Foliar Application of Protein Hydrolysate-Based Biostimulant and Herbal Extracts with Antifungal Properties in Winter Wheat Cultivation as a Strategy to Enhance Cereal Yield
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening Tests (Selection of Optimal Concentration for Pot Experiments)
2.1.1. Phytotoxicity Assessment
2.1.2. Antifungal Properties Evaluation
2.2. Results of Pots Experiments
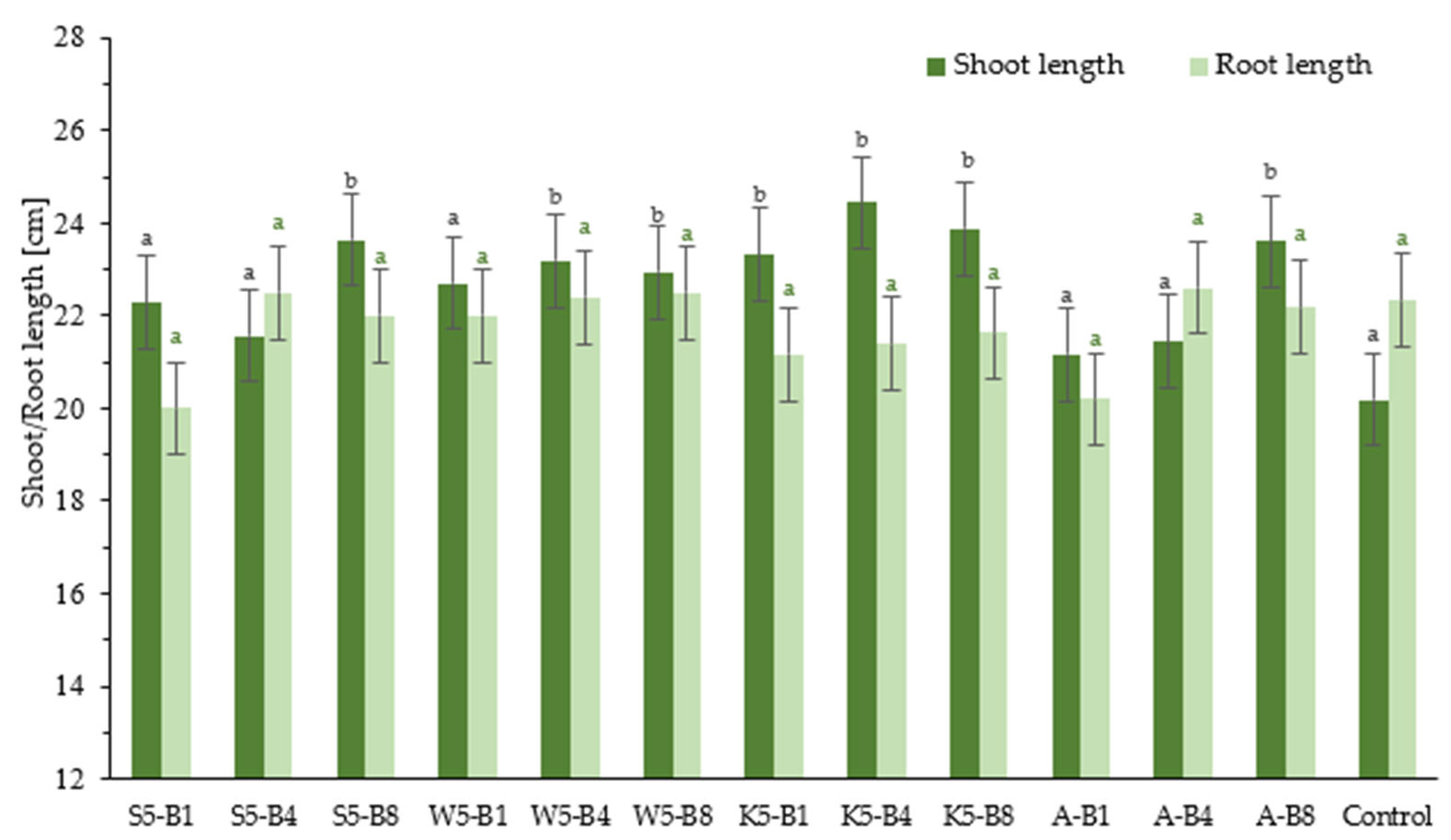
2.2.1. Effect of Foliar Application of Tested Preparation on Winter Wheat Growth
2.2.2. Effect of Foliar Application of Tested Preparation on FW and DW Contents of Winter Wheat Seedlings
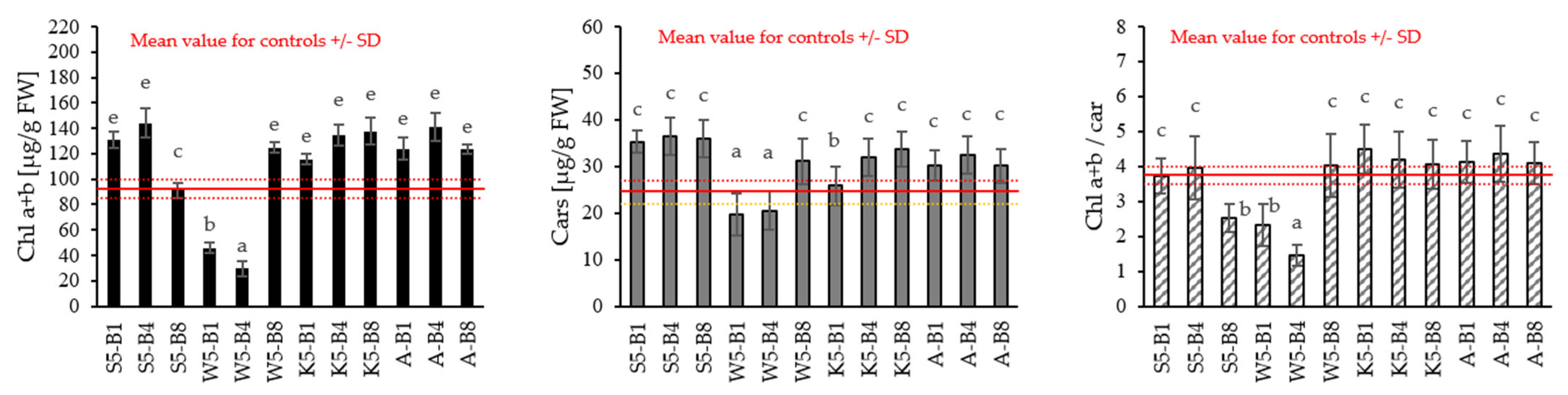
2.2.3. Variations in the Content of Photosynthetic Pigments
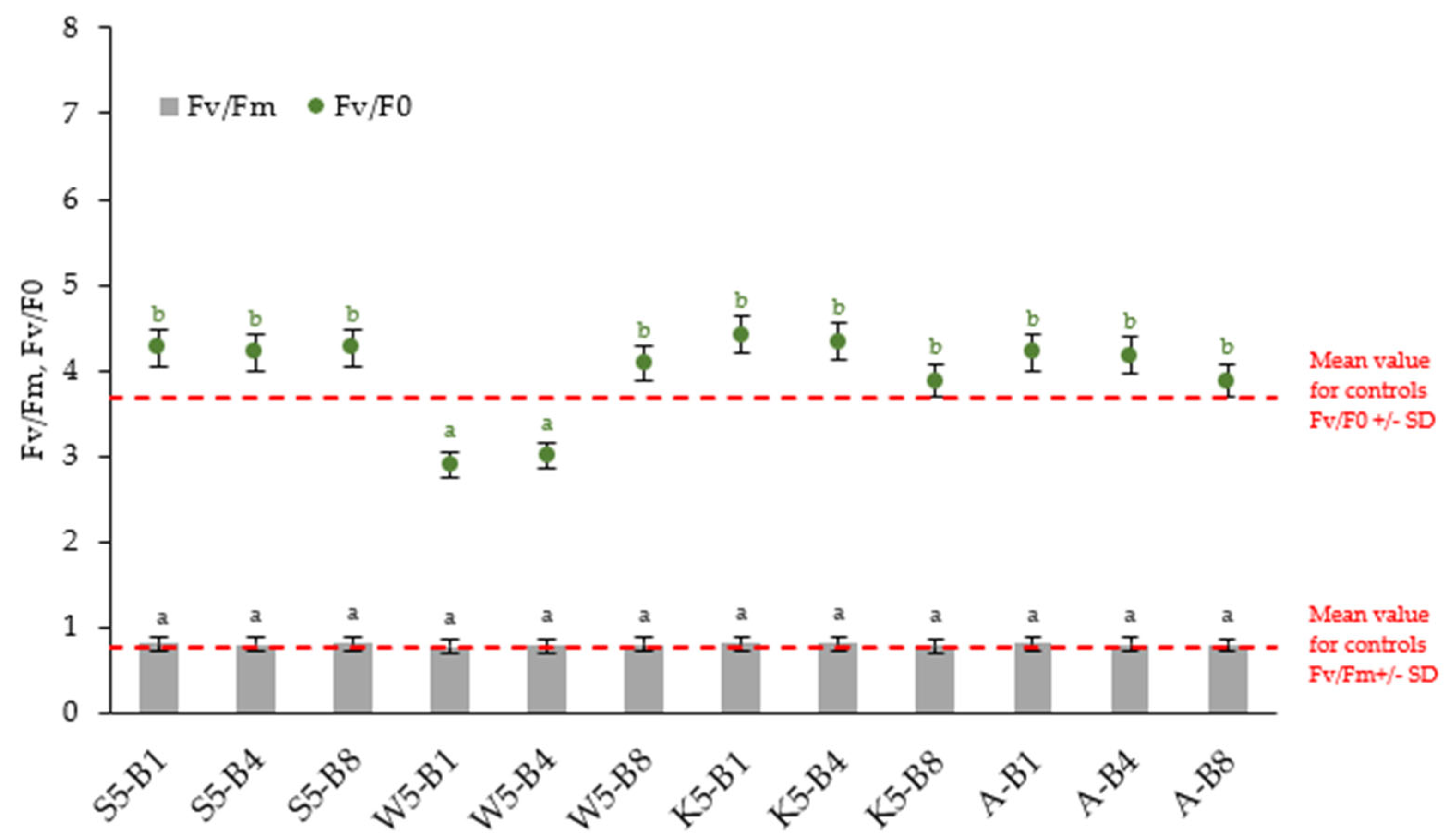
2.2.4. Variations in the Chlorophyll Fluorescence Parameters
2.2.5. Variations in the Dehydrogenase (DHs) Activity
3. Materials and Methods
3.1. Screening Tests (Selection of Optimal Concentration for Pot Experiments)
3.1.1. Preparation of Herbal Extracts
3.1.2. Phytotoxicity Assessment
3.1.3. Antifungal Properties Evaluation
3.2. Pot Experiments—Growth Studies on Winter Wheat
3.2.1. Plant Material
3.2.2. Preparations Tested
3.2.3. Experimental Setup
3.2.4. Growth Measurements
3.2.5. Photosynthetic Pigment Content Determination
3.2.6. Chlorophyll Fluorescence Analysis
3.2.7. Dehydrogenase (DHs) Activity Test
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anielak, A.M.; Kłeczek, A.; Łuszczek, B. Innovative Method of Extraction of Humic Substances from Digested Sludge and Assessment of the Impact of Their on the Growth of Selected Plants. Energies 2023, 16, 1283. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable Sources of Plant Biostimulation: Microalgae as a Sustainable Means to Improve Crop Performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, B.K.; Rivas-Castillo, A.M.; Valdez-Calderón, A.; Gayosso-Morales, M.A. Microalgae as Biostimulants: A New Approach in Agriculture. World J. Microbiol. Biotechnol. 2022, 38, 4. [Google Scholar] [CrossRef]
- Prajapati, A.; Patel, C.K.; Singh, N.; Jain, S.K.; Chongtham, S.K.; Maheshwari, M.N.; Patel, R.N. Evaluation of Seaweed Extract on Growth and Yield of Potato. Environ. Ecol. 2016, 34, 605–608. [Google Scholar]
- Avis, T.J.; Gravel, V.; Antoun, H.; Tweddell, R.J. Multifaceted Beneficial Effects of Rhizosphere Microorganisms on Plant Health and Productivity. Soil Biol. Bioch. 2008, 40, 1733–1740. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.; Farooq, M.; Xue, L.; Ali, S. Fulvic Acid Application Improves the Maize Performance Under Well-Watered and Drought Conditions. J. Agron. Crop. Sci. 2011, 197, 409–417. [Google Scholar] [CrossRef]
- Pipiak, P.; Sieczyńska, K.; Gendaszewska, D.; Skwarek-Fadecka, M. Effect of Pre-Sowing Seed Stimulation on Maize Seedling Vigour. Int. J. Mol. Sci. 2024, 25, 12480. [Google Scholar] [CrossRef]
