.; Chinnaperumal, K.; Nakouti, I.; El-Sheikh, M.A.; Shaik, J.P. Green synthesized silver nanoparticles using Andrographis macrobotrys Nees leaf extract and its potential to antibacterial, antioxidant, anti-inflammatory and lung cancer cells cytotoxicity effects. Inorg. Chem. Commun. 2023, 153, 110787. [Google Scholar] [CrossRef]
- Ghasemi, S.; Dabirian, S.; Kariminejad, F.; Koohi, D.E.; Nemattalab, M.; Majidimoghadam, S.; Yousefbeyk, F. Process optimization for green synthesis of silver nanoparticles using Rubus discolor leaves extract and its biological activities against multi-drug resistant bacteria and cancer cells. Sci. Rep. 2024, 14, 4130. [Google Scholar] [CrossRef]
- Martin, P.; Zhang, P.; Rodger, P.M.; Valsami-Jones, E. Simulations of morphological transformation in silver nanoparticles as a tool for assessing their reactivity and potential toxicity. NanoImpact 2019, 14, 100147. [Google Scholar] [CrossRef]
- Huong, V.T.L.; Nguyen, N.T. Green synthesis, characterization and antibacterial activity of silver nanoparticles using Sapindus mukorossi fruit pericarp extract. Mater. Today-Proc. 2021, 42, 88–93. [Google Scholar] [CrossRef]
- Chugh, D.; Viswamalya, V.S.; Das, B. Green synthesis of silver nanoparticles with algae and the importance of capping agents in the process. J. Genet. Eng. Biotechnol. 2021, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, N.H.; Buazar, F.; Matroodi, S. Synergistic effects of combinatorial chitosan and polyphenol biomolecules on enhanced antibacterial activity of biofunctionalized silver nanoparticles. Sci. Rep. 2020, 10, 19615. [Google Scholar] [CrossRef]
- Baker, S.; Satish, S. Molecular and biomolecular spectroscopy biosynthesis of gold nanoparticles by Pseudomonas veronii AS41G inhabiting Annona squamosa L. Spectrochim. Acta A 2015, 150, 91–95. [Google Scholar] [CrossRef]
- Hać, A.; Brokowska, J.; Rintz, E.; Bartkowski, M.; Węgrzyn, G.; Herman-Antosiewicz, A. Mechanism of selective anti-cancer activity of isothiocyanates relies on differences in DNA damage repair between cancer and healthy cells. Eur. J. Nutr. 2020, 59, 1421–1432. [Google Scholar] [CrossRef]
- Richa, K.; Temsurenla; Supong, A.; Ajungla, T.; Sinha, U.B. Mechanistic insight into the antibacterial activity of isothiocyanates via cell membrane permeability alteration. Pharm. Chem. J. 2022, 56, 300–308. [Google Scholar] [CrossRef]
- Shaheen, I.; Ahmad, K.S. Chromatographic identification of “green capping agents” extracted from Nasturtium officinale (Brassicaceae) leaves for the synthesis of MoO3 nanoparticles. J. Sep. Sci. 2020, 43, 598–605. [Google Scholar] [CrossRef]
- Nayaka, S.; Bhat, M.P.; Udayashankar, A.C.; Lakshmeesha, T.R.; Geetha, N.; Jogaiah, S. Biosynthesis and characterization of Dillenia indica-mediated silver nanoparticles and their biological activity. Appl. Organomet. Chem. 2020, 34, e5567. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Strong antimicrobial activity of silver nanoparticles obtained by the green synthesis in Viridibacillus sp. extracts. Front. Microbiol. 2022, 13, 820048. [Google Scholar] [CrossRef]
- Kumar, R.; Panwar, H. Synthesis and pharmacological evaluation: Antimicrobial, anti-inflammatory, analgesic, ulcerogenic properties of several bis-heterocyclic derivatives. Indones. J. Pharm. 2015, 26, 14499. [Google Scholar] [CrossRef][Green Version]
- Moghaddam, A.J.; Dávila-Céspedes, A.; Kehraus, S.; Crüsemann, M.; Köse, M.; Müller, C.E.; König, G.M. Cyclopropane-containing fatty acids from the marine bacterium Labrenzia sp. 011 with antimicrobial and GPR84 activity. Mar. Drugs 2018, 16, 369. [Google Scholar] [CrossRef] [PubMed]
