Morphological Alterations of Conal Ridges and Differential Expression of AP2α in the Offspring Hearts of Experimental Diabetic Rats
Abstract
1. Introduction
2. Results
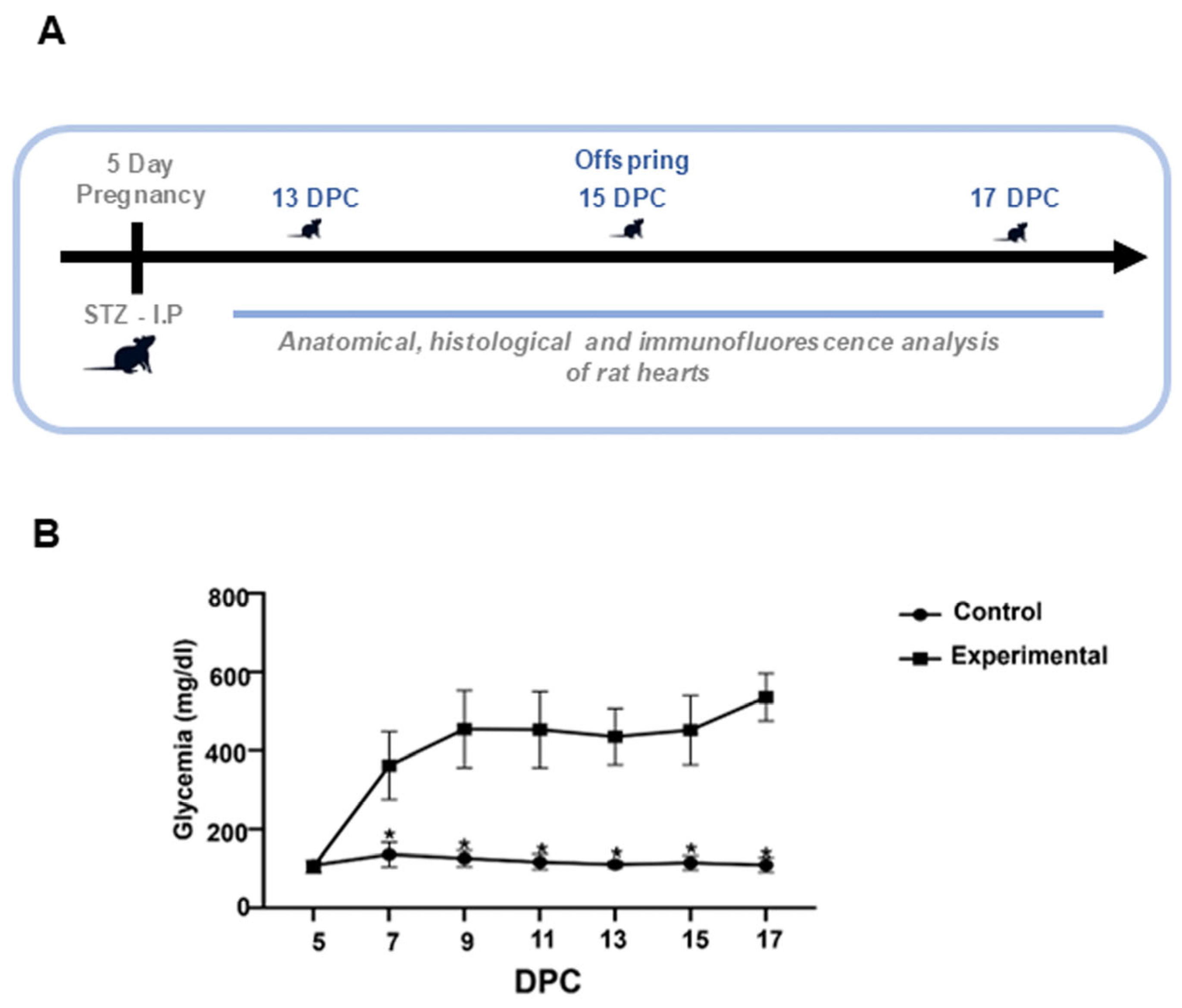
2.1. Glucose Levels of Rats with STZ-Induced Diabetes
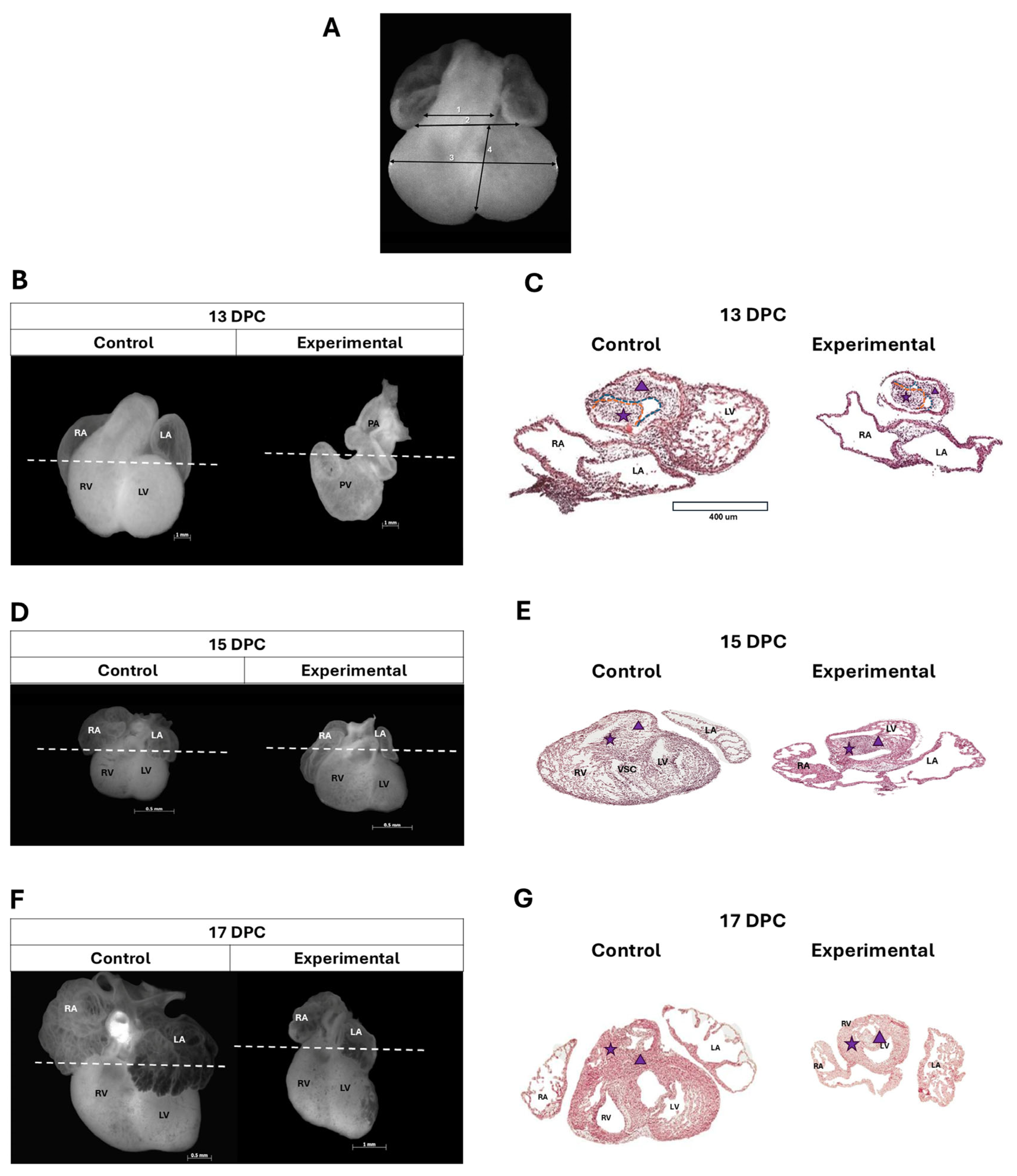
2.2. Anatomical and Histological Changes of Conal Ridges in the Offspring of Diabetic Rats
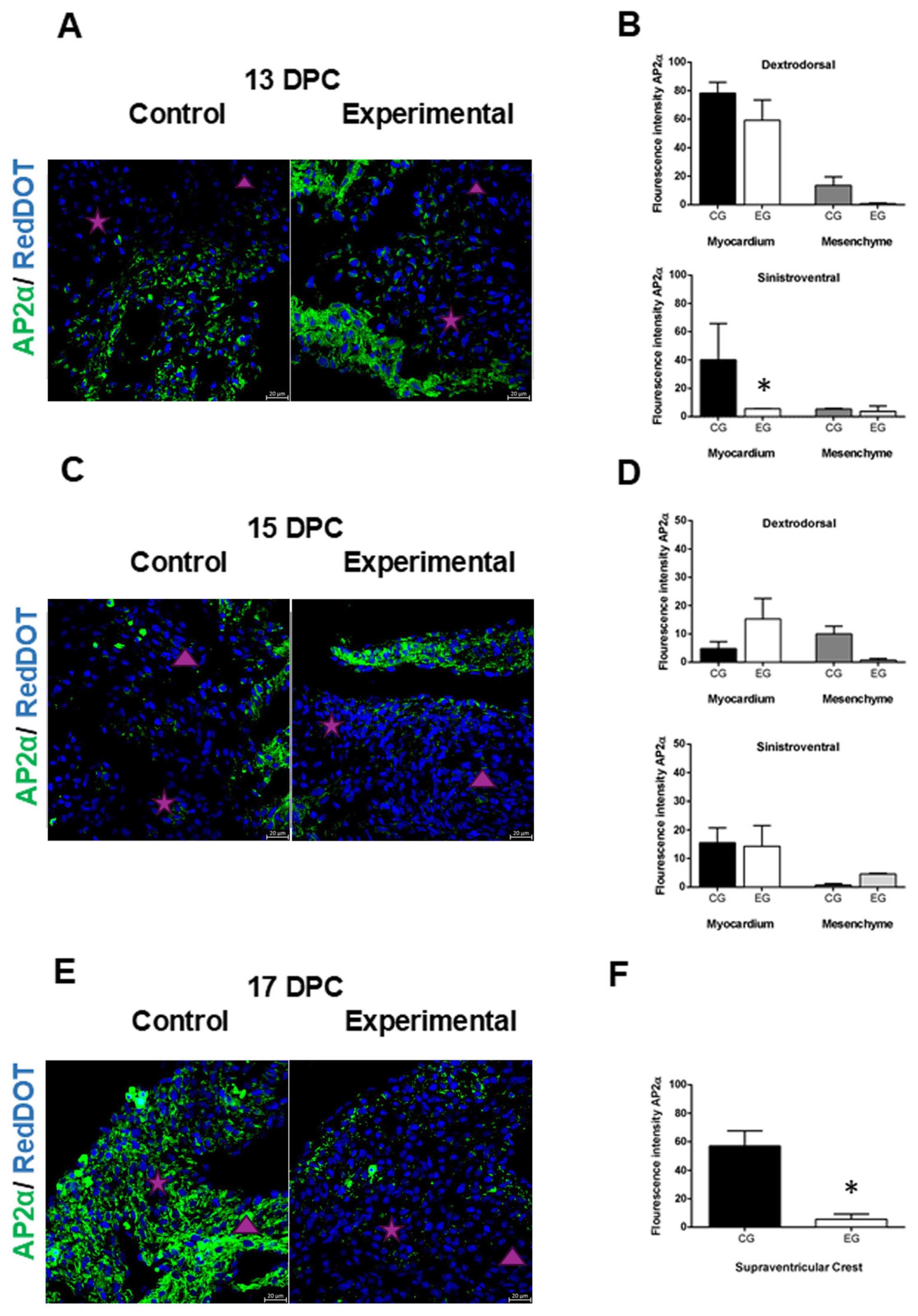
2.3. Alterations in the Expression of AP2α in the Heart of the Offspring of Diabetic Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Induction of Hyperglycemia in Rats
4.3. Obtaining Embryos and Fetuses of Rats with STZ-Induced Diabetes
4.4. Anatomical and Histological Analysis
4.5. Immunofluorescence of AP2α
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AV | Atrioventricular canal |
| AP2α | Transcription factor activating protein |
| DPC | Days post coitum |
| DDR | Dextrodorsal ridge |
| cNCCs | Cardiac neural crest cells |
| H/E | Hematoxylin/eosin |
| IP | Infundibular primordium |
| LV | Left ventricle |
| LA | Left atrium |
| OFTs | Outflow tracts |
| NCCs | Neural crest cells |
| STZ | Streptozotocin |
| SVR | Sinistroventral ridge |
| RA | Right atrium |
| RV | Right ventricle |
| PV | Pulmonary vein |
| PA | Pulmonary artery |
| VSC | Ventrosuperior cushion |
References
- De la Cruz, M.V. Embryological development of the outlet of each ventricle. In Living Morphogenesis of the Heart; Cruz, M.V., Markwald, R.R., Eds.; Springer: Boston, MA, USA, 1998; pp. 131–156. [Google Scholar]