- Wieczorek, D.; Gendaszewska, D.; Miśkiewicz, K.; Słubik, A.; Ławińska, K. Biotransformation of Protein-Rich Waste by Yarrowia lipolytica IPS21 to High-Value Products—Amino Acid Supernatants. Microbiol. Spectr. 2023, 11, e02749-23. [Google Scholar] [CrossRef]
- Famielec, S. Environmental Effects of Tannery Waste Incineration in a Tunnel Furnace System. Proc. ECOpole 2015, 9, 363–368. [Google Scholar] [CrossRef]
- Skwarek, M.; Nawrocka, J.; Lasoń-Rydel, M.; Ławińska, K. Diversity of Plant Biostimulants in Plant Growth Promotion and Stress Protection in Crop and Fibrous Plants. Fibres Text. East. Eur. 2020, 28, 34–41. [Google Scholar]
- Gendaszewska, D.; Lasoń-Rydel, M.; Ławińska, K.; Grzesiak, E.; Pipiak, P. Characteristics of Collagen Preparations from Leather Wastes by the High-Pressure Liquid Chromatography Method. Fibres Text. East. Eur. 2021, 29, 75–79. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Kuang, Y.; Wang, N. Amino Acids Biostimulants and Protein Hydrolysates in Agricultural Sciences. Plants 2024, 13, 210. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Ravazzolo, L.; Franceschi, C.; Quaggiotti, S. mRNA-Sequencing Analysis Reveals Transcriptional Changes in Root of Maize Seedlings Treated with Two Increasing Concentrations of a New Biostimulant. J. Agric. Food Chem. 2017, 65, 9956–9969. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and the Council Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009L0128 (accessed on 2 April 2025).
- Gendaszewska, D.; Pipiak, P.; Wieczorek, D.; Miśkiewicz, K.; Ławińska, K.; Popińska, W. Utilization of Protein Hydrolysates from Animal Waste for the Production of Biostimulants in Wheat Cultivation (Triticum aestivum L.). Fibres Text. East. Eur. 2025; in press. [Google Scholar]
- Kursa, W.; Jamiołkowska, A.; Skwaryło-Bednarz, B.; Kowalska, G.; Gałązka, A. Impact of Selected Plant Extracts on Winter Wheat (Triticum aestivum L.) Seedlings: Growth, Plant Health Status and Soil Activity. Agriculture 2024, 14, 959. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Depciuch, J.; Drygaś, B.; Jańczak-Pieniążek, M.; Mazurek, K.; Pawlak, R. The Influence of Biostimulants Used in Sustainable Agriculture for Antifungal Protection on the Chemical Composition of Winter Wheat Grain. Int. J. Environ. Res. Public Health 2022, 19, 12998. [Google Scholar] [CrossRef]
- Kursa, W.; Jamiołkowska, A.; Wyrostek, J.; Kowalski, R. Antifungal Effect of Plant Extracts on the Growth of the Cereal Pathogen Fusarium spp. An In Vitro Study. Agronomy 2022, 12, 3204. [Google Scholar] [CrossRef]
- Gendaszewska, D.; Wionczyk, B.; Bednarek, A.; Boniecki, P. Antifungal Activity of Finished Chromium Tanned Leather Containing Thyme and Tea Tree Essential Oils. Fibres Text. East. Eur. 2022, 30, 41–45. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Mohamed, A.A.; Ali, H.M.; Al Farraj, D.A. Characterization of Phytoconstituents from Alcoholic Extracts of Four Woody Species and Their Potential Uses for Management of Six Fusarium oxysporum Isolates Identified from Some Plant Hosts. Plants 2021, 10, 1325. [Google Scholar] [CrossRef]
