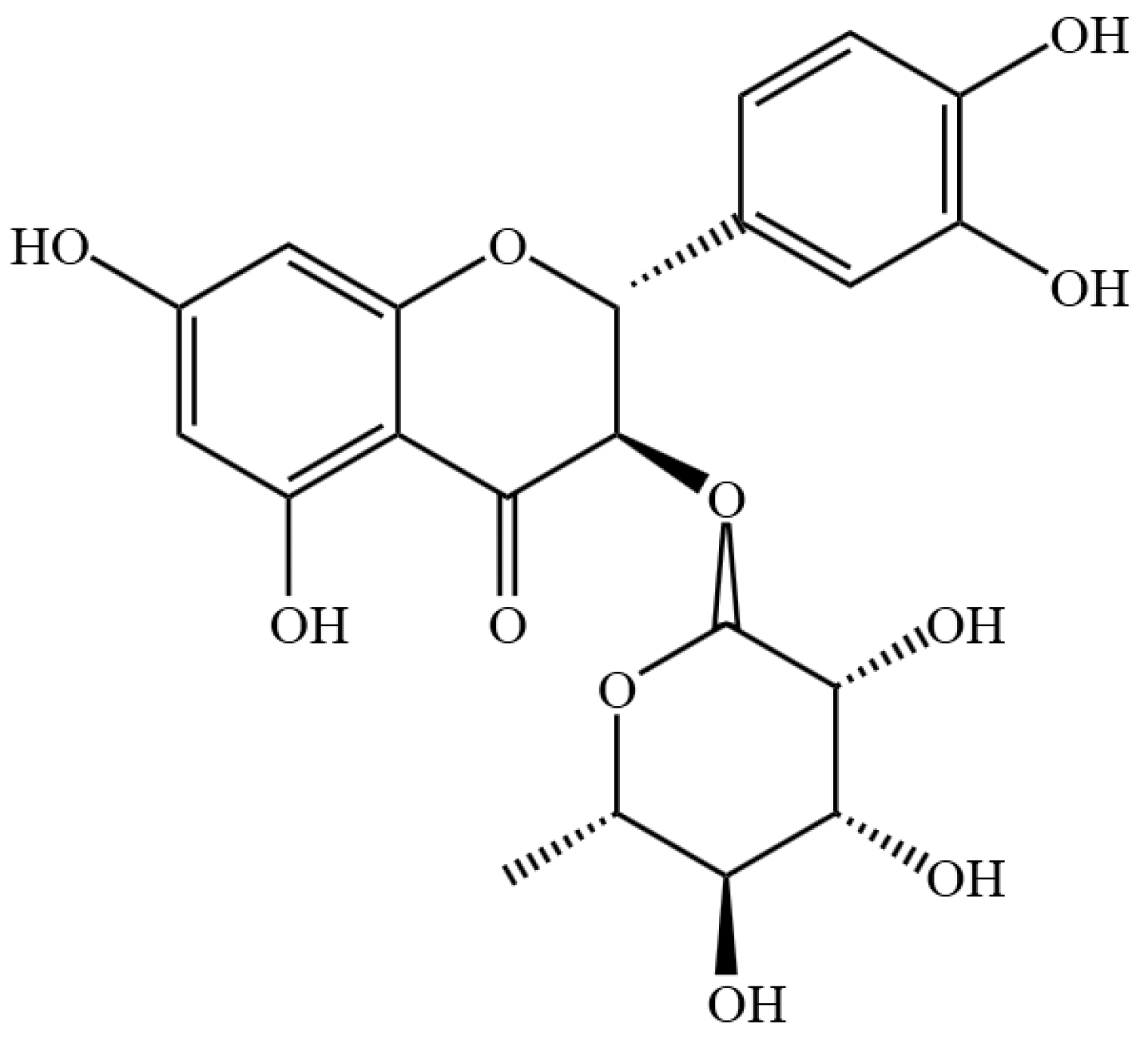
Astilbin Alleviates IL-17-Induced Hyperproliferation and Inflammation in HaCaT Cells via Inhibiting Ferroptosis Through the cGAS-STING Pathway
Abstract
1. Introduction
2. Results
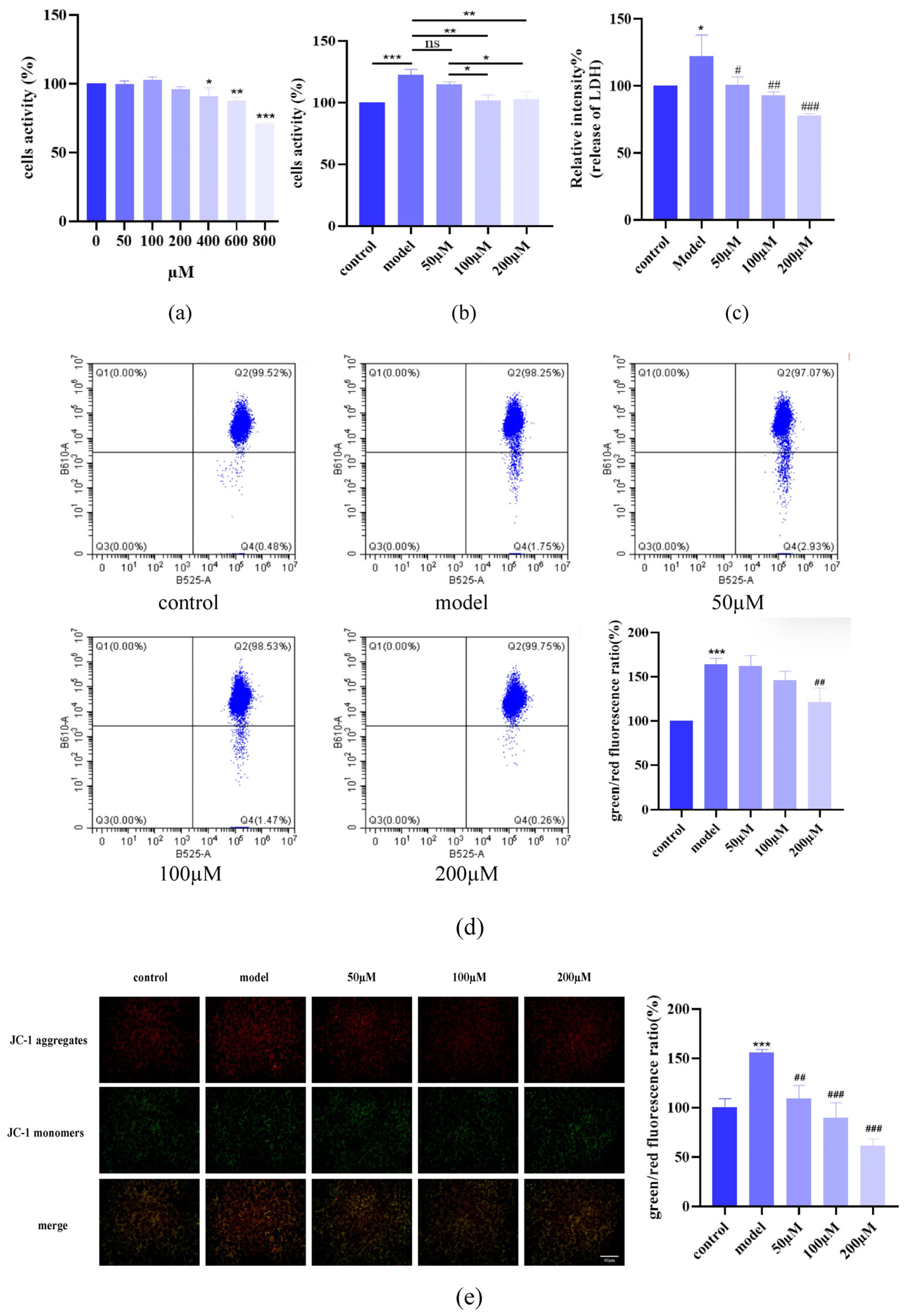
2.1. AST Attenuated IL-17-Induced Keratinocyte Damage
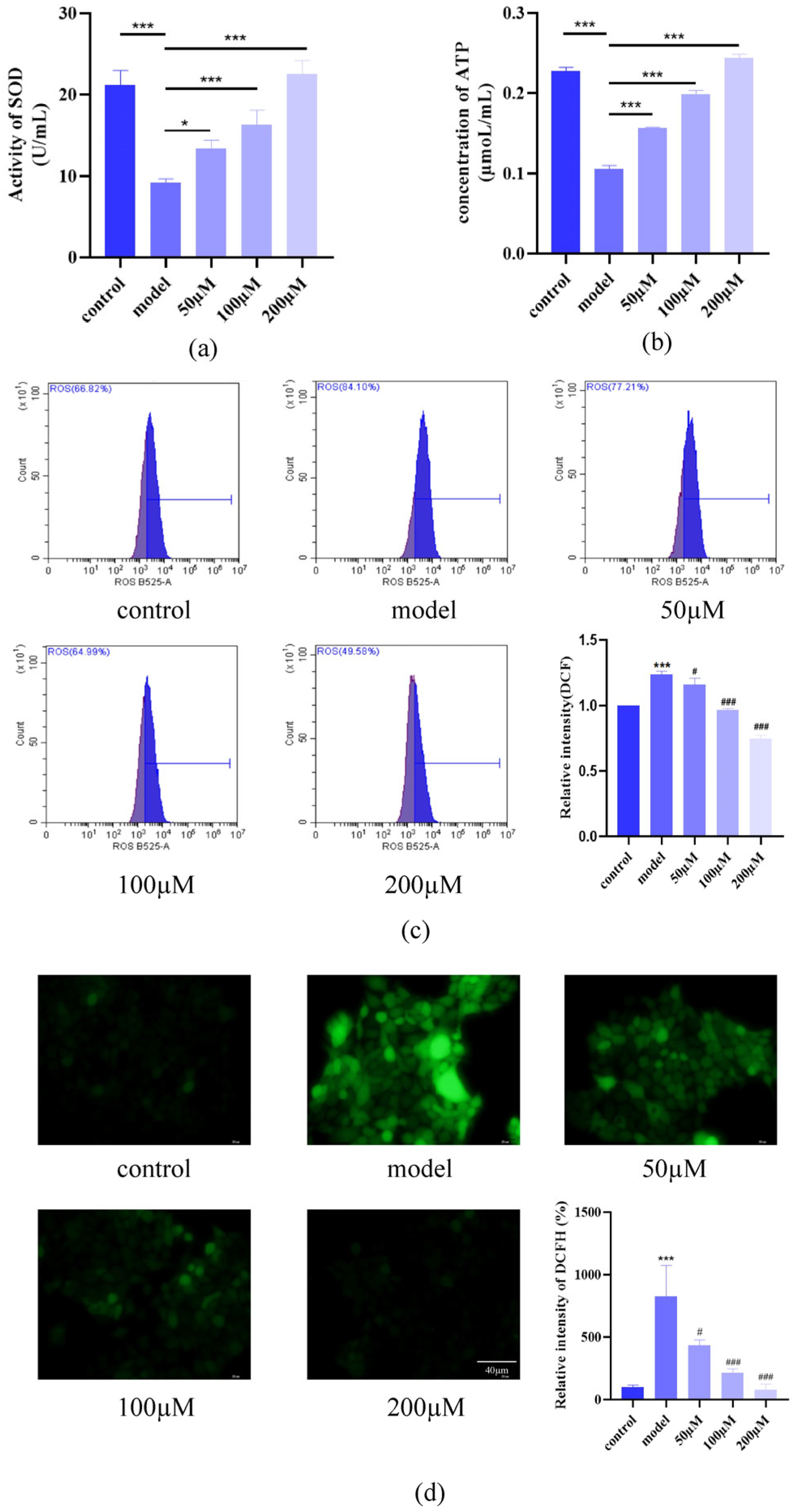
2.2. AST Reduced Oxidative Stress in IL-17-Stimulated Keratinocytes
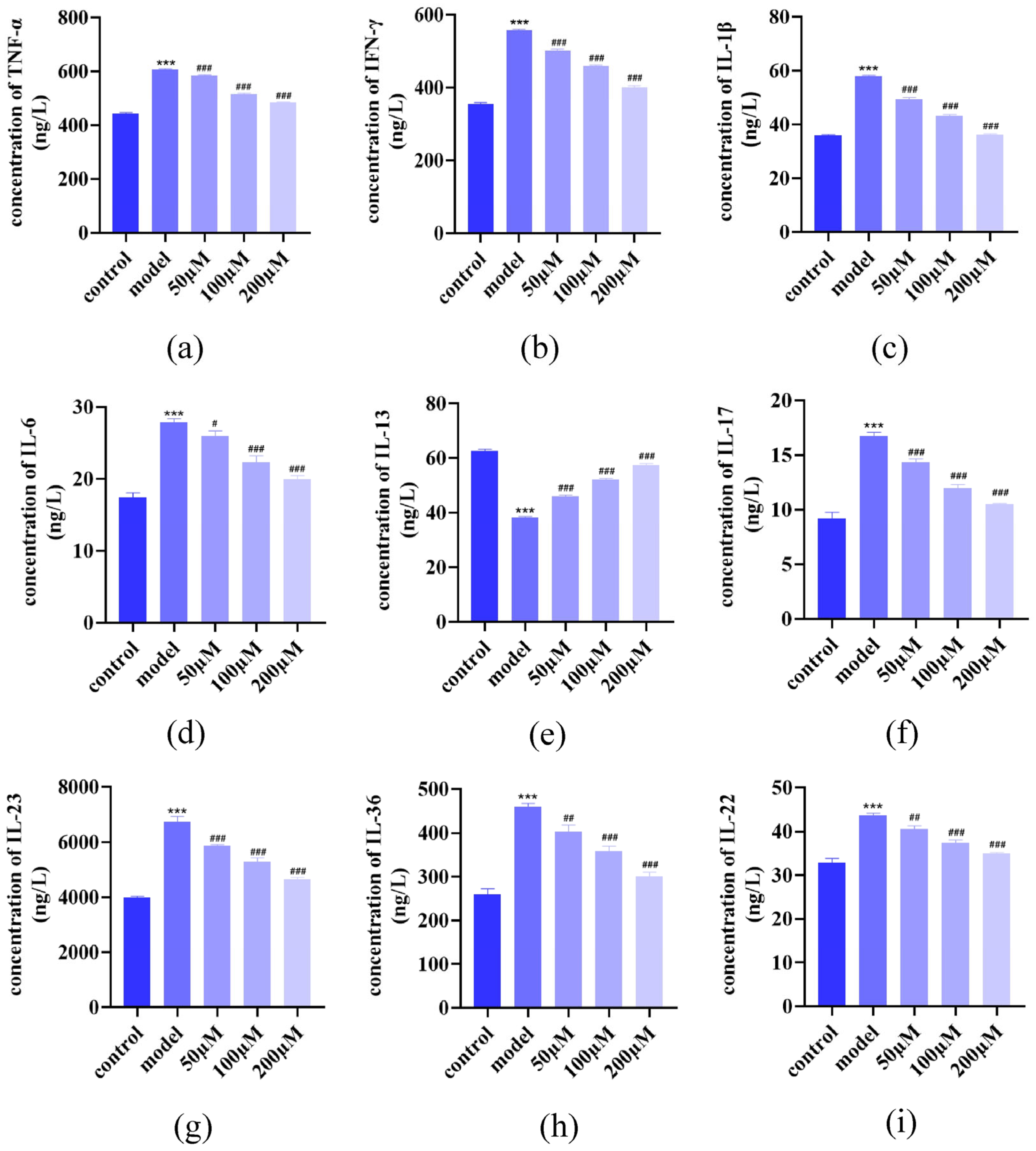
2.3. AST Modulated Inflammatory Cytokine Secretion
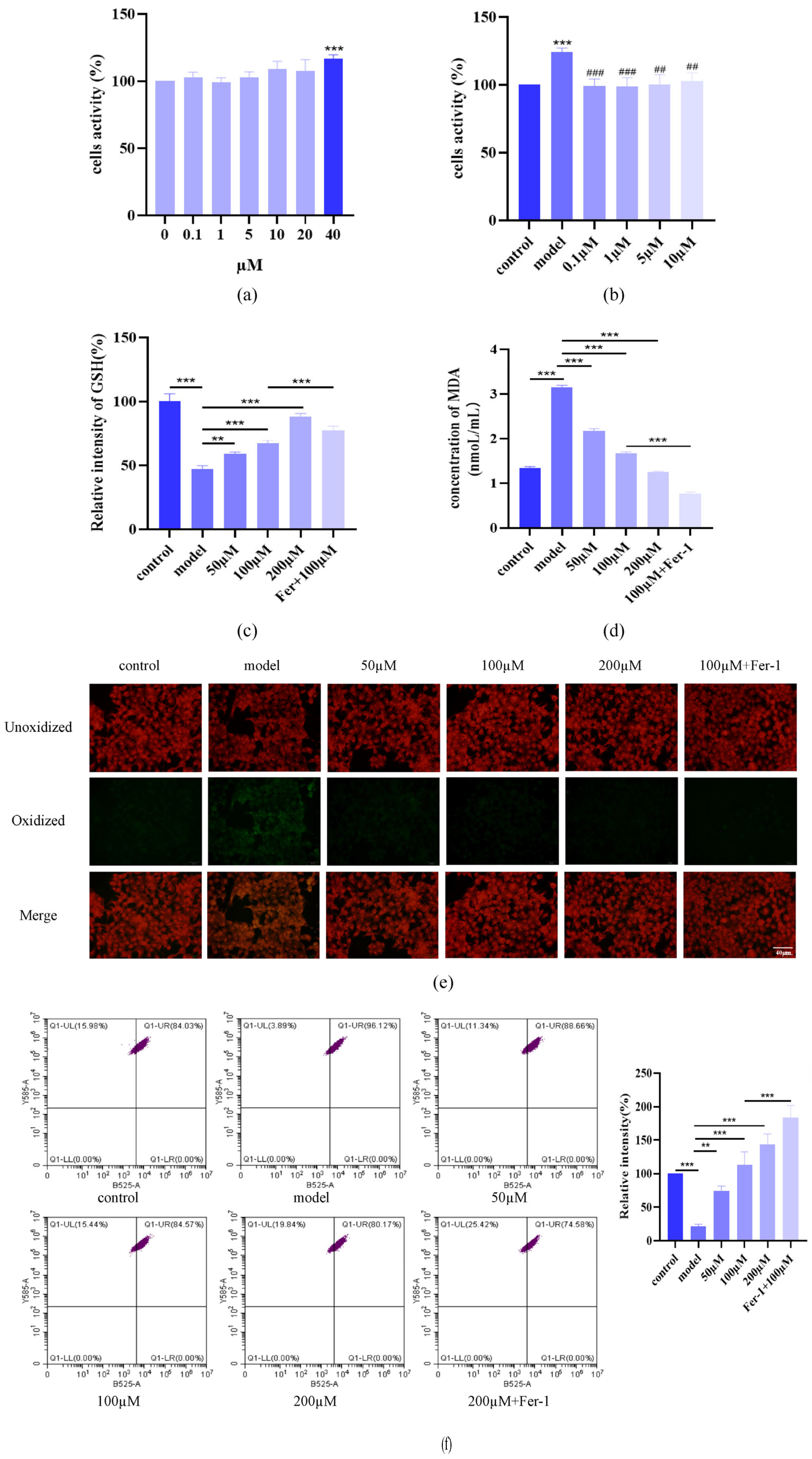
2.4. AST Restored GSH, MDA, and C11 Levels in IL-17-Damaged HacaT Cells
2.5. AST Restored Fe2+ Levels, Mitochondrial Structure, and Protein Levels in IL-17-Damaged HaCaT Cells
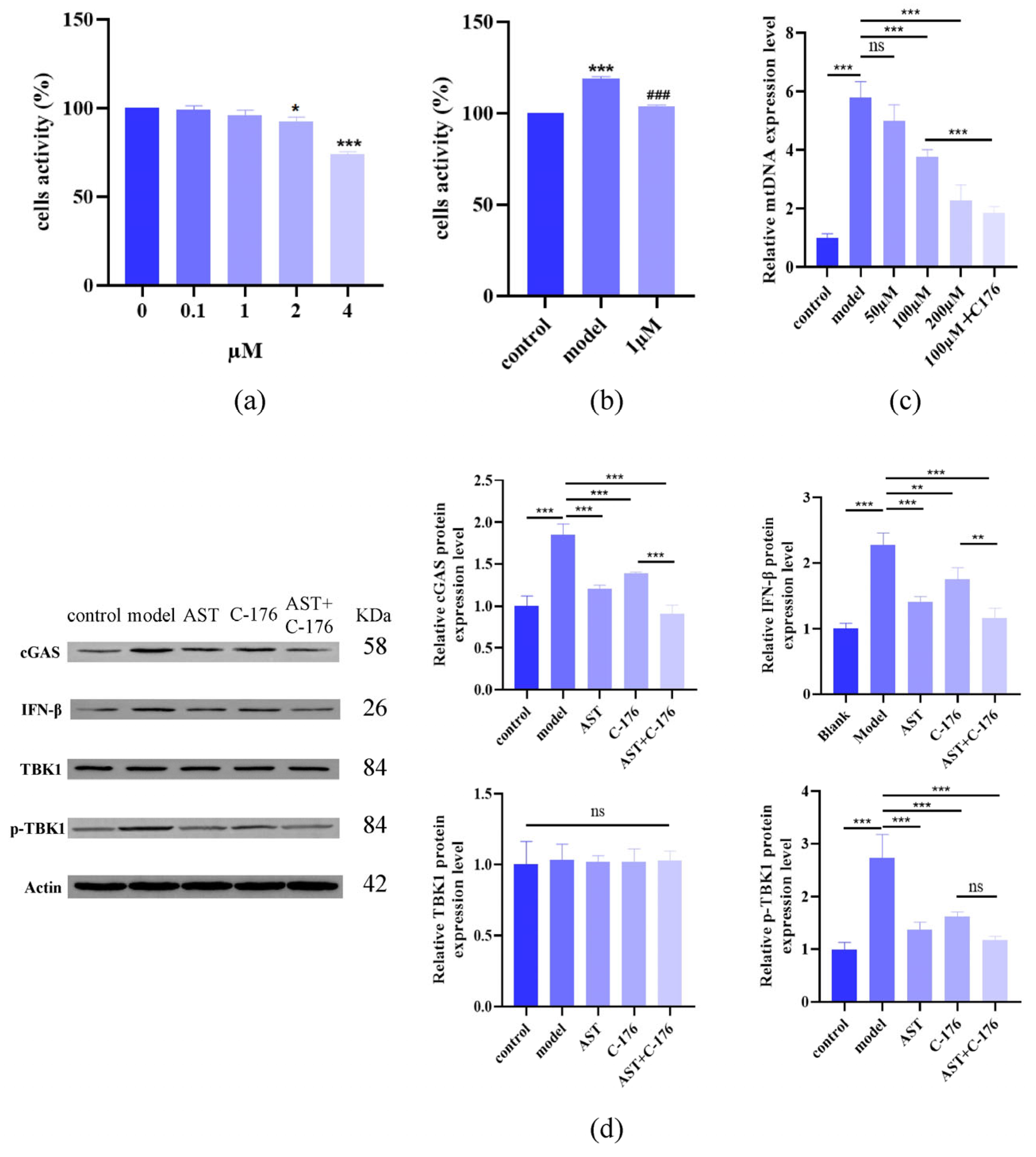
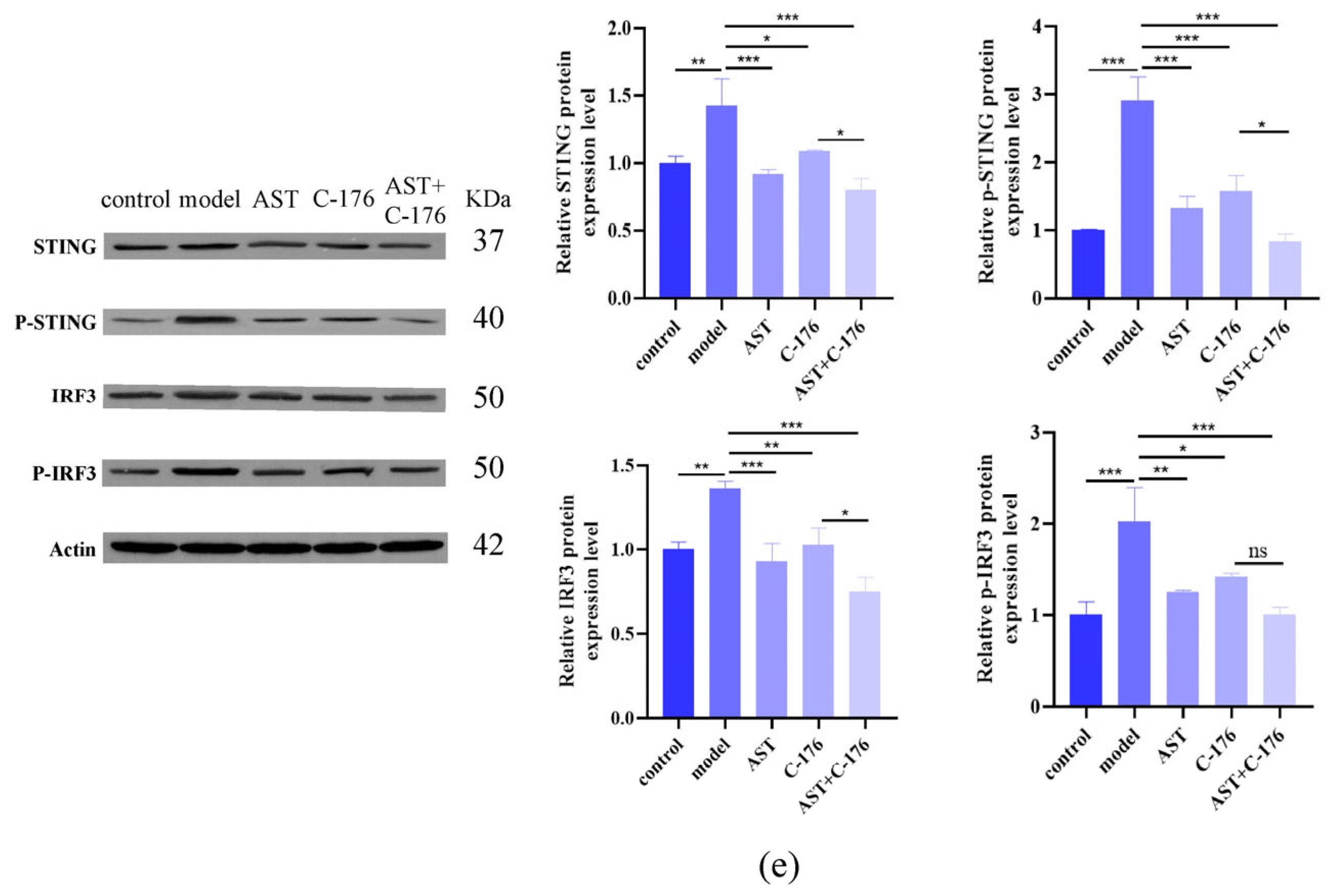
2.6. AST Inhibited cGAS-STING Pathway Activation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture, Treatments, and Inflammation Model Establishment
4.3. MTT Assay for Cell Viability
4.4. Lactate Dehydrogenase (LDH) Release Assay
4.5. Intracellular SOD and ATP Quantification
4.6. Detection of Intracellular ROS Levels
4.7. ELISA Detection of Cytokines
4.8. Measurement of Mitochondrial Membrane Potential
4.9. Observation of Mitochondrial Morphology
4.10. Quantification of Intracellular MDA and GSH Levels
4.11. Detection of Intracellular Lipid Peroxides (C11-BODIPY)
4.12. Detection of Intracellular Fe2+ Levels
4.13. Quantitative PCR (qPCR) Detection of mtDNA
4.14. Western Blot Analysis
4.15. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AST | Astilbin |
| cGAS-STING | GMP-AMP synthase-stimulator of interferon genes |
| MDA | malondialdehyde |
| GSH | glutathione |
| mtDNA | mitochondrial DNA |
| DAMPs | damage-associated molecular patterns |
| DMSO | Dimethyl sulfoxide |
| FBS | fetal bovine serum |
| LDH | Lactate Dehydrogenase |
| qPCR | quantitative PCR |
| TBK1 | TANK-binding kinase 1 |
| IRF3 | interferon regulatory factor 3 |
| Th17 | T helper 17 cell |
| IL-23 | interleukin-23 |
| IL-17 | interleukin-17 |
| ROS | reactive oxygen species |
| TNF-α | tumor necrosis factor-α |
| MTT | Methylthiazolyldiphenyl-tetrazolium bromide |
| SOD | Superoxide dismutase |
| ATP | Adenosine triphosphate |
| ELISA | Enzyme-linked immunosorbent assay |
| IFN | interferon |
| Fer-1 | ferrostatin-1 |
| TEM | transmission electron microscopy |
| CCL26 | C-C motif chemokine ligand 26 |
References
