SPINT1 Expressed in Epithelial Cells of Choroid Plexus in Human and Mouse Brains
Abstract
1. Introduction
2. Results
2.1. SPINT1 Expression in Mouse Choroid Plexus
2.2. SPINT1 Expression in Human Choroid Plexus
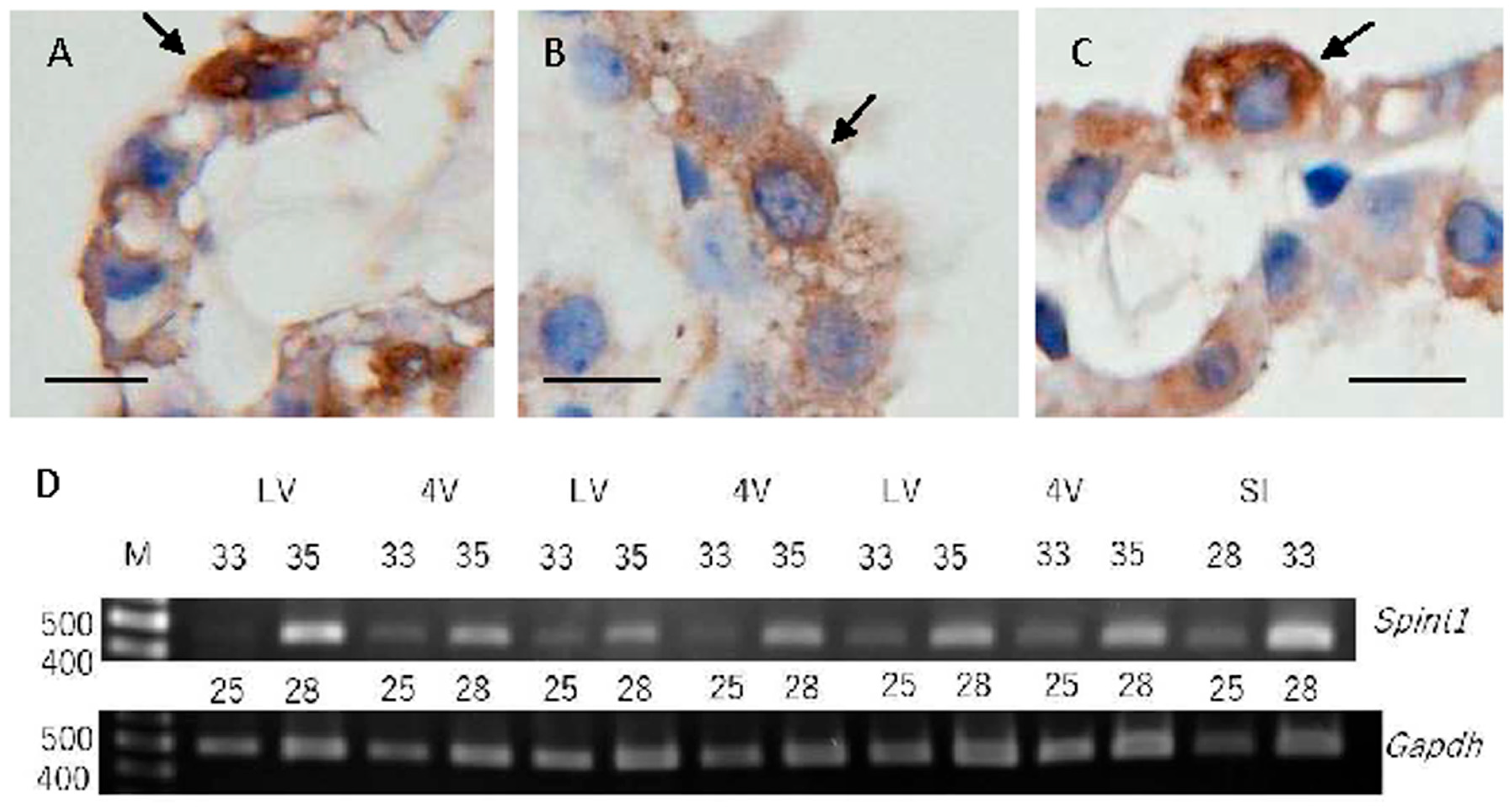
2.2.1. Classical Immunohistochemical SPINT1 Expression
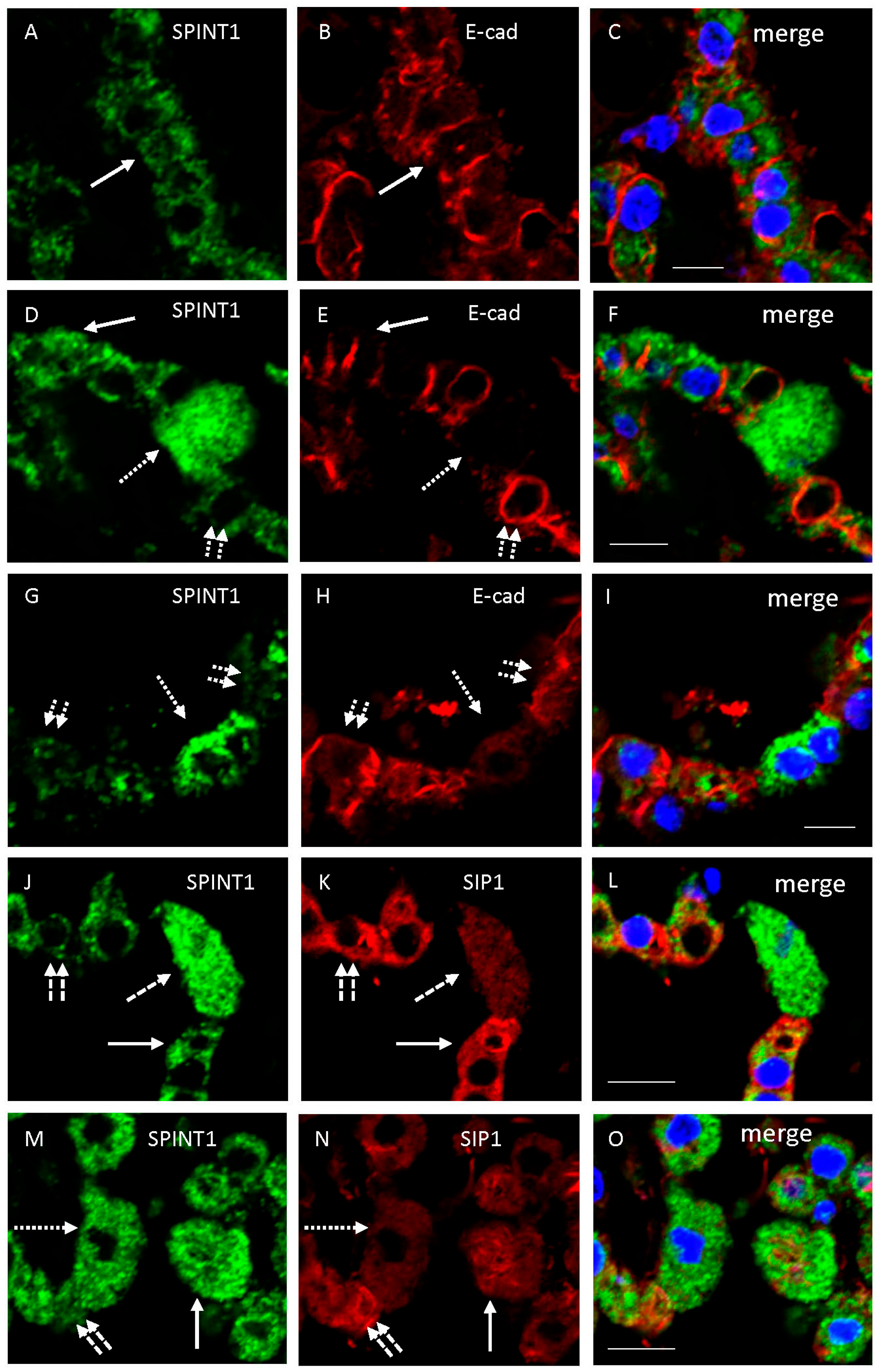
2.2.2. Double Immunofluorescence Examination of SPINT1 Expression
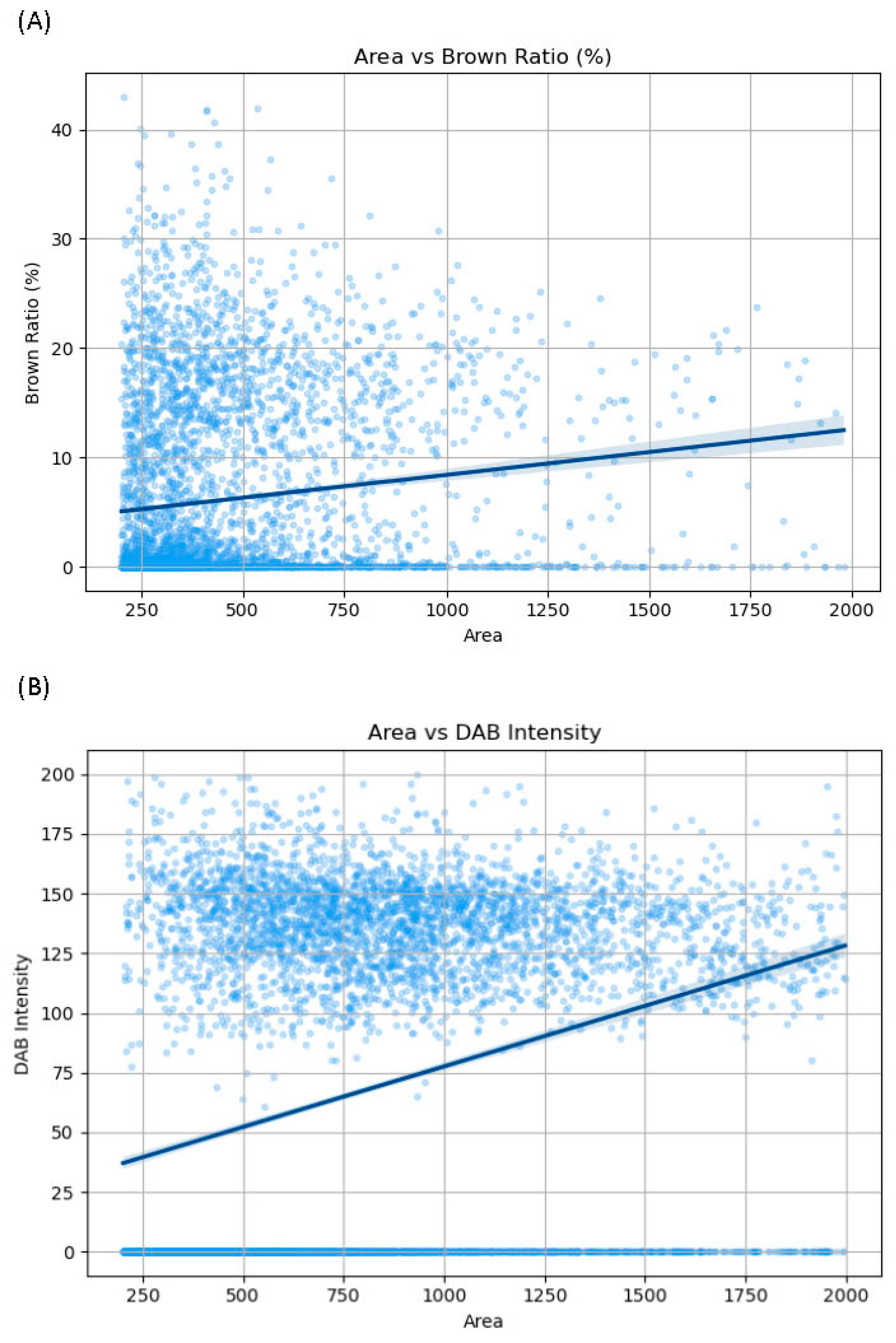
2.2.3. Morphometrical Analysis
3. Discussion
4. Materials and Methods
4.1. Human Tissues
4.2. Animals
4.3. Immunohistochemistry
4.4. Morphometry
4.5. RT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CP | Choroid plexus |
| CPE | Choroid plexus epithelium |
| HAI-1 | Hepatocyte growth factor activator inhibitor type 1 |
| SI | Small intestine |
| SIP1 | Smad-interacting protein 1 |
| SPINT1 | Serine protease inhibitor Kunitz type 1 |
References
- Lun, M.P.; Johnson, M.B.; Broadbelt, K.G.; Watanabe, M.; Kang, Y.-J.; Chau, K.F.; Springel, M.W.; Malesz, A.; Sousa, A.M.M.; Pletikos, M.; et al. Spatially heterogeneous choroid plexus transcriptomes encode positional identity and contribute to regional CSF production. J. Neurosci. 2015, 35, 4903–4916. [Google Scholar] [CrossRef] [PubMed]
- Bryniarski, M.A.; Ren, T.; Rizvi, A.R.; Snyder, A.M.; Morris, M.E. Targeting the choroid plexuses for protein drug delivery. Pharmaceutics 2020, 12, 963. [Google Scholar] [CrossRef] [PubMed]