- Al-Snafi, A. The Antifungal Spectrum of Medicinal Plants: A Review. GSC Biol. Pharm. Sci. 2023, 24, 118–146. [Google Scholar] [CrossRef]
- Beni, C.; Casorri, L.; Masciarelli, E.; Ficociello, B.; Masetti, O.; Neri, U.; Aromolo, R.; Rinaldi, S.; Papetti, P.; Cichelli, A. Characterization of Thyme and Tansy Extracts Used as Basic Substances in Zucchini Crop Protection. J. Agric. Stud. 2020, 8, 95–110. [Google Scholar] [CrossRef]
- Taylor, A.; Bonafos, R.; Chovelon, M.; Parvaud, C.E.; Furet, A.; Aveline, N.; Marchand, P.A. Equisetum arvense (Horsetail) Extract: The First Approved Basic Substance Allowed for EU Crop Protection. Int. J. Bio-Resour. Stress Manag. 2022, 13, 566–577. [Google Scholar] [CrossRef]
- Ivanescu, B.; Tuchiluș, C.; Corciovă, A.; Apetrei, C.; Mihai, C.T.; Vlase, A.-M.; Vlase, L. Antioxidant, Antimicrobial, and Cytotoxic Activity of Tanacetum vulgare, Tanacetum corymbosum, and Tanacetum macrophyllum Extracts. Farmacia 2018, 66, 282–288. [Google Scholar]
- Devrnja, N.; Anđelković, B.; Aranđelović, S.; Radulović, S.; Soković, M.; Krstić-Milošević, D.; Ristić, M.; Ćalić, D. Comparative Studies on the Antimicrobial and Cytotoxic Activities of Tanaceum vulgare L. Essential Oil and Methanol Extracts. S. Afr. J. Bot. 2017, 111, 212–221. [Google Scholar] [CrossRef]
- Marchand, P. Valerian and Yarrow: Two Medicinal Plants as Crop Protectants Against Late Frost. Int. J. Econ. Plants 2018, 5, 192–196. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical Composition of Wild and Commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E. Phytotoxicity Assessment of Biodegradable and Non-Biodegradable Plastics Using Seed Germination and Early Growth Tests. Chemosphere 2022, 289, 133132. [Google Scholar] [CrossRef]
- Eghlima, G.; Esmaeili, H.; Farzaneh, M.; Mirjalili, M.H. Multivariate Analysis of Equisetum arvense L. Ecotypes Based on Silicon Content, Phytochemical, and Morphological Characterization. Silicon 2024, 16, 115–122. [Google Scholar] [CrossRef]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of Silicon in Plant Stress Tolerance: Opportunities to Achieve a Sustainable Cropping System. 3 Biotech. 2019, 9, 73. [Google Scholar] [CrossRef]
- Eghlima, G.; Chegini, K.G.; Farzaneh, M.; Aghamir, F. Effect of Common Horsetail Extract on Growth Characteristics, Essential Oil Yield, and Chemical Compositions of Basil (Ocimum basilicum L.). Sci. Rep. 2024, 14, 11082. [Google Scholar] [CrossRef]
- Judžentienė, A.; Būdienė, J.; Stancelytė, D.; Nedveckytė, I. Phytochemistry and Allelopathic Effects of Tanacetum vulgare L. (Tansy) Extracts on Lepidium sativum L. (Garden Pepper Cress) and Lactuca sativa L. (Lettuce). Horticulturae 2024, 10, 538. [Google Scholar] [CrossRef]