- Rana, M.S.; Horsten, N.C.; Tesink-Taekema, S.; Lamers, W.H.; Moorman, A.F.; van den Hoff, M.J. Trabeculated right ventricular free wall in the chicken heart forms by ventricularization of the myocardium initially forming the outflow tract. Circ. Res. 2007, 100, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, R.; Gomez-Quiroz, L.E.; Gonzalez-Marquez, H.; Villavicencio-Guzman, L.; Salazar-Garcia, M.; Sanchez-Gomez, C. The proximal segment of the embryonic outflow (conus) does not participate in aortic vestibule development. PLoS ONE 2018, 13, e0209930. [Google Scholar] [CrossRef] [PubMed]
- Ya, J.; van den Hoff, M.J.; de Boer, P.A.; Tesink-Taekema, S.; Franco, D.; Moorman, A.F.; Lamers, W.H. Normal development of the outflow tract in the rat. Circ. Res. 1998, 82, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Martínez, M.; García-Peláez, I.; Sánchez-Gómez, C. Desarrollo del Sistema Cardiovascular. In Embriología humana y biología del desarrollo; Médica Panamericana: Mexico City, Mexico, 2021; pp. 339–395. [Google Scholar]
- Gómez, C.S.; Pliego, L.P.; Ramos, A.C.; Rosas, M.Á.M.; García, M.S.; Romero, H.L.G.; Jiménez, M.A.G. Histological study of the proximal and distal segments of the embryonic outflow tract and great arteries. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2005, 283, 202–211. [Google Scholar] [CrossRef]
- Villavicencio-Guzman, L.; Sanchez-Gomez, C.; Jaime-Cruz, R.; Ramirez-Fuentes, T.C.; Patino-Morales, C.C.; Salazar-Garcia, M. Human Heart Morphogenesis: A New Vision Based on In Vivo Labeling and Cell Tracking. Life 2023, 13, 165. [Google Scholar] [CrossRef]
- Hutson, M.R.; Kirby, M.L. Model systems for the study of heart development and disease. Cardiac neural crest and conotruncal malformations. Semin. Cell Dev. Biol. 2007, 18, 101–110. [Google Scholar] [CrossRef]
- Brewer, S.; Jiang, X.; Donaldson, S.; Williams, T.; Sucov, H.M. Requirement for AP-2alpha in cardiac outflow tract morphogenesis. Mech. Dev. 2002, 110, 139–149. [Google Scholar] [CrossRef]
