Ergolide Regulates Microglial Activation and Inflammatory-Mediated Dysfunction: A Role for the Cysteinyl Leukotriene Pathway
Abstract
1. Introduction
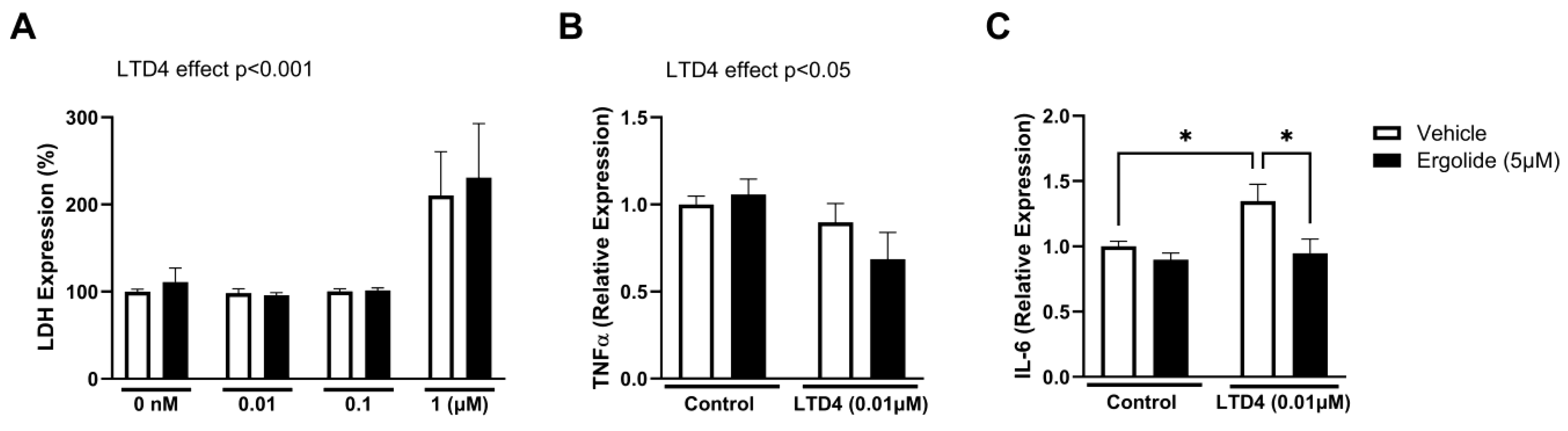
2. Results
3. Discussion
4. Materials and Methods
4.1. Maintenance and Treatment of BV2, N9, and SH-SY5Y Cells
4.2. NFκB Luciferase Assay
4.3. Cell Viability and Cytotoxicity Analysis
4.4. Reactive Oxygen Species (ROS) Analysis
4.5. Analysis of Cytokine and Nitrite Concentration
4.6. Western Immunoblot Analysis
4.7. Analysis of Gene Expression
4.8. Zebrafish Maintenance
4.9. LPS Stimulation in Zebrafish Larvae
4.10. Survival and Touch Startle Response Assay
4.11. Assessment of PTZ-Induced Hyperactivity
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartman, S.; Coppotelli, G.; Ross, J.M. Mitochondrial Dysfunction: A Key Player in Brain Aging and Diseases. Curr. Issues Mol. Biol. 2024, 46, 1987–2026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, H.S.; Wang, X.; Dumont, A.S.; Liu, Q. Cellular senescence, DNA damage, and neuroinflammation in the aging brain. Trends Neurosci. 2024, 47, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Golde, T.E. Disease-Modifying Therapies for Alzheimer’s Disease: More Questions than Answers. Neurotherapeutics 2022, 19, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.X.; Tan, E.K.; Zhou, Z.D. Passive immunotherapy for Alzheimer’s disease: Challenges & future directions. J. Transl. Med. 2024, 22, 430. [Google Scholar] [CrossRef]
- Hanke, M.L.; Kielian, T. Toll-like receptors in health and disease in the brain: Mechanisms and therapeutic potential. Clin. Sci. 2011, 121, 367–387. [Google Scholar] [CrossRef]
- Dabi, Y.T.; Ajagbe, A.O.; Degechisa, S.T. Toll-like receptors in pathogenesis of neurodegenerative diseases and their therapeutic potential. Immun. Inflamm. Dis. 2023, 11, e839. [Google Scholar] [CrossRef]
- Howe, A.M.; Burke, S.; O’Reilly, M.E.; McGillicuddy, F.C.; Costello, D.A. Palmitic Acid and Oleic Acid Differently Modulate TLR2-Mediated Inflammatory Responses in Microglia and Macrophages. Mol. Neurobiol. 2022, 59, 2348–2362. [Google Scholar] [CrossRef]
- Howe, A.M.; Cosgrave, A.; O’Murchu, M.; Britchfield, C.; Mulvagh, A.; Fernandez-Perez, I.; Dykstra, M.; Jones, A.C.; Costello, D.A. Characterising lipoteichoic acid as an in vitro model of acute neuroinflammation. Int. Immunopharmacol. 2020, 85, 106619. [Google Scholar] [CrossRef]
- Costello, D.A.; Lyons, A.; Denieffe, S.; Browne, T.C.; Cox, F.F.; Lynch, M.A. Long term potentiation is impaired in membrane glycoprotein CD200-deficient mice: A role for Toll-like receptor activation. J. Biol. Chem. 2011, 286, 34722–34732. [Google Scholar] [CrossRef]
- Costello, D.A.; Lynch, M.A. Toll-like receptor 3 activation modulates hippocampal network excitability, via glial production of interferon-β. Hippocampus 2013, 23, 696–707. [Google Scholar] [CrossRef]
- Costello, D.A.; Carney, D.G.; Lynch, M.A. α-TLR2 antibody attenuates the Aβ-mediated inflammatory response in microglia through enhanced expression of SIGIRR. Brain Behav. Immun. 2015, 46, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Hao, W.; Wolf, L.; Kiliaan, A.J.; Penke, B.; Rube, C.E.; Walter, J.; Heneka, M.T.; Hartmann, T.; et al. TLR2 is a primary receptor for Alzheimer’s amyloid β peptide to trigger neuroinflammatory activation. J. Immunol. 2012, 188, 1098–1107. [Google Scholar] [CrossRef]
- Richard, K.L.; Filali, M.; Prefontaine, P.; Rivest, S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid β 1-42 and delay the cognitive decline in a mouse model of Alzheimer’s disease. J. Neurosci. 2008, 28, 5784–5793. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Ho, D.H.; Suk, J.E.; You, S.; Michael, S.; Kang, J.; Joong Lee, S.; Masliah, E.; Hwang, D.; Lee, H.J.; et al. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 2013, 4, 1562. [Google Scholar] [CrossRef] [PubMed]
- Soraci, L.; Gambuzza, M.E.; Biscetti, L.; Lagana, P.; Lo Russo, C.; Buda, A.; Barresi, G.; Corsonello, A.; Lattanzio, F.; Lorello, G.; et al. Toll-like receptors and NLRP3 inflammasome-dependent pathways in Parkinson’s disease: Mechanisms and therapeutic implications. J. Neurol. 2023, 270, 1346–1360. [Google Scholar] [CrossRef]