- Frattini, A.; Pellegri, N.; Nicastro, D.; De Sanctis, O. Effect of amine groups in the synthesis of Ag nanoparticles using aminosilanes. Mater. Chem. Phys. 2005, 94, 148–152. [Google Scholar] [CrossRef]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An overview of their antimicrobial activity against human infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef]
- Patil, S.B.; Basrani, S.T.; Chougule, S.A.; Gavandi, T.C.; Karuppayil, S.M.; Jadhav, A.K. Butyl isothiocyanate exhibits antifungal and anti-biofilm activity against Candida albicans by targeting cell membrane integr 10.3390/molecules23030624 ity, cell cycle progression and oxidative stress. Arch. Microbiol. 2024, 206, 251. [Google Scholar] [CrossRef]
- Mitsiogianni, M.; Koutsidis, G.; Mavroudis, N.; Trafalis, D.T.; Botaitis, S.; Franco, R.; Panayiotidis, M.I. The role of isothiocyanates as cancer chemo-preventive, chemo-therapeutic and anti-melanoma agents. Antioxidants 2019, 8, 106. [Google Scholar] [CrossRef]
- Ozdal, M.; Gurkok, S. Recent advances in nanoparticles as antibacterial agent. ADMET DMPK 2022, 10, 115–129. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.; Zeng, Y.; Zhu, J.; Du, X.; Cai, Z.; Zhou, J. The rhodamine isothiocyanate analogue as a quorum sensing inhibitor has the potential to control microbially-induced biofouling. Mar. Drugs 2020, 18, 484. [Google Scholar] [CrossRef]
- Rajivgandhi, G.; Vijayan, R.; Maruthupandy, M.; Vaseeharan, B.; Manoharan, N. Antibiofilm effect of Nocardiopsis sp. GRG 1 (KT235640) compound against biofilm forming Gram negative bacteria on UTIs. Microb. Pathog. 2018, 118, 190–198. [Google Scholar] [CrossRef]
- Abdelgawad, H.; Magdy Korany, S.; Reyad, A.M.; Zahid, I.; Akhter, N.; Alsherif, E.; Crecchio, C. Synergistic impacts of plant-growth-promoting bacteria and selenium nanoparticles on improving the nutritional value and biological activities of three cultivars of Brassica sprouts. ACS Omega 2023, 8, 26414–26424. [Google Scholar] [CrossRef]
- Sidhu, A.K.; Verma, N.; Kaushal, P. Role of biogenic capping agents in the synthesis of metallic nanoparticles and evaluation of their therapeutic potential. Front. Nanotechnol. 2022, 3, 801620. [Google Scholar] [CrossRef]
- Iqbal, N.; Anastasiou, A.; Aslam, Z.; Raif, E.M.; Do, T.; Giannoudis, P.V.; Jha, A. Interrelationships between the structural, spectroscopic, and antibacterial properties of nanoscale (<50 nm) cerium oxides. Sci. Rep. 2021, 11, 20875. [Google Scholar] [CrossRef]
- Wahab, M.A.; Li, L.; Li, H.; Abdala, A. Silver nanoparticle-based nanocomposites for combating infectious pathogens: Recent advances and future prospects. Nanomaterials 2021, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Memon, A.G.; Shaikh, A.A.; Ismail, T.; Giwa, A.S.; Mahmood, A. Insight into single-element noble metal anisotropic silver nanoparticle shape-dependent selective ROS generation and quantification. RSC Adv. 2021, 11, 8314–8322. [Google Scholar] [CrossRef]
- Hinsch, J.J.; Liu, J.; White, J.J.; Wang, Y. The role of steps on silver nanoparticles in electrocatalytic oxygen reduction. Catalysts 2022, 12, 576. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Mashrur, F.R.; Chhoan, A.P.; Shahriar, S.M.; Haidere, M.F.; Runa, N.J.; Cho, J.Y. Silver nanoparticles as potential antiviral agents. Pharmaceutics 2021, 13, 2034. [Google Scholar] [CrossRef]
