The Combination of CD300c Antibody with PD-1 Blockade Suppresses Tumor Growth and Metastasis by Remodeling the Tumor Microenvironment in Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Results
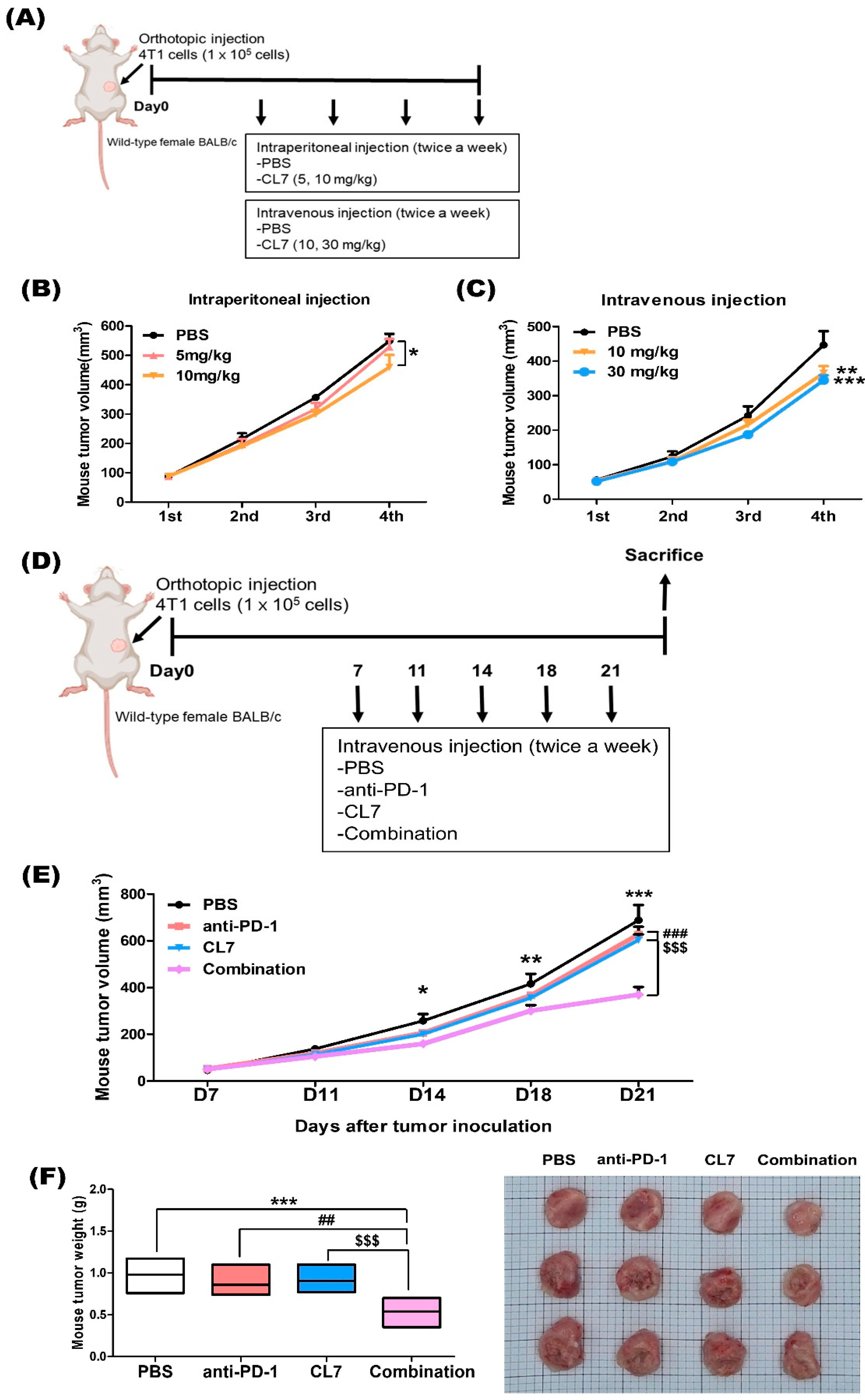
2.1. Combination Treatment of CL7 and Anti-PD-1 Synergistically Reduced the Tumor Growth in 4T1 TNBC Model
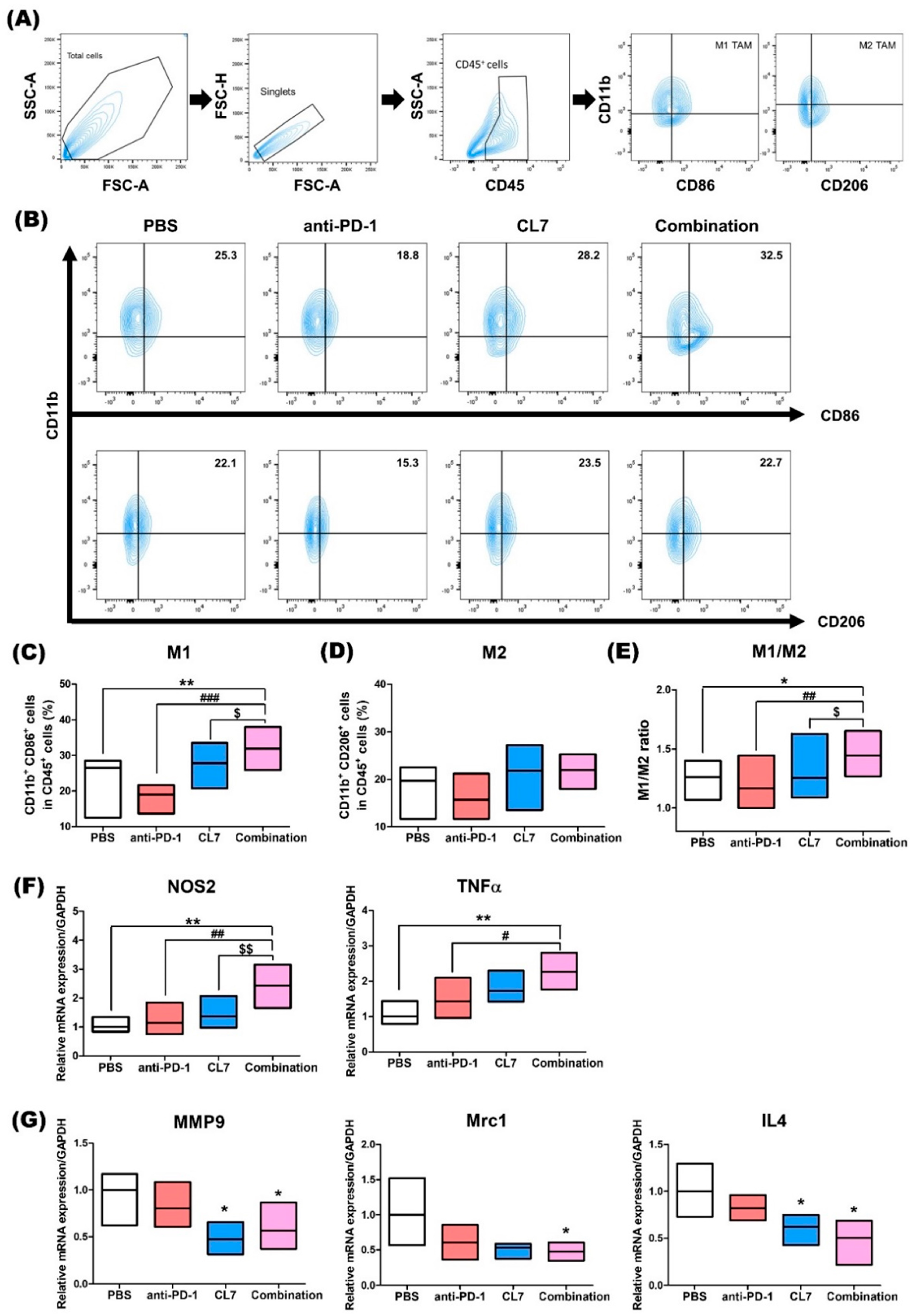
2.2. Combination Treatment of CL7 and Anti-PD-1 Has Effects on the Macrophage Population in the Tumor Microenvironment of TNBC
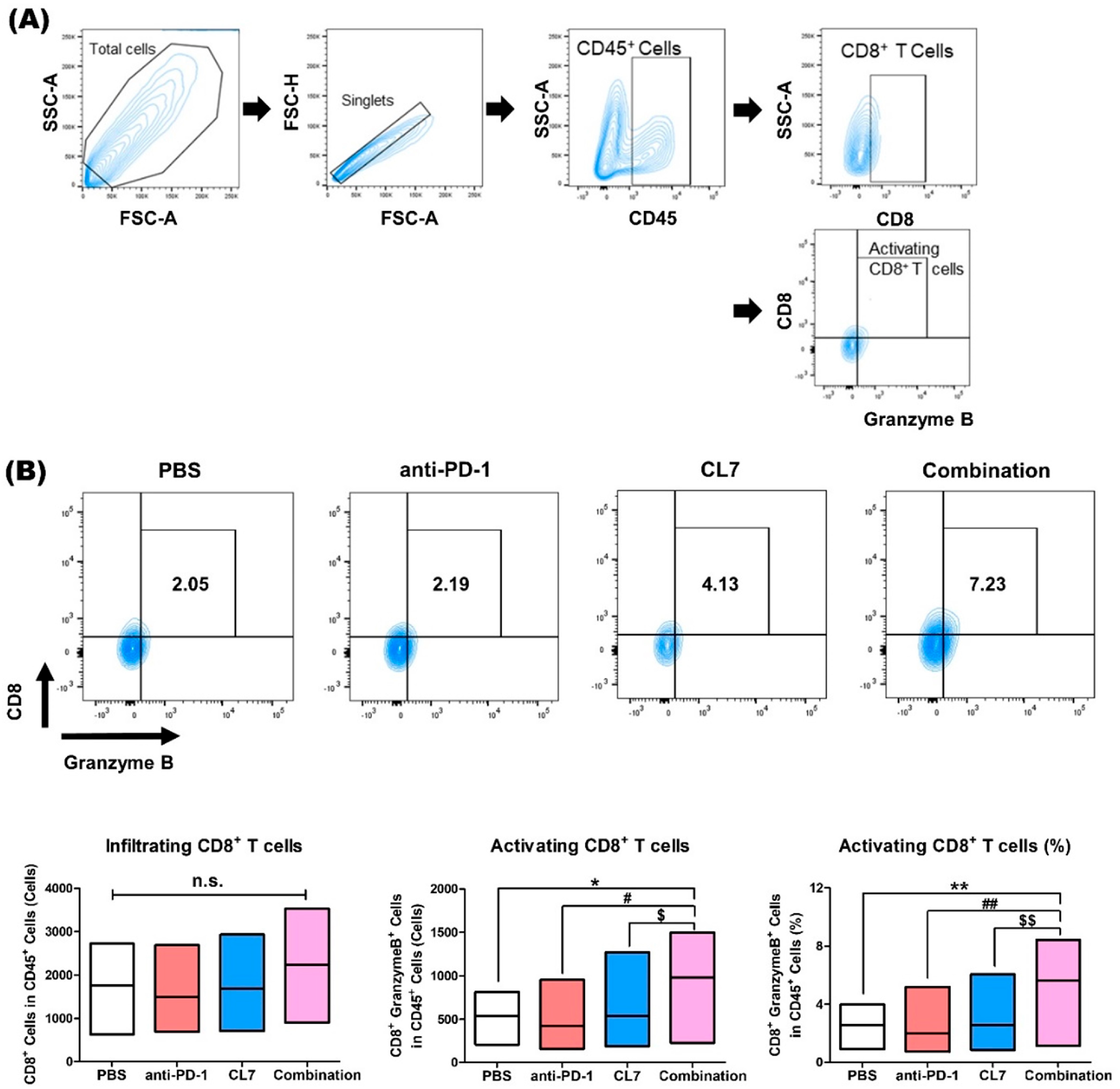
2.3. Combination Treatment of CL7 and Anti-PD-1 Significantly Enhanced the Activating CD8+ T Cells in the Breast Cancer Tissue
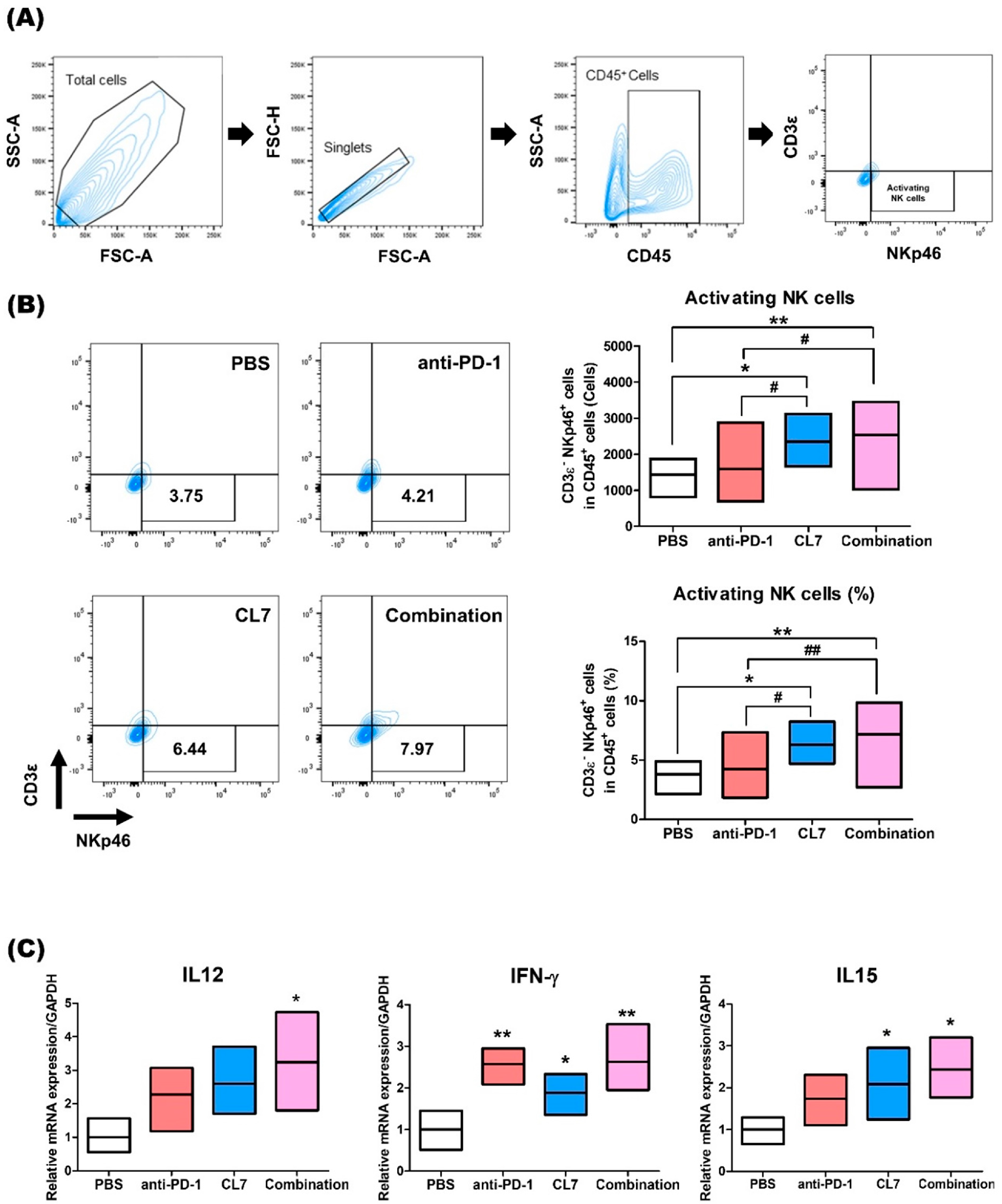
2.4. CL7 and Anti-PD-1 Combination Treatment Promotes NK Cell Activation in the Breast Cancer
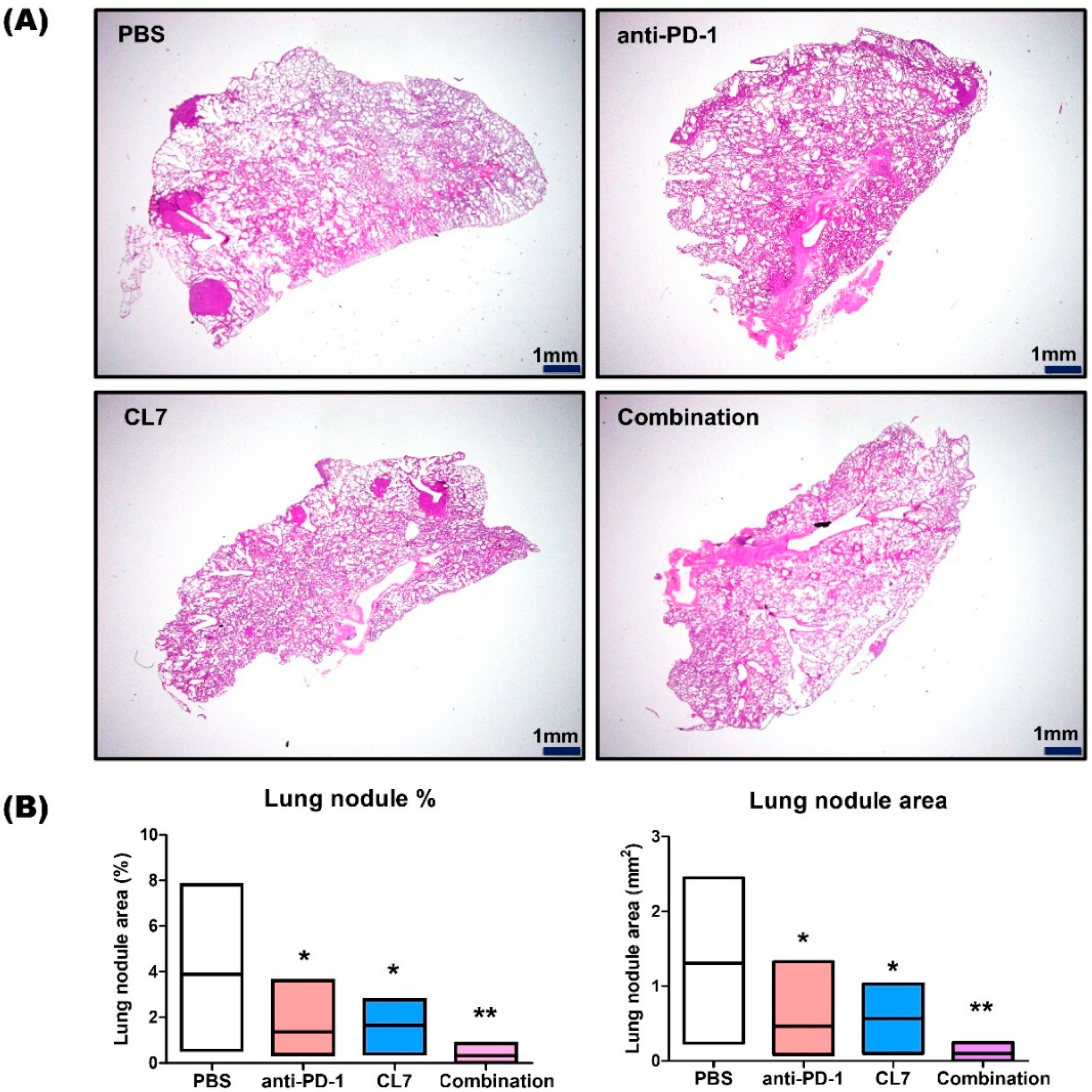
2.5. CL7 and Anti-PD-1 Suppressed the Lung Metastasis in the TNBC Model
3. Discussion
4. Materials and Methods
4.1. Reagents and Cell Lines
4.2. Animal Study
4.3. Flow Cytometry
4.4. Quantitative Real-Time PCR (qRT-PCR)
4.5. Hematoxylin and Eosin Staining
4.6. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.; Choi, I.; Han, I.H.; Bae, H. Enhanced Therapeutic Effect of Optimized Melittin-dKLA, a Peptide Agent Targeting M2-like Tumor-Associated Macrophages in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2022, 23, 15751. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.; Bekele, F.; Fekadu, G. Treatment Strategies Against Triple-Negative Breast Cancer: An Updated Review. Breast Cancer 2022, 14, 15–24. [Google Scholar] [CrossRef]
- Wang, H.; Tian, T.; Zhang, J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int. J. Mol. Sci. 2021, 22, 8470. [Google Scholar] [CrossRef]
- Huang, R.; Kang, T.; Chen, S. The role of tumor-associated macrophages in tumor immune evasion. J. Cancer Res. Clin. Oncol. 2024, 150, 238. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Chen, X.; Song, E. The theory of tumor ecosystem. Cancer Commun. 2022, 42, 587–608. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Gatti-Mays, M.E.; Kalinsky, K.; Korde, L.A.; Sharon, E.; Amiri-Kordestani, L.; Bear, H.; McArthur, H.L.; Frank, E.; Perlmutter, J.; et al. Current Landscape of Immunotherapy in Breast Cancer: A Review. JAMA Oncol. 2019, 5, 1205–1214. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, Z.; Lin, X.; Wang, S.; Wen, Y.; Wang, L.; Zhi, L.; Zhou, J. Tumor microenvironment and immunotherapy for Triple-negative breast cancer. Biomark. Res. 2024, 12, 166. [Google Scholar] [CrossRef]
