3.1. GABA Enhances Antioxidant Enzyme Activity in Tomato Seedlings
GABA is a recently discovered amino acid with many biological functions. It has been shown to stimulate plant growth and development and mediate adaptive responses to biotic and abiotic sources of stress [
13]. Studies have confirmed that a treatment with 5 mM of GABA can reduce the levels of Superoxide anions and Hydrogen Peroxide in strawberries under salt stress conditions, while simultaneously enhancing the antioxidant capacity of the strawberries [
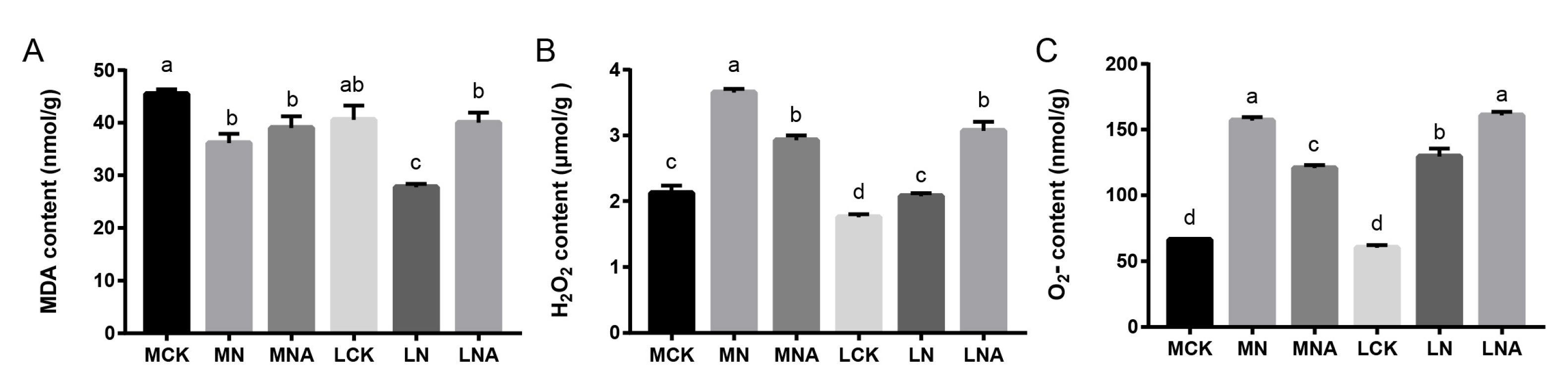
14]. In this study, exogenous GABA was applied to tomato seedlings to study the response mechanism of tolerant tomato varieties. The results showed that the H
2O
2 and O
2− content in the leaves of M82 seedlings was significantly lower (
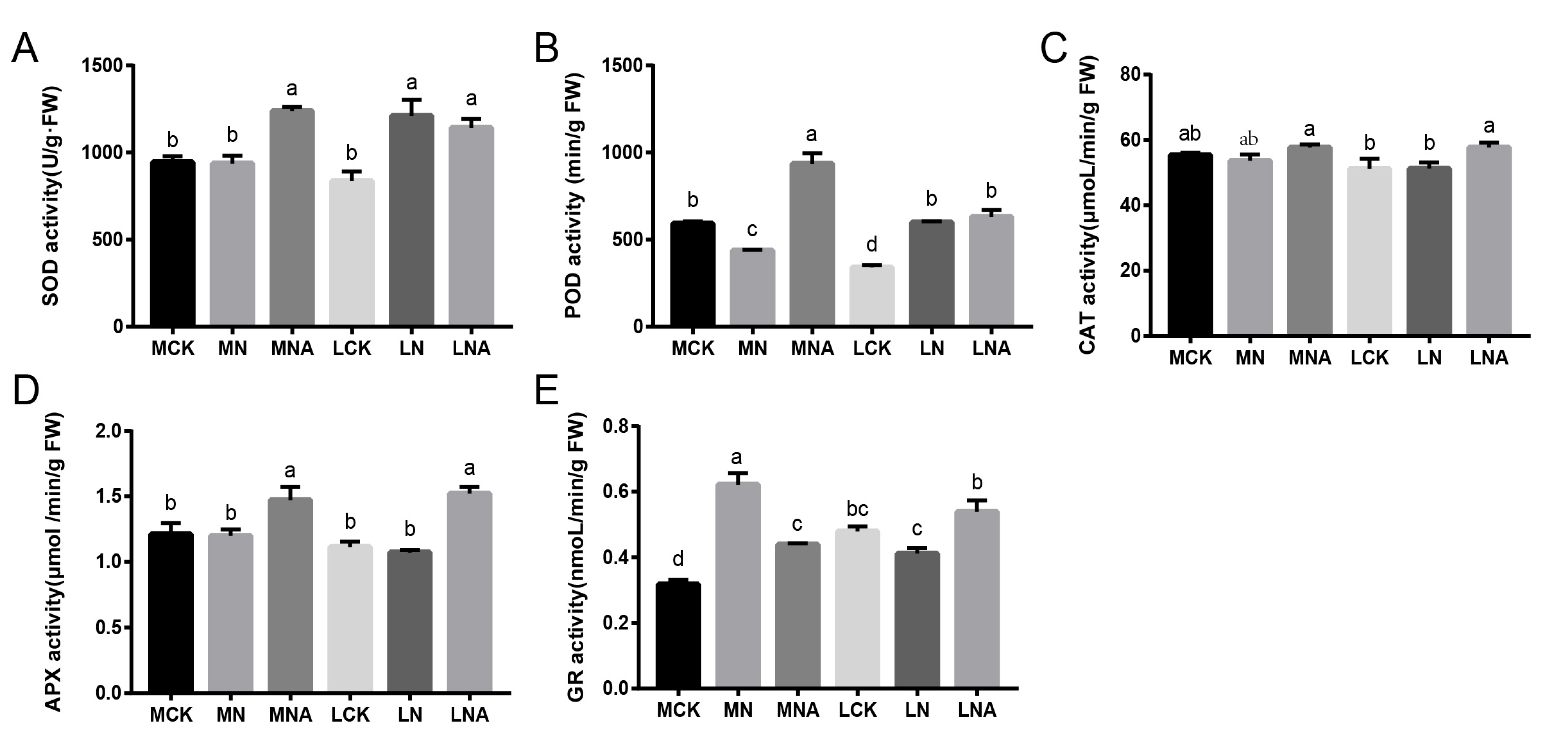
Figure 1B,C), while the SOD, POD, and APX activity was significantly higher (
Figure 2A,B,D) and that of GR was significantly lower after GABA was added (
Figure 2E). The MDA, H
2O
2, and O
2− content in the leaves of IL7-5-5 seedlings was significantly higher (
Figure 1A–C), as was the CAT, APX, and GR activity (
Figure 2C–E). Our study indicated that GABA could improve the active oxygen scavenging ability of tomato seedlings by increasing the activity of antioxidant enzymes, and the response mechanisms of the two tomato varieties were different. These results are consistent with those findings by Ahmad S. in tomatoes and SHU C. in rice [
15,
16].