- Christensen, I.B.; Mogensen, E.N.; Damkier, H.H.; Praetorius, J. Choroid plexus epithelial cells express the adhesion protein P-cadherin at cell-cell contacts and syntaxin-4 in the luminal membrane domain. Am. J. Physiol. Cell Physiol. 2018, 314, C519–C533. [Google Scholar] [CrossRef] [PubMed]
- Damkier, H.; Praetorius, J. Structure of the mammalian choroid plexus. In Role of the Choroid Plexus in Health and Disease; Praetorius, J., Blazer-Yost, B., Damkier, H., Eds.; Springer: New York, NY, USA, 2020; pp. 1–33. [Google Scholar]
- Szmydynger-Chodobska, J.; Pascale, C.L.; Pfeffer, A.N.; Coulter, C.; Chodobski, A. Expression of junctional proteins in choroid plexus epithelial cell lines: A comparative study. Cerebrospinal Fluid Res. 2007, 4, 11. [Google Scholar] [CrossRef]
- Myung, J.; Schmal, C.; Hong, S.; Tsukizawa, Y.; Rose, P.; Zhang, Y.; Holtzman, M.J.; Schutter, E.D.; Herzel, H.; Bordyugov, G.; et al. The choroid plexus is an important circadian clock component. Nat. Commun. 2018, 9, 1062. [Google Scholar] [CrossRef]
- Dong, Y.; Lam, S.M.; Li, Y.; Li, M.-D.; Shui, G. The circadian clock at the intersection of metabolism and aging—Emerging roles of metabolites. J. Genet. Genom. 2025, S1673-8527(25)00123-7. [Google Scholar] [CrossRef]
- Mishima, K.; Tozawa, T.; Satoh, K.; Matsumoto, Y.; Hishikawa, Y.; Okawa, M. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep-waking. Biol. Psychiatry 1999, 45, 417–421. [Google Scholar] [CrossRef]
- Takebayashi, G.; Chiba, Y.; Wakamatsu, K.; Murakami, R.; Miyai, Y.; Matsumoto, K.; Uemura, N.; Yanase, K.; Shirakami, G.; Ogino, Y.; et al. E-cadherin is expressed in epithelial cells of the choroid plexus in human and mouse brains. Curr. Issues Mol. Biol. 2023, 45, 7813–7826. [Google Scholar] [CrossRef]