- Deepak, K.G.K.; Vempati, R.; Nagaraju, G.P.; Dasari, V.R.; S, N.; Rao, D.N.; Malla, R.R. Tumor microenvironment: Challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol. Res. 2020, 153, 104683. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. New. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Mao, Y.; Qu, Q.; Chen, X.; Huang, O.; Wu, J.; Shen, K. The Prognostic Value of Tumor-Infiltrating Lymphocytes in Breast Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0152500. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Dieras, V.; Barrios, C.H.; Winer, E.P.; Schneeweiss, A.; Iwata, H.; Loi, S.; Patel, S.; Henschel, V.; Chui, S.Y.; et al. Patient-reported outcomes from the phase III IMpassion130 trial of atezolizumab plus nab-paclitaxel in metastatic Triple-negative breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 582–589. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, R.; Wu, X.; Zhang, M.; Jin, T. PD-L1 mediates triple-negative breast cancer evolution via the regulation of TAM/M2 polarization. Int. J. Oncol. 2022, 61, 150. [Google Scholar] [CrossRef]
- Simhadri, V.R.; Mariano, J.L.; Gil-Krzewska, A.; Zhou, Q.; Borrego, F. CD300c is an activating receptor expressed on human monocytes. J. Innate Immun. 2013, 5, 389–400. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, H.; Lim, C.K.; Kim, J.D.; Heo, J.S.; Jung, J.; Kim, C.; Chon, H.J.; Jeon, J.W. Engagement of CD300c by a novel monoclonal antibody induces the differentiation of monocytes to M1 macrophages. Immunobiology 2024, 229, 152780. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gogenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- St Paul, M.; Ohashi, P.S. The Roles of CD8(+) T Cell Subsets in Antitumor Immunity. Trends Cell Biol. 2020, 30, 695–704. [Google Scholar] [CrossRef]
- Lee, C.; Lee, H.; Cho, H.; Kim, S.; Choi, I.; Hwang, Y.S.; Jeong, H.; Jang, H.; Pak, S.; Hwang, D.S.; et al. Combination of anti-PD-L1 antibody with peptide MEL-dKLA targeting M2 tumor-associated macrophages suppresses breast cancer metastasis. Cancer Commun. 2022, 42, 345–349. [Google Scholar] [CrossRef]
- Mandapati, A.; Lukong, K.E. Triple negative breast cancer: Approved treatment options and their mechanisms of action. J. Cancer Res. Clin. Oncol. 2023, 149, 3701–3719. [Google Scholar] [CrossRef]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Zhang, G.; Yan, X.; Xiao, X.; Ma, X.; Lin, S.; Wu, J.; Li, X.; Wang, W.; Liu, J.; et al. Prospects of Immunotherapy for Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 797092. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Qureshi, S.; Chan, N.; George, M.; Ganesan, S.; Toppmeyer, D.; Omene, C. Immune Checkpoint Inhibitors in Triple Negative Breast Cancer: The Search for the Optimal Biomarker. Biomark. Insights 2022, 17, 11772719221078774. [Google Scholar] [CrossRef]