- Figueras, M.T.F.; Puig, L. Histopathological diagnosis of psoriasis and psoriasiform dermatitides. Diagn. Histopathol. 2025, 31, 87–97. [Google Scholar] [CrossRef]
- Xia, L.; Li, H.; Long, L.; Ruan, W.; Ma, J.; Xu, S.; Qiao, D. Research progress on the pathogenesis of psoriasis and its small molecule inhibitors. Arch. Pharmazie 2025, 358, e2400621. [Google Scholar] [CrossRef] [PubMed]
- Yongjun, Z.; Yanqiang, S.; Jingxia, L.; Xuefei, L.; Bin, Y.; Jiajian, Z. Immune Cell Infiltration Analysis Demonstrates Excessive Mast Cell Activation in Psoriasis. Front. Immunol. 2021, 12, 773280. [Google Scholar]
- Chen, J.; Zhu, Z.; Li, Q.; Lin, Y.; Dang, E.; Meng, H.; Sha, N.; Bai, H.; Wang, G.; An, S.; et al. Neutrophils Enhance Cutaneous Vascular Dilation and Permeability to Aggravate Psoriasis by Releasing Matrix Metallopeptidase 9. J. Investig. Dermatol. 2020, 141, 787–799. [Google Scholar] [CrossRef]
- Juanjuan, W.; Ling, Z.; Hui, H.; Jiao, L.; Xincheng, Z.; Jiajie, L.; Junqin, L.; Xuping, N.; Ruixia, H.; Kaiming, Z. IL-17A is involved in the hyperplasia of blood vessels in local lesions of psoriasis by inhibiting autophagy. J. Cosmet. Dermatol. 2023, 23, 326–338. [Google Scholar]
- Arrom, L.M.; Puig, L. Genetic and Epigenetic Mechanisms of Psoriasis. Genes 2023, 14, 1619. [Google Scholar] [CrossRef]
- Gao, Y.; Gong, B.; Chen, Z.; Song, J.; Xu, N.; Weng, Z. Damage-Associated Molecular Patterns, a Class of Potential Psoriasis Drug Targets. Int. J. Mol. Sci. 2024, 25, 771. [Google Scholar] [CrossRef]
- Emma, G.-Y.; Krueger, J.M.; Lebwohl, G.M. Systemic immune mechanisms in atopic dermatitis and psoriasis with implications for treatment. Exp. Dermatol. 2018, 27, 409–417. [Google Scholar]
- Karl-Peter, H.; Veit, H. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar]
- Dandan, G.; Qianqian, W.; Aifang, L.; Shuxuan, L.; Baiyan, W.; Yalan, L.; Juan, Y.; Tao, G.; Shuying, F. Liquiritin targeting Th17 cells differentiation and abnormal proliferation of keratinocytes alleviates psoriasis via NF-κB and AP-1 pathway. Phytother. Res. PTR 2023, 38, 174–186. [Google Scholar]
- Wenjie, H.; Xuyu, Z.; Qi, H.; Danlin, W.; Shifei, Y.; Cui, Z.; Qian, L.; Yulian, H.; Wenchun, X.; Kun, H. Protein Kinase CK2 Promotes Proliferation, Abnormal Differentiation, and Proinflammatory Cytokine Production of Keratinocytes via Regulation of STAT3 and Akt Pathways in Psoriasis. Am. J. Pathol. 2023, 193, 567–578. [Google Scholar]