- Kataoka, H.; Suganuma, T.; Shimomura, T.; Itoh, H.; Kitamura, N.; Nabeshima, K.; Koono, M. Distribution of hepatocyte growth factor activator inhibitor type 1 (HAI-1) in human tissues. Cellular surface localization of HAI-1 in simple columnar epithelium and its modulated expression in injures and regenerative tissues. J. Histochem. Cytochem. 1999, 47, 673–682. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Kanemaru, A.; Sawaguchi, A.; Yamamoto, K.; Baba, T.; Lin, C.-Y.; Johnson, M.D.; Fukushima, T.; Kataoka, H. Hepatocyte growth factor activator inhibitor type 1 maintains the assembly of keratin into desmosomes in keratinocytes by regulating protease-activated receptor 2-dependent p38 signaling. Am. J. Pathol. 2015, 185, 1610–1623. [Google Scholar] [CrossRef]
- Kataoka, H.; Meng, J.-Y.; Itoh, H.; Hamasuna, R.; Shimomura, T.; Suganuma, T.; Koono, M. Localization of hepatocyte growth factor activator inhibitor type 1 in Langhans’ cells of human placenta. Histochem. Cell Biol. 2000, 114, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Kozai, A.; Murakami, R.; Chiba, Y.; Miyai, Y.; Matsumoto, K.; Kanenishi, K.; Ueno, M. Immunohistochemical localization of HCA1 receptor in placenta in presence of fetal growth restriction. Placenta 2024, 154, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Fukushima, T.; Takahashi, N.; Tanaka, H.; Kataoka, H. Hepatocyte growth factor activator inhibitor type 1 regulates epithelial to mesenchymal transition through membrane-bound serine proteinases. Cancer Res. 2009, 69, 1828–1835. [Google Scholar] [CrossRef]