- Sousa, S.M.; Viccini, L.F. Cytotoxic and Genotoxic Activity of Achillea millefolium L., Asteraceae, Aqueous Extracts. Rev. Bras. Farmacogn. 2011, 21, 98–104. [Google Scholar] [CrossRef]
- Kursa, W.; Jamiołkowska, A.; Skwaryło-Bednarz, B.; Kowalski, R.; Wyrostek, J.; Patkowska, E.; Kopacki, M. In Vitro Efficacy of Herbal Plant Extracts on Some Phytopathogenic Fungi. Acta Sci. Pol. Hortorum Cultus 2022, 21, 79–90. [Google Scholar] [CrossRef]
- Boligłowa, E.; Znój, K. The Effect of Plant Preparations on Growth of Selected Phytopathogenic Fungi. J. Res. Appl. Agric. Eng. 2003, 48, 24–27. (In Polish) [Google Scholar]
- Gaidau, C.; Stanca, M.; Niculescu, M.D.; Alexe, C.A.; Becheritu, M.; Horoias, R.; Stanculescu, I.R. Wool Keratin Hydrolysates for Bioactive Additives Preparation. Materials 2021, 14, 4696. [Google Scholar] [CrossRef]
- Metomo, F.N.N.N.; Tayi, F.; Younes, E.; Amadine, O.; Zahouily, M. Production of Sheep Wool Keratin Hydrolysate and Evaluation of its Effectiveness in Promoting Maize Cultivation. J. Environ. Manag. 2024, 366, 121648. [Google Scholar] [CrossRef]
- Kocoń, A. Efficiency of Nitrogen Utilization from Urea (15N) Applied to Soil or Leaves by Winter Wheat and Faba Bean Plants. Acta Agrophysica 2003, 85, 55–63. [Google Scholar]
- Haitova, D. A Review of Foliar Fertilization of Some Vegetable Crops. Annu. Res. Rev. Biol. 2013, 3, 455–465. [Google Scholar]
- Jaskulski, D. Comparison of the Effect of Foliar Application of Fertilizers on the Production and Economic Effects of Cultivating Some Field Crops. Fragm. Agron. 2007, 1, 106–112. [Google Scholar]
- Możejko, M.; Bohacz, J. Effect of Keratin Hydrolysates Obtained from Feather Decomposition by Trichophyton ajelloi on Plant Germination, Growth, and Biological Activity of Selected Arable Soils under Model Conditions. Agronomy 2023, 13, 187. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll Fluorescence, Photoinhibition and Abiotic Stress: Does It Make Any Difference the Fact to Be a C3 or C4 Species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Szafrańska, K.; Reiter, R.J.; Posmyk, M.M. Melatonin Application to Pisum sativum L. Seeds Positively Influences the Function of the Photosynthetic Apparatus in Growing Seedlings during Paraquat-Induced Oxidative Stress. Front. Plant Sci. 2016, 7, 1663. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to Correctly Determine the Different Chlorophyll Fluorescence Parameters and the Chlorophyll Fluorescence Decrease Ratio R Fd of Leaves with the PAM Fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Maiti, S.K. Different Soil Factors Influencing Dehydrogenase Activity in Mine Degraded Lands—State-of-Art Review. Water Air Soil Pollut. 2021, 232, 360. [Google Scholar] [CrossRef]
- Kenarova, A.; Boteva, S. Fungicides in Agriculture and Their Side Effects on Soil Enzyme Activities: A Review. Bulg. J. Agric. Sci. 2023, 29, 33–42. [Google Scholar]