3.2. Transcription Factors Play a Key Role in Plant Salt Stress Responses
Li et al. showed that transcription factors can improve plants’ stress resistance under high salt stress [
17]. Currently, the WRKY, NAC, MYB, ZIP, AP2/ERF, and HSF families are mainly known to be involved in the salt stress response [
18,
19,
20]. A large number of studies have found that genes, such as CabZIP25 in peppers [
21], GmbZIP2 in soybeans [
22], and AhbZIP in peanuts [
23], are induced by salt stress, and their expression levels become significantly different when exposed to high salt (150 mmol/L NaCl) treatments. Studies have also confirmed that the UNE and TGA transcription factor family members are present in tobacco (
Nicotiana tabacum L., NtbHLH123), wheat (
Triticum aestivum L., TabHLH1), soybeans (
Glycine max L., NPR1-TGA2), and Arabidopsis (
Arabidopsis thaliana, AtbHLH18, AtbHLH34, and AtbHLH115) [
24,
25,
26,
27,
28]. These factors can regulate the expression of a series of stress-related genes to improve the salt tolerance of plants. In our experiment, MYB, bHLH, AP2, C2H2, NAC, WRKY, LAPA, and other transcription factors were involved in regulating the salt tolerance in tomatoes. Compared with the control seedlings, the MYB and LAPA1 expression in the salt-sensitive and salt-tolerant varieties were up-regulated. LAPA1 is a candidate gene that regulates the tomato’s salt tolerance by adding GABA under salt stress, and the MYB12 transcription factor plays an important role in the tomato’s adaptation to salt stress.