- Aviña, F.J.A.; Hernández, A.D.A. Embriopatía Congénita en los Niños de Madres Diabéticas. Rev. Mex. De Pediatría 2014, 81, 79–83. [Google Scholar]
- Linder, J.; Johnson, J. Fetal Conotruncal Defects. In Pediatric Cardiology; Abdulla, R.-I., Anderson, R.H., Backer, C.L., Berger, S., Blom, N.A., Holzer, R.J., Robinson, J.D., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- García, M.S.; Maldonado, E.R.; Monsalve, M.C.R.; Guzmán, L.V.; López, A.R.; Sánchez-Gómez, C. Importance of maternal diabetes on the chronological deregulation of the intrauterine development: An experimental study in rat. J. Diabetes Res. 2015, 2015, 1–10. [Google Scholar] [CrossRef][Green Version]
- Salazar-Garcia, M.; Villavicencio-Guzman, L.; Revilla-Monsalve, C.; Patino-Morales, C.C.; Jaime-Cruz, R.; Ramirez-Fuentes, T.C.; Corona, J.C. Harmful Effects on the Hippocampal Morpho-Histology and on Learning and Memory in the Offspring of Rats with Streptozotocin-Induced Diabetes. Int. J. Mol. Sci. 2024, 25, 11335. [Google Scholar] [CrossRef]
- Roest, P.A.; van Iperen, L.; Vis, S.; Wisse, L.J.; Poelmann, R.E.; Steegers-Theunissen, R.P.; Molin, D.G.; Eriksson, U.J.; Gittenberger-De Groot, A.C. Exposure of neural crest cells to elevated glucose leads to congenital heart defects, an effect that can be prevented by N-acetylcysteine. Birth Defects Res. A Clin. Mol. Teratol. 2007, 79, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Helle, E.I.T.; Biegley, P.; Knowles, J.W.; Leader, J.B.; Pendergrass, S.; Yang, W.; Reaven, G.R.; Shaw, G.M.; Ritchie, M.; Priest, J.R. First Trimester Plasma Glucose Values in Women without Diabetes are Associated with Risk for Congenital Heart Disease in Offspring. J. Pediatr. 2018, 195, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Gu, S.; Karunamuni, G.H.; Jenkins, M.W.; Watanabe, M.; Rollins, A.M. Cardiac neural crest ablation results in early endocardial cushion and hemodynamic flow abnormalities. Am. J. Physiol. Heart Circ. 2016, 311, H1150–H1159. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.; Garg, V. Maternal hyperglycemia and fetal cardiac development: Clinical impact and underlying mechanisms. Birth Defects Res. 2018, 110, 1504–1516. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, B.; Sun, Y.; Du, Y.; Santillan, M.K.; Santillan, D.A.; Snetselaar, L.G.; Bao, W. Association of Maternal Prepregnancy Diabetes and Gestational Diabetes Mellitus With Congenital Anomalies of the Newborn. Diabetes Care 2020, 43, 2983–2990. [Google Scholar] [CrossRef]
- Turunen, R.; Pulakka, A.; Metsala, J.; Vahlberg, T.; Ojala, T.; Gissler, M.; Kajantie, E.; Helle, E. Maternal Diabetes and Overweight and Congenital Heart Defects in Offspring. JAMA Netw. Open 2024, 7, e2350579. [Google Scholar] [CrossRef]
- Hufnagel, A.; Dearden, L.; Fernandez-Twinn, D.S.; Ozanne, S.E. Programming of cardiometabolic health: The role of maternal and fetal hyperinsulinaemia. J. Endocrinol. 2022, 253, R47–R63. [Google Scholar] [CrossRef]
- Chen, A.; Tan, B.; Du, R.; Chong, Y.S.; Zhang, C.; Koh, A.S.; Li, L.J. Gestational diabetes mellitus and development of intergenerational overall and subtypes of cardiovascular diseases: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2024, 23, 320. [Google Scholar] [CrossRef]
- Krusteva, M.; Malinova, M. The anthropometric indices, morbidity and mortality of newborn infants with diabetic fetopathy. Akush. Ginekol. (Sofiia) 2000, 39, 14–17. [Google Scholar]
- Jaime-Cruz, R.; Sanchez-Gomez, C.; Villavicencio-Guzman, L.; Lazzarini-Lechuga, R.; Patino-Morales, C.C.; Garcia-Lorenzana, M.; Ramirez-Fuentes, T.C.; Salazar-Garcia, M. Embryonic Hyperglycemia Disrupts Myocardial Growth, Morphological Development, and Cellular Organization: An In Vivo Experimental Study. Life 2023, 13, 768. [Google Scholar] [CrossRef]
- Wang, G.; Shen, W.B.; Chen, A.W.; Reece, E.A.; Yang, P. Diabetes and Early Development: Epigenetics, Biological Stress, and Aging. Am. J. Perinatol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Reece, E.A.; Wang, F.; Gabbay-Benziv, R. Decoding the oxidative stress hypothesis in diabetic embryopathy through proapoptotic kinase signaling. Am. J. Obstet. Gynecol. 2015, 212, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Minami, I.; Braas, D.; Pappoe, H.; Wu, X.; Sagadevan, A.; Vergnes, L.; Fu, K.; Morselli, M.; Dunham, C.; et al. Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis. eLife 2017, 6, e29330. [Google Scholar] [CrossRef] [PubMed]
- Kodo, K.; Shibata, S.; Miyagawa-Tomita, S.; Ong, S.; Takahashi, H.; Kume, T.; Okano, H.; Matsuoka, R.; Yamagishi, H. Regulation of Sema3c and the Interaction between Cardiac Neural Crest and Second Heart Field during Outflow Tract Development. Sci. Rep. 2017, 7, 6771. [Google Scholar] [CrossRef]
- Tang, W.; Martik, M.L.; Li, Y.; Bronner, M.E. Cardiac neural crest contributes to cardiomyocytes in amniotes and heart regeneration in zebrafish. eLife 2019, 8, e47929. [Google Scholar] [CrossRef]
- Suzuki, N.; Svensson, K.; Eriksson, U.J. High glucose concentration inhibits migration of rat cranial neural crest cells in vitro. Diabetologia 1996, 39, 401–411. [Google Scholar] [CrossRef]
- Sato, M.; Yost, H.J. Cardiac neural crest contributes to cardiomyogenesis in zebrafish. Dev. Biol. 2003, 257, 127–139. [Google Scholar] [CrossRef]
- NOM-062-Z00-1999; Norma Oficial Mexicana, Especificaciones Tecnicas para la Produccion, Cuidado y Uso de los Animales de Laboratorio. Publicado en el Diario Oficial: Mexico City, Mexico, 2001.