- Heneka, M.T. Inflammasome activation and innate immunity in Alzheimer’s disease. Brain Pathol. 2017, 27, 220–222. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Raghani, N.; Chorawala, M.; Bhattacharya, S.; Prajapati, B.G.; Elossaily, G.M.; Chaiyasut, C. NF-κB Pathway and Its Inhibitors: A Promising Frontier in the Management of Alzheimer’s Disease. Biomedicines 2023, 11, 2587. [Google Scholar] [CrossRef]
- Valera, E.; Mante, M.; Anderson, S.; Rockenstein, E.; Masliah, E. Lenalidomide reduces microglial activation and behavioral deficits in a transgenic model of Parkinson’s disease. J. Neuroinflamm. 2015, 12, 93. [Google Scholar] [CrossRef]
- Kong, F.; Jiang, X.; Wang, R.; Zhai, S.; Zhang, Y.; Wang, D. Forsythoside B attenuates memory impairment and neuroinflammation via inhibition on NF-κB signaling in Alzheimer’s disease. J. Neuroinflamm. 2020, 17, 305. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Y.; Yin, S.; Wan, F.; Hu, J.; Kou, L.; Sun, Y.; Wu, J.; Zhou, Q.; Huang, J.; et al. Targeting Microglial α-Synuclein/TLRs/NF-κB/NLRP3 Inflammasome Axis in Parkinson’s Disease. Front. Immunol. 2021, 12, 719807. [Google Scholar] [CrossRef]
- Kacimi, R.; Giffard, R.G.; Yenari, M.A. Endotoxin-activated microglia injure brain derived endothelial cells via NF-κB, JAK-STAT and JNK stress kinase pathways. J. Inflamm. 2011, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory Effects of Curcumin in Microglial Cells. Front. Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Floyd, R.A.; Zheng, N.Y.; Nael, R.; Robinson, K.A.; Nguyen, X.; Pye, Q.N.; Stewart, C.A.; Geddes, J.; Markesbery, W.R.; et al. p38 kinase is activated in the Alzheimer’s disease brain. J. Neurochem. 1999, 72, 2053–2058. [Google Scholar] [CrossRef]
- Kheiri, G.; Dolatshahi, M.; Rahmani, F.; Rezaei, N. Role of p38/MAPKs in Alzheimer’s disease: Implications for amyloid β toxicity targeted therapy. Rev. Neurosci. 2018, 30, 9–30. [Google Scholar] [CrossRef] [PubMed]
- Ashabi, G.; Ramin, M.; Azizi, P.; Taslimi, Z.; Alamdary, S.Z.; Haghparast, A.; Ansari, N.; Motamedi, F.; Khodagholi, F. ERK and p38 inhibitors attenuate memory deficits and increase CREB phosphorylation and PGC-1α levels in Aβ-injected rats. Behav. Brain Res. 2012, 232, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Karpenko, M.N.; Vasilishina, A.A.; Gromova, E.A.; Muruzheva, Z.M.; Miliukhina, I.V.; Bernadotte, A. Interleukin-1β, interleukin-1 receptor antagonist, interleukin-6, interleukin-10, and tumor necrosis factor-α levels in CSF and serum in relation to the clinical diversity of Parkinson’s disease. Cell Immunol. 2018, 327, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Lerche, S.; Zimmermann, M.; Wurster, I.; Roeben, B.; Fries, F.L.; Deuschle, C.; Waniek, K.; Lachmann, I.; Gasser, T.; Jakobi, M.; et al. CSF and Serum Levels of Inflammatory Markers in PD: Sparse Correlation, Sex Differences and Association With Neurodegenerative Biomarkers. Front. Neurol. 2022, 13, 834580. [Google Scholar] [CrossRef]
- Llano, D.A.; Li, J.; Waring, J.F.; Ellis, T.; Devanarayan, V.; Witte, D.G.; Lenz, R.A. Cerebrospinal fluid cytokine dynamics differ between Alzheimer disease patients and elderly controls. Alzheimer Dis. Assoc. Disord. 2012, 26, 322–328. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef] [PubMed]
- Schuitemaker, A.; Dik, M.G.; Veerhuis, R.; Scheltens, P.; Schoonenboom, N.S.; Hack, C.E.; Blankenstein, M.A.; Jonker, C. Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol. Aging 2009, 30, 1885–1889. [Google Scholar] [CrossRef]
- Duro, M.V.; Ebright, B.; Yassine, H.N. Lipids and brain inflammation in APOE4-associated dementia. Curr. Opin. Lipidol. 2022, 33, 16–24. [Google Scholar] [CrossRef]
- Guan, P.P.; Liang, Y.Y.; Cao, L.L.; Yu, X.; Wang, P. Cyclooxygenase-2 Induced the β-Amyloid Protein Deposition and Neuronal Apoptosis Via Upregulating the Synthesis of Prostaglandin E2 and 15-Deoxy-Δ12,14-prostaglandin J2. Neurotherapeutics 2019, 16, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Ikeda-Matsuo, Y.; Miyata, H.; Mizoguchi, T.; Ohama, E.; Naito, Y.; Uematsu, S.; Akira, S.; Sasaki, Y.; Tanabe, M. Microsomal prostaglandin E synthase-1 is a critical factor in dopaminergic neurodegeneration in Parkinson’s disease. Neurobiol. Dis. 2019, 124, 81–92. [Google Scholar] [CrossRef]
- Currais, A.; Quehenberger, O.; Armando, A.M.; Daugherty, D.; Maher, P.; Schubert, D. Amyloid proteotoxicity initiates an inflammatory response blocked by cannabinoids. NPJ Aging Mech. Dis. 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W. Leukotrienes and inflammation. Am. J. Respir. Crit. Care Med. 1998, 157, S210–S213. [Google Scholar] [CrossRef]
- Gelosa, P.; Colazzo, F.; Tremoli, E.; Sironi, L.; Castiglioni, L. Cysteinyl Leukotrienes as Potential Pharmacological Targets for Cerebral Diseases. Mediat. Inflamm. 2017, 2017, 3454212. [Google Scholar] [CrossRef]
- Yu, S.Y.; Zhang, X.Y.; Wang, X.R.; Xu, D.M.; Chen, L.; Zhang, L.H.; Fang, S.H.; Lu, Y.B.; Zhang, W.P.; Wei, E.Q. Cysteinyl leukotriene receptor 1 mediates LTD4-induced activation of mouse microglial cells in vitro. Acta Pharmacol. Sin. 2014, 35, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Wang, X.R.; Xu, D.M.; Yu, S.Y.; Shi, Q.J.; Zhang, L.H.; Chen, L.; Fang, S.H.; Lu, Y.B.; Zhang, W.P.; et al. HAMI 3379, a CysLT2 receptor antagonist, attenuates ischemia-like neuronal injury by inhibiting microglial activation. J. Pharmacol. Exp. Ther. 2013, 346, 328–341. [Google Scholar] [CrossRef]
- Zhao, R.; Ying, M.; Gu, S.; Yin, W.; Li, Y.; Yuan, H.; Fang, S.; Li, M. Cysteinyl Leukotriene Receptor 2 is Involved in Inflammation and Neuronal Damage by Mediating Microglia M1/M2 Polarization through NF-κB Pathway. Neuroscience 2019, 422, 99–118. [Google Scholar] [CrossRef]
- Neu, I.; Mallinger, J.; Wildfeuer, A.; Mehlber, L. Leukotrienes in the cerebrospinal fluid of multiple sclerosis patients. Acta Neurol. Scand. 1992, 86, 586–587. [Google Scholar] [CrossRef]
- Whitney, L.W.; Ludwin, S.K.; McFarland, H.F.; Biddison, W.E. Microarray analysis of gene expression in multiple sclerosis and EAE identifies 5-lipoxygenase as a component of inflammatory lesions. J. Neuroimmunol. 2001, 121, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Du, C.; Lv, J.; Wei, W.; Cui, Y.; Xie, X. Antiasthmatic drugs targeting the cysteinyl leukotriene receptor 1 alleviate central nervous system inflammatory cell infiltration and pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol. 2011, 187, 2336–2345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.T.; Lin, J.R.; Wu, F.; Ghosh, A.; Tang, S.S.; Hu, M.; Long, Y.; Sun, H.B.; Hong, H. Montelukast ameliorates streptozotocin-induced cognitive impairment and neurotoxicity in mice. Neurotoxicology 2016, 57, 214–222. [Google Scholar] [CrossRef]
- Ghosh, A.; Chen, F.; Wu, F.; Tang, S.S.; Hu, M.; Long, Y.; Sun, H.B.; Kong, L.Y.; Hong, H. CysLT1R downregulation reverses ntracerebroventricular streptozotocin-induced memory impairment via modulation of neuroinflammation in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 73, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Cevik, B.; Solmaz, V.; Aksoy, D.; Erbas, O. Montelukast inhibits pentylenetetrazol-induced seizures in rats. Med. Sci. Monit. 2015, 21, 869–874. [Google Scholar] [CrossRef]
- Tang, S.S.; Hong, H.; Chen, L.; Mei, Z.L.; Ji, M.J.; Xiang, G.Q.; Li, N.; Ji, H. Involvement of cysteinyl leukotriene receptor 1 in Aβ1-42-induced neurotoxicity in vitro and in vivo. Neurobiol. Aging 2014, 35, 590–599. [Google Scholar] [CrossRef]
- Tang, S.S.; Wang, X.Y.; Hong, H.; Long, Y.; Li, Y.Q.; Xiang, G.Q.; Jiang, L.Y.; Zhang, H.T.; Liu, L.P.; Miao, M.X.; et al. Leukotriene D4 induces cognitive impairment through enhancement of CysLT1 R-mediated amyloid-β generation in mice. Neuropharmacology 2013, 65, 182–192. [Google Scholar] [CrossRef]
- Fang, S.C.; Wang, J.J.; Chen, F.; Tang, S.S.; Mu, R.H.; Yuan, D.H.; Zhao, J.J.; Hong, H.; Long, Y. Hippocampal CysLT1R overexpression or activation accelerates memory deficits, synaptic dysfunction, and amyloidogenesis in young APP/PS1 transgenic mice. Ann. Transl. Med. 2021, 9, 1531. [Google Scholar] [CrossRef]
- Samanta, S.; Chakraborty, S.; Bagchi, D. Pathogenesis of Neurodegenerative Diseases and the Protective Role of Natural Bioactive Components. J. Am. Nutr. Assoc. 2024, 43, 20–32. [Google Scholar] [CrossRef]
- An, J.; Chen, B.; Kang, X.; Zhang, R.; Guo, Y.; Zhao, J.; Yang, H. Neuroprotective effects of natural compounds on LPS-induced inflammatory responses in microglia. Am. J. Transl. Res. 2020, 12, 2353–2378. [Google Scholar]