- Wolf, M.; Shnyra, A.; Cobb, C.M. Autoimmune Mechanisms of Psoriasis: Pathogenic Role of the IL-23/IL-17 Axis. J. Autoimmune Disord. 2018, 4, 1–6. [Google Scholar] [CrossRef][Green Version]
- Marijana, V.; Marija, K.; Ines, B.; Vlatka, S.; Prpić, M.L. Current Concepts of Psoriasis Immunopathogenesis. Int. J. Mol. Sci. 2021, 22, 11574. [Google Scholar]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Chen, J.; Yin, C.; Zhang, Y.; Lai, X.; Liu, C.; Luo, Y.; Luo, J.; He, J.; Yu, B.; Wang, Q.; et al. EGCG Alleviates DSS-Induced Colitis by Inhibiting Ferroptosis Through the Activation of the Nrf2-GPX4 Pathway and Enhancing Iron Metabolism. Nutrients 2025, 17, 547. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, Y.; Huang, Y.; Zhang, L.; Zhang, C.; Gao, H.; Yan, Q. Inhibition of tubular epithelial cells ferroptosis alleviates renal interstitial fibrosis by reducing lipid hydroperoxides and TGF-β/Smad signaling. Cell Commun. Signal. 2025, 23, 81. [Google Scholar] [CrossRef]
- Udayakumar, K.; Suma, E.; Sung, M.J.; Chang, W.K. c-Abl Tyrosine Kinase Inhibition Attenuate Oxidative Stress-Induced Pancreatic β-Cell Dysfunction via Glutathione Antioxidant System. Transl. Res. J. Lab. Clin. Med. 2022, 249, 74–87. [Google Scholar]
- Ni, J.; Zhang, L.; Feng, G.; Bao, W.; Wang, Y.; Huang, Y.; Chen, T.; Chen, J.; Cao, X.; You, K.; et al. Vanillic acid restores homeostasis of intestinal epithelium in colitis through inhibiting CA9/STIM1-mediated ferroptosis. Pharmacol. Res. 2024, 202, 107128. [Google Scholar] [CrossRef]
- Minkyung, P.; Sujeong, P.; Yumin, C.; Lai, C.Y.; Jeong, K.M.; Jun, P.Y.; Wol, C.S.; Heedoo, L.; Jin, L.S. The mechanism underlying correlation of particulate matter-induced ferroptosis with inflammasome activation and iron accumulation in macrophages. Cell Death Discov. 2024, 10, 144. [Google Scholar]
- Wang, C.; Chu, Q.; Dong, W.; Wang, X.; Zhao, W.; Dai, X.; Liu, W.; Wang, B.; Liu, T.; Zhong, W.; et al. Microbial metabolite deoxycholic acid-mediated ferroptosis exacerbates high-fat diet-induced colonic inflammation. Mol. Metab. 2024, 84, 101944. [Google Scholar] [CrossRef]
- Zhou, F.; Li, D.; Liu, C.; Li, C.; Li, K.; Shi, L.; Zhou, F. m6A-activated BACH1 exacerbates ferroptosis by epigenetic suppression HSPB1 in severe acute pancreatitis. Drug Dev. Res. 2024, 85, e22256. [Google Scholar] [CrossRef] [PubMed]