- Veider, F.; Sanchez Armengol, E.; Bernkop-Schnürch, A. Charge-reversible nanoparticles: Advanced delivery systems for therapy and diagnosis. Small 2024, 20, 2304713. [Google Scholar] [CrossRef]
- Joseph, E.; Singhvi, G. Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. In Nanomaterials for Drug Delivery and Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Amsterdam, The Netherlands, 2019; pp. 91–116. [Google Scholar]
- Rambaran, N.; Naidoo, Y.; Mohamed, F.; Chenia, H.Y.; Baijnath, H. Antibacterial and anti-quorum sensing properties of silver nanoparticles phytosynthesized using Embelia ruminata. Plants 2024, 13, 168. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Green silver nanoparticles: An antibacterial mechanism. Antibiotics 2024, 14, 5. [Google Scholar] [CrossRef]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Felgueiras, H.P. Activity of specialized biomolecules against Gram-positive and Gram-negative bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Mammari, N.; Lamouroux, E.; Boudier, A.; Duval, R.E. Current knowledge on the oxidative-stress-mediated antimicrobial properties of metal-based nanoparticles. Microorganisms 2022, 10, 437. [Google Scholar] [CrossRef]
- Nisar, P.; Ali, N.; Rahman, L.; Ali, M.; Shinwari, Z.K. Antimicrobial activities of biologically synthesized metal nanoparticles: An insight into the mechanism of action. J. Biol. Inorg. Chem. 2019, 24, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Arellano, U.; Wang, J.A.; Balcázar, L.M.; Chen, L.; Salmones, J.; Solís, S.; González, J. Ag/CeO2/SBA-15 hybrid catalysts for the elimination of E. coli in potable water system. J. Appl. Res. Technol. 2020, 18, 315–332. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Morohoshi, T.; Kato, M.; Fukamachi, K.; Kato, N.; Ikeda, T. N-acylhomoserine lactone regulates violacein production in Chromobacterium violaceum type strain ATCC 12472. FEMS Microbiol. Lett. 2008, 279, 124–130. [Google Scholar] [CrossRef]
- Abudoleh, S.M.; Mahasneh, A.M. Anti-quorum sensing activity of substances isolated from wild berry associated bacteria. Avicenna J. Med. Biotechnol. 2017, 9, 23. [Google Scholar]
- Qais, F.A.; Ahmad, I.; Altaf, M.; Manoharadas, S.; Al-Rayes, B.F.; Abuhasil, M.S.A.; Almaroai, Y.A. Biofabricated silver nanoparticles exhibit broad-spectrum antibiofilm and antiquorum sensing activity against Gram-negative bacteria. RSC Adv. 2021, 11, 13700–13710. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Li, J.; Qin, G. New strategies for biocontrol of bacterial toxins and virulence: Focusing on quorum-sensing interference and biofilm inhibition. Toxins 2023, 15, 570. [Google Scholar] [CrossRef]
- Santos, R.A.; Monteiro, M.; Rangel, F.; Jerusik, R.; Saavedra, M.J.; Carvalho, A.P.; Serra, C.R. Bacillus spp. inhibit Edwardsiella tarda quorum-sensing and fish infection. Mar. Drugs 2021, 19, 602. [Google Scholar] [CrossRef]
- Bulut, G.; Yaşa, İ.; Eren Eroğlu, A.E. Selection and molecular response of AHL-lactonase (aiia) producing Bacillus sp. under penicillin G-induced conditions. Protein J. 2023, 42, 427–436. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, W.J.; Bhatt, K.; Zhou, Z.; Huang, Y.; Zhang, L.H.; Wang, J. Innovative microbial disease biocontrol strategies mediated by quorum quenching and their multifaceted applications: A review. Front. Plant Sci. 2023, 13, 1063393. [Google Scholar] [CrossRef] [PubMed]
- D’Aquila, P.; De Rose, E.; Sena, G.; Scorza, A.; Cretella, B.; Passarino, G.; Bellizzi, D. Quorum quenching approaches against bacterial-biofilm-induced antibiotic resistance. Antibiotics 2024, 13, 619. [Google Scholar] [CrossRef] [PubMed]