- Park, H.Y.; Park, C.; Hwang, H.J.; Kim, B.W.; Kim, G.Y.; Kim, C.M.; Kim, N.D.; Choi, Y.H. 7,8-Dihydroxyflavone attenuates the release of pro-inflammatory mediators and cytokines in lipopolysaccharide-stimulated BV2 microglial cells through the suppression of the NF-κB and MAPK signaling pathways. Int. J. Mol. Med. 2014, 33, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chang, Y.Y.; Huang, S.; Xiao, L.H.; Zhou, W.; Zhang, L.Y.; Li, C.; Zhou, R.P.; Tang, J.; Lin, L.; et al. Aromatic-Turmerone Attenuates LPS-Induced Neuroinflammation and Consequent Memory Impairment by Targeting TLR4-Dependent Signaling Pathway. Mol. Nutr. Food Res. 2018, 62, 201700281. [Google Scholar] [CrossRef]
- Hori, Y.; Tsutsumi, R.; Nasu, K.; Boateng, A.; Ashikari, Y.; Sugiura, M.; Nakajima, M.; Kurauchi, Y.; Hisatsune, A.; Katsuki, H.; et al. Aromatic-Turmerone Analogs Protect Dopaminergic Neurons in Midbrain Slice Cultures through Their Neuroprotective Activities. Cells 2021, 10, 1090. [Google Scholar] [CrossRef]
- Mazzantini, C.; El Bourji, Z.; Parisio, C.; Davolio, P.L.; Cocchi, A.; Pellegrini-Giampietro, D.E.; Landucci, E. Anti-Inflammatory Properties of Cannabidiol and Β-Caryophyllene Alone or Combined in an In Vitro Inflammation Model. Pharmaceuticals 2024, 17, 467. [Google Scholar] [CrossRef] [PubMed]
- Askari, V.R.; Shafiee-Nick, R. The protective effects of β-caryophyllene on LPS-induced primary microglia M1/M2 imbalance: A mechanistic evaluation. Life Sci. 2019, 219, 40–73. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- Han, J.W.; Lee, B.G.; Kim, Y.K.; Yoon, J.W.; Jin, H.K.; Hong, S.; Lee, H.Y.; Lee, K.R.; Lee, H.W. Ergolide, sesquiterpene lactone from Inula britannica, inhibits inducible nitric oxide synthase and cyclo-oxygenase-2 expression in RAW 264.7 macrophages through the inactivation of NF-κB. Br. J. Pharmacol. 2001, 133, 503–512. [Google Scholar] [CrossRef]
- Song, Y.J.; Lee, D.Y.; Kim, S.N.; Lee, K.R.; Lee, H.W.; Han, J.W.; Kang, D.W.; Lee, H.Y.; Kim, Y.K. Apoptotic potential of sesquiterpene lactone ergolide through the inhibition of NF-κB signaling pathway. J. Pharm. Pharmacol. 2005, 57, 1591–1597. [Google Scholar] [CrossRef]
- Meng, X.; Sun, L.; Meng, X.; Bi, Q. The protective effect of Ergolide in osteoarthritis: In vitro and in vivo studies. Int. Immunopharmacol. 2024, 127, 111355. [Google Scholar] [CrossRef]
- Yami, A.; Hamzeloo-Moghadam, M.; Darbandi, A.; Karami, A.; Mashati, P.; Takhviji, V.; Gharehbaghian, A. Ergolide, a potent sesquiterpene lactone induces cell cycle arrest along with ROS-dependent apoptosis and potentiates vincristine cytotoxicity in ALL cell lines. J. Ethnopharmacol. 2020, 253, 112504. [Google Scholar] [CrossRef] [PubMed]
- Sundaramurthi, H.; Tonelotto, V.; Wynne, K.; O’Connell, F.; O’Reilly, E.; Costa-Garcia, M.; Kovacshazi, C.; Kittel, A.; Marcone, S.; Blanco, A.; et al. Ergolide mediates anti-cancer effects on metastatic uveal melanoma cells and modulates their cellular and extracellular vesicle proteomes. Open Res. Eur. 2023, 3, 88. [Google Scholar] [CrossRef] [PubMed]
- Berghmans, S.; Hunt, J.; Roach, A.; Goldsmith, P. Zebrafish offer the potential for a primary screen to identify a wide variety of potential anticonvulsants. Epilepsy Res. 2007, 75, 18–28. [Google Scholar] [CrossRef]
- Bashirzade, A.A.; Zabegalov, K.N.; Volgin, A.D.; Belova, A.S.; Demin, K.A.; de Abreu, M.S.; Babchenko, V.Y.; Bashirzade, K.A.; Yenkoyan, K.B.; Tikhonova, M.A.; et al. Modeling neurodegenerative disorders in zebrafish. Neurosci. Biobehav. Rev. 2022, 138, 104679. [Google Scholar] [CrossRef]
- Razali, K.; Othman, N.; Mohd Nasir, M.H.; Doolaanea, A.A.; Kumar, J.; Ibrahim, W.N.; Mohamed Ibrahim, N.; Mohamed, W.M.Y. The Promise of the Zebrafish Model for Parkinson’s Disease: Today’s Science and Tomorrow’s Treatment. Front. Genet. 2021, 12, 655550. [Google Scholar] [CrossRef]
- Liu, Y. Zebrafish as a Model Organism for Studying Pathologic Mechanisms of Neurodegenerative Diseases and other Neural Disorders. Cell Mol. Neurobiol. 2023, 43, 2603–2620. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Meijer, A.H.; Schaaf, M.J.M. Modeling Inflammation in Zebrafish for the Development of Anti-inflammatory Drugs. Front. Cell Dev. Biol. 2020, 8, 620984. [Google Scholar] [CrossRef]