- Nanda, R.; Chow, L.Q.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for Triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Pathria, P.; Louis, T.L.; Varner, J.A. Targeting Tumor-Associated Macrophages in Cancer. Trends Immunol. 2019, 40, 310–327. [Google Scholar] [CrossRef]
- Fu, L.Q.; Du, W.L.; Cai, M.H.; Yao, J.Y.; Zhao, Y.Y.; Mou, X.Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, H.; Song, Y.; Huang, T.; Cao, J.; Tang, Q.; Zou, Z. Targeting tumor-associated macrophages: A potential treatment for solid tumors. J. Cell. Physiol. 2021, 236, 3445–3465. [Google Scholar] [CrossRef]
- Mantuano, N.R.; Oliveira-Nunes, M.C.; Alisson-Silva, F.; Dias, W.B.; Todeschini, A.R. Emerging role of glycosylation in the polarization of tumor-associated macrophages. Pharmacol. Res. 2019, 146, 104285. [Google Scholar] [CrossRef]
- Li, M.; He, L.; Zhu, J.; Zhang, P.; Liang, S. Targeting tumor-associated macrophages for cancer treatment. Cell Biosci. 2022, 12, 85. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Cao, Y.; Gu, Y.; Lin, C.; Liu, X.; Lv, K.; He, X.; Fang, H.; Jin, K.; et al. Poor Clinical Outcomes and Immunoevasive Contexture in Intratumoral IL-10-Producing Macrophages Enriched Gastric Cancer Patients. Ann. Surg. 2022, 275, e626–e635. [Google Scholar] [CrossRef]