- Roopashree, P.G.; Shetty, S.S.; Shetty, V.V.; Nalilu, S.K. Medium-chain fatty acids and breast cancer risk by receptor and pathological subtypes. Nutrients 2022, 14, 5351. [Google Scholar] [CrossRef] [PubMed]
- Millaty, I.N.K.; Wijayanti, N.; Hidayati, L.; Nuringtyas, T.R. Identification of anti-cancer compounds in leaves extracts of agarwood (Aquilaria malaccensis (Lamk.)). IOP Conf. Ser. Earth Environ. 2020, 457, 12036. [Google Scholar] [CrossRef]
- Balaji, M.; Thamilvanan, D.; Vinayagam, S.C.; Balakumar, B.S. Anti-cancer, antioxidant activity and GC-MS analysis of selected micro algal members of Chlorophyceae. Int. J. Pharm. Sci. Res. 2017, 8, 3302–3314. [Google Scholar] [CrossRef]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green synthesized silver nanoparticles: Antibacterial and anticancer activities, biocompatibility, and analyses of surface-attached proteins. Front. Microbiol. 2021, 12, 632505. [Google Scholar] [CrossRef]
- Heravi, R.E.; Zakeri, S.; Nazari, P. Anti-cancer activity evaluation of green synthesized gold-silver alloy nanoparticles on colorectal HT-29 and prostate DU-145 carcinoma cell lines. Micro Nano Lett. 2018, 13, 1475–1479. [Google Scholar] [CrossRef]
- Satyanarayana, B.M.; Reddy, N.V.; Kommula, S.K.R.; Rao, J.V. Biogenesis of silver nanoparticles using leaf extracts of Asparagus racemosus and Sophora interrupta: Structure characterization, antibacterial and anti-cancer studies. SN Appl. Sci. 2020, 2, 1857. [Google Scholar] [CrossRef]
- Kitimu, S.R.; Kirira, P.; Sokei, J.; Ochwangi, D.; Mwitari, P.; Maina, N. Biogenic synthesis of silver nanoparticles using Azadirachta indica methanolic bark extract and their anti-proliferative activities against DU-145 human prostate cancer cells. Afr. J. Biotechno. 2022, 21, 64–72. [Google Scholar]
- Majeed, S.; Danish, M.; Zakariya, N.A.; Hashim, R.; Ansari, M.T.; Alkahtani, S.; Hasnain, M.S. In vitro evaluation of antibacterial, antioxidant, and antidiabetic activities and glucose uptake through 2-NBDG by Hep-2 liver cancer cells treated with green synthesized silver nanoparticles. Oxidative Med. Cell. Longev. 2022, 2022, 1646687. [Google Scholar] [CrossRef]
- Almarwani, B.; Hamada, Y.Z.; Phambu, N.; Sunda-Meya, A. Investigating the insertion mechanism of cell-penetrating peptide penetration into cell membranes: Implications for targeted drug delivery. Biophysica 2023, 3, 620–635. [Google Scholar] [CrossRef]
- Kaewkla, O.; Franco, C.M. Rational approaches to improving the isolation of endophytic actinobacteria from Australian native trees. Microb. Ecol. 2013, 65, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Khathi, S. Screening of Bacterial Endophytes from Kenyan Medicinal Plants for Anti-Quorum Sensing and Anti-Biofilm Activities. Honours Thesis, University of KwaZulu-Natal, Durban, South Africa, 2015. [Google Scholar]
- Wink, J. Methods for the taxonomic description of the Actinobacteria. In Compendium of Actinobacteria; University of Braunschweig: Braunschweig, Germany, 2012; Available online: http://www.dsmz.de/fileadmin/Bereiche/Microbiology/Dateien/Bacterial_Nomenclature_uptodate/Actinomethods.pdf (accessed on 15 February 2018).