- Chenuet, P.; Mellier, M.; Nacer, Y.M.; Culerier, E.; Marquant, Q.; Fauconnier, L.; Rouxel, N.; Ledru, A.; Rose, S.; Ryffel, B.; et al. Birch pollen allergen-induced dsDNA release activates cGAS-STING signaling and type 2 immune response in mice. iScience 2025, 28, 112324. [Google Scholar] [CrossRef] [PubMed]
- Weijie, O.; Shoubi, W.; Dan, Y.; Jieli, W.; Yunuo, Z.; Wei, L.; Jiaoyue, H.; Zuguo, L. The cGAS-STING pathway-dependent sensing of mitochondrial DNA mediates ocular surface inflammation. Signal Transduct. Target. Ther. 2023, 8, 371. [Google Scholar]
- Yu, X.; Li, B.; Yan, J.; Li, W.; Tian, H.; Wang, G.; Zhou, S.; Dai, Y. Cuproptotic nanoinducer-driven proteotoxic stress potentiates cancer immunotherapy by activating the mtDNA-cGAS-STING signaling. Biomaterials 2024, 307, 122512. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, R.; Du, W.; Wang, Q.; Pei, D. The role of cGAS-STING signaling pathway in ferroptosis. J. Adv. Res. 2024. [Google Scholar] [CrossRef]
- Sui, H.; Sun, Z.; Liu, C.; Xi, H. Ferritinophagy promotes microglia ferroptosis to aggravate neuroinflammation induced by cerebral ischemia-reperfusion injury via activation of the cGAS-STING signaling pathway. Neurochem. Int. 2024, 183, 105920. [Google Scholar] [CrossRef]
- Di, T.-T.; Ruan, Z.-T.; Zhao, J.-X.; Wang, Y.; Liu, X.; Wang, Y.; Li, P. Astilbin inhibits Th17 cell differentiation and ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice via Jak3/Stat3 signaling pathway. Int. Immunopharmacol. 2016, 32, 32–38. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Xue, L.; Yang, Y.; Zhi, F. Astilbin protects against cerebral ischaemia/reperfusion injury by inhibiting cellular apoptosis and ROS-NLRP3 inflammasome axis activation. Int. Immunopharmacol. 2020, 84, 106571. [Google Scholar] [CrossRef]
- Shizhen, D.; Guotao, L.; Biying, W.; Jie, X.; Chunxia, H.; Zhijie, L.; Yanbing, D.; Weiming, X.; Weijuan, G. Astilbin Activates the Reactive Oxidative Species/PPARγ Pathway to Suppress Effector CD4+ T Cell Activities via Direct Binding with Cytochrome P450 1B1. Front. Pharmacol. 2022, 13, 848957. [Google Scholar]
- Shen, Z.; Deng, X.; Zheng, S.; Jiang, X.; Ding, R.; Wu, Y.; Luo, B.; Guo, J.; Jie, W. Astilbin inhibits high glucose-induced vascular smooth muscle cells proliferation and migration. FASEB J. 2013, 27, lb483. [Google Scholar] [CrossRef]
- Xiaoqi, S.; Hong, Z.; Yingyu, Z.; Qi, Y.; Shujie, Z. Caspase-dependent mitochondrial apoptotic pathway is involved in astilbin-mediated cytotoxicity in breast carcinoma cells. Oncol. Rep. 2018, 40, 2278–2286. [Google Scholar]
