Circulating Monocytes Contribute to Erythrocyte Clearance in Polycythemia Vera
Abstract
1. Introduction
2. Results
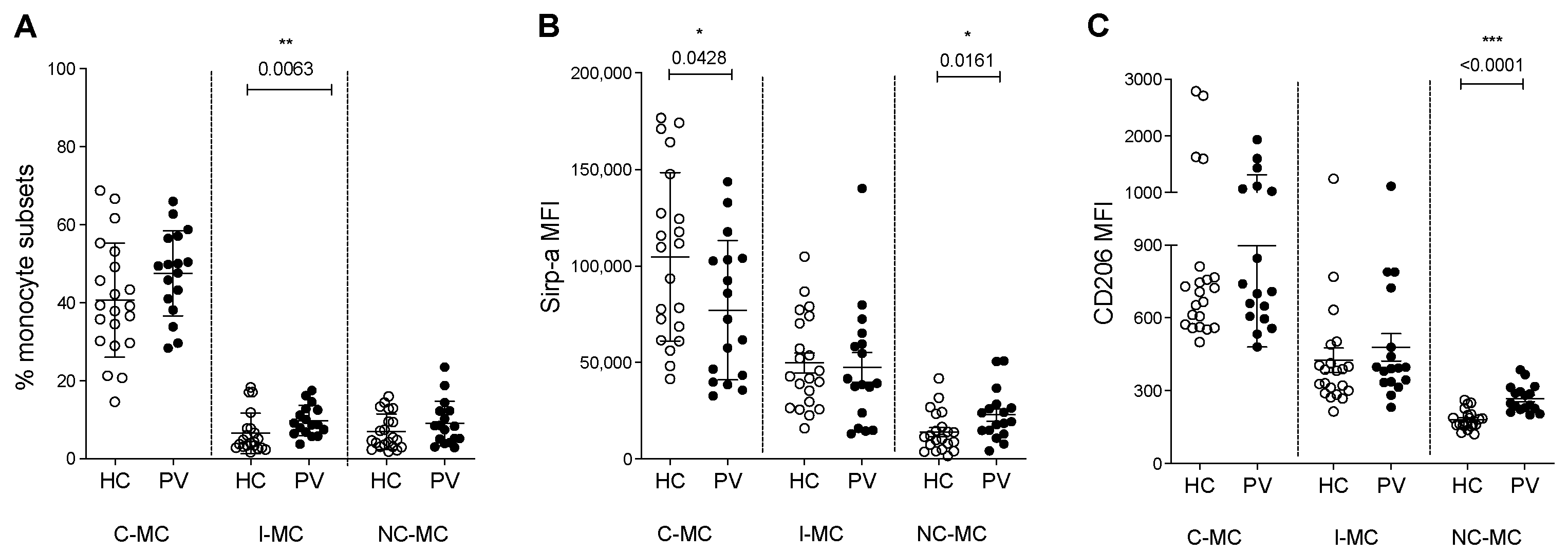
2.1. Circulating Monocyte Subsets and Phagocytosis Markers Are Changed in PV Patients
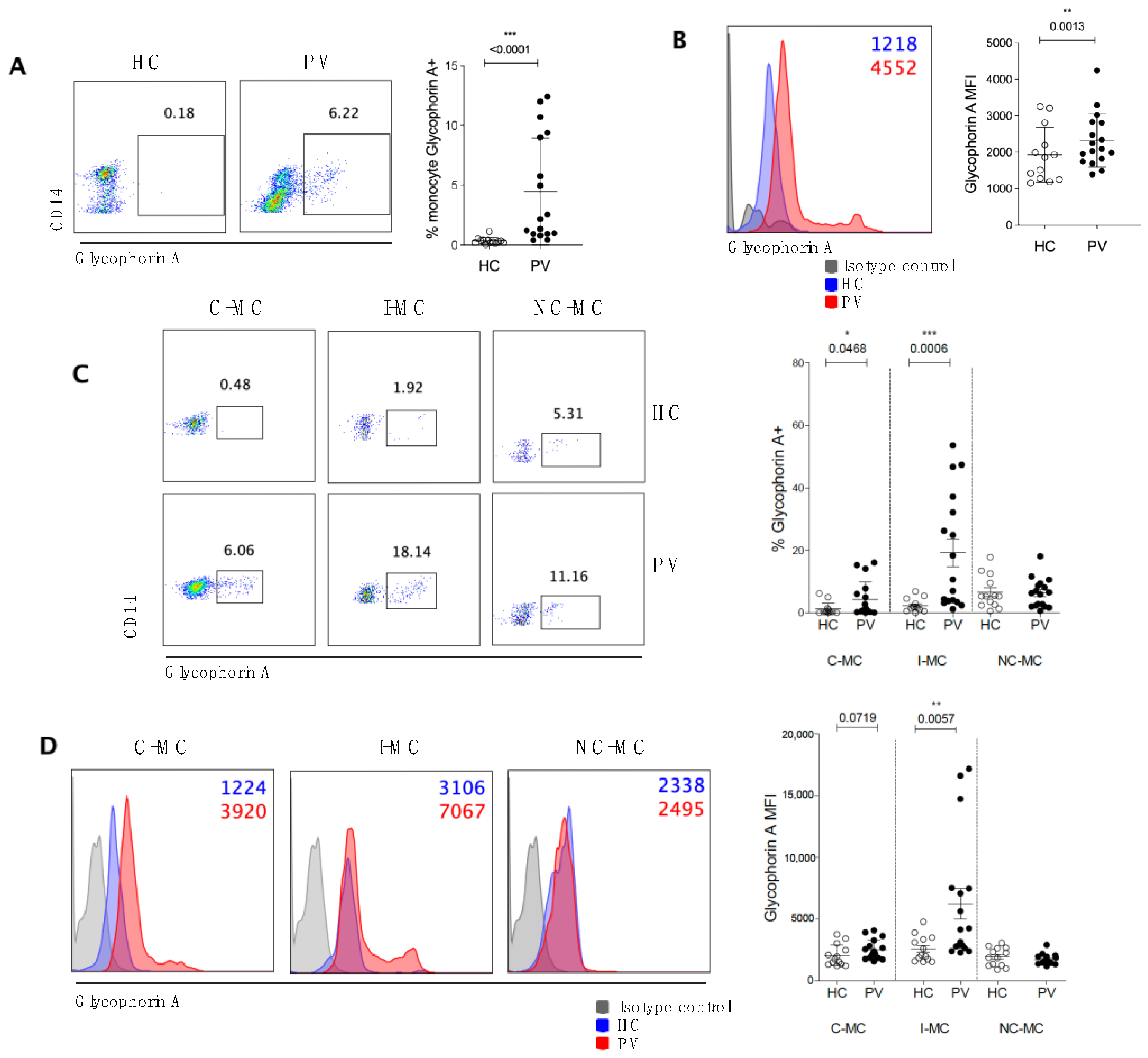
2.2. Circulating Monocytes Play a Role in RBC Clearance in PV Patients
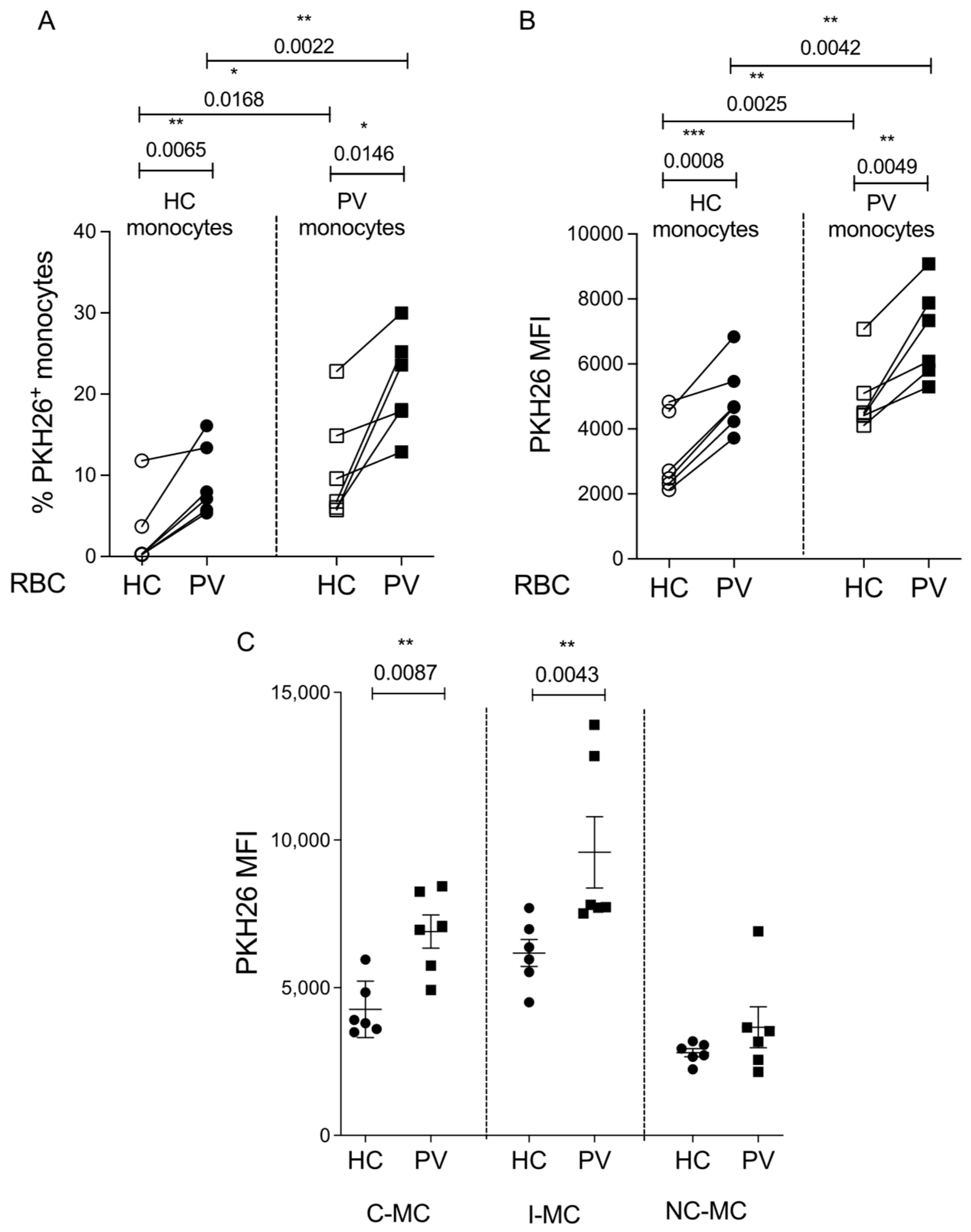
2.3. Erythrophagocytosis Is Influenced by Both the Monocytes and RBCs of PV Patients
2.4. Expressions of HO-1 and FPN Are Increased Post-Erythrophagocytosis
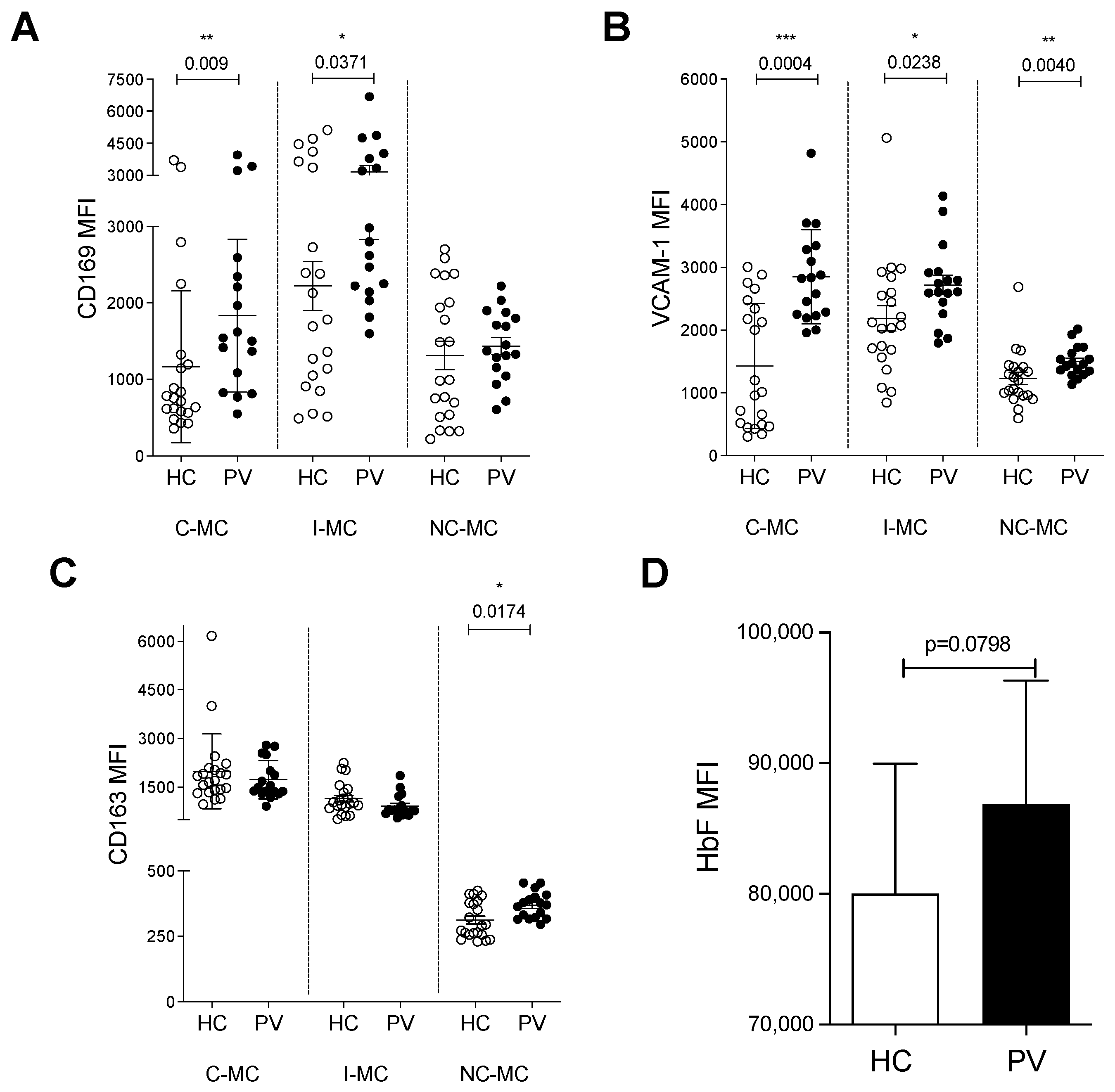
2.5. Monocytes from PV Patients Express Adhesion Molecules for Erythroid Cells and May Interfere with Erythropoiesis
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Peripheral Blood Mononuclear Cells’ Isolation
4.3. Monocyte Phenotyping via Flow Cytometry (FC)
4.4. In Vitro Phagocytosis Assay
4.5. K562 Culture
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spivak, J.L. Polycythemia Vera. Curr. Treat. Options Oncol. 2018, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.M.; Tong, W.; Levine, R.L.; Scott, M.A.; Beer, P.A.; Stratton, M.R.; Futreal, P.A.; Erber, W.N.; McMullin, M.F.; Harrison, C.N.; et al. JAK2 Exon 12 Mutations in Polycythemia Vera and Idiopathic Erythrocytosis. N. Engl. J. Med. 2007, 356, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Vainchenker, W.; Kralovics, R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood 2017, 129, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.J.; Scott, L.M.; Campbell, P.J.; East, C.; Fourouclas, N.; Swanton, S.; Vassiliou, G.S.; Bench, A.J.; Boyd, E.M.; Curtin, N.; et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005, 365, 1054–1061. [Google Scholar] [CrossRef]
- James, C.; Ugo, V.; Le Couedic, J.P.; Staerk, J.; Delhommeau, F.; Lacout, C.; Garçon, L.; Raslova, H.; Berger, R.; Bennaceur-Griscelli, A.; et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Med. Sci. 2005, 21, 669–670. [Google Scholar] [CrossRef]