- Coram, N.J.; Rawlings, D.E. Molecular relationship between two groups of the genus Leptospirillum and the finding that Leptospirillum ferriphilum sp. nov. dominates South African commercial biooxidation tanks that operate at 40 °C. Appl. Environ. Microb. 2002, 68, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Baltes, M.R.H.; Dubois, J.G.; Hanocq, M. Ethyl acetate extraction procedure and isocratic high-performance liquid chromatographic assay for testosterone metabolites in cell microsomes. J. Chromatogr. B Biomed. Appl. 1998, 706, 201–207. [Google Scholar] [CrossRef]
- Singh, D.; Rathod, V.; Ninganagouda, S.; Hiremath, J.; Singh, A.K.; Mathew, J. Optimization and characterization of silver nanoparticle by endophytic fungi Penicillium sp. isolated from Curcuma longa (Turmeric) and application studies against MDR E. coli and S. aureus. Bioinorg. Chem. Appl. 2014, 2014, 408021. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Biswas, K.; Jena, S.K.; Hashem, A.; Abdallah, E.F.; Mohanta, T.K. Anti-biofilm and antibacterial activities of silver nanoparticles synthesized by the reducing activity of phytoconstituents present in the Indian medicinal plants. Front. Microbiol. 2020, 11, 1143. [Google Scholar] [CrossRef]
- Manivasagan, P.; Kang, K.H.; Kim, D.G.; Kim, S.K. Production of polysaccharide-based bioflocculant for the synthesis of silver nanoparticles by Streptomyces sp. Int. J. Biol. Macromol. 2015, 77, 159–167. [Google Scholar] [CrossRef]
- Gu, J.; Hu, C.; Zhang, W.; Dichiara, A.B. Reagentless preparation of shape memory cellulose nanofibril aerogels decorated with Pd nanoparticles and their application in dye discoloration. Appl. Catal. B Environ. 2018, 237, 482–490. [Google Scholar] [CrossRef]
- Chenia, H.Y. Anti-quorum sensing potential of crude Kigelia africana fruit extracts. Sensors 2013, 13, 2802–2817. [Google Scholar] [CrossRef]
- Segeritz, C.P.; Vallier, L. In: Cell culture: Growing cells as model systems in vitro. In Basic Science Methods for Clinical Researchers; Elsevier: Cambridge, UK, 2017; pp. 151–172. [Google Scholar]
- Ravi, K.; Dhoddi, B.N.; Pochampally, J.; Matta, R. Synthesis of benzothiazole/benzimidazole-conjugated imidazoles as anti-cancer agents and their molecular modeling. Russ. J. Org. Chem. 2023, 59, 915–923. [Google Scholar] [CrossRef]
- Nakashima, M.; Adachi, S.; Yasuda, I.; Yamauchi, T.; Kawaguchi, J.; Hanamatsu, T.; Moriwaki, H. Inhibition of Rho-associated coiled-coil containing protein kinase enhances the activation of epidermal growth factor receptor in pancreatic cancer cells. Mol. Cancer 2011, 10, 79. [Google Scholar] [CrossRef][Green Version]




| Crude Extract Samples | Functional Groups |
|---|---|
| KE4D | |
| Mannitol | Hydroxyl (O-H), carbon dioxide (O=C=O), bonded stretching of amines/amides (N-H/C-H/O-H), alkene (C=C), isothiocyanate (N=C=S), alkyl halides (R-X). |
| 5294 | Hydroxyl (O-H), carbon dioxide (O=C=O), alkane (C-H), carboxylic acid (R-COOH), alkene (C=C), isothiocyanate (N=C=S), alkyl halides( R-X). |
| 5333 | Hydroxyl (O-H), carbon dioxide (O=C=O), alkane (C-H), cyanide (C-N), alkene (C=C), isothiocyanate (N=C=S), alkyl halides(R-X). |
| KE4K | |
| Mannitol | Hydroxyl (O-H), carbon dioxide (O=C=O), alkane (C-H), alkene (C=C), isothiocyanate (N=C=S), alkyl amine (R-NH2). |
| 5294 | Hydroxyl (O-H), carbon dioxide (O=C=O), alkene (C=C), nitrile (CΞN), aromatic compound (C-H), alkyl amine (R-NH2). |
| 5333 | Hydroxyl (O-H), carbon dioxide (O=C=O), alkene (C=C), nitrile (CΞN), aromatic compound (C-H), alkyl amine (R-NH2). |
| AgNPs | Functional groups |
| KE4D | |
| Mannitol | Hydroxyl (O-H), carbon dioxide (O=C=O), alkyne (CΞC), isothiocyanate (N=C=S), nitro- (N-O), alkane (C-H), alcohol (C-O). |