- Wang, W.; Yuhai; Wang, H.; Chasuna; Bagenna. Astilbin reduces ROS accumulation and VEGF expression through Nrf2 in psoriasis-like skin disease. Biol. Res. 2019, 52, 49. [Google Scholar] [CrossRef] [PubMed]
- Yixing, W.; Xuping, Z.; Jin, Z.; Shilei, X.; Xiaodan, K.; Yuan, C. Preparation of a nanoemulsified drug delivery system of astilbin using edible formulation components: In vivo evaluation of oxaliplatin-induced cirrhotic liver injury. Pharmacogn. Mag. 2022, 18, 321–327. [Google Scholar]
- Wang, S.-w.; Xu, Y.; Weng, Y.-y.; Fan, X.-y.; Bai, Y.-f.; Zheng, X.-y.; Lou, L.-j.; Zhang, F. Astilbin ameliorates cisplatin-induced nephrotoxicity through reducing oxidative stress and inflammation. Food Chem. Toxicol. 2018, 114, 227–236. [Google Scholar] [CrossRef]
- Zhang, Q.-F.; Zhang, Z.-R.; Cheung, H.-Y. Antioxidant activity of Rhizoma Smilacis Glabrae extracts and its key constituent-astilbin. Food Chem. 2008, 115, 297–303. [Google Scholar] [CrossRef]
- Meng, Q.F.; Zhang, Z.; Wang, Y.J.; Chen, W.; Li, F.F.; Yue, L.T.; Zhang, C.J.; Li, H.; Zhang, M.; Wang, C.C.; et al. Astilbin ameliorates experimental autoimmune myasthenia gravis by decreased Th17 cytokines and up-regulated T regulatory cells. J. Neuroimmunol. 2016, 298, 138–145. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Liu, Z.Y.; Cao, Z.Q.; Shi, Y.J.; Yang, N.; Cao, G.S.; Zhang, C.M.; Sun, R.; Zhang, C.H. Topical astilbin ameliorates imiquimod-induced psoriasis-like skin lesions in SKH-1 mice via suppression dendritic cell-Th17 inflammation axis. J. Cell. Mol. Med. 2022, 26, 1281–1292. [Google Scholar] [CrossRef]
- Jamil, H.A.; Karim, N.A. Unraveling Mitochondrial Reactive Oxygen Species Involvement in Psoriasis: The Promise of Antioxidant Therapies. Antioxidants 2024, 13, 1222. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Yu, Y.; Cai, W.; Xu, X.; Xie, K.; Lin, X.; Lin, L.; Lu, J.; Chen, P. Activation of the STING-IRF3 pathway involved in psoriasis with diabetes mellitus. J. Cell. Mol. Med. 2022, 26, 2139–2151. [Google Scholar]
- Sugumaran, D.; Yong, A.C.H.; Stanslas, J. Advances in psoriasis research: From pathogenesis to therapeutics. Life Sci. 2024, 355, 122991. [Google Scholar] [CrossRef]
- Stober, C.B.; Ellis, L.; Goodall, J.C.; Veldhoen, M.; Gaston, J.S.H. Metabolic Stress expands Polyfunctional, Proinflammatory Th17 cells in Psoriatic Arthritis, where there is IL-23-independent IL-17 production. Arthritis Rheumatol. 2024. [Google Scholar] [CrossRef]