- Khan, K.M.; Collier, A.D.; Meshalkina, D.A.; Kysil, E.V.; Khatsko, S.L.; Kolesnikova, T.; Morzherin, Y.Y.; Warnick, J.E.; Kalueff, A.V.; Echevarria, D.J. Zebrafish models in neuropsychopharmacology and CNS drug discovery. Br. J. Pharmacol. 2017, 174, 1925–1944. [Google Scholar] [CrossRef]
- Henn, A.; Lund, S.; Hedtjarn, M.; Schrattenholz, A.; Porzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 2009, 26, 83–94. [Google Scholar] [CrossRef]
- Jiang, S.; Wan, Q.; Wang, X.; Di, L.; Li, X.; Kang, R.; Li, S.; Huang, L. LXA4 attenuates perioperative neurocognitive disorders by suppressing neuroinflammation and oxidative stress. Int. Immunopharmacol. 2023, 123, 110788. [Google Scholar] [CrossRef]
- Al-Thani, N.A.; Zinck, D.; Stewart, G.S.; Costello, D.A. Modulation of Urea Transport Attenuates TLR2-Mediated Microglial Activation and Upregulates Microglial Metabolism In Vitro. Metabolites 2024, 14, 634. [Google Scholar] [CrossRef] [PubMed]
- Tikhanovich, I.; Kuravi, S.; Artigues, A.; Villar, M.T.; Dorko, K.; Nawabi, A.; Roberts, B.; Weinman, S.A. Dynamic Arginine Methylation of Tumor Necrosis Factor (TNF) Receptor-associated Factor 6 Regulates Toll-like Receptor Signaling. J. Biol. Chem. 2015, 290, 22236–22249. [Google Scholar] [CrossRef]
- Medrano-Jimenez, E.; Jimenez-Ferrer Carrillo, I.; Pedraza-Escalona, M.; Ramirez-Serrano, C.E.; Alvarez-Arellano, L.; Cortes-Mendoza, J.; Herrera-Ruiz, M.; Jimenez-Ferrer, E.; Zamilpa, A.; Tortoriello, J.; et al. Malva parviflora extract ameliorates the deleterious effects of a high fat diet on the cognitive deficit in a mouse model of Alzheimer’s disease by restoring microglial function via a PPAR-γ-dependent mechanism. J. Neuroinflamm. 2019, 16, 143. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, X.; Liu, B.; Liu, A.J.; Li, H.; Mao, X.; Wu, B.; Bi, K.S.; Jia, Y. Jujuboside A, a neuroprotective agent from semen Ziziphi Spinosae ameliorates behavioral disorders of the dementia mouse model induced by Aβ 1-42. Eur. J. Pharmacol. 2014, 738, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Xie, W.; Sun, Y. Oridonin inhibits LPS-induced inflammation in human gingival fibroblasts by activating PPARγ. Int. Immunopharmacol. 2019, 72, 301–307. [Google Scholar] [CrossRef]
- Li-Hua, D.; Yan, L.; Shi-Ji, W.; Guang, W.; Lu-Lu, S.; Xue-Feng, P.; Pengda, S. Esculentoside A inhibits LPS-induced BV2 microglia activation through activating PPAR-γ. Eur. J. Pharmacol. 2017, 813, 61–65. [Google Scholar] [CrossRef]
- Huang, C.; Yang, Y.; Li, W.X.; Wu, X.Q.; Li, X.F.; Ma, T.T.; Zhang, L.; Meng, X.M.; Li, J. Hyperin attenuates inflammation by activating PPAR-γ in mice with acute liver injury (ALI) and LPS-induced RAW264.7 cells. Int. Immunopharmacol. 2015, 29, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Hu, M.; Wang, H.; Hu, M.; Long, Y.; Miao, M.X.; Li, J.C.; Wang, X.B.; Kong, L.Y.; Hong, H. Montelukast targeting the cysteinyl leukotriene receptor 1 ameliorates Aβ1-42-induced memory impairment and neuroinflammatory and apoptotic responses in mice. Neuropharmacology 2014, 79, 707–714. [Google Scholar] [CrossRef]
- Attaluri, S.; Upadhya, R.; Kodali, M.; Madhu, L.N.; Upadhya, D.; Shuai, B.; Shetty, A.K. Brain-Specific Increase in Leukotriene Signaling Accompanies Chronic Neuroinflammation and Cognitive Impairment in a Model of Gulf War Illness. Front. Immunol. 2022, 13, 853000. [Google Scholar] [CrossRef]
- Kanaoka, Y.; Austen, K.F. Roles of cysteinyl leukotrienes and their receptors in immune cell-related functions. Adv. Immunol. 2019, 142, 65–84. [Google Scholar] [CrossRef]
- Baraban, S.C.; Taylor, M.R.; Castro, P.A.; Baier, H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 2005, 131, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Novoa, B.; Bowman, T.V.; Zon, L.; Figueras, A. LPS response and tolerance in the zebrafish (Danio rerio). Fish. Shellfish. Immunol. 2009, 26, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Widder, M.; Carbaugh, C.; van der Schalie, W.; Miller, R., Jr.; Brennan, L.; Moore, A.; Campbell, R.; Akers, K.; Ressner, R.; Martin, M.; et al. Identification of Potential Sepsis Therapeutic Drugs Using a Zebrafish Rapid Screening Approach. Life 2024, 14, 1689. [Google Scholar] [CrossRef]
- Yu, L.; Yan, J.; Sun, Z. D-limonene exhibits anti-inflammatory and antioxidant properties in an ulcerative colitis rat model via regulation of iNOS, COX-2, PGE2 and ERK signaling pathways. Mol. Med. Rep. 2017, 15, 2339–2346. [Google Scholar] [CrossRef]