3.3. Combined Transcriptomics and Metabolomics Reveal GABA’s Role in Salt Stress Adaptation
Transcriptomics and metabolomics data were combined to explore the molecular mechanism of GABA regulation to induce salt tolerance in tomatoes. GABA has been reported to have many roles during the plant’s response to abiotic stress [
29,
30]. We performed a KEGG annotation using both metabolome (
Figure 4) and transcriptome (
Figure 5) data and then conducted the annotation through the integration of the two omics analyses (
Table 6 and
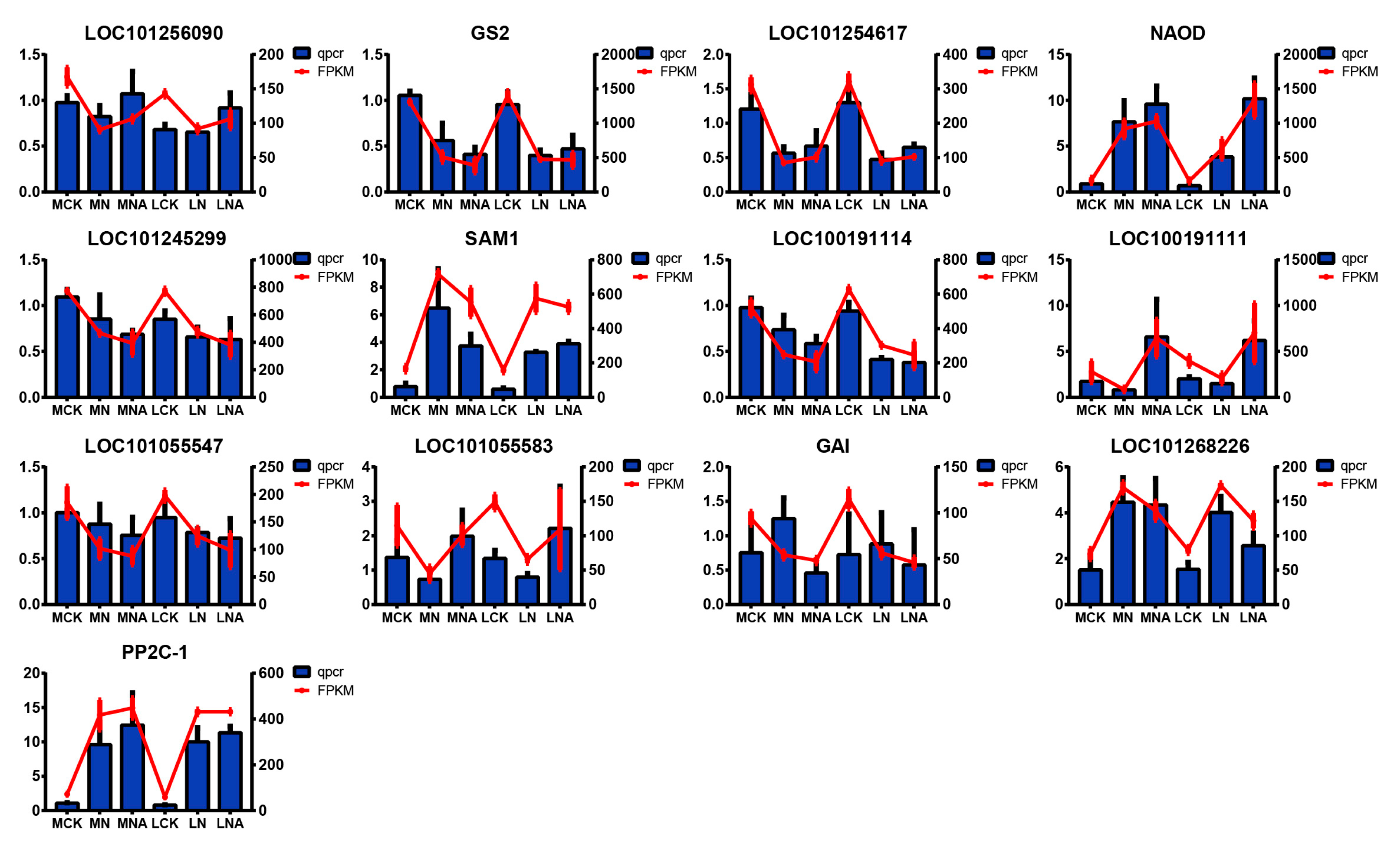
Table 7). Adding GABA regulated the salt tolerance in both salt-sensitive and salt-tolerant tomatoes. The KEGG pathway enrichment analysis of differential genes and metabolites in MNA and LNA showed that M82 was related to plant hormone signal transduction, including the auxin response genes GH3-8, GH3-10, and small auxin-up protein 58. The small auxin-up protein 58 gene and other genes have been validated in the qRT-PCR data (
Figure 6). The differentially expressed gene Auxin-Responsive protein SAUR32 in the plant hormone signal transduction pathway in IL7-5-5 indicated that adding GABA could counteract salt stress by regulating various genes in the auxin signaling pathway in both tomato varieties.