- Andreassen, S.N.; Toft-Bertelsen, T.L.; Wardman, J.H.; Villadsen, R.; MacAulay, N. Transcriptional profiling of transport mechanisms and regulatory pathways in rat choroid plexus. Fluids Barriers CNS 2022, 19, 44. [Google Scholar] [CrossRef]
- Oberst, M.; Anders, J.; Xie, B.; Singh, B.; Ossandon, M.; Johnson, M.; Dickson, R.B.; Lin, C.-Y. Matriptase and HAI-1 are expressed by normal and malignant epithelial cells in vitro and in vivo. Am. J. Pathol. 2001, 158, 1301–1311. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Takeda, N.; Hoshiko, S.; Yorita, K.; Baba, T.; Sawaguchi, A.; Nezu, Y.; Yoshikawa, T.; Fukushima, T.; Kataoka, H. Membrane-bound serine protease inhibitor HAI-1 is required for maintenance of intestinal epithelial integrity. Am. J. Pathol. 2011, 179, 1815–1826. [Google Scholar] [CrossRef]
- Murakami, R.; Chiba, Y.; Miyatake, N.; Miyai, Y.; Matsumoto, K.; Wakamatsu, K.; Saito, Y.; Hara, M.; Murayama, S.; Ueno, M. Morphometry of choroid plexus epithelial cells in neurodegenerative diseases. Neuropathology 2024. [Google Scholar] [CrossRef]
- Chiba, Y.; Sugiyama, Y.; Nishi, N.; Nonaka, W.; Murakami, R.; Ueno, M. Sodium/glucose cotransporter 2 is expressed in choroid plexus epithelial cells and ependymal cells in human and mouse brain. Neuropathology 2020, 40, 482–491. [Google Scholar] [CrossRef]