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Soliman, K.F.A. Thymoquinone increases the expression of neuroprotective proteins while decreasing the expression of pro-inflammatory cytokines and the gene expression NFκB pathway signaling targets in LPS/IFNγ -activated BV-2 microglia cells. J. Neuroimmunol. 2018, 320, 87–97. [Google Scholar] [CrossRef]
- Yang, R.; Liu, S.; Zhou, J.; Bu, S.; Zhang, J. Andrographolide attenuates microglia-mediated Aβ neurotoxicity partially through inhibiting NF-κB and JNK MAPK signaling pathway. Immunopharmacol. Immunotoxicol. 2017, 39, 276–284. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic. Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Porres-Martinez, M.; Gonzalez-Burgos, E.; Carretero, M.E.; Gomez-Serranillos, M.P. Major selected monoterpenes α-pinene and 1,8-cineole found in Salvia lavandulifolia (Spanish sage) essential oil as regulators of cellular redox balance. Pharm. Biol. 2015, 53, 921–929. [Google Scholar] [CrossRef]
- Tiwari, M.; Kakkar, P. Plant derived antioxidants—Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. Vitr. 2009, 23, 295–301. [Google Scholar] [CrossRef]
- Staton Laws Iii, J.; Smid, S.D. Sesquiterpene-evoked phytochemical toxicity in PC12 neuronal cells reveals a variable degree of oxidative stress and α-tocopherol and glutathione-dependent protection. Curr. Res. Toxicol. 2024, 6, 100144. [Google Scholar] [CrossRef]
- Huang, Y.; Xiang, P.; Chen, Y.; Pan, Q.; Yuan, K. Alantolactone facilitates ferroptosis in non-small cell lung cancer through promoting FTH1 ubiquitination and degradation. Chem. Biol. Drug Des. 2024, 104, e14560. [Google Scholar] [CrossRef] [PubMed]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, R.G.; Sikorska, M.; Sandhu, J.K.; Lanthier, P.; Ribecco-Lutkiewicz, M.; Bani-Yaghoub, M. Differentiation of mouse Neuro 2A cells into dopamine neurons. J. Neurosci. Methods 2010, 186, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Bocchini, V.; Mazzolla, R.; Barluzzi, R.; Blasi, E.; Sick, P.; Kettenmann, H. An immortalized cell line expresses properties of activated microglial cells. J. Neurosci. Res. 1992, 31, 616–621. [Google Scholar] [CrossRef]
- Zhang, M.; Qian, C.; Zheng, Z.G.; Qian, F.; Wang, Y.; Thu, P.M.; Zhang, X.; Zhou, Y.; Tu, L.; Liu, Q.; et al. Jujuboside A promotes Aβ clearance and ameliorates cognitive deficiency in Alzheimer’s disease through activating Axl/HSP90/PPARγ pathway. Theranostics 2018, 8, 4262–4278. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Withers, C.M.; Bartz, R.R.; MacGarvey, N.C.; Fu, P.; Sweeney, T.E.; Welty-Wolf, K.E.; Suliman, H.B. Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J. Biol. Chem. 2011, 286, 16374–16385. [Google Scholar] [CrossRef]
- Choi, S.; Nguyen, V.T.; Tae, N.; Lee, S.; Ryoo, S.; Min, B.S.; Lee, J.H. Anti-inflammatory and heme oxygenase-1 inducing activities of lanostane triterpenes isolated from mushroom Ganoderma lucidum in RAW264.7 cells. Toxicol. Appl. Pharmacol. 2014, 280, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, C.; Lee, H.S.; Park, J.; Choe, J.; Lee, J.H. The anti-inflammatory effect of tussilagone, from Tussilago farfara, is mediated by the induction of heme oxygenase-1 in murine macrophages. Int. Immunopharmacol. 2009, 9, 1578–1584. [Google Scholar] [CrossRef]
- Lee, W.Y.; Chen, Y.C.; Shih, C.M.; Lin, C.M.; Cheng, C.H.; Chen, K.C.; Lin, C.W. The induction of heme oxygenase-1 suppresses heat shock protein 90 and the proliferation of human breast cancer cells through its byproduct carbon monoxide. Toxicol. Appl. Pharmacol. 2014, 274, 55–62. [Google Scholar] [CrossRef]
- Na, J.Y.; Song, K.; Lee, J.W.; Kim, S.; Kwon, J. 6-Shogaol has anti-amyloidogenic activity and ameliorates Alzheimer’s disease via CysLT1R-mediated inhibition of cathepsin B. Biochem. Biophys. Res. Commun. 2016, 477, 96–102. [Google Scholar] [CrossRef]
- Wang, X.; Gan, W.; Kang, M.; Lv, C.; Zhao, Z.; Wu, Y.; Zhang, X.; Wang, R. Asthma aggravates alzheimer’s disease by up-regulating NF-κB signaling pathway through LTD4. Brain Res. 2024, 1825, 148711. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.; Hartung, T. Ltd4 Augments Tnf Release in-Vivo and in-Vitro. Agents Actions 1994, 41, C155–C156. [Google Scholar] [CrossRef]