- Cui, C.; Su, M.; Lin, Y.; Lai, L. A CD300c-Fc Fusion Protein Inhibits T Cell Immunity. Front. Immunol. 2018, 9, 2657. [Google Scholar] [CrossRef]
- Lankry, D.; Simic, H.; Klieger, Y.; Levi-Schaffer, F.; Jonjic, S.; Mandelboim, O. Expression and function of CD300 in NK cells. J. Immunol. 2010, 185, 2877–2886. [Google Scholar] [CrossRef]
- Raggi, F.; Pelassa, S.; Pierobon, D.; Penco, F.; Gattorno, M.; Novelli, F.; Eva, A.; Varesio, L.; Giovarelli, M.; Bosco, M.C. Regulation of Human Macrophage M1-M2 Polarization Balance by Hypoxia and the Triggering Receptor Expressed on Myeloid Cells-1. Front. Immunol. 2017, 8, 1097. [Google Scholar] [CrossRef]
- Dimitrova, M.; Zenarruzabeitia, O.; Borrego, F.; Simhadri, V.R. CD300c is uniquely expressed on CD56 bright Natural Killer Cells and differs from CD300a upon ligand recognition. Sci. Rep. 2016, 6, 23942. [Google Scholar] [CrossRef]
- Oshi, M.; Asaoka, M.; Tokumaru, Y.; Yan, L.; Matsuyama, R.; Ishikawa, T.; Endo, I.; Takabe, K. CD8 T Cell Score as a Prognostic Biomarker for Triple Negative Breast Cancer. Int. J. Mol. Sci. 2020, 21, 6968. [Google Scholar] [CrossRef]