Amino acids in plants are indispensable osmotic substances, and enhancing their synthesis promotes osmotic regulation and the maintenance of cell membrane homeostasis in plants under stress conditions. Secondly, they are important antioxidants in plants, effectively removing excess free radicals and reducing salt stress-induced damage to plants [
31]. These two functions complement each other and jointly contribute to improving the resilience of plants under salt stress. In pepper seedlings, exogenous GABA can improve the plants’ tolerance to salt stress by regulating the concentrations of proline and other substances in their leaves. Metabolizing proline and arginine also plays an important role when coping with salt- and alkalinity-induced stress [
32]. In our experiment, the amino acid biosynthesis pathway showed a significant up-regulation in the expression of nine amino acids and metabolic enzymes in the M82 seedlings. These enzymes included aspartic acid synthetase, alanine aminotransferase 2, aspartate aminotransferase, cytoplasm arginase 1, cysteine synthase, threonine dehydrase 2, chloroplast threonine deaminase 1 precursor, phospho-2-deoxy-3-deoxyheptanoate aldolase 1 (chloroplast), and branch chain amino acid aminotransferase 2 (chloroplast). Among them, cysteine synthase is a key enzyme in the L-cysteine biosynthesis pathway in bacteria, plants, and protozoa. It plays a crucial role in essential biological processes, such as the sulfur assimilation metabolic pathway, the regulation of the REDOX balance, and the response to environmental stress. Other enzymes, such as aspartic acid synthase, regulate the osmotic pressure in tomato cells, maintain osmotic balance, and resist salt stress by influencing the synthesis and degradation of amino acids. Significant changes were observed in the expression levels of eight amino acid synthases and metabolic enzymes in IL7-5-5 seedlings. These included branched amino acid aminotransferase 2, arginase 2, asparagine synthase, 3-phosphoshikimic acid 1-carboxyvinyltransferase (chloroplast), bifunctional L-3-cyanoalanine synthase/cysteine synthase 1 (mitochondria), and tryptophan synthase β-chain 1. Among them, the branched-chain amino acid transaminase is involved in the metabolism of branched-chain amino acids (leucine, isoleucine, and valine). It may act as an osmoregulatory substance in plants responding to salt stress and help maintain the intracellular osmotic balance. This is consistent with the research conclusions of Sun Y. (2024) [
33] and Wu K. (2024) [
34]. Asparagine synthetase (AS) is a kind of aminotransferase that is commonly found in organisms. It uses ammonia and/or aspartic acid as a substrate to catalyze the biosynthesis of asparagine and plays an important role in the plant nitrogen metabolism and protein synthesis, which, in turn, directly affects the growth and development of plants.