- Chiba, Y.; Murakami, R.; Matsumoto, K.; Wakamatsu, K.; Nonaka, W.; Uemura, N.; Yanase, K.; Kamada, M.; Ueno, M. Glucose, fructose, and urate transporters in the choroid plexus epithelium. Int. J. Mol. Sci. 2020, 21, 7230. [Google Scholar] [CrossRef]
- Ko, C.-J.; Hsu, T.-W.; Wu, S.-R.; Lan, S.-W.; Hsiao, T.-F.; Lin, H.-Y.; Lin, H.-H.; Tu, H.-F.; Huang, C.-C.; Chen, M.-J.; et al. Inhibition of TMPRSS2 by HAI-2 reduces prostate cancer invasion and metastasis. Oncogene 2020, 39, 5950–5963. [Google Scholar] [CrossRef]
- He, S.; Zhao, Z.; Yang, Y.; O’Connell, D.; Zhang, X.; Oh, S.; Ma, B.; Lee, J.-H.; Zhang, T.; Varghese, B.; et al. Truncating mutation in the autophagy gene UVRAG confers oncogenic properties and chemosensitivity in colorectal cancers. Nat. Commun. 2015, 6, 7839. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhai, X.; Shi, B.; Luo, D.; Jin, B. KIAA1199 overexpression is associated with abnormal expression on EMT markers and is a novel independent prognostic biomarker for hepatocellular carcinoma. OncoTargets Ther. 2018, 11, 8341–8348. [Google Scholar] [CrossRef] [PubMed]
- Yanase, K.; Uemura, N.; Chiba, Y.; Murakami, R.; Fujihara, R.; Matsumoto, K.; Shirakami, G.; Araki, N.; Ueno, M. Immunoreactivities for hepcidin, ferroportin, and hephaestin in astrocytes and choroid plexus epithelium of human brains. Neuropathology 2020, 40, 75–83. [Google Scholar] [CrossRef] [PubMed]

| (No.) | Age/Sex | Main Diagnosis | PMD (h) |
|---|---|---|---|
| 1 | 42/M | Pulmonary hypertension, Heart failure | 10 |
| 2 | 57/F | Psychiatric disorder, Liver abscess, Sepsis | 12 |
| 3 | 64/M | Multiple system atrophy, Pneumonia | 5 |
| 4 | 68/F | Thalamic hemorrhage | 5 |