- Ceci, C.; Atzori, M.G.; Lacal, P.M.; Graziani, G. Targeting Tumor-Associated Macrophages to Increase the Efficacy of Immune Checkpoint Inhibitors: A Glimpse into Novel Therapeutic Approaches for Metastatic Melanoma. Cancers 2020, 12, 3401. [Google Scholar] [CrossRef]
- Eisinger, S.; Sarhan, D.; Boura, V.F.; Ibarlucea-Benitez, I.; Tyystjarvi, S.; Oliynyk, G.; Arsenian-Henriksson, M.; Lane, D.; Wikstrom, S.L.; Kiessling, R.; et al. Targeting a scavenger receptor on tumor-associated macrophages activates tumor cell killing by natural killer cells. Proc. Natl. Acad. Sci. USA 2020, 117, 32005–32016. [Google Scholar] [CrossRef]
- Georgoudaki, A.M.; Prokopec, K.E.; Boura, V.F.; Hellqvist, E.; Sohn, S.; Ostling, J.; Dahan, R.; Harris, R.A.; Rantalainen, M.; Klevebring, D.; et al. Reprogramming Tumor-Associated Macrophages by Antibody Targeting Inhibits Cancer Progression and Metastasis. Cell Rep. 2016, 15, 2000–2011. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, F.; Liu, Z.; Fan, Z. Immunotherapy for Triple-Negative Breast Cancer: Combination Strategies to Improve Outcome. Cancers 2023, 15, 321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, Y.; Xue, J.; Li, J.; Yi, J.; Bu, J.; Zhang, Z.; Qiu, P.; Gu, X. Advances in immunotherapy for Triple-negative breast cancer. Mol. Cancer 2023, 22, 145. [Google Scholar] [CrossRef]
- Gao, D.; Du, J.; Cong, L.; Liu, Q. Risk factors for initial lung metastasis from breast invasive ductal carcinoma in stages I-III of operable patients. Jpn. J. Clin. Oncol. 2009, 39, 97–104. [Google Scholar] [CrossRef]