| 5294 | Hydroxyl (O-H), alkane (C-H), carbon dioxide (O=C=O), alkyne (CΞC), isothiocyanate (N=C=S), alkene (C=C), nitro- (N-O), alkyl aryl ether (C-O), sulfoxide (S=O). |
| 5333 | Amine (N-H), carbon dioxide (O=C=O), alkyne (CΞC), isothiocyanate (N=C=S), nitro- (N-O), fluoro-compound (C-F), sulfonamide (S=O), aromatic amine (C-N), phenol (O-H), trisubstituted alkene (C=C). |
| KE4K | |
| Mannitol | Hydroxyl (O-H), amine salt (N-H), carbon dioxide (O=C=O), isothiocyanate (N=C=S), nitro- (N-O), ether (C-O-C). |
| 5294 | Hydroxyl (O-H), amine salt (N-H), carbon dioxide (O=C=O), alkyne (CΞC), aromatic compound (C-H), alkene (C=C), sulfonamide (S=O). |
| 5333 | Hydroxyl (O-H), carbon dioxide (O=C=O), nitrile (CΞN), aromatic compound (C-H), sulfoxide (S=O). |
| Fermentation media control | |
| Mannitol | Hydroxyl (O-H), carbon dioxide (O=C=O), alkane (C-H), alkene (C=C), carboxylic acid (R-COOH), ester (R-COO-R). |
| 5294 | Hydroxyl (O-H), carbon dioxide (O=C=O), ester (R-COO-R), carboxylic acid (R-COOH), nitrile (CΞN), ketone RC(=O)R. |
| 5333 | Hydroxyl (O-H), carbon dioxide (O=C=O), ester (R-COO-R), sulfoxide (S=O), carboxylic acid (R-COOH), ketone RC(=O)R. |
| Compound | Medium Mannitol Control | KE4D Mannitol | KE4K Mannitol | |||
|---|---|---|---|---|---|---|
| Rt (min) | %Area | Rt (min) | %Area | Rt (min) | %Area | |
| 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl | 16.614 | 57.04 | ND | ND | ND | ND |
| 2,5-Hexanedione, 3,4-dihydroxy-3,4-dimethyl- | ND | ND | ND | ND | 6.347 | 18.36 |
| 2,5-Hexanedione, 3,4-dihydroxy-3,4-dimethyl- | ND | ND | ND | ND | 7.973 | 1.26 |
| Acetamide, N-(2-methylpropyl)- | ND | ND | ND | ND | 6.413 | 6.41 |
| cis-10-Heptadecenoic acid | 23.272 | 4.87 | ND | ND | ND | ND |
| Cyclopropaneacetic acid, 2-hexyl- | ND | ND | 12.484 | 2.09 | ND | ND |
| Dodecanoic acid, isooctyl ester | 21.65 | 2.42 | ND | ND | ND | ND |
| Eicosanoic acid | ND | ND | 20.418 | 3.17 | ND | ND |
| Hexadecanoic acid | ND | ND | 18.728 | 7.83 | ND | ND |
| Hexadecanoic acid, methyl ester | ND | ND | 17.151 | 1.69 | ND | ND |
| Hexanedioic acid, bis(2-methylpropyl) ester | 14.392 | 6.84 | ND | ND | ND | ND |
| Isovaline, 3-hydroxy- | ND | ND | ND | ND | 6.347 | 18.36 |
| Isovaline, 3-hydroxy- | ND | ND | ND | ND | 6.413 | 6.41 |
| Malic Acid | ND | ND | ND | ND | 7.244 | 4.23 |
| N(1),N(1)-Diethyl-1,2-butanediamine | ND | ND | ND | ND | 6.478 | 4.03 |
| N-(3-Methylbutyl)acetamide | ND | ND | ND | ND | 7.705 | 6.61 |
| Pentadecanoic acid | ND | ND | 16.337 | 2.14 | ND | ND |
| Pentadecanoic acid | ND | ND | 16.49 | 11.86 | ND | ND |
| Pentadecanoic acid, 14-methyl-, methyl ester | ND | ND | 17.796 | 1.27 | ND | ND |
| Pyrazine, tetramethyl- | ND | ND | ND | ND | 8.017 | 0.47 |
| Pyrrolo [1,2-a]pyrazine-1,4-dione, hexahydro-3-(2 | ND | ND | 20.21 | 35.08 | ND | ND |
| Tetradecanoic acid | ND | ND | 15.11 | 0.92 | ND | ND |
| Tetradecanoic acid, 5,9,13-trimethyl-, methyl ester | ND | ND | 19.301 | 2.13 | ND | ND |
| Tridecanoic acid, 12-methyl-, methyl ester | ND | ND | 15.813 | 6.01 | ND | ND |
| Compound | Medium 5294 Control | KE4D 5294 | KE4K 5294 | |||
|---|---|---|---|---|---|---|
| Rt (min) | %Area | Rt (min) | %Area | Rt (min) | %Area | |
| 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 16.838 | 66.45 | ND | ND | ND | ND |
| 1-Methyl-2-morpholin-4-ylethyl acetate | ND | ND | ND | ND | 13.68 | 3.55 |
| 1-Penten-3-one, 1-(2,6,6-trimethyl-1-cyclohexen-1-yl) | ND | ND | 13.996 | 1.84 | ND | ND |
| 2,4-Imidazolidinedione, 5-(2-methylpropyl)-, (S)- | ND | ND | 13.552 | 5.3 | ND | ND |
| 3,7-Cyclodecadiene-1-methanol,. alpha.,.alpha.,4,8 tetramethyl | ND | ND | 14.05 | 1.22 | ND | ND |
| 4-Methyloctanoic acid | ND | ND | 9.787 | 3.01 | ND | ND |
| 6-Octadecenoic acid, (Z)- | 23.717 | 2.51 | ND | ND | ND | ND |
| Butanoic acid, 3-methyl- | ND | ND | ND | ND | 5.375 | 23.59 |
| Dibutyl phthalate | ND | ND | 17.959 | 8.33 | 17.964 | 2.26 |
| Dioxane-2,5-dimethanol | ND | ND | 8.977 | 2.17 | ND | ND |
| Dodecanoic acid, isooctyl ester | 22.059 | 2.85 | ND | ND | ND | ND |
| Eicosane | ND | ND | 18.713 | 2.25 | ND | ND |
| Eicosanoic acid | ND | ND | 19.658 | 2.32 | ND | ND |
| Glutaric acid, di(isobutyl) ester | 13.472 | 2.4 | ND | ND | ND | ND |
| Heptadecane, 2,6,10,15-tetramethyl- | ND | ND | 13.47 | 3.62 | ND | ND |
| Hexanedioic acid, bis(2-methylpropyl) ester | 14.529 | 11.29 | ND | ND | ND | ND |
| i-Propyl 12-methyltetradecanoate | ND | ND | 16.073 | 6.08 | ND | ND |
| Isobutyl isothiocyanate | ND | ND | ND | ND | 6.482 | 2.17 |
| -Isopropyl-2,4-imidazolidinedione | ND | ND | 12.553 | 1.72 | ND | ND |
| l-(+)-Ascorbic acid 2,6-dihexadecanoate | ND | ND | 18.103 | 3.96 | ND | ND |
| N-Methyl-3-hydroxymethylpyrrolidin-2-one | ND | ND | ND | ND | 13.773 | 3.5 |
| Propanamide, N-methyl- | ND | ND | ND | ND | 5.455 | 19.62 |
| Propanol, 2,2-dimethyl-, acetate | ND | ND | 3.818 | 1.32 | ND | ND |
| Pyrazine, tetramethyl- | ND | ND | ND | ND | 7.67 | 4.1 |
| Pyrrolo [1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl) | ND | ND | 17.528 | 2 | 17.869 | 0.41 |
| Pyrrolo [1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl) | ND | ND | 17.833 | 2.29 | ND | ND |
| Tetradecanoic acid | ND | ND | 11.38 | 1.64 | ND | ND |
| Compound | Medium 5333 Control | KE4D 5333 | KE4K 5333 | |||
|---|---|---|---|---|---|---|
| Rt (min) | %Area | Rt (min) | %Area | Rt (min) | %Area | |
| 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) | 16.878 | 42.95 | ND | ND | ND | ND |
| 2,4-Imidazolidinedione, 5-(2-methylpropyl)-, (S)- | 13.881 | 15.59 | ND | ND | ND | ND |
| 2,4-Imidazolidinedione, 5-methyl- | 11.658 | 4.88 | ND | ND | ND | ND |
| 5-Isopropyl-2,4-imidazolidinedione | 12.853 | 3.1 | ND | ND | ND | ND |
| 5-n-Propylhydantoin | ND | ND | 12.603 | 5.5 | ND | ND |
| 6,19-Cycloandrostane-3,7-diol, 3.beta.-methoxy- | ND | ND | ND | ND | 29.23 | 4 |
| Benzonitrile, 3-benzyloxy- | ND | ND | 18.687 | 14.76 | ND | ND |
| Dibutyl phthalate | ND | ND | 18.077 | 15.54 | 18.087 | 3.48 |
| Diisooctyl phthalate | ND | ND | ND | ND | 29.388 | 17.17 |
| Dodecane, 2,6,11-trimethyl- | ND | ND | ND | ND | 11.464 | 1.48 |
| Eicosane | ND | ND | 14.474 | 0.73 | 15.602 | 1.97 |
| Glutaric acid, isobutyl undecyl ester | 13.51 | 1.34 | ND | ND | ND | ND |
| Hexanedioic acid, bis(2-methylpropyl) ester | 14.567 | 6.16 | ND | ND | ND | ND |
| Phthalic acid, di(4,4-dimethylpent-2-yl) ester | ND | ND | ND | ND | 29.31 | 4.87 |
| Propanoic acid, 2-methyl-, 3-hydroxy-2,4,4-trimethylpentyl | 11.419 | 1.5 | ND | ND | ND | ND |
| Pyrrolo [1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- | ND | ND | 17.619 | 3.84 | ND | ND |
| Pyrrolo [1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- | ND | ND | 17.935 | 2.54 | ND | ND |
| Tricosane-2,4-dione | ND | ND | ND | ND | 13.542 | 18.48 |
| Characteristic | Medium Mannitol | Medium 5294 | Medium 5333 | |||
|---|---|---|---|---|---|---|
| KE4D | KE4K | KE4D | KE4K | KE4D | KE4K | |
| Size range | 5–55 nm | 5–55 nm | 5–49 nm | 4–55 nm | 4–49 nm | 4–49 nm |