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [CrossRef]
| Rat Heart Lengths (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | Experimental | |||||||
| DPC | OFT | AV | MD | mD | OFT | AV | MD | mD |
| 13 | 0.5 ± 0.27 | 0.64 ± 0.3 | 1.36 ± 1.36 | 0.71 ± 0.34 | 0.32 ± 0.06 * | 0.38 ± 0.1 * | 0.81 ± 0.04 * | 0.5 ± 0.04 |
| 15 | 0.58 ± 0.1 | 0.96 ± 0.12 | 1.68 ± 0.03 | 0.94 ± 0.07 | 0.56 ± 0.03 * | 1.1 ± 0.09 * | 1.66 ± 0.05 * | 0.9 ± 0.08 * |
| 17 | 0.54 ± 0.09 | 1.54 ± 0.17 | 2.34 ± 0.26 | 1.51 ± 0.21 | 0.54 ± 0.16 * | 1.06 ± 0.32 * | 1.83 ± 0.39 * | 1.5 ± 0.19 * |
| Cardiopathy | Incidence |
|---|---|
| Side-by-side artery | 9 |
| Double outflow of the RV | 7 |
| AV canal | 7 |
| PA stenosis | 5 |
| RV dilation | 5 |
| Normal | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Fuentes, T.C.; Jaime-Cruz, R.; Patiño-Morales, C.C.; Villavicencio-Guzmán, L.; Corona, J.C.; Revilla-Monsalve, M.C.; Jarillo-Luna, R.A.; Salazar-García, M. Morphological Alterations of Conal Ridges and Differential Expression of AP2α in the Offspring Hearts of Experimental Diabetic Rats. Int. J. Mol. Sci. 2025, 26, 5061. https://doi.org/10.3390/ijms26115061
Ramírez-Fuentes TC, Jaime-Cruz R, Patiño-Morales CC, Villavicencio-Guzmán L, Corona JC, Revilla-Monsalve MC, Jarillo-Luna RA, Salazar-García M. Morphological Alterations of Conal Ridges and Differential Expression of AP2α in the Offspring Hearts of Experimental Diabetic Rats. International Journal of Molecular Sciences. 2025; 26(11):5061. https://doi.org/10.3390/ijms26115061
Chicago/Turabian StyleRamírez-Fuentes, Tania Cristina, Ricardo Jaime-Cruz, Carlos César Patiño-Morales, Laura Villavicencio-Guzmán, Juan Carlos Corona, María Cristina Revilla-Monsalve, Rosa Adriana Jarillo-Luna, and Marcela Salazar-García. 2025. "Morphological Alterations of Conal Ridges and Differential Expression of AP2α in the Offspring Hearts of Experimental Diabetic Rats" International Journal of Molecular Sciences 26, no. 11: 5061. https://doi.org/10.3390/ijms26115061
APA StyleRamírez-Fuentes, T. C., Jaime-Cruz, R., Patiño-Morales, C. C., Villavicencio-Guzmán, L., Corona, J. C., Revilla-Monsalve, M. C., Jarillo-Luna, R. A., & Salazar-García, M. (2025). Morphological Alterations of Conal Ridges and Differential Expression of AP2α in the Offspring Hearts of Experimental Diabetic Rats. International Journal of Molecular Sciences, 26(11), 5061. https://doi.org/10.3390/ijms26115061