- Dai, C.; Krantz, S.; Dessypris, E.; Means, R.J.; Horn, S.; Gilbert, H. Polycythemia vera. II. Hypersensitivity of bone marrow erythroid, granulocyte-macrophage, and megakaryocyte progenitor cells to interleukin-3 and granulocyte-macrophage colony-stimulating factor. Blood 1992, 80, 891–899. [Google Scholar] [CrossRef]
- Bassan, V.L.; Barretto, G.D.; de Almeida, F.C.; Palma, P.V.B.; Binelli, L.S.; da Silva, J.P.L.; Fontanari, C.; Castro, R.C.; Pontes, L.L.d.F.; Frantz, F.G.; et al. Philadelphia-negative myeloproliferative neoplasms display alterations in monocyte subpopulations frequency and immunophenotype. Med. Oncol. 2022, 39, 223. [Google Scholar] [CrossRef]
- Ishii, T.; Bruno, E.; Hoffman, R.; Xu, M. Involvement of various hematopoietic-cell lineages by the JAK2 V617F mutation in polycythemia vera. Blood 2006, 108, 3128–3134. [Google Scholar] [CrossRef]
- Delhommeau, F.; Dupont, S.; Tonetti, C.; Massé, A.; Godin, I.; Le Couedic, J.P.; Debili, N.; Saulnier, P.; Casadevall, N.; Vainchenker, W.; et al. Evidence that the JAK2 G1849T (V617F) mutation occurs in a lymphomyeloid progenitor in polycythemia vera and idiopathic myelofibrosis. Blood 2007, 109, 71–77. [Google Scholar] [CrossRef]
- Bessis, M. Erythroblastic Island, functional unity of bone marrow. Rev. Hematol. 1958, 13, 8–11. [Google Scholar]
- Li, W.; Guo, R.; Song, Y.; Jiang, Z. Erythroblastic Island Macrophages Shape Normal Erythropoiesis and Drive Associated Disorders in Erythroid Hematopoietic Diseases. Front. Cell Dev. Biol. 2021, 8, 613885. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.H.; Lam, Y.W.; Fraser, S.T. Cellular dynamics of mammalian red blood cell production in the erythroblastic island niche. Biophys. Rev. 2019, 11, 873–894. [Google Scholar] [CrossRef]