- Mattarozzi, M.; Di Zinno, J.; Montanini, B.; Manfredi, M.; Marengo, E.; Fornasier, F.; Ferrarini, A.; Careri, M.; Visioli, G. Biostimulants Applied to Maize Seeds Modulate the Enzymatic Activity and Metaproteome of the Rhizosphere. Appl. Soil Ecol. 2020, 148, 103480. [Google Scholar] [CrossRef]
- Furtak, K.; Gajda, A.M. Activity of Dehydrogenases as an Indicator of Soil Environment Quality. Pol. J. Soil Sci. 2017, 50, 33–43. [Google Scholar] [CrossRef]
- Wang, Z.; Yun, S.; An, Y.; Shu, L.; Li, S.; Sun, K.; Zhang, W. Effect of Fungicides on Soil Respiration, Microbial Community, and Enzyme Activity: A Global Meta-Analysis (1975–2024). Ecotoxicol. Environ. Saf. 2025, 289, 117433. [Google Scholar] [CrossRef]
- Lloyd, A.W.; Percival, D.; Langille, M.G.I.; Yurgel, S.N. Changes to Soil Microbiome Resulting from Synergetic Effects of Fungistatic Compounds Pyrimethanil and Fluopyram in Lowbush Blueberry Agriculture, with Nine Fungicide Products Tested. Microorganisms 2023, 11, 410. [Google Scholar] [CrossRef]
- Dancewicz, K.; Gabrys, B. Effect of Extracts of Garlic (Allium sativum L.), Wormwood (Artemisia absinthium L.), and Tanacetum vulgare L. on the Behavior of the Peach Potato Aphid Myzus persicae (Sulzer) During Settling on Plants. Pesticides 2008, 3–4, 93–99. [Google Scholar]
- Nashwa, S.M.A.; Abo-Elyousr, K.A.M. Evaluation of Various Plant Extracts Against the Early Blight Disease of Tomato Plants Under Greenhouse and Field Conditions. Plant Protect. Sci. 2012, 48, 74–79. [Google Scholar] [CrossRef]
- Hough-Goldstein, J.; Hahn, S.P. Antifeedant and Oviposition Deterrent Activity of an Aqueous Extract of Tanacetum vulgare L. on Two Cabbage Pests. Environ. Entomol. 1992, 21, 837–844. [Google Scholar] [CrossRef]
- Męczarska, K.; Cyboran-Mikołajczyk, S.; Solarska-Ściuk, K.; Oszmiański, J.; Siejak, K.; Bonarska-Kujawa, D. Protective Effect of Field Horsetail Polyphenolic Extract on Erythrocytes and Their Membranes. Int. J. Mol. Sci. 2025, 26, 3213. [Google Scholar] [CrossRef] [PubMed]
- ISO 18763:2016; Soil Quality—Determination of the Toxic Effects of Pollutants on Germination and Early Growth of Higher Plants. Test No. 208: Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test. ISO: Geneva, Switzerland, 2016.
- Baran, Ş.; Alazzawi, S.; Semerci, A.B. The Effects of Equisetum arvense L. Extracts Prepared Using Different Solvents and Extraction Methods for Antioxidant and Antimicrobial Activity. Food Health 2024, 10, 1–11. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls a and b Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Smirnov, A.A.; Semenova, N.A.; Dorokhov, A.S.; Proshkin, Y.A.; Godyaeva, M.M.; Vodeneev, V.; Sukhov, V.; Panchenko, V.; Chilingaryan, N.O. Influence of Pulsed, Scanning and Constant (16- and 24-h) Modes of LED Irradiation on the Physiological, Biochemical and Morphometric Parameters of Lettuce Plants (Lactuca sativa L.) while Cultivated in Vertical Farms. Agriculture 2022, 12, 1988. [Google Scholar] [CrossRef]