| 5 | 70/M | Myocardial infarction | 2 |
| 6 | 72/F | Pneumonia | 4 |
| 7 | 74/M | Lung cancer | 10 |
| 8 | 75/M | Gastric cancer | 1.5 |
| 9 | 75/M | Multiple system atrophy, Pneumonia | 1 |
| 10 | 84/M | Myocardial infarction, Cerebral infarction | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, R.; Chiba, Y.; Takebayashi, G.; Wakamatsu, K.; Miyai, Y.; Matsumoto, K.; Uemura, N.; Yanase, K.; Ogino, Y.; Ueno, M. SPINT1 Expressed in Epithelial Cells of Choroid Plexus in Human and Mouse Brains. Int. J. Mol. Sci. 2025, 26, 5130. https://doi.org/10.3390/ijms26115130
Murakami R, Chiba Y, Takebayashi G, Wakamatsu K, Miyai Y, Matsumoto K, Uemura N, Yanase K, Ogino Y, Ueno M. SPINT1 Expressed in Epithelial Cells of Choroid Plexus in Human and Mouse Brains. International Journal of Molecular Sciences. 2025; 26(11):5130. https://doi.org/10.3390/ijms26115130
Chicago/Turabian StyleMurakami, Ryuta, Yoichi Chiba, Genta Takebayashi, Keiji Wakamatsu, Yumi Miyai, Koichi Matsumoto, Naoya Uemura, Ken Yanase, Yuichi Ogino, and Masaki Ueno. 2025. "SPINT1 Expressed in Epithelial Cells of Choroid Plexus in Human and Mouse Brains" International Journal of Molecular Sciences 26, no. 11: 5130. https://doi.org/10.3390/ijms26115130
APA StyleMurakami, R., Chiba, Y., Takebayashi, G., Wakamatsu, K., Miyai, Y., Matsumoto, K., Uemura, N., Yanase, K., Ogino, Y., & Ueno, M. (2025). SPINT1 Expressed in Epithelial Cells of Choroid Plexus in Human and Mouse Brains. International Journal of Molecular Sciences, 26(11), 5130. https://doi.org/10.3390/ijms26115130