- Rhodes, M.M.; Kopsombut, P.; Bondurant, M.C.; Price, J.O.; Koury, M.J. Adherence to macrophages in erythroblastic islands enhances erythroblast proliferation and increases erythrocyte production by a different mechanism than erythropoietin. Blood 2008, 111, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Molitor, D.C.A.; Boor, P.; Buness, A.; Schneider, R.K.; Teichmann, L.L.; Körber, R.M.; Horvath, G.L.; Koschmieder, S.; Gütgemann, I. Macrophage frequency in the bone marrow correlates with morphologic subtype of myeloproliferative neoplasm. Ann. Hematol. 2021, 100, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Casu, C.; Gardenghi, S.; Breda, L.; Crielaard, B.J.; Guy, E.; Marongiu, M.F.; Gupta, R.; Levine, R.L.; Abdel-Wahab, O.; et al. Macrophages support pathological erythropoiesis in polycythemia vera and β-thalassemia. Nat. Med. 2013, 19, 437–445. [Google Scholar] [CrossRef]
- Chow, A.; Huggins, M.; Ahmed, J.; Hashimoto, D.; Lucas, D.; Kunisaki, Y.; Pinho, S.; Leboeuf, M.; Noizat, C.; van Rooijen, N.; et al. CD169+ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat. Med. 2013, 19, 429–436. [Google Scholar] [CrossRef]
- Haschka, D.; Petzer, V.; Kocher, F.; Tschurtschenthaler, C.; Schaefer, B.; Seifert, M.; Sopper, S.; Sonnweber, T.; Feistritzer, C.; Arvedson, T.L.; et al. Classical and intermediate monocytes scavenge non-transferrin-bound iron and damaged erythrocytes. JCI Insight 2019, 4, e98867. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, H.; Bao, W.; Mendelson, A.; An, X.; Shi, P.; Chou, S.T.; Manwani, D.; Yazdanbakhsh, K. Patrolling monocytes scavenge endothelial-adherent sickle RBCs: A novel mechanism of inhibition of vaso-occlusion in SCD. Blood 2019, 134, 579–590. [Google Scholar] [CrossRef]
- Fabriek, B.O.; Polfliet, M.M.J.; Vloet, R.P.M.; Van Der Schors, R.C.; Ligtenberg, A.J.M.; Weaver, L.K.; Geest, C.; Matsuno, K.; Moestrup, S.K.; Dijkstra, C.D.; et al. The macrophage CD163 surface glycoprotein is an erythroblast adhesion receptor. Blood 2007, 109, 5223–5229. [Google Scholar] [CrossRef]
- van den Akker, E.; Satchwell, T.J.; Pellegrin, S.; Daniels, G.; Toye, A.M. The majority of the in vitro erythroid expansion potential resides in CD34− cells, outweighing the contribution of CD34+ cells and significantly increasing the erythroblast yield from peripheral blood samples. Haematologica 2010, 95, 1594–1598. [Google Scholar] [CrossRef]
- Heideveld, E.; Masiello, F.; Marra, M.; Esteghamat, F.; Yağcı, N.; von Lindern, M.; Migliaccio, A.R.F.; Akker, E.v.D.; Yağcı, N. CD14+ cells from peripheral blood positively regulate hematopoietic stem and progenitor cell survival resulting in increased erythroid yield. Haematologica 2015, 100, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Morsia, E.; Gangat, N. Myeloproliferative Neoplasms with Monocytosis. Curr. Hematol. Malig. Rep. 2022, 17, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Londino, J.D.; Gulick, D.; Isenberg, J.S.; Mallampalli, R.K. Cleavage of signal regulatory protein α (sirpα) enhances inflammatory signaling. J. Biol. Chem. 2015, 290, 31113–31125. [Google Scholar] [CrossRef]
- Wang, J.; Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J. 2011, 434, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, P.B.; Eggert, T.; Zhu, Y.P.; Marcovecchio, P.; Meyer, M.A.; Wu, R.; Hedrick, C.C. Patrolling Monocytes Control NK Cell Expression of Activating and Stimulatory Receptors to Curtail Lung Metastases. J. Immunol. 2020, 204, 192–198. [Google Scholar] [CrossRef]
- Wang, W.; Liu, W.; Fidler, T.; Wang, Y.; Tang, Y.; Woods, B.; Welch, C.; Cai, B.; Silvestre-Roig, C.; Ai, D.; et al. Macrophage inflammation, erytrhophagocytosis and accelerated atherosclerosis in Jak2 V617F mice. Circ. Res. 2018, 123, e35–e47. [Google Scholar] [CrossRef]
- Lysenko, V.; Schürch, P.M.; Tuzlak, S.; van Wijk, N.W.V.; Kovtonyuk, L.V.; Becher, B.; Manz, M.G.; Kreutmair, S.; Theocharides, A.P.A. Blocking the CD47-SIRPα interaction reverses the disease phenotype in a polycythemia vera mouse model. Leukemia 2023, 37, 1277–1286. [Google Scholar] [CrossRef]
- Baliga, B.S.; Mankad, M.; Shah, A.K.; Mankad, V.N. Mechanism of differentiation of human erythroleukaemic cell line K562 by hemin. Cell Prolif. 1993, 26, 519–529. [Google Scholar] [CrossRef]
- Uchida, N.; Haro-Mora, J.J.; Demirci, S.; Fujita, A.; Raines, L.; Hsieh, M.M.; Tisdale, J.F. High-level embryonic globin production with efficient erythroid differentiation from a K562 erythroleukemia cell line. Exp. Hematol. 2018, 62, 7–16.e1. [Google Scholar] [CrossRef]
- Rowley, P.T.; Ohlsson-Wilhelm, B.M.; Farley, B.A. K562 Human Erythroleukemia Cells Demonstrate Commitment. Blood 1985, 65, 862–868. [Google Scholar] [CrossRef]
- Ding, L.; Chen, D.; Li, Y.; Xie, Y.; Sun, X.; Wang, D. Saracatinib prompts hemin-induced K562 erythroid differentiation but suppresses erythropoiesis of hematopoietic stem cells. Hum. Cell 2024, 37, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Addya, S.; Keller, M.A.; Delgrosso, K.; Ponte, C.M.; Vadigepalli, R.; Gonye, G.E.; Surrey, S. Erythroid-induced commitment of K562 cells results in clusters of differentially expressed genes enriched for specific transcription regulatory elements. Physiol. Genom. 2004, 19, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, Z.; Li, X.; Xiao, J.; Li, C. Regulation of erythroid differentiation in K562 cells by the EPAS1-IRS2 axis under hypoxic conditions. Front. Cell Dev. Biol. 2023, 11, 1161541. [Google Scholar] [CrossRef]
- Wang, D.; Si, S.; Wang, Q.; Luo, G.; Du, Q.; Liang, Q.; Guo, X.; Zhang, G.; Feng, J.; Leng, Z. MiR-27a Promotes Hemin-Induced Erythroid Differentiation of K562 Cells by Targeting CDC25B. Cell. Physiol. Biochem. 2018, 46, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, J.; Yang, G.H.; Wang, X.S.; Zhang, J.W. Regulation of erythroid differentiation by miR-376a and its targets. Cell Res. 2011, 21, 1196–1209. [Google Scholar] [CrossRef]
- Toobiak, S.; Shaklai, M.; Shaklai, N. Carbon monoxide induced erythroid differentiation of K562 cells mimics the central macrophage milieu in erythroblastic islands. PLoS ONE 2012, 7, e33940. [Google Scholar] [CrossRef]
- Manwani, D.; Bieker, J.J. The Erythroblastic Island. Curr. Top. Dev. Biol. 2008, 82, 23–53. [Google Scholar]
- Yoshida, H.; Kawane, K.; Koike, M.; Mori, Y.; Uchiyama, Y.; Nagata, S. Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature 2005, 437, 754–758. [Google Scholar] [CrossRef]
- Sadahira, Y.; Yasuda, T.; Yoshino, T.; Manabe, T.; Takeishi, T. Impaired splenic erythropoiesis in phlebotomized mice injected with CL2MDP-liposome: An experimental model for studying the role of stromal macrophages in erythropoiesis Abstract: Erythropoiesis occurs in the presence of erythropoietin (EPO) without m. J. Leukoc. Biol. 2000, 68, 464–470. [Google Scholar] [CrossRef]
- Hanspal, M.; Hanspal, J.S. The association of erythroblasts with macrophages promotes erythroid proliferation and maturation: A 30-kD heparin-binding protein is involved in this contact. Blood 1994, 84, 3494–3504. [Google Scholar] [CrossRef]
- Morris, L.; Crockerf, P.R.; Fraser, I.; Hill, M.; Gordon, S. Expression of a divalent cation-dependent erythroblast adhesion receptor by stromal macrophages from murine bone marrow. J. Cell Sci. 1991, 99, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Paulson, R.F.; Shi, L.; Wu, D.C. Stress Erythropoiesis: New signals and new stress progenitor cells. Curr. Opin. Hematol. 2011, 18, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Van Egeren, D.; Kamaz, B.; Liu, S.; Nguyen, M.; Reilly, C.R.; Kalyva, M.; DeAngelo, D.J.; Galinsky, I.; Wadleigh, M.; Winer, E.S.; et al. Transcriptional differences between JAK2-V617F and wild-type bone marrow cells in patients with myeloproliferative neoplasms. Exp. Hematol. 2022, 107, 14–19. [Google Scholar] [CrossRef]
- Fan, W.; Cao, W.; Shi, J.; Gao, F.; Wang, M.; Xu, L.; Wang, F.; Li, Y.; Guo, R.; Bian, Z.; et al. Contributions of bone marrow monocytes/macrophages in myeloproliferative neoplasms with JAK2V617F mutation. Ann. Hematol. 2023, 102, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Prabhu, K.S.; Paulson, R.F. Monocyte-derived macrophages expand the murine stress erythropoietic niche during the recovery from anemia. Blood 2018, 132, 2580–2593. [Google Scholar] [CrossRef]
- Guarda, C.C.; Silveira-Mattos, P.S.M.; Yahouédéhou, S.C.M.A.; Santiago, R.P.; Aleluia, M.M.; Figueiredo, C.V.B.; Fiuza, L.M.; Carvalho, S.P.; Oliveira, R.M.; Nascimento, V.M.L.; et al. Hydroxyurea alters circulating monocyte subsets and dampens its inflammatory potential in sickle cell anemia patients. Sci. Rep. 2019, 9, 14829. [Google Scholar] [CrossRef]
- Gambero, S.; Canalli, A.A.; Traina, F.; Albuquerque, D.M.; Saad, S.T.O.; Costa, F.F.; Conran, N. Therapy with hydroxyurea is associated with reduced adhesion molecule gene and protein expression in sickle red cells with a concomitant reduction in adhesive properties. Eur. J. Haematol. 2007, 78, 144–151. [Google Scholar] [CrossRef]
- Cartron, J.P.; Elion, J. Erythroid adhesion molecules in sickle cell disease: Effect of hydroxyurea. Transfus. Clin. Biol. 2008, 15, 39–50. [Google Scholar] [CrossRef]
- Odièvre, M.H.; Bony, V.; Benkerrou, M.; Lapouméroulie, C.; Alberti, C.; Ducrocq, R.; Jacqz-Aigrain, E.; Elion, J.; Cartron, J.-P. Modulation of erythroid adhesion receptor expression by hydroxyurea in children with sickle cell disease. Haematologica 2008, 93, 502–509. [Google Scholar] [CrossRef]

| Median (Min–Max) | |
| Age (n = 37) | 63 (24–77) |
| RBC (3.96 × 106/μL) (n = 37) | 4 (2.7–8.1) |
| Platelets (130–400 × 103/μL) (n = 37) | 318 (137–898) |
| WBC (3.7–11.1 × 103/μL) (n = 37) | 5.47 (2.4–16.3) |
| MHC (27.3–32.6 pg) (n = 37) | 33.8 (17.8–43.3) |
| MCV (82–98 fL) (n = 37) | 104.6 (65.4–124.6) |
| HGB (11.8–16.7 g/dL) (n = 37) | 14 (10.2–17.7) |
| HCT (36–50%) (n = 37) | 42.75 (33.8–55) |
| Monocytes (0.2–0.92 × 103/μL) (n = 37) | 0.25 (0.09–0.7) |
| Serum iron (70–180 μg/dL) (n = 18) | 75.5 (23–143) |
| Ferritin (m: 30–400; f: 13–150 ng/dL) (n = 18) | 58.5 (12.4–429.8) |
| TIBC (255–450 μg/dL) (n = 18) | 359.5 (261–630) |
| Transferrin saturation (20–45%) (n = 18) | 20 (4–37) |
| n | |
| Sex (male/female) (n = 37) | 19/18 |
| Hydroxyrea (n = 37) | 34 |
| Phlebotomy (n = 37) | 3 |
| Presence of JAK2 V617F mutation (n = 37) | 35 |
| Presence of JAK2 exon12 mutation (n = 37) | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, M.D.; Paes, I.F.; Leonardo, D.P.; Souza, C.M.; Albuquerque, D.M.; Lanaro, C.; Pagnano, K.B.B.; Conran, N.; Sesti-Costa, R.; Costa, F.F. Circulating Monocytes Contribute to Erythrocyte Clearance in Polycythemia Vera. Int. J. Mol. Sci. 2025, 26, 5133. https://doi.org/10.3390/ijms26115133
Borges MD, Paes IF, Leonardo DP, Souza CM, Albuquerque DM, Lanaro C, Pagnano KBB, Conran N, Sesti-Costa R, Costa FF. Circulating Monocytes Contribute to Erythrocyte Clearance in Polycythemia Vera. International Journal of Molecular Sciences. 2025; 26(11):5133. https://doi.org/10.3390/ijms26115133
Chicago/Turabian StyleBorges, Marina D., Izabela F. Paes, Daniela P. Leonardo, Cristiane M. Souza, Dulcinéia M. Albuquerque, Carolina Lanaro, Katia B. B. Pagnano, Nicola Conran, Renata Sesti-Costa, and Fernando F. Costa. 2025. "Circulating Monocytes Contribute to Erythrocyte Clearance in Polycythemia Vera" International Journal of Molecular Sciences 26, no. 11: 5133. https://doi.org/10.3390/ijms26115133
APA StyleBorges, M. D., Paes, I. F., Leonardo, D. P., Souza, C. M., Albuquerque, D. M., Lanaro, C., Pagnano, K. B. B., Conran, N., Sesti-Costa, R., & Costa, F. F. (2025). Circulating Monocytes Contribute to Erythrocyte Clearance in Polycythemia Vera. International Journal of Molecular Sciences, 26(11), 5133. https://doi.org/10.3390/ijms26115133