ABC transporters are membrane proteins that transport various types of substances across membranes using active or passive transport [
35]. They help tolerate biological and abiotic sources of stress and maintain the cell’s osmotic homeostasis, signal transduction, and lipid homeostasis [
36]. In this experiment, the KEGG pathways of the two tomato varieties were examined, and the ABC transporters pathway activity was shown to be significantly different in the two tomato varieties. Between the MN and MNA treatment groups, the expression levels of the genes coding ABCB9, ABCC5, ABCG3, ABCG22, and other transporters changed significantly, and the metabolites raffinosaccharide, sulfate, and adenine (hemisulfate) were significantly up-regulated. Studies have shown that most of the identified ABCB members primarily participate in the transport and regulation of hormones (auxin), and some members are functionally redundant and can also transport auxin [
37,
38]. The currently identified functions of ABCC members include the transport of metabolites (e.g., anthocyanins, phytic acid, and folic acid) [
39,
40] and detoxification [
41,
42]. ABCG is the largest subfamily among the ABC transporters. At present, there are 70 known ABCG transporters in tomatoes [
43] that are involved in important biological functions and can improve the plants’ resilience against abiotic sources of stress, like heavy metals [
44]. In this experiment, significant changes in the expression levels of transporter genes were detected in all three subfamilies in the M82 seedlings, suggesting that GABA regulates the transport efficiency of this pathway in M82 seedlings to maintain the cell osmotic balance and actively respond to salt stress. In M82 seedlings, the up-regulation of ABCB9 is synchronized with the expression changes in the auxin-responsive gene GH3-8. Auxin-related genes, such as SAUR6, SAUR14, and SAUR16, have been proven to regulate the activity of POD or CAT, thereby affecting the cellular antioxidant capacity [
45]. This suggests that GABA may indirectly regulate the antioxidant enzyme system through ABC transporters and auxin.
There were significant differences in the levels of the ABCG22 transporter gene and 18 different metabolites between the LN and LNA treatment groups, including mannitol, sorbitol, glutamine, sulfate, and L-lysine hydrochloride, which participate in various biochemical processes via different pathways. Among them, mannitol and sorbitol are osmoregulatory substances that help plants maintain an intracellular water balance under salt stress, reduce water loss by reducing the intracellular osmotic pressure, and, thus, improve salt tolerance in plants. Glutamine is an important form of nitrogen storage and transport in plants. Sulfate is a source of sulfur in plants, and its metabolism is essential in antioxidant defense and protein synthesis in plants. When studying the effects of salt on sesame, Zhang et al. found that the differentially expressed genes and differential metabolites were highly correlated with the amino acid metabolism. Compared with the salt-sensitive sesame, the accumulation of various amino acids in the salt-tolerant sesame was greater, and the expression levels of multiple genes related to amino acid synthesis were higher than those in the salt-sensitive sesame. Therefore, this is likely to be the key mechanism behind salt tolerance in sesame [
46]. After adding GABA, mannitol and sorbitol significantly accumulated in IL7-5-5 seedlings, and the expression of ABCG3 and ABCG22 was up-regulated. This was consistent with the greater increase in the SOD activity (
Figure 2A). This correlation suggests that GABA may enhance the oxidative stress defense capability of IL7-5-5 tomatoes by up-regulating the expression of ABCG3/ABCG22, thereby promoting the transmembrane transport of mannitol and sorbitol. The accumulation of polyols not only maintains the cellular osmotic balance but also may synergistically alleviate the oxidative damage induced by salt stress by directly scavenging hydroxyl radicals and indirectly supporting SOD activity [
47,
48].
Carbon metabolism is an extremely complex process involving multiple biochemical steps. It is essential for the synthesis, decomposition, and conversion of carbohydrate compounds in plants. It not only affects the growth and development process of plants but also determines the ability of plants to cope with external sources of stress [
49]. In this study, the differentially expressed genes and metabolites that belonged to the carbon metabolism pathway in both tomato varieties were heavily enriched. Among them, those in the M82 seedlings included fructose diphosphate aldolase, threonine dehydrase 2, enolase, cisaconite hydratase, formate dehydrogenase, succinate dehydrogenase, and malic acid. In the IL7-5-5 seedlings, the differentially expressed genes and metabolites included glycine cracking system H protein, threonine dehydrase 2, enolase, formate dehydrogenase, D-gluconic acid, aspartate, and serine. Accumulated carbohydrates can not only effectively alleviate the impact of osmotic stress on plants but also act as an important source of energy. Bao et al. also found that sugar accumulation played a key role in maintaining the cell’s osmotic balance and cell membrane structure’s stability at low temperatures. These results indicate that regulating the carbon metabolism may be one of the main mechanisms of the GABA-induced positive response of tomato seedlings to salt stress and the different response mechanisms observed in tomato seedlings with different degrees of salt tolerance.