- Casida, L.; Klein, D.; Santoro, T. Soil Dehydrogenase Activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Rostocki, A.; Lasoń-Rydel, M.; Wieczorek, D.; Ławińska, K.; Obraniak, A. From Protein Waste to Agriculture or the Building Sector: Exploring the Environmental Impact of New Granulates on Soil and Water Ecosystems. Ecol. Indic. 2024, 162, 112020. [Google Scholar] [CrossRef]
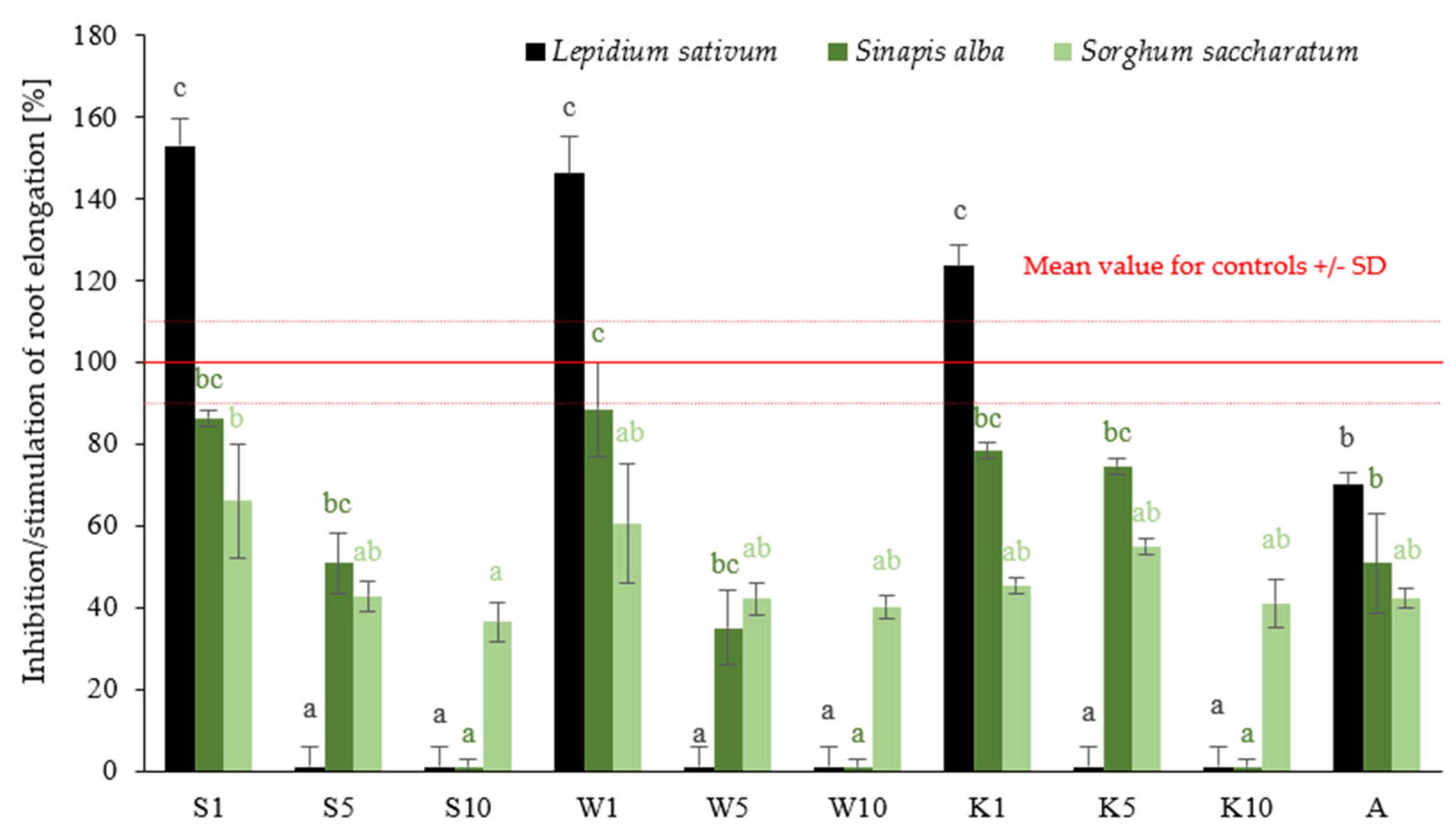

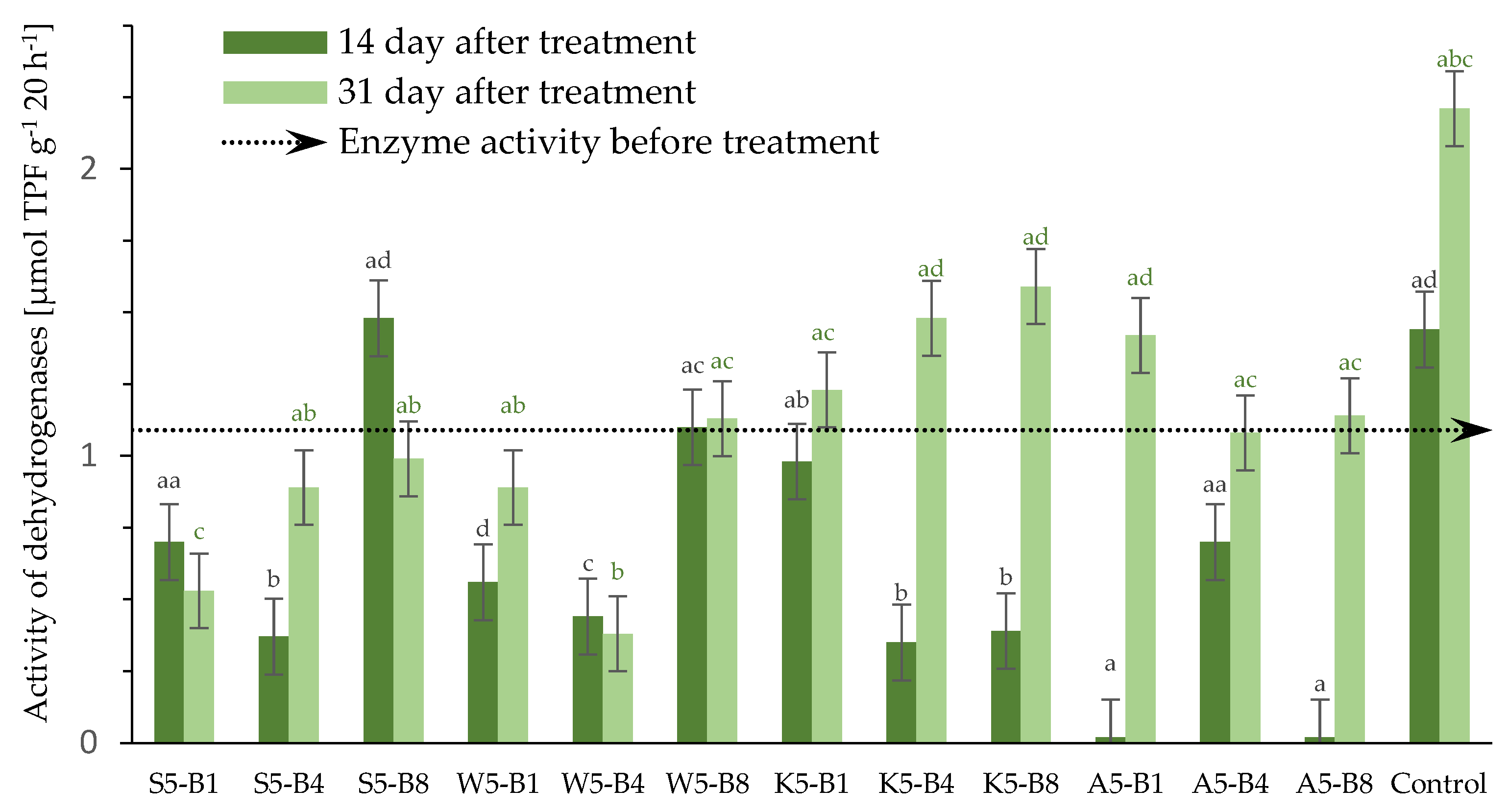
| Microorganism | Extracts/Fungicide | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S5 | S10 | W1 | W5 | W10 | K1 | K5 | K10 | A | |
| S. griseus | <6 | TGI | TGI | <6 | TGI | TGI | <6 | TGI | TGI | <6 |
| Alternaria spp. | <6 | 16.33 | 19.5 | <6 | 20 | 23 | <6 | 19 | 23.33 | 15 |
| F. keratoplasticum | <6 | <6 | <6 | <6 | 9.58 | 13.66 | <6 | 13.45 | 17.5 | 10 |
| Experimental Treatment | Shoot FW [g] | Shoot DW [g] | Root FW [g] | Root DW [g] |
|---|---|---|---|---|
| S5-B1 | 3.5 ± 0.5 b | 0.4 ± 0.2 a | 0.9 ± 0.2 a | 0.3 ± 0.1 a |
| S5-B4 | 2.4 ± 0.4 ab | 0.2 ± 0.0 a | 0.5 ± 0.1 a | 0.2 ± 0.1 a |
| S5-B8 | 2.7 ± 0.6 ab | 0.3 ± 0.1 a | 0.9 ± 0.2 a | 0.3 ± 0.1 a |
| W5-B1 | 2.6 ± 0.6 ab | 0.3 ± 0.1 a | 0.9 ± 0.1 a | 0.3 ± 0.1 a |
| W5-B4 | 2.5 ± 0.5 ab | 0.3 ± 0.1 a | 0.9 ± 0.2 a | 0.3 ± 0.1 a |
| W5-B8 | 2.6 ± 0.7 ab | 0.3 ± 0.1 a | 0.5 ± 0.1 a | 0.3 ± 0.1 a |
| K5-B1 | 2.3 ± 0.3 ab | 0.2 ± 0.1 a | 0.9 ± 0.2 a | 0.3 ± 0.1 a |
| K5-B4 | 2.7 ± 0.3 ab | 0.3 ± 0.1 a | 0.9 ± 0.2 a | 0.4 ± 0.1 a |
| K5-B8 | 2.9 ± 0.4 ab | 0.4 ± 0.1 a | 0.7 ± 0.2 a | 0.3 ± 0.1 a |
| A-B1 | 1.9 ± 0.3 a | 0.2 ± 0.0 a | 0.8 ± 0.2 a | 0.4 ± 0.1 a |
| A-B4 | 2.6 ± 0.5 ab | 0.3 ± 0.2 a | 0.9 ± 0.2 a | 0.3 ± 0.1 a |
| A-B8 | 3.0 ± 0.4 ab | 0.3 ± 0.2 a | 0.6 ± 0.1 a | 0.2 ± 0.0 a |
| Control | 2.5 ± 0.2 ab | 0.3 ± 0.1 a | 0.8 ± 0.2 a | 0.3 ± 0.0 a |
| Plant Name | Experiment Condition | Methods | Composition | Ref. |
|---|---|---|---|---|
| Horsetail (Equisetum arvense L.) | Dried leaves were ground into a powder and extracted with water containing 200 ppm SO2 at a 3:1 solvent-to-material ratio. | UPLC–ESI–MS–MS | Caffeoyl tartrate isomer, Caffeoylshikimic acid isomer, Dicaffeoyl tartaric acid isomer, Kaempherol diglycoside, Kaempferol-3-O-6-acetylglucoside, Quercetin dihexoside, Quercetin-glucoside, Quercetin-3-O-6-acetylglucoside isomer | [55] |
| Tansy (Tanacetum vulgare L.) | Fifteen grams of crushed herbal material with 200 mL distilled water was macerated in an ultrasonic bath at 21–24 °C for 45 min. The mixture was filtered and divided into three 25 mL portions. | HPLC-DAD-TOF | Succinic acid, Quinic acid, 3-Dehydrocaffeoyl-5-caffeoylquinic acid, 4-Dehydrocaffeoyl-5-caffeoylquinic acid, Feruloylquinic acid, Diferulic acid, Protocatechuic/gentisic acid, Ferulic (hydroxycinnamic) acid, Isochlorogenic (3,5-dicaffeoylquinic) acid A, Isochlorogenic (3,4-dicaffeoylquinic) acid B, p-Hydroxyphenylacetic acid 1-O-hexoside, Luteolin, Kaempferol, Quercetin, Acacetin, Ludovicin C, Hydroxyarbusculin, 6-Methoxykaempferol/Isorhamnetin, Isorhamnetin 3-O-glucoside, Tanacetin/armefolin, 5,7,3′-Trihydroxy-3,6,4′,5′-tetramethoxyflavone | [33] |