- Zhu, W.; Qi, C.; Shi, C.; Yang, H.; Shi, F.; Ding, Y. Indoleamine-2,3-dioxygenase (IDO) Mediates the Suppression of T Cells by IFN-γ Primed Mesenchymal Stromal Cells in the Treatment of Psoriasis-Like Inflammation. Front. Biosci.-Landmark 2024, 29, 411. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Yuan, R.; Wang, S.; Yang, S.; Zhang, Q. Effects of silencing NLRP3 gene on proliferation of psoriasis-like HaCaT cells and expressions of cytokines. Cell. Mol. Biol. 2024, 70, 85–89. [Google Scholar] [PubMed]
- LiLi, Y.; ChunYu, C. Rehmannioside A Inhibits TRAF6/MAPK Pathway and Improves Psoriasis by Interfering with the Interaction of HaCaT Cells with IL-17A. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2585–2596. [Google Scholar]
- Zhao, S.S.; Hyrich, K.; Yiu, Z.; Barton, A.; Bowes, J. Genetically proxied IL-13 inhibition is associated with risk of psoriatic disease: Mendelian randomization study. Arthritis Rheumatol. 2024, 76, 1602–1610. [Google Scholar] [CrossRef]
- Hijnen, D.; Knol, E.F.; Gent, Y.Y.; Giovannone, B.; Beijn, S.J.P.; Kupper, T.S.; Bruijnzeel-Koomen, C.A.F.M.; Clark, R.A. CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-γ, IL-13, IL-17, and IL-22. J. Investig. Dermatol. 2013, 133, 973–979. [Google Scholar] [CrossRef]
- Takeshima, H.; Horie, M.; Mikami, Y.; Makita, K.; Miyashita, N.; Matsuzaki, H.; Noguchi, S.; Urushiyama, H.; Hiraishi, Y.; Mitani, A.; et al. CISH is a negative regulator of IL-13-induced CCL26 production in lung fibroblasts. Allergol. Int. 2018, 68, 101–109. [Google Scholar] [CrossRef]
- Khalili, M.; Kooshesh, A.; Meymandi, S.S.; Mehrolhasani, N.; Amiri, R.; Rukerd, M.R.Z.; Aflatoonian, M. Exploring the Significance of Eosinophil Infiltration in Diagnosis of Psoriasis: A Cross-sectional Analysis. Iran. J. Pathol. 2025, 20, 18–23. [Google Scholar] [CrossRef]
- Larose, M.-C.; Chakir, J.; Archambault, A.-S.; Joubert, P.; Provost, V.; Laviolette, M.; Flamand, N. Correlation between CCL26 production by human bronchial epithelial cells and airway eosinophils: Involvement in patients with severe eosinophilic asthma. J. Allergy Clin. Immunol. 2015, 136, 904–913. [Google Scholar] [CrossRef]
- Alzahrani, K.R.; Cardona, E.G.; Gandhi, V.; Palikhe, N.S.; Laratta, C.; Julien, O.; Vliagoftis, H. German cockroach extract prevents IL-13-induced CCL26 expression in airway epithelial cells through IL-13 degradation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2024, 38, e23531. [Google Scholar] [CrossRef]