| Morphology | Spherical | Spherical, triangular, rod-shaped | Spherical | Spherical | Spherical | Spherical |
| Aggregation | +++ | +++ | + | ++ | ++ | +++ |
| ζ-potential (mV) | −17.0 | +16.2 | −17.0 | −16.8 | −17.1 | −12.1 |
| AgNPs (400 μg) | Diameter of Inhibition Zone (mm) | |||||
|---|---|---|---|---|---|---|
| E. faecalis ATCC 51299 | L. monocytogenes ATCC 19111 | S. aureus ATCC 43300 | A. baumannii ATCC 19606 | E. coli ATCC 35218 | P. aeruginosa ATCC 27853 | |
| KE4D Mannitol | 12 | 6 | 14 | 14 | 13 | 14 |
| KE4D 5294 | 10 | 0 | 16 | 14 | 16 | 14 |
| KE4D 5333 | 7 | 5 | 15 | 15 | 15 | 13 |
| KE4K Mannitol | 17.5 | 10 | 16 | 14.5 | 15 | 6 |
| KE4K 5294 | 15.5 | 0 | 0 | 11.5 | 13.5 | 0 |
| KE4K 5333 | 0 | 0 | 0 | 8 | 9 | 0 |
| Ampicillin (AMP10) | 17 | 10 | 12 | 0 | 15 | 0 |
| Gentamicin (CN10) | 11 | 0 | 13 | 0 | 12 | 18 |
| Tetracycline (TET30) | 0 | 15 | 16 | 15 | 9 | 0 |
| AgNPs | 100 µg/mL | 120 µg/mL | 140 µg/mL | 160 µg/mL | 180 µg/mL | 200 µg/mL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %GI | %VI | %GI | %VI | %GI | %VI | %GI | %VI | %GI | %VI | %GI | %VI | |
| KE4D Mannitol | 3.54 | 37.08 | 11.36 | 41.90 | 11.69 | 44.56 | 11.99 | 50.08 | 11.88 | 50.55 | 18.68 | 50.06 |
| KE4D 5294 | 8.04 | 18.19 | 8.71 | 21.48 | 8.25 | 85.12 | 76.49 | 87.34 | 79.52 | 92.00 | 89.32 | 98.61 |
| KE4D 5333 | 4.64 | 14.93 | −4.47 | 13.18 | 4.53 | 14.02 | 8.35 | 14.91 | 18.01 | 20.57 | −4.51 | 20.80 |
| KE4K Mannitol | 6.05 | 25.38 | 11.80 | 33.25 | 31.45 | 55.94 | 83.59 | 75.07 | 90.32 | 69.22 | 92.20 | 80.88 |
| KE4K 5294 | 14.65 | 15.98 | 21.21 | 26.01 | 25.36 | 49.84 | 90.95 | 80.85 | 91.59 | 83.79 | 91.59 | 86.50 |
| KE4K 5333 | 26.61 | 42.25 | 28.67 | 43.74 | 28.11 | 47.26 | 33.33 | 52.02 | 54.94 | 72.43 | 91.74 | 78.03 |
| 50 µg/mL | 100 µg/mL | 200 µg/mL | 400 µg/mL | 800 µg/mL | ||||||||
| %GI | %VI | %GI | %VI | %GI | %VI | %GI | %VI | %GI | %VI | |||
| Vanillin control | 6.26 | −2.76 | 2.77 | 14.48 | 8.70 | 36.57 | 12.03 | 56.98 | 17.88 | 68.29 | ||
| AgNP Sample | MCF-7 IC50 Value (µg/mL) | DU-145 IC50 Value (µg/mL) |
|---|---|---|
| KE4D medium Mannitol | 3.000 | 2.277 |
| KE4D medium 5294 | 3.003 | 2.059 |
| KE4D medium 5333 | 2.034 | 2.335 |
| KE4K medium Mannitol | 2.237 | 1.938 |
| KE4K medium 5294 | 2.990 | 2.020 |
| KE4K medium 5333 | 3.007 | 2.399 |
| 5-fluorouracil control | 21.750 | 43.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, F.; Chenia, H.Y. Antimicrobial, Quorum Sensing Inhibition, and Anti-Cancer Activities of Silver Nanoparticles Synthesized from Kenyan Bacterial Endophytes of Teclea nobilis. Int. J. Mol. Sci. 2025, 26, 3306. https://doi.org/10.3390/ijms26073306
Mohamed F, Chenia HY. Antimicrobial, Quorum Sensing Inhibition, and Anti-Cancer Activities of Silver Nanoparticles Synthesized from Kenyan Bacterial Endophytes of Teclea nobilis. International Journal of Molecular Sciences. 2025; 26(7):3306. https://doi.org/10.3390/ijms26073306
Chicago/Turabian StyleMohamed, Farzana, and Hafizah Yousuf Chenia. 2025. "Antimicrobial, Quorum Sensing Inhibition, and Anti-Cancer Activities of Silver Nanoparticles Synthesized from Kenyan Bacterial Endophytes of Teclea nobilis" International Journal of Molecular Sciences 26, no. 7: 3306. https://doi.org/10.3390/ijms26073306
APA StyleMohamed, F., & Chenia, H. Y. (2025). Antimicrobial, Quorum Sensing Inhibition, and Anti-Cancer Activities of Silver Nanoparticles Synthesized from Kenyan Bacterial Endophytes of Teclea nobilis. International Journal of Molecular Sciences, 26(7), 3306. https://doi.org/10.3390/ijms26073306