| Yarrow (Achillea millefolium L.) | Lyophilized plant material (1 g) was added to 200 mL distilled water, then heated and boiled for 5 min. The mixture was left to stand for 5 min, then filtered under reduced pressure. Infusions and decoctions were then frozen and lyophilized for further analysis. | HPLC | 3-O-Caffeoylquinic acid, Caffeic acid hexoside, 4-O-Caffeoylquinic acid, 5-O-Caffeoylquinic acid, Apigenin C-hexoside-C-hexoside, Apigenin C-hexoside-C-pentoside, Apigenin C-glucose-C-pentoside, Luteolin 6-C-glucoside, Quercetin O-pentosyl-hexoside, Quercetin O-hexoside, Quercetin O-malonylhexosyl-rhamnoside, Kaempferol O-pentosyl-hexoside, Quercetin 3-O-rutinoside, Apigenin O-dihexoside, Isorhamnetin O-hexoside, 3,4-O-dicaffeoylquinic acid, Quercetin O-acetylhexoside, cis 3,5-O-dicaffeoylquinic acid, trans 3,5-O-dicaffeoylquinic acid, 4,5-O-dicaffeoylquinic acid, Apigenin 7-O-glucoside, Luteolin O-acetylhexoside, Isorhamnetin O-acetylhexoside, Apigenin O-acetylhexoside | [28] |
| Code | Name | Concentration [%] | pH [-] | Density [g/cm3] | Turbidity [NTU] |
|---|---|---|---|---|---|
| S1 | Horsetail extract (Equisetum arvense L.) | 1 | 5.26 | 0.992 | 16 |
| S5 | Horsetail extract (Equisetum arvense L.) | 5 | 4.40 | 0.995 | 841 |
| S10 | Horsetail extract (Equisetum arvense L.) | 10 | 4.32 | 0.998 | >1000 |
| W1 | Tansy extract (Tanacetum vulgare L.) | 1 | 6.37 | 0.991 | 20 |
| W5 | Tansy extract (Tanacetum vulgare L.) | 5 | 4.94 | 0.996 | 464 |
| W10 | Tansy extract (Tanacetum vulgare L.) | 10 | 4.23 | 0.997 | >1000 |
| K1 | Yarrow extract (Achillea millefolium L.) | 1 | 5.80 | 0.990 | 56.1 |
| K5 | Yarrow extract (Achillea millefolium L.) | 5 | 4.08 | 0.992 | 377 |
| K10 | Yarrow extract (Achillea millefolium L.) | 10 | 3.99 | 0.998 | >1000 |
| A | Afrodyta 250 SC | 0.5 | 7.21 | 0.992 | >1000 |
| No. | Code | Name of Fungicide | Composition of Biostimulant | pH [-] | Density [g/cm3] | Turbidity [NTU] |
|---|---|---|---|---|---|---|
| 1 | S5-B1 | Horsetail extract 5% (Equisetum arvense L.) | Biostimulant 1: Collagen hydrolysate 0.5% Sodium salicylate 0.03% | 4.33 | 1.01 | 181 |
| 2 | S5-B4 | Horsetail extract 5% (Equisetum arvense L.) | Biostimulant 4a: Collagen hydrolysate 0.5% Titanium ascorbate 0.01% | 4.32 | 1.01 | 212 |
| 3 | S5-B8 | Horsetail extract 5% (Equisetum arvense L.) | Biostimulant 8: Collagen hydrolysate 0.5% Keratin hydrolysate 0.5% Sodium salicylate 0.03% | 4.32 | 1.02 | 276 |
| 4 | W5-B1 | Tansy extract 5% (Tanacetum vulgare L.) | Biostimulant 1: Collagen hydrolysate 0.5% Sodium salicylate 0.03% | 5.00 | 1.02 | 30.5 |
| 5 | W5-B4 | Tansy extract 5% (Tanacetum vulgare L.) | Biostimulant 4a: Collagen hydrolysate 0.5% Titanium ascorbate 0.01% | 4.99 | 1.02 | 37.0 |
| 6 | W5-B8 | Tansy extract 5% (Tanacetum vulgare L.) | Biostimulant 8: Collagen hydrolysate 0.5% Keratin hydrolysate 0.5% Sodium salicylate 0.03% | 5.02 | 1.03 | 58.0 |
| 7 | K5-B1 | Yarrow extract 5% (Achillea millefolium L.) | Biostimulant 1: Collagen hydrolysate 0.5% Sodium salicylate 0.03% | 4.04 | 1.02 | 471 |
| 8 | K5-B4 | Yarrow extract 5% (Achillea millefolium L.) | Biostimulant 4a: Collagen hydrolysate 0.5% Titanium ascorbate 0.01% | 3.97 | 1.02 | 192 |
| 9 | K5-B8 | Yarrow extract 5% (Achillea millefolium L.) | Biostimulant 8: Collagen hydrolysate 0.5% Keratin hydrolysate 0.5% Sodium salicylate 0.03% | 4.03 | 1.03 | 181 |
| 10 | A-B1 | Afrodyta 250 SC 0.5% | Biostimulant 1: Collagen hydrolysate 0.5% Sodium salicylate 0.03% | 7.26 | 0.998 | >1000 |
| 11 | A-B4 | Afrodyta 250 SC 0.5% | Biostimulant 4a: Collagen hydrolysate 0.5% Titanium ascorbate 0.01% | 7.40 | 0.999 | >1000 |
| 12 | A-B8 | Afrodyta 250 SC 0.5% | Biostimulant 8: Collagen hydrolysate 0.5% Keratin hydrolysate 0.5% Sodium salicylate 0.03% | 7.25 | 0.999 | >1000 |
| 13 | Control | - | Water | 7.00 | 0.998 | 0 |
| Salinity [g NaCl/dm3] | pH in H2O [-] | N-NO3 [mg/dm3] | P [mg/dm3] | K [mg/dm3] | Cu [mg/dm3] | Fe [mg/dm3] | Zn [mg/dm3] | Mg [mg/dm3] | C [%] | Nt [%] | OM [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| <3.0 | 6.5 | 5.59 | 122 | 736 | 0.71 | 1.29 | 0.93 | 207 | 13.9 | 0.91 | 23.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gendaszewska, D.; Wieczorek, D.; Pipiak, P.; Miśkiewicz, K.; Zacharska, K.; Ławińska, K. Foliar Application of Protein Hydrolysate-Based Biostimulant and Herbal Extracts with Antifungal Properties in Winter Wheat Cultivation as a Strategy to Enhance Cereal Yield. Int. J. Mol. Sci. 2025, 26, 5089. https://doi.org/10.3390/ijms26115089
Gendaszewska D, Wieczorek D, Pipiak P, Miśkiewicz K, Zacharska K, Ławińska K. Foliar Application of Protein Hydrolysate-Based Biostimulant and Herbal Extracts with Antifungal Properties in Winter Wheat Cultivation as a Strategy to Enhance Cereal Yield. International Journal of Molecular Sciences. 2025; 26(11):5089. https://doi.org/10.3390/ijms26115089
Chicago/Turabian StyleGendaszewska, Dorota, Dorota Wieczorek, Paulina Pipiak, Katarzyna Miśkiewicz, Katarzyna Zacharska, and Katarzyna Ławińska. 2025. "Foliar Application of Protein Hydrolysate-Based Biostimulant and Herbal Extracts with Antifungal Properties in Winter Wheat Cultivation as a Strategy to Enhance Cereal Yield" International Journal of Molecular Sciences 26, no. 11: 5089. https://doi.org/10.3390/ijms26115089
APA StyleGendaszewska, D., Wieczorek, D., Pipiak, P., Miśkiewicz, K., Zacharska, K., & Ławińska, K. (2025). Foliar Application of Protein Hydrolysate-Based Biostimulant and Herbal Extracts with Antifungal Properties in Winter Wheat Cultivation as a Strategy to Enhance Cereal Yield. International Journal of Molecular Sciences, 26(11), 5089. https://doi.org/10.3390/ijms26115089