- Kamran, A.; Liming, W.; YunMi, Q.; Menghua, L. Case report: Clinical and histopathological characteristics of psoriasiform erythema and de novo IL-17A cytokines expression on lesioned skin in atopic dermatitis children treated with dupilumab. Front. Med. 2022, 9, 932766. [Google Scholar]
- Schaap, J.M.J.; Bruins, F.M.; He, X.; Orro, K.; Peppelman, M.; van Erp, P.E.J.; de Jong, E.M.G.J.; Koenen, H.J.P.M.; van den Bogaard, E.H.; Seyger, M.M.B. Skin Surface Protein Detection by Transdermal Analysis Patches in Pediatric Psoriasis. Ski. Pharmacol. Physiol. 2021, 34, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Haneya, A.A.; Warda, K.; Azza, A.; Soheir, M.; Hoda, K.; Dina, S.; Mai, S. Frequency of genotypes and allelic polymorphisms of Vitamin D receptor in egyptian psoriatic patients and their association with disease severity, immune modulation of IL-22 levels and the response to topical calcipotriol treatment: A case control study. Indian J. Dermatol. 2022, 67, 37–44. [Google Scholar]
- Helena, I.; Lluís, P. Exploring the Role of IL-36 Cytokines as a New Target in Psoriatic Disease. Int. J. Mol. Sci. 2021, 22, 4344. [Google Scholar]
- Sharma, R.K.; Sharma, M.R.; Mahendra, A.; Singh, S.; Sood, S.; Upadhyay, S.K.; Sharma, A.K. Hyposalivation in patients with psoriasis: Association with severity, inflammatory, and anti-inflammatory cytokine biomarkers of the disease. Biotechnol. Appl. Biochem. 2024, 71, 1170–1180. [Google Scholar] [CrossRef]
- Liu, Q.; Han, Z.; Li, T.; Meng, J.; Zhu, C.; Wang, J.; Wang, J.; Zhang, Z.; Wu, H. Microglial HO-1 aggravates neuronal ferroptosis via regulating iron metabolism and inflammation in the early stage after intracerebral hemorrhage. Int. Immunopharmacol. 2024, 147, 113942. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, B.; Zhang, Y.; Fan, W.; Xue, Q.; Chen, X.; Wang, J.; Qi, X. Mitochondria-mediated ferroptosis contributes to the inflammatory responses of bovine viral diarrhea virus (BVDV) in vitro. J. Virol. 2024, 98, e0188023. [Google Scholar] [CrossRef]
- Wang, X.; Pan, L.; Niu, D.; Zhou, J.; Shen, M.; Zeng, Z.; Gong, W.; Yang, E.; Tang, Y.; Cheng, G.; et al. Jingfang Granules alleviates the lipid peroxidation induced ferroptosis in rheumatoid arthritis rats by regulating gut microbiota and metabolism of short chain fatty acids. J. Ethnopharmacol. 2025, 339, 119160. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, Y.; Xu, B.; Wen, H.; Chen, K.; Lin, J.; Wang, Y.; Xu, J.; Wang, H.; Shi, F.; et al. TDP43 augments astrocyte inflammatory activity through mtDNA-cGAS-STING axis in NMOSD. J. Neuroinflamm. 2025, 22, 14. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, W.; Wang, J.; Zhao, D.; Tian, H.; Qiu, Y.; Ji, S.; Wang, S.; Fu, Q.; Zhang, F.; et al. Oxidative stress induces ferroptosis in tendon stem cells by regulating mitophagy through cGAS-STING pathway. Int. Immunopharmacol. 2024, 138, 112652. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, X.; Gao, X.; Chen, H.; Geng, L. Shikonin suppresses IL-17-induced VEGF expression via blockage of JAK2/STAT3 pathway. Int. Immunopharmacol. 2014, 19, 327–333. [Google Scholar] [CrossRef]
- Jie, Z.; Junbin, Z.; Qingxia, L.; Lamei, C.; Yali, S. IL-17 promotes proliferation, inflammation and inhibits apoptosis of HaCaT cells via interacting with the TRAF3 interacting protein 2. Exp. Ther. Med. 2021, 21, 49. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Zhang, H.; Chang, A.; Peng, H.; Li, S.; Zhang, K.; Wang, W.; Yin, X.; Qu, C.; Dong, X.; et al. Astilbin Alleviates IL-17-Induced Hyperproliferation and Inflammation in HaCaT Cells via Inhibiting Ferroptosis Through the cGAS-STING Pathway. Int. J. Mol. Sci. 2025, 26, 5075. https://doi.org/10.3390/ijms26115075
Xu X, Zhang H, Chang A, Peng H, Li S, Zhang K, Wang W, Yin X, Qu C, Dong X, et al. Astilbin Alleviates IL-17-Induced Hyperproliferation and Inflammation in HaCaT Cells via Inhibiting Ferroptosis Through the cGAS-STING Pathway. International Journal of Molecular Sciences. 2025; 26(11):5075. https://doi.org/10.3390/ijms26115075
Chicago/Turabian StyleXu, Xiaohan, Huizhong Zhang, Aqian Chang, Hulinyue Peng, Shiman Li, Ke Zhang, Wenqi Wang, Xingbin Yin, Changhai Qu, Xiaoxv Dong, and et al. 2025. "Astilbin Alleviates IL-17-Induced Hyperproliferation and Inflammation in HaCaT Cells via Inhibiting Ferroptosis Through the cGAS-STING Pathway" International Journal of Molecular Sciences 26, no. 11: 5075. https://doi.org/10.3390/ijms26115075
APA StyleXu, X., Zhang, H., Chang, A., Peng, H., Li, S., Zhang, K., Wang, W., Yin, X., Qu, C., Dong, X., & Ni, J. (2025). Astilbin Alleviates IL-17-Induced Hyperproliferation and Inflammation in HaCaT Cells via Inhibiting Ferroptosis Through the cGAS-STING Pathway. International Journal of Molecular Sciences, 26(11), 5075. https://doi.org/10.3390/ijms26115075