- Kaneda, M.M.; Messer, K.S.; Ralainirina, N.; Li, H.; Leem, C.J.; Gorjestani, S.; Woo, G.; Nguyen, A.V.; Figueiredo, C.C.; Foubert, P.; et al. Corrigendum: PI3Kgamma is a molecular switch that controls immune suppression. Nature 2017, 542, 124. [Google Scholar] [CrossRef]
- Ungricht, R.; Kutay, U. Mechanisms and functions of nuclear envelope remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 229–245. [Google Scholar] [CrossRef]
- Zheng, J.; Long, X.; Chen, H.; Ji, Z.; Shu, B.; Yue, R.; Liao, Y.; Ma, S.; Qiao, K.; Liu, Y.; et al. Photoclick Reaction Constructs Glutathione-Responsive Theranostic System for Anti-Tuberculosis. Front. Mol. Biosci. 2022, 9, 845179. [Google Scholar] [CrossRef]
- Takeda, A.J.; Maher, T.J.; Zhang, Y.; Lanahan, S.M.; Bucklin, M.L.; Compton, S.R.; Tyler, P.M.; Comrie, W.A.; Matsuda, M.; Olivier, K.N.; et al. Human PI3Kgamma deficiency and its microbiota-dependent mouse model reveal immunodeficiency and tissue immunopathology. Nat. Commun. 2019, 10, 4364. [Google Scholar] [CrossRef]
| Gene | F/R | Primer Sequence |
| GAPDH | F | 5′-ACC CAG AAG ACT GTG GAT GG-3′ |
| R | 5′-CAC ATT GGG GGT AGG AAC AC-3′ | |
| Mrc1 | F | 5′-TTC GGT GGA CTG TGG ACG AGC-3′ |
| R | 5′-ATA AGC CAC CTