Therapeutic Peptides: Recent Advances in Discovery, Synthesis, and Clinical Translation
Abstract
1. Introduction
2. Therapeutic Peptides: Advantages and Limitations
3. Peptide Drug Discovery
3.1. Peptide Hormones in the Human Body and Their Analogues
3.2. Peptide Drugs Derived from Natural Products
3.3. Phase Display for the Identification of Peptide Candidates
3.4. Computer-Aided Drug Design (CADD) for Peptide Drug Discovery
4. Synthesis of Therapeutic Peptides and Quality Control
4.1. Chemical Synthesis of Peptides
- (1)
- Pseudoproline Dipeptide Integration: Disruption of β-sheet aggregation via conformation-disrupting pseudoproline motifs [47];
- (2)
- Advanced Resin Matrices: High-performance resins (e.g., ChemMatrix®) optimized for hydrophobic or extended sequences, enhancing solvation and reducing steric hindrance [48];
- (3)
- Microwave-Assisted Synthesis: Accelerated coupling kinetics and reduced reaction times through controlled microwave irradiation, improving efficiency and yield [49].
4.2. Biosynthesis of Peptides
4.2.1. Recombinant DNA Technology: Precision Engineering for Complex Peptides
4.2.2. Enzymatic Synthesis: Catalytic Precision for Tailored Therapeutics
4.2.3. Microbial Cell Factories: Sustainable Platforms for Industrial-Scale Production
4.3. Quality Control of Peptides: Regulatory Framework and Future Perspectives
5. Clinical Application of Therapeutic Peptides
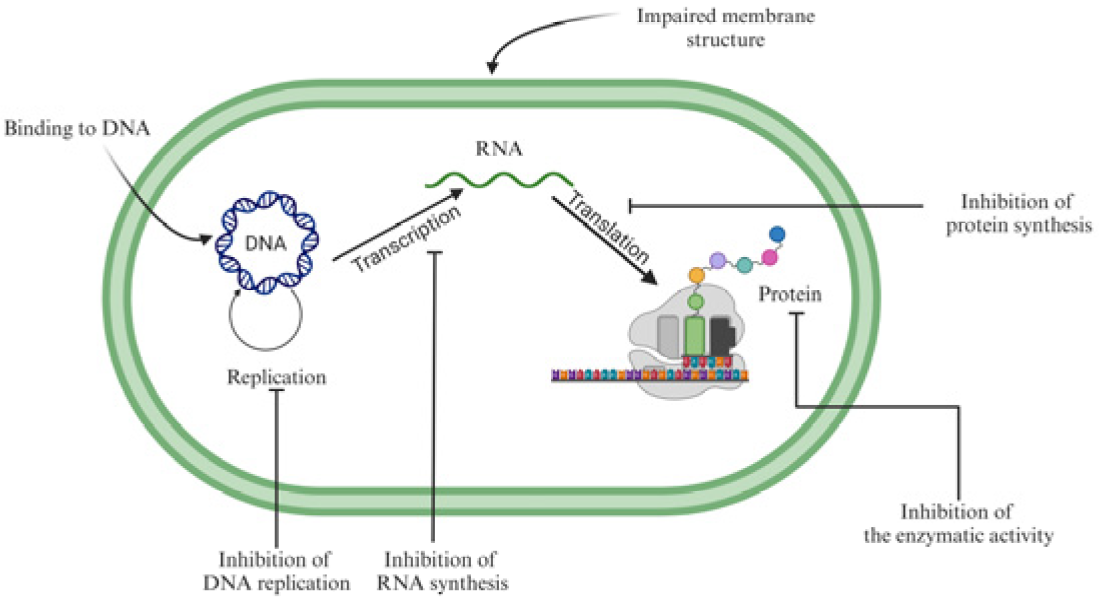
5.1. Antimicrobial Peptides
5.2. Anticancer Peptides and Peptide–Drug Conjugates
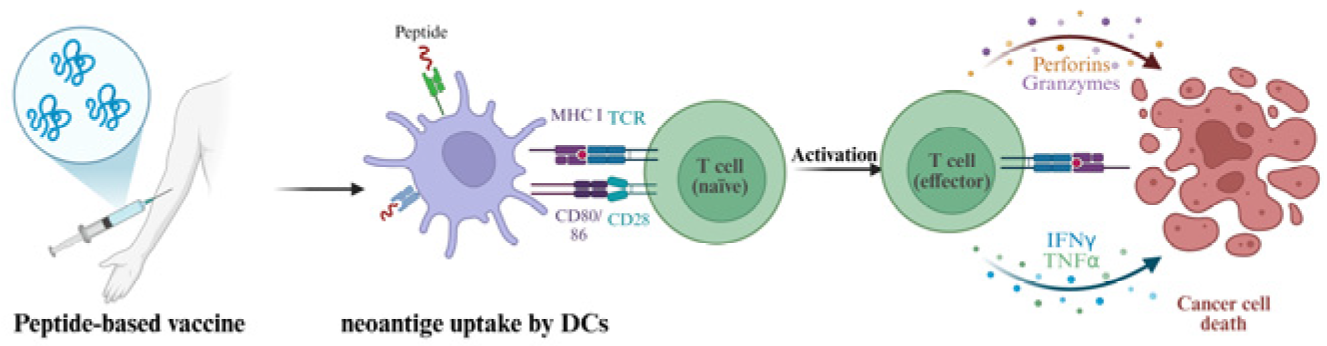
5.3. Peptide-Based Vaccines
5.4. Therapeutic Peptides in Cardiometabolic Medicine
6. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MDR | Multidrug-Resistant |
| AI | Artificial Intelligence |
| GLP-1 | Glucagon-Like Peptide-1 |
| PPIs | Protein–Protein Interactions |
| CADD | Computer-Aided Drug Design |
| SPSS | Solid-Phase Peptide Synthesis |
| TFA | Trifluoroacetic Acid |
| LPSS | Liquid-Phase Peptide Synthesis |
| CuAAC | Copper-catalyzed Azide–Alkyne Cycloaddition |
| SMAP | Staphylococcus Aureus Antimicrobial Peptide |
| HRMS | High-Resolution Mass Spectrometry |
| MAM | Multi-Attribute Monitoring |
| NMR | Nuclear Magnetic Resonance |
| CD | Circular Dichroism |
| AMP | Antimicrobial Peptide |
| ACP | Anticancer Peptide |
| PDCs | Peptide–Drug Conjugates |
| APCs | Antigen-Presenting Cells |
| DC | Dendritic Cell |
| CTL | Cytotoxic T Lymphocyte |
| GM-CSF | Granulocyte–Macrophage Colony-Stimulating Factor |
| AML | Acute Myeloid Leukemia |
| NSCLC | Non-Small Cell Lung Cancer |
| STING | Stimulator of Interferon Genes |
| CVDs | Cardiovascular Diseases |
| NP | Natriuretic Peptide |
| RAAS | Renin–Angiotensin–Aldosterone System |
Appendix
| No. | Peptide Sequences | Design Modification |
|---|---|---|
| 1 | HGVSGHGQHGVHG | Native alloferon (Reference) |
| 2 | HGVSGHGQHGVHG (D-enantiomer) | Reduced hemolytic activity via D-amino acid substitution |
| 3 | HIVSGHGQHGVHI | Enhanced hydrophobicity through residue replacement (H→I) |
| 4 | HGVSGHGQHKVHK | Increased net charge (+2) by lysine substitution |
| 5 | RGVSGRGQRGVRG | Maximized net charge (+4) via arginine enrichment |
| 6 | NH2-HGVSGHGQHGVHG-COOH | Conformational restriction via terminal capping |
| 7 | HGCVSGHGQHGVCHG | Structural stabilization by disulfide bridge |
| 8 | IIKKIHGVSGHGQHGVHG | N-terminal fusion with cervical cancer-targeting motif (IIKKI) |
| 9 | HGVSGHGQHGVHGIIKKI | C-terminal fusion with cervical cancer-targeting motif (IIKKI) |
| 10 | (VSGHGV) × 3 | Tandem repeat of core functional motif |
| 11 | (VSGHGQHGV) × 3 | Extended motif repetition |
| 12 | (HGVSGHGQHGVHG) × 3 | Full-sequence repetition |
| 13 | Sialic aicd-HGVSGHGQHGVHG | Glycosylation for improved receptor targeting |
| 14 | PEG(1 kDa)-HGVSGHGQHGVHG | PEGylation to enhance pharmacokinetic stability |
| 15 | CPP-HGVSGHGQHGVHG | Conjugation with a cell-penetrating peptide (CPP) motif |
References
- Rossino, G.; Marchese, E.; Galli, G.; Verde, F.; Finizio, M.; Serra, M.; Linciano, P.; Collina, S. Peptides as therapeutic agents: Challenges and opportunities in the green transition Era. Molecules 2023, 28, 7165. [Google Scholar] [CrossRef] [PubMed]
- Chandarana, C.; Juwarwala, I.; Shetty, S.; Bose, A. Peptide drugs: Current status and it’s applications in the treatment of various diseases. Curr. Drug Res. Rev. 2024, 16, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, M.; Ha-Duong, T. Computational design of cyclic peptides to inhibit protein-peptide interactions. Biophys. Chem. 2023, 296, 106987. [Google Scholar] [CrossRef]
- Liu, W.Q.; Ji, X.; Ba, F.; Zhang, Y.; Xu, H.; Huang, S.; Zheng, X.; Liu, Y.; Ling, S.; Jewett, M.C.; et al. Cell-free biosynthesis and engineering of ribosomally synthesized lanthipeptides. Nat. Commun. 2024, 15, 4336. [Google Scholar] [CrossRef]
- Buonaguro, L.; Tagliamonte, M. Peptide-based vaccine for cancer therapies. Front. Immunol. 2023, 14, 1210044. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Nada, H.; Choi, Y.; Kim, S.; Jeong, K.S.; Meanwell, N.A.; Lee, K. New insights into protein–protein interaction modulators in drug discovery and therapeutic advance. Signal Transduct. Target. Ther. 2024, 9, 341. [Google Scholar] [CrossRef]
- deGruyter, J.N.; Malins, L.R.; Baran, P.S. Residue-specific peptide modification: A chemist’s guide. Biochemistry 2017, 56, 3863–3873. [Google Scholar] [CrossRef]
- Asmani, A.Z.A.; Zainuddin, A.F.F.; Azmi Murad, N.A.; Mohd Darwis, N.H.; Suhaimi, N.S.; Zaini, E.; Taher, M.; Susanti, D.; Khotib, J. Immunogenicity of monoclonal antibody: Causes, consequences, and control strategies. Pathol. Res. Pract. 2024, 263, 155627. [Google Scholar] [CrossRef]
- Haggag, Y.A.; Donia, A.A.; Osman, M.A.; El-Gizawy, S.A. Peptides as drug candidates: Limitations and recent development perspectives. Biomed. J. Sci. Tech. Res. 2018, 8, 1–4. [Google Scholar] [CrossRef]
- Bellmann-Sickert, K.; Beck-Sickinger, A.G. Peptide drugs to target G protein-coupled receptors. Trends Pharmacol. Sci. 2010, 31, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Lv, Z.; Guo, S.; Jiang, G.; Liu, H. An update—prolonging the action of protein and peptide drugs. J. Drug Deliv. Sci. Technol. 2021, 61, 102124. [Google Scholar] [CrossRef]
- Binder, U.; Skerra, A. Strategies for extending the half-life of biotherapeutics: Successes and complications. Expert Opin. Biol. Ther. 2024, 25, 93–118. [Google Scholar] [CrossRef]
- Zhu, N.; Dong, F.; Shi, G.; Lao, X.; Zheng, H. HORDB a comprehensive database of peptide hormones. Sci. Data 2022, 9, 187. [Google Scholar] [CrossRef]
- Gong, B.; Yao, Z.; Zhou, C.; Wang, W.; Sun, L.; Han, J. Glucagon-like peptide-1 analogs: Miracle drugs are blooming? Eur. J. Med. Chem. 2024, 269, 116342. [Google Scholar] [CrossRef]
- Lau, J.; Bloch, P.; Schäffer, L.; Pettersson, I.; Spetzler, J.; Kofoed, J.; Madsen, K.; Knudsen, L.B.; McGuire, J.; Steensgaard, D.B.; et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J. Med. Chem. 2015, 58, 7370–7380. [Google Scholar] [CrossRef]
- Ladenheim, E. Liraglutide and obesity: A review of the data so far. Drug Des. Devel. Ther. 2015, 30, 1867–1875. [Google Scholar] [CrossRef]
- Iovino, M.; Messana, T.; De Pergola, G.; Iovino, E.; Dicuonzo, F.; Guastamacchia, E.; Giagulli, V.A.; Triggiani, V. The role of neurohypophyseal hormones vasopressin and oxytocin in neuropsychiatric disorders. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 341–347. [Google Scholar] [CrossRef]
- Arshad, M.F.; Solanki, S.; Dancyger-Stevens, L.; Karunanayaka, M.; Loh, E.W.; Htoon, K.N.; Turki, M.; Munir, A. Desmopressin prescription safety in adult inpatients: A real-world tertiary centre experience. Endocr. Connect. 2025, 14, e240441. [Google Scholar] [CrossRef]
- Yang, X.; Feng, P.; Yin, Y.; Bushley, K.; Spatafora, J.W.; Wang, C. Cyclosporine biosynthesis in tolypocladium inflatum benefits fungal adaptation to the environment. mBio 2018, 9, e01211–e01218. [Google Scholar] [CrossRef] [PubMed]
- Alqadi, R.; Alqumia, A.; Alhomoud, I.S.; Alhowail, A.; Aldubayan, M.; Mohammed, H.A.; Alhmoud, H.; Khan, R.A. Cyclosporine: Immunosuppressive effects, entwined toxicity, and clinical modulations of an organ transplant drug. Transpl. Immunol. 2025, 88, 102147. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Asgher, M.; Sher, F.; Hussain, S.M.; Nazish, N.; Joshi, N.; Sharma, A.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M.N. Exploring marine as a rich source of bioactive peptides: Challenges and opportunities from marine pharmacology. Mar. Drugs 2022, 20, 208. [Google Scholar] [CrossRef]
- Deer, T.R.; Pope, J.E.; Hanes, M.C.; McDowell, G.C. Intrathecal therapy for chronic pain: A review of morphine and ziconotide as firstline options. Pain Med. 2019, 20, 784–798. [Google Scholar] [CrossRef]
- Leisch, M.; Egle, A.; Greil, R. Plitidepsin: A potential new treatment for relapsed/refractory multiple myeloma. Future Oncol. 2018, 15, 109–120. [Google Scholar] [CrossRef]
- Fan, Y.; Feng, R.; Zhang, X.; Wang, Z.L.; Xiong, F.; Zhang, S.; Zhong, Z.F.; Yu, H.; Zhang, Q.W.; Zhang, Z.; et al. Encoding and display technologies for combinatorial libraries in drug discovery: The coming of age from biology to therapy. Acta Pharm. Sin. B 2024, 14, 3362–3384. [Google Scholar] [CrossRef]
- Hamzeh-Mivehroud, M.; Alizadeh, A.A.; Morris, M.B.; Church, W.B.; Dastmalchi, S. Phage display as a technology delivering on the promise of peptide drug discovery. Drug Discov. Today 2013, 18, 1144–1157. [Google Scholar] [CrossRef]
- Hermanson, T.; Bennett, C.L.; Macdougall, I.C. Peginesatide for the treatment of anemia due to chronic kidney disease—An unfulfilled promise. Expert. Opin. Drug Saf. 2016, 15, 1421–1426. [Google Scholar] [CrossRef]
- Josephson, K.; Ricardo, A.; Szostak, J.W. mRNA display: From basic principles to macrocycle drug discovery. Drug Discov. Today 2014, 19, 388–399. [Google Scholar] [CrossRef]
- Malhis, M.; Funke, S.A. Mirror-image phage display for the selection of D-amino acid peptide ligands as potential therapeutics. Curr. Protoc. 2024, 4, e957. [Google Scholar] [CrossRef]
- Gurung, S.; Khan, F.; Gunassekaran, G.R.; Yoo, J.D.; Poongkavithai Vadevoo, S.M.; Permpoon, U.; Kim, S.H.; Kim, H.J.; Kim, I.S.; Han, H.; et al. Phage display-identified PD-L1-binding peptides reinvigorate T-cell activity and inhibit tumor progression. Biomaterials 2020, 247, 119984. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Chen, P.C.; Hampton, J.T.; Tharp, J.M.; Reed, C.A.; Das, S.K.; Wang, D.S.; Hayatshahi, H.S.; Shen, Y.; Liu, J.; et al. A genetically encoded, phage-displayed cyclic-peptide library. Angew. Chem. Int. Ed. Engl. 2019, 58, 15904–15909. [Google Scholar] [CrossRef] [PubMed]
- Lagoutte, P. La présentation sur ribosome. Med. Sci. 2020, 36, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Diller, D.J.; Swanson, J.; Bayden, A.S.; Jarosinski, M.; Audie, J. Rational, computer-enabled peptide drug design: Principles, methods, applications and future directions. Future Med. Chem. 2015, 7, 2173–2193. [Google Scholar] [CrossRef]
- Zhai, S.; Liu, T.; Lin, S.; Li, D.; Liu, H.; Yao, X.; Hou, T. Artificial intelligence in peptide-based drug design. Drug Discov. Today 2025, 30, 104300. [Google Scholar] [CrossRef]
- Desai, D.; Kantliwala, S.V.; Vybhavi, J.; Ravi, R.; Patel, H.; Patel, J. Review of alphaFold 3: Transformative advances in drug design and therapeutics. Cureus 2024, 16, e63646. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Fu, Y.; Xue, J.; Yuan, F.; Qu, T.; Rissanou, A.N.; Wang, Y.; Li, X.; Hu, H. Therapeutic stapled peptides: Efficacy and molecular targets. Pharmacol. Res. 2024, 203, 107137. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, R.; Gao, S.; Li, W.; Liu, Y.; Su, G.; Song, M.; Jiang, M.; Jiang, C.; Zhang, X. Artificial intelligence applied in neoantigen identification facilitates personalized cancer immunotherapy. Front. Oncol. 2023, 12, 1054231. [Google Scholar] [CrossRef]
- Roy, R.; Al-Hashimi, H.M. AlphaFold3 takes a step toward decoding molecular behavior and biological computation. Nat. Struct. Mol. Biol. 2024, 31, 997–1000. [Google Scholar] [CrossRef]
- Belaid, W.F.; Dekhira, A.; Lesot, P.; Ferroukhi, O. Development of deep learning software to improve HPLC and GC predictions using a new crown-ether based mesogenic stationary phase and beyond. J. Chromatogr. A 2025, 1739, 465476. [Google Scholar] [CrossRef]
- Hou, W.; Zhang, X.; Liu, C.-F. Progress in chemical synthesis of peptides and proteins. Trans. Tianjin Univ. 2017, 23, 401–419. [Google Scholar] [CrossRef]
- Hickey, J.L.; Sindhikara, D.; Zultanski, S.L.; Schultz, D.M. Beyond 20 in the 21st century: Prospects and challenges of non-canonical amino acids in peptide drug discovery. ACS Med. Chem. Lett. 2023, 14, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Shimogawa, M.; Huang, Y.; Pan, B.; Petersson, E.J. Synthesis of peptides and proteins with site-specific glutamate arginylation. Methods Mol. Biol. 2023, 2620, 177–207. [Google Scholar]
- Coin, I.; Beyermann, M.; Bienert, M. Solid-phase peptide synthesis: From standard procedures to the synthesis of difficult sequences. Nat. Protoc. 2007, 2, 3247–3256. [Google Scholar] [CrossRef]
- Al Musaimi, O.; de la Torre, B.G.; Albericio, F.J.G.C. Greening Fmoc/tBu solid-phase peptide synthesis. Green. Chem. 2020, 22, 996–1018. [Google Scholar] [CrossRef]
- Behrendt, R.; White, P.; Offer, J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 2016, 22, 4–27. [Google Scholar] [CrossRef]
- Manne, S.R.; Rustler, K.; Bruckdorfer, T.; de la Torre, B.G.; Albericio, F. Incorporation of pseudoproline monomer (Fmoc-Thr[ψMe,Mepro]–OH) facilitates efficient solid-phase synthesis of difficult peptides. Tetrahedron Lett. 2023, 115, 154301. [Google Scholar] [CrossRef]
- Mueller, L.K.; Baumruck, A.C.; Zhdanova, H.; Tietze, A.A. Challenges and perspectives in chemical synthesis of highly hydrophobic peptides. Front. Bioeng. Biotechnol. 2020, 8, 162. [Google Scholar] [CrossRef]
- Hojo, K.; Shinozaki, N.; Nozawa, Y.; Fukumori, Y.; Ichikawa, H. Aqueous microwave-assisted solid-phase synthesis using Boc-amino acid nanoparticles. Appl. Sci. 2013, 3, 614–623. [Google Scholar] [CrossRef]
- Kiss, K.; Ránky, S.; Gyulai, Z.; Molnár, L. Development of a novel, automated, robotic system for rapid, high-throughput, parallel, solid-phase peptide synthesis. SLAS Technol. 2023, 28, 89–97. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; de la Torre, B.G.; Albericio, F. Liquid-phase peptide synthesis (LPPS): A third wave for the preparation of peptides. Chem. Rev. 2022, 122, 13516–13546. [Google Scholar] [CrossRef] [PubMed]
- Testa, C.; Papini, A.M.; Chorev, M.; Rovero, P. Copper-catalyzed azide-alkyne cycloaddition (CuAAC)-mediated macrocyclization of peptides: Impact on conformation and biological activity. Curr. Top. Med. Chem. 2018, 18, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; Chavhan, S.; Sapkal, S.; Shrikhande, V. Recombinant DNA technology and its applications: A review. Int. J. Medipharm Res. 2018, 4, 79–88. [Google Scholar]
- Sahoo, A.; Das, P.K.; Dasu, V.V.; Patra, S. Insulin evolution: A holistic view of recombinant production advancements. Int. J. Biol. Macromol. 2024, 277, 133951. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.S.; Kim, H.J.; Park, E.J.; Na, D.H. Peptide mapping analysis of synthetic semaglutide and liraglutide for generic development of drugs originating from recombinant DNA technology. J. Pharm. Biomed. Anal. 2025, 256, 116682. [Google Scholar] [CrossRef]
- Moreno-Cid, J.A. Scale-Up of E. coli Culture for Optimal Fed-Batch Strategy Using Exponential Feeding. 2021. Available online: https://bionet.com/scale-up-of-e-coli-culture-for-optimal-fed-batch-strategy-using-exponential-feeding/ (accessed on 14 May 2025).
- Ko, H.; Kang, M.; Kim, M.J.; Yi, J.; Kang, J.; Bae, J.H.; Sohn, J.H.; Sung, B.H. A novel protein fusion partner, carbohydrate-binding module family 66, to enhance heterologous protein expression in Escherichia coli. Microb. Cell Fact. 2021, 20, 232. [Google Scholar] [CrossRef]
- Xu, G.; Micklefield, J. Enzymatic synthesis of peptide therapeutics. Nat. Chem. Biol. 2024, 20, 1256–1257. [Google Scholar]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic synthesis for industrial applications. Angew. Chem. Int. Ed. Engl. 2020, 60, 88–119. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Qin, S.; Xie, M.; Luo, S.Z.; Li, W. Advances in biosynthesis of peptide drugs: Technology and industrialization. Biotechnol. J. 2024, 19, e2300256. [Google Scholar] [CrossRef]
- Li, R.; Schmidt, M.; Zhu, T.; Yang, X.; Feng, J.; Tian, Y.; Cui, Y.; Nuijens, T.; Wu, B. Traceless enzymatic protein synthesis without ligation sites constraint. Natl. Sci. Rev. 2021, 9, nwab158. [Google Scholar] [CrossRef]
- Gohil, N.; Bhattacharjee, G.; Singh, V. Chapter 1—An introduction to microbial cell factories for production of biomolecules. In Microbial Cell Factories Engineering for Production of Biomolecules; Singh, V., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 1–19. [Google Scholar]
- Munirah, M.; Hardianto, D.; Martius, E.; Nasution, U.J.; Safarrida, A. Insulin production in Pichia pastoris: Mini-review of biotechnological advancements and process optimization. Process Biochem. 2025, 149, 277–287. [Google Scholar] [CrossRef]
- Vijayakumar, V.E.; Venkataraman, K. A systematic review of the potential of Pichia pastoris (Komagataella phaffii) as an alternative host for biologics production. Mol. Biotechnol. 2023, 66, 1621–1639. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, S.; Wang, P.; Zhang, Y.; Kang, X.; Pan, X.; Li, L.; Li, D.; Gao, P.; An, Y.; et al. Protective RBD-dimer vaccines against SARS-CoV-2 and its variants produced in glycoengineered Pichia pastoris. PLoS Pathog. 2024, 20, e1012487. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.G.; Zhu, C.H.; Zhang, J.; Fan, D.D. Endotoxin removal from human-like collagen using Triton X-114 two-phase extraction and affinity chromatography resin. Chem. Eng. 2017, 45, 6–11. [Google Scholar]
- İncir, İ.; Kaplan, Ö. Escherichia coli as a versatile cell factory: Advances and challenges in recombinant protein production. Protein Expr. Purif. 2024, 219, 106463. [Google Scholar] [CrossRef]
- Gabba, A.; Attariya, R.; Behren, S.; Pett, C.; van der Horst, J.C.; Yurugi, H.; Yu, J.; Urschbach, M.; Sabin, J.; Birrane, G.; et al. MUC1 glycopeptide vaccine modified with a GalNAc glycocluster targets the macrophage galactose C-type lectin on dendritic cells to elicit an improved humoral response. J. Am. Chem. Soc. 2023, 145, 13027–13037. [Google Scholar] [CrossRef]
- Abbood, A. Insights into Therapeutic Peptides and their Quality Control. Int. J. Adv. Pharm. Sci. Res. 2024, 5, 20–27. [Google Scholar] [CrossRef]
- Elsayed, Y.Y.; Kühl, T.; Imhof, D. Regulatory guidelines for the analysis of therapeutic peptides and proteins. J. Pept. Sci. 2025, 31, e70001. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, M.; Guo, H.; Wang, Y.; Zhang, Y.; He, M.; Cai, Z. Stimuli-responsive hydrogels based on protein/peptide and their sensing applications. Prog. Mater. Sci. 2025, 148, 101355. [Google Scholar] [CrossRef]
- Santhanakrishnan, K.R.; Koilpillai, J.; Narayanasamy, D. PEGylation in pharmaceutical development: Current status and emerging trends in macromolecular and immunotherapeutic drugs. Cureus 2024, 16, e66669. [Google Scholar] [CrossRef]
- Martagan, T.; Baaijens, M.; Dirckx, C.; Holman, J.; Meyer, R.; Repping, O.; Ravenstein, B. MSD: Continuous pharmaceutical manufacturing data for the 2024 MSOM data-driven research challenge. Manuf. Serv. Oper. Manag. 2024, 26, 1587–1604. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, Q.; Ji, J. Antimicrobial peptides and their mimetics: Promising candidates of next-generation therapeutic agents combating multidrug-resistant bacteria. Adv. Biol. 2025, 9, e2400461. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, Q.; Ren, K.; Xu, T.; Zhang, Z.; Xu, M.; Rao, Z.; Zhang, X. A Review of Antimicrobial Peptides: Structure, Mechanism of Action, and Molecular Optimization Strategies. Fermentation 2024, 10, 540. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Song, Z.; Tan, Z.; Cheng, J. Recent advances in design of antimicrobial peptides and polypeptides toward clinical translation. Adv. Drug Deliv. Rev. 2021, 170, 261–280. [Google Scholar] [CrossRef]
- Dlozi, P.N.; Gladchuk, A.; Crutchley, R.D.; Keuler, N.; Coetzee, R.; Dube, A. Cathelicidins and defensins antimicrobial host defense peptides in the treatment of TB and HIV: Pharmacogenomic and nanomedicine approaches towards improved therapeutic outcomes. Biomed. Pharmacother. 2022, 151, 113189. [Google Scholar] [CrossRef]
- Keshri, A.K.; Rawat, S.S.; Chaudhary, A.; Sharma, S.; Kapoor, A.; Mehra, P.; Kaur, R.; Mishra, A.; Prasad, A. LL-37, the master antimicrobial peptide, its multifaceted role from combating infections to cancer immunity. Int. J. Antimicrob. Agents 2025, 65, 107398. [Google Scholar] [CrossRef]
- Fu, J.; Zong, X.; Jin, M.; Min, J.; Wang, F.; Wang, Y. Mechanisms and regulation of defensins in host defense. Signal Transduct. Target. Ther. 2023, 8, 300. [Google Scholar] [CrossRef]
- Brady, D.; Grapputo, A.; Romoli, O.; Sandrelli, F. Insect Cecropins, Antimicrobial Peptides with Potential Therapeutic Applications. Int. J. Mol. Sci. 2019, 20, 5862. [Google Scholar] [CrossRef]
- Sokolenko, M.; Sokolenko, L.; Honchar, H.; Sokolenko, A.; Andrushchak, M. The advancements in treatment of HIV-infected patients with herpetic infection. Georgian Med. News 2020, 304–305, 56–61. [Google Scholar]
- Bae, S.; Oh, K.; Kim, H.; Kim, Y.; Kim, H.R.; Hwang, Y.I.; Lee, D.S.; Kang, J.S.; Lee, W.J. The effect of alloferon on the enhancement of NK cell cytotoxicity against cancer via the up-regulation of perforin/granzyme B secretion. Immunobiology 2013, 218, 102610–102633. [Google Scholar] [CrossRef]
- Li, C.M.; Haratipour, P.; Lingeman, R.G.; Perry, J.J.P.; Gu, L.; Hickey, R.J.; Malkas, L.H. Novel peptide therapeutic approaches for cancer treatment. Cells 2021, 10, 2908. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, R.K.; Khan, N.; Meghwanshi, G.K.; Ponne, S.; Althobiti, M.; Kumar, R. Current research status of anti-cancer peptides: Mechanism of action, production, and clinical applications. Biomed. Pharmacother. 2023, 164, 114996. [Google Scholar] [CrossRef] [PubMed]
- Barrett, R.; Birch, B. Triptorelin therapy for lower urinary tract symptoms (LUTS) in prostate cancer patients: A systematic meta-analysis. BJUI Compass 2023, 5, 17–28. [Google Scholar] [CrossRef]
- Veal, G.J.; Cole, M.; Errington, J.; Parry, A.; Hale, J.; Pearson, A.D.; Howe, K.; Chisholm, J.C.; Beane, C.; Brennan, B.; et al. Pharmacokinetics of dactinomycin in a pediatric patient population: A United Kingdom children’s cancer study group study. Clin. Cancer Res. 2005, 11, 5893–5899. [Google Scholar] [CrossRef]
- Silberman, J.; Dalbey, K.; Torre, C.; David, E.; Bergsagel, L.; Durden, D.; Garlich, J.; Schwertschlag, U.; Lonial, S. A novel pan-PI3K/Akt inhibitor, SF1126, inhibits in vitro growth of multiple myeloma cells. Blood 2007, 110, 4806. [Google Scholar] [CrossRef]
- Cianfrocca, M.E.; Kimmel, K.A.; Gallo, J.; Cardoso, T.; Brown, M.M.; Hudes, G.; Lewis, N.; Weiner, L.; Lam, G.N.; Brown, S.C.; et al. Phase 1 trial of the antiangiogenic peptide ATN-161 (Ac-PHSCN-NH2), a beta integrin antagonist, in patients with solid tumours. Br. J. Cancer 2006, 94, 1621–1626. [Google Scholar] [CrossRef]
- Rajeshkumar, N.V.; Rai, A.; Gulati, A. Endothelin B receptor agonist, IRL 1620, enhances the anti-tumor efficacy of paclitaxel in breast tumor rats. Breast Cancer Res. Treat. 2005, 94, 237–247. [Google Scholar] [CrossRef]
- He, H.; Deng, X.; Wang, Z.; Chen, J. Recent progress in the development of peptide-drug conjugates (PDCs) for cancer therapy. Eur. J. Med. Chem. 2025, 284, 117204. [Google Scholar] [CrossRef]
- Baldini, C.; Goldschmidt, V.; Brana, I.; Doger, B.; Italiano, A.; Cousin, S.; Falchook, G.S.; Necchi, A.; Reig, O.; Carteret, L.; et al. BT8009-100: A phase I/II study of novel bicyclic peptide and MMAE conjugate BT8009 in patients (pts) with advanced malignancies associated with nectin-4 expression, including urothelial cancer (UC). J. Clin. Oncol. 2023, 41, 498. [Google Scholar] [CrossRef]
- Kumthekar, P.; Tang, S.C.; Brenner, A.J.; Kesari, S.; Piccioni, D.E.; Anders, C.; Carrillo, J.; Chalasani, P.; Kabos, P.; Puhalla, S.; et al. ANG1005, a brain-penetrating peptide-drug conjugate, shows activity in patients with breast cancer with leptomeningeal carcinomatosis and recurrent brain metastases. Clin. Cancer Res. 2020, 26, 2789–2799. [Google Scholar] [CrossRef]
- Liu, W.; Tang, H.; Li, L.; Wang, X.; Yu, Z.; Li, J. Peptide-based therapeutic cancer vaccine: Current trends in clinical application. Cell Prolif. 2021, 54, e13025. [Google Scholar] [CrossRef] [PubMed]
- Khong, H.; Overwijk, W.W. Adjuvants for peptide-based cancer vaccines. J. Immunother. Cancer 2016, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.G.; Talati, C.; Pinilla-Ibarz, J. Galinpepimut-S (GPS): An investigational agent for the treatment of acute myeloid leukemia. Expert. Opin. Investig. Drugs 2021, 30, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Schneble, E.J.; Berry, J.S.; Trappey, F.A.; Clifton, G.T.; Ponniah, S.; Mittendorf, E.; Peoples, G.E. The HER2 peptide nelipepimut-S (E75) vaccine (NeuVax™) in breast cancer patients at risk for recurrence: Correlation of immunologic data with clinical response. Immunotherapy 2014, 6, 519–531. [Google Scholar] [CrossRef]
- Tagliamento, M.; Rijavec, E.; Barletta, G.; Biello, F.; Rossi, G.; Grossi, F.; Genova, C. CIMAvax-EGF, a therapeutic non-small cell lung cancer vaccine. Expert. Opin. Biol. Ther. 2018, 18, 829–835. [Google Scholar] [CrossRef]
- Jiang, N.; Yu, Y.; Wu, D.; Wang, S.; Fang, Y.; Miao, H.; Ma, P.; Huang, H.; Zhang, M.; Zhang, Y.; et al. HLA and tumour immunology: Immune escape, immunotherapy and immune-related adverse events. J. Cancer Res. Clin. Oncol. 2023, 149, 737–747. [Google Scholar] [CrossRef]
- Singh, A.; Thakur, M.; Sharma, L.K.; Chandra, K. Designing a multi-epitope peptide based vaccine against SARS-CoV-2. Sci. Rep. 2020, 10, 16219. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Wang, L.; Liu, B. Personalized neoantigen vaccination with synthetic long peptides: Recent advances and future perspectives. Theranostics 2020, 10, 6011–6023. [Google Scholar] [CrossRef]
- Malli Cetinbas, N.; Monnell, T.; Soomer-James, J.; Shaw, P.; Lancaster, K.; Catcott, K.C.; Dolan, M.; Mosher, R.; Routhier, C.; Chin, C.N.; et al. Tumor cell-directed STING agonist antibody-drug conjugates induce type III interferons and anti-tumor innate immune responses. Nat. Commun. 2024, 15, 5842. [Google Scholar] [CrossRef]
- Lee, E.C.Z.; Anand, V.V.; Razavi, A.C.; Alebna, P.L.; Muthiah, M.D.; Siddiqui, M.S.; Chew, N.W.S.; Mehta, A. The global epidemic of metabolic fatty liver disease. Curr. Cardiol. Rep. 2024, 26, 199–210. [Google Scholar] [CrossRef]
- Westermeier, F.; Fisman, E.Z. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) and cardiometabolic protection: Historical development and future challenges. Cardiovasc. Diabetol. 2025, 24, 44. [Google Scholar] [CrossRef] [PubMed]
- Frías, J.P.; Auerbach, P.; Bajaj, H.S.; Fukushima, Y.; Lingvay, I.; Macura, S.; Søndergaard, A.L.; Tankova, T.I.; Tentolouris, N.; Buse, J.B. Efficacy and safety of once-weekly semaglutide 20 mg versus 10 mg in patients with type 2 diabetes (SUSTAIN FORTE): A double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol. 2021, 9, 563–574. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, P.M.; Birkenfeld, A.L.; McGowan, B.; Mosenzon, O.; Pedersen, S.D.; Wharton, S.; Carson, C.G.; Jepsen, C.H.; Kabisch, M.; Wilding, J.P.H. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: A randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018, 392, 637–649. [Google Scholar] [CrossRef]
- Kerkelä, R.; Ulvila, J.; Magga, J. Natriuretic peptides in the regulation of cardiovascular physiology and metabolic events. J. Am. Heart Assoc. 2015, 4, e002423. [Google Scholar] [CrossRef]
- Maisel, A.S. Nesiritide: A new therapy for the treatment of heart failure. Cardiovasc. Toxicol. 2003, 3, 37–42. [Google Scholar] [CrossRef]
- Ichiki, T.; Dzhoyashvili, N.; Burnett, J.C. Natriuretic peptide based therapeutics for heart failure: Cenderitide: A novel first-in-class designer natriuretic peptide. Int. J. Cardiol. 2019, 281, 166–171. [Google Scholar] [CrossRef]
- Miguel, M.; Aleixandre, A. Antihypertensive peptides derived from egg proteins. J. Nutr. 2006, 136, 1457–1460. [Google Scholar] [CrossRef]

| Name of Peptide Drug | Target | Indications |
|---|---|---|
| Yorvipath | Parathyroid hormone receptor | Hypoparathyroidism in adults |
| Trofinetide | Insulin-like growth factor 1 (IGF-1) | Rett syndrome in patients aged ≥2 years |
| Rezafungin | β-1,3-glucan synthase inhibitor | Adult patients with candidemia and invasive candidiasis |
| Flotufolastat F18 | Prostate-specific membrane antigen (PSMA) | Metastatic prostate cancer |
| Motixafortide | CXCR4 antagonist | Stem cell mobilization for autologous transplantation in multiple myeloma |
| Zilucoplan | Complement C5 inhibitor | Generalized myasthenia gravis (anti-AChR antibody-positive adults) |
| Tirzepatide | GIP and GLP-1 receptors | T2DM and Obesity |
| Terlipressin | V1 and V2 receptors | Hepatorenal syndrome with rapid reduction in kidney function |
| Vosoritide | Natriuretic peptide receptor B (NPR-B) | Achondroplasia |
| Melphalan flufenamide | Exerts anti-tumor activity through cross-linking of DNA | Multiple myeloma (MM) and amyloid light-chain amyloidosis |
| Voclosporin | T-cells | Lupus nephritis |
| Pegcetacoplan | Complement protein C3 and its activation product C3b | Paroxysmal nocturnal hemoglobinuria |
| Parameters | Chemical Synthesis | Biological Synthesis |
|---|---|---|
| Peptide Length | Optimal for short peptides (≤50 amino acids) | Suitable for long peptides(>50 amino acids) |
| Cost Efficiency | High reagent costs; limit large-scale feasibility; poor scalability | Cost-effective at scale; highly scalable via fermentation/bioreactor systems |
| Purity and Impurities | Truncated sequences, racemization, and side reactions necessitate HPLC purification | Host-cell proteins, endotoxins, and misfolding require stringent downstream purification |
| Modification Flexibility | Enables non-natural amino acids, isotopic labeling, and site-specific modifications | Limited to natural amino acids; modifications demand genetic/enzymatic engineering |
| Production Time | Shorter cycles (days to weeks) for simple peptides | Extended timelines (weeks to months) due to biological system complexity |
| Batch Consistency | High reproducibility for short sequences; variability escalates with length/complexity | Potential batch variability from biological instability (e.g., mutations, expression drift) |
| Environmental Impact | Solvent-intensive processes generate hazardous waste | Reduced solvent use; more sustainable for large-scale manufacturing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, B.; Wang, X.; Guo, M.; Tzeng, C.-M. Therapeutic Peptides: Recent Advances in Discovery, Synthesis, and Clinical Translation. Int. J. Mol. Sci. 2025, 26, 5131. https://doi.org/10.3390/ijms26115131
Zheng B, Wang X, Guo M, Tzeng C-M. Therapeutic Peptides: Recent Advances in Discovery, Synthesis, and Clinical Translation. International Journal of Molecular Sciences. 2025; 26(11):5131. https://doi.org/10.3390/ijms26115131
Chicago/Turabian StyleZheng, Bingyi, Xueting Wang, Meizhai Guo, and Chi-Meng Tzeng. 2025. "Therapeutic Peptides: Recent Advances in Discovery, Synthesis, and Clinical Translation" International Journal of Molecular Sciences 26, no. 11: 5131. https://doi.org/10.3390/ijms26115131
APA StyleZheng, B., Wang, X., Guo, M., & Tzeng, C.-M. (2025). Therapeutic Peptides: Recent Advances in Discovery, Synthesis, and Clinical Translation. International Journal of Molecular Sciences, 26(11), 5131. https://doi.org/10.3390/ijms26115131