- Thompson, C.; Cloutier, A.; Bosse, Y.; Thivierge, M.; Gouill, C.L.; Larivee, P.; McDonald, P.P.; Stankova, J.; Rola-Pleszczynski, M. CysLT1 receptor engagement induces activator protein-1- and NF-κB-dependent IL-8 expression. Am. J. Respir. Cell Mol. Biol. 2006, 35, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsih, S.; Youns, M.; El-Readi, M.Z.; Ashour, M.L.; Nibret, E.; Sporer, F.; Herrmann, F.; Reichling, J.; Wink, M. Biological activity of the essential oil of Kadsura longipedunculata (Schisandraceae) and its major components. J. Pharm. Pharmacol. 2010, 62, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Mascayano, C.; Munoz-Osses, M.; Navarrete, E.; Torres, P.; Torres-Gonzalez, S.; Morales, P.; Huidobro-Toro, J.P. Natural pentacyclic triterpenoid as allosteric modulators of human 5-lipoxygenase with potential anti-inflammatory activity. J. Biomol. Struct. Dyn. 2024, 42, 13529–13537. [Google Scholar] [CrossRef]
- El-Dakhakhny, M.; Madi, N.J.; Lembert, N.; Ammon, H.P. Nigella sativa oil, nigellone and derived thymoquinone inhibit synthesis of 5-lipoxygenase products in polymorphonuclear leukocytes from rats. J. Ethnopharmacol. 2002, 81, 161–164. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Li, C.T.; Zhang, S.R.; Zheng, W.; Wei, E.Q.; Zhang, L.H. CysLT2 receptor mediates lipopolysaccharide-induced microglial inflammation and consequent neurotoxicity in vitro. Brain Res. 2015, 1624, 433–445. [Google Scholar] [CrossRef]
- Kyritsis, N.; Kizil, C.; Zocher, S.; Kroehne, V.; Kaslin, J.; Freudenreich, D.; Iltzsche, A.; Brand, M. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 2012, 338, 1353–1356. [Google Scholar] [CrossRef]
- Butler, C.T.; Reynolds, A.L.; Tosetto, M.; Dillon, E.T.; Guiry, P.J.; Cagney, G.; O’Sullivan, J.; Kennedy, B.N. A Quininib Analogue and Cysteinyl Leukotriene Receptor Antagonist Inhibits Vascular Endothelial Growth Factor (VEGF)-independent Angiogenesis and Exerts an Additive Antiangiogenic Response with Bevacizumab. J. Biol. Chem. 2017, 292, 3552–3567. [Google Scholar] [CrossRef]
- Slater, K.; Bosch, R.; Smith, K.F.; Jahangir, C.A.; Garcia-Mulero, S.; Rahman, A.; O’Connell, F.; Piulats, J.M.; O’Neill, V.; Horgan, N.; et al. 1,4-dihydroxy quininib modulates the secretome of uveal melanoma tumour explants and a marker of oxidative phosphorylation in a metastatic xenograft model. Front Med. 2022, 9, 1036322. [Google Scholar] [CrossRef]
- Tesfaye, B.A.; Hailu, H.G.; Zewdie, K.A.; Ayza, M.A.; Berhe, D.F. Montelukast: The New Therapeutic Option for the Treatment of Epilepsy. J. Exp. Pharmacol. 2021, 13, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Lenz, Q.F.; Arroyo, D.S.; Temp, F.R.; Poersch, A.B.; Masson, C.J.; Jesse, A.C.; Marafiga, J.R.; Reschke, C.R.; Iribarren, P.; Mello, C.F. Cysteinyl leukotriene receptor (CysLT) antagonists decrease pentylenetetrazol-induced seizures and blood-brain barrier dysfunction. Neuroscience 2014, 277, 859–871. [Google Scholar] [CrossRef]
- Fleck, J.; Temp, F.R.; Marafiga, J.R.; Jesse, A.C.; Milanesi, L.H.; Rambo, L.M.; Mello, C.F. Montelukast reduces seizures in pentylenetetrazol-kindled mice. Braz. J. Med. Biol. Res. 2016, 49, e5031. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.A.G.; Panda, S.K.; Stanca, A.; Luyten, W. Optimization of a locomotion-based zebrafish seizure model. J. Neurosci. Methods 2022, 375, 109594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, L.; Ji, X.; Zhang, S.; Sik, A.; Liu, K.; Jin, M. Anti-Inflammation Associated Protective Mechanism of Berberine and its Derivatives on Attenuating Pentylenetetrazole-Induced Seizures in Zebrafish. J. Neuroimmune Pharmacol. 2020, 15, 309–325. [Google Scholar] [CrossRef]
- Sturgeon, M.L.; Langton, R.; Sharma, S.; Cornell, R.A.; Glykys, J.; Bassuk, A.G. The opioid antagonist naltrexone decreases seizure-like activity in genetic and chemically induced epilepsy models. Epilepsia Open 2021, 6, 528–538. [Google Scholar] [CrossRef]
- Murugan, R.; Ramya Ranjan Nayak, S.P.; Haridevamuthu, B.; Priya, D.; Chitra, V.; Almutairi, B.O.; Arokiyaraj, S.; Saravanan, M.; Kathiravan, M.K.; Arockiaraj, J. Neuroprotective potential of pyrazole benzenesulfonamide derivative T1 in targeted intervention against PTZ-induced epilepsy-like condition in in vivo zebrafish model. Int. Immunopharmacol. 2024, 131, 111859. [Google Scholar] [CrossRef]
- Oosterhof, N.; Boddeke, E.; van Ham, T.J. Immune cell dynamics in the CNS: Learning from the zebrafish. Glia 2015, 63, 719–735. [Google Scholar] [CrossRef]
- Ji, S.Y.; Cha, H.J.; Molagoda, I.M.N.; Kim, M.Y.; Kim, S.Y.; Hwangbo, H.; Lee, H.; Kim, G.Y.; Kim, D.H.; Hyun, J.W.; et al. Suppression of Lipopolysaccharide-Induced Inflammatory and Oxidative Response by 5-Aminolevulinic Acid in RAW 264.7 Macrophages and Zebrafish Larvae. Biomol Ther 2021, 29, 685–696. [Google Scholar] [CrossRef]
- Kanwal, Z.; Wiegertjes, G.F.; Veneman, W.J.; Meijer, A.H.; Spaink, H.P. Comparative studies of Toll-like receptor signalling using zebrafish. Dev. Comp. Immunol. 2014, 46, 35–52. [Google Scholar] [CrossRef]
- Loes, A.N.; Hinman, M.N.; Farnsworth, D.R.; Miller, A.C.; Guillemin, K.; Harms, M.J. Identification and Characterization of Zebrafish Tlr4 Coreceptor Md-2. J. Immunol. 2021, 206, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Bartikova, H.; Hanusova, V.; Skalova, L.; Ambroz, M.; Bousova, I. Antioxidant, pro-oxidant and other biological activities of sesquiterpenes. Curr. Top. Med. Chem. 2014, 14, 2478–2494. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Pinki, F.; Stewart, G.S.; Costello, D.A. Inhibition of Urea Transporter (UT)-B Modulates LPS-Induced Inflammatory Responses in BV2 Microglia and N2a Neuroblastoma Cells. Neurochem. Res. 2021, 46, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
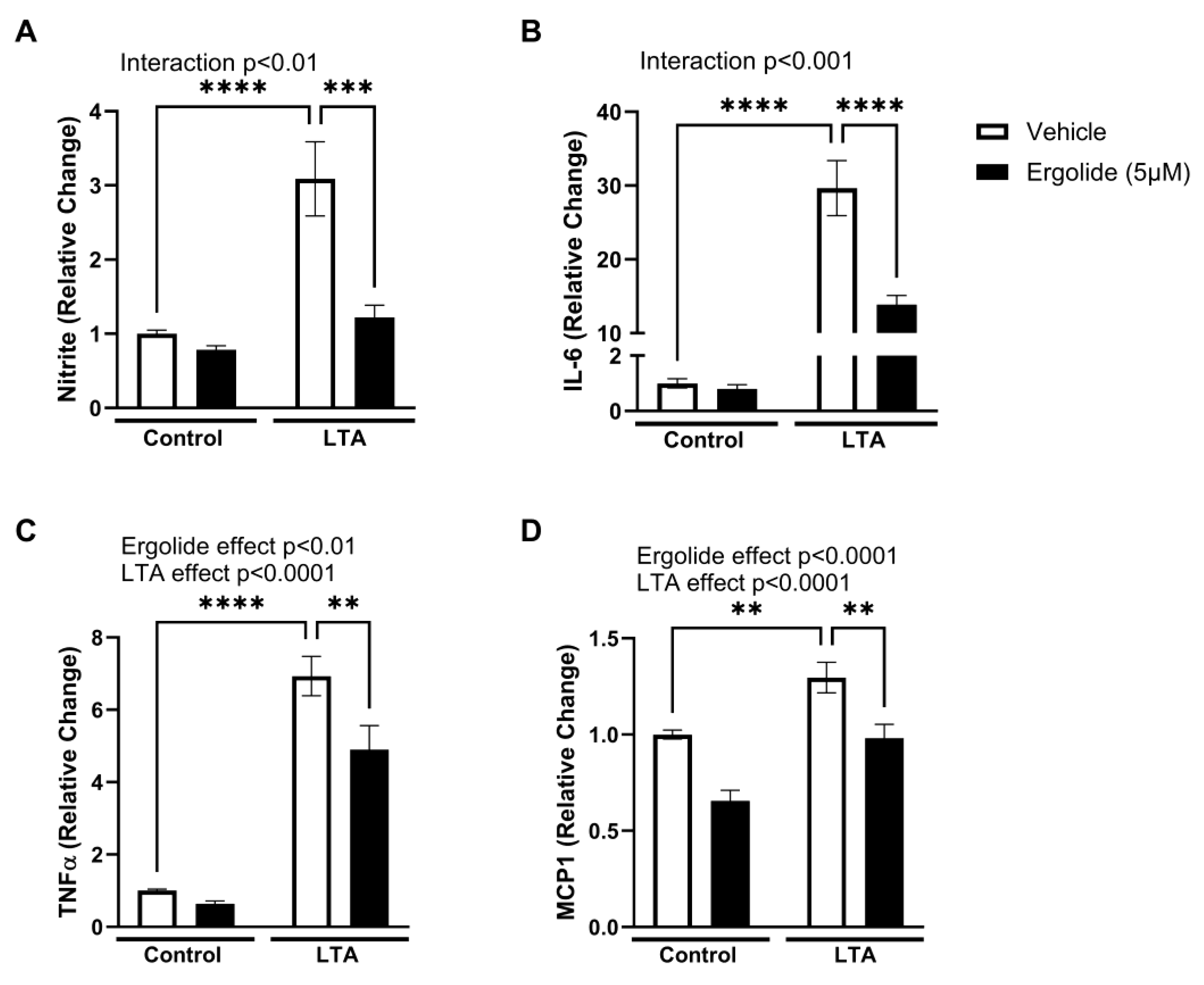
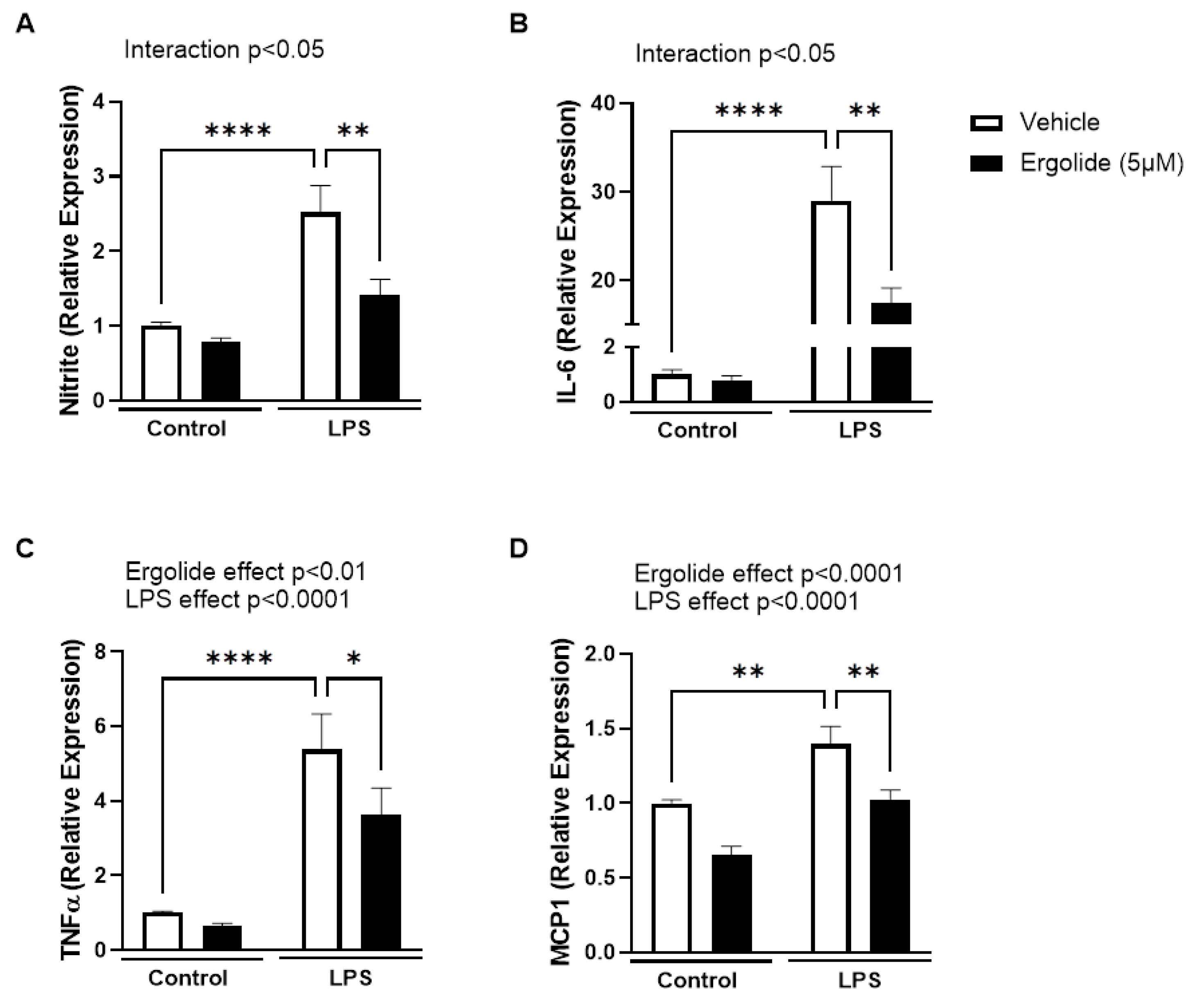
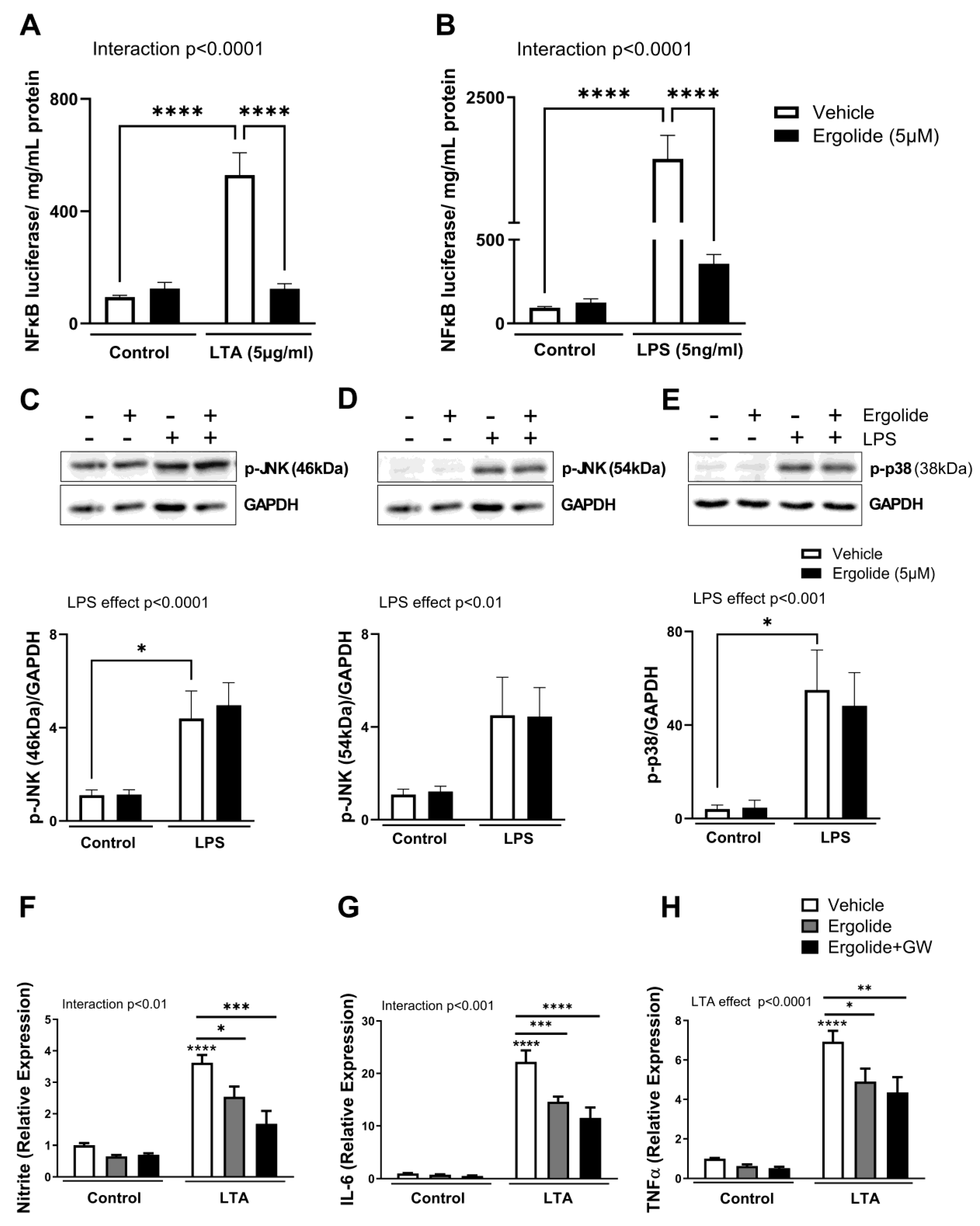
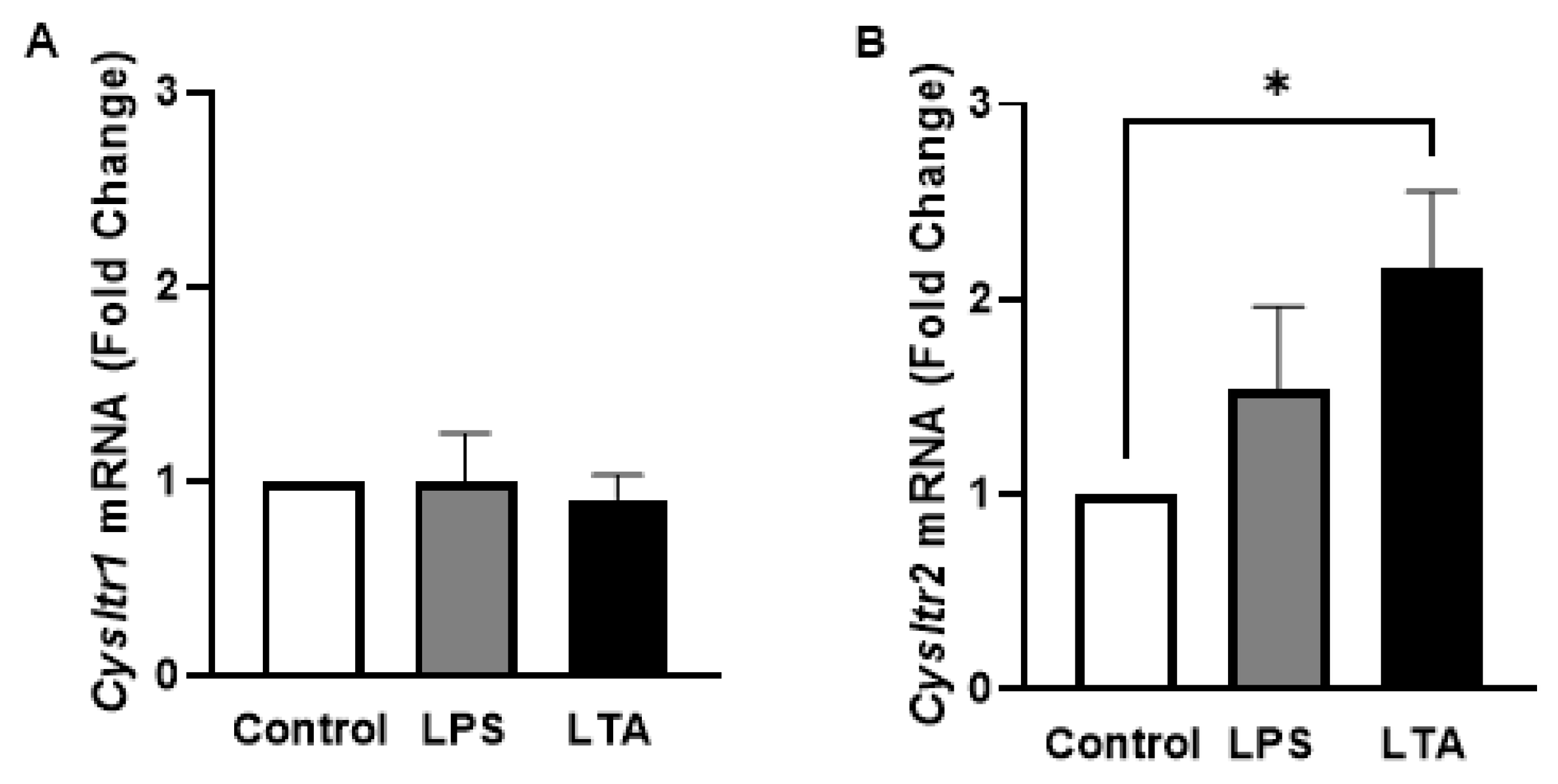




| LTA (Mean ± SEM) | LTA + Ergolide (Mean ± SEM) | p | LPS (Mean ± SEM) | LPS + Ergolide (Mean ± SEM) | p | |
|---|---|---|---|---|---|---|
| Nitrite (µM) | 21.86 ± 3.89 | 9.80 ± 0.96 | * | 19.24 ± 1.32 | 11.54 ± 1.66 | *** |
| TNFα (pg/mL) | 145.21 ± 21.96 | 102.73 ± 23.63 | - | 155.07 ± 18.72 | 101.05 ± 15.6 | * |
| IL-6 (pg/mL) | 9005.73 ± 867.27 | 5245.29 ± 1244.85 | * | 8894.52 ± 1039.8 | 5505.03 ± 795.16 | * |
| MCP1 (pg/mL) | 2102.51 ± 173.55 | 1659.62 ± 131.09 | ** | 2244.45 ± 189.88 | 1680.56 ± 176.14 | ** |
| Species | Gene | Forward Primer (5′->3′) | Reverse Primer (5′->3′) |
|---|---|---|---|
| danio rerio | cysltr1 | ACTTTTTGTGAGTGGAAATGTGTT | ACCACTCATGGAGGTCCTGTT |
| danio rerio | cysltr2 | TGTTTGGAGCTCGCACATGA | ATGATCACCAAAGCGCAAGC |
| danio rerio | gapdh | AAGTCAGTGGACACAACCTGG | CAAGAAAGTCGTCAAGGCTGC |
| danio rerio | il1β | TGTGTTATTGTTTTCCTGGCATTTC | GCGACAGCGTGGATCTACAG |
| danio rerio | il8 | CACAAAGGCTGCCATTCACT | GATTGATGGTGTGGCTCAGGT |
| danio rerio | tnfα | CACAAAGGCTGCCATTCACT | GATTGATGGTGTGGCTCAGGT |
| mouse | Cysltr1 | AAACTGTAAGCTTGGAATC | TACATTTCTGCAATCACAGC |
| mouse | Cysltr2 | TCTGTTAGAGCCACTTGTAG | CACATGATGGACATCTTATCTC |
| mouse | Gapdh | CTAATGACCACAGTCCATTC | GTGGGATGATGTTTTGGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvin, D.M.; Fernandez-Garcia, S.; Dawson, E.; Pryce, C.; Egan, B.P.; Clarke, N.C.; Reynolds, A.L.; Costello, D.A. Ergolide Regulates Microglial Activation and Inflammatory-Mediated Dysfunction: A Role for the Cysteinyl Leukotriene Pathway. Int. J. Mol. Sci. 2025, 26, 5050. https://doi.org/10.3390/ijms26115050
Galvin DM, Fernandez-Garcia S, Dawson E, Pryce C, Egan BP, Clarke NC, Reynolds AL, Costello DA. Ergolide Regulates Microglial Activation and Inflammatory-Mediated Dysfunction: A Role for the Cysteinyl Leukotriene Pathway. International Journal of Molecular Sciences. 2025; 26(11):5050. https://doi.org/10.3390/ijms26115050
Chicago/Turabian StyleGalvin, Danielle M., Sara Fernandez-Garcia, Emma Dawson, Ciara Pryce, Billy P. Egan, Niamh C. Clarke, Alison L. Reynolds, and Derek A. Costello. 2025. "Ergolide Regulates Microglial Activation and Inflammatory-Mediated Dysfunction: A Role for the Cysteinyl Leukotriene Pathway" International Journal of Molecular Sciences 26, no. 11: 5050. https://doi.org/10.3390/ijms26115050
APA StyleGalvin, D. M., Fernandez-Garcia, S., Dawson, E., Pryce, C., Egan, B. P., Clarke, N. C., Reynolds, A. L., & Costello, D. A. (2025). Ergolide Regulates Microglial Activation and Inflammatory-Mediated Dysfunction: A Role for the Cysteinyl Leukotriene Pathway. International Journal of Molecular Sciences, 26(11), 5050. https://doi.org/10.3390/ijms26115050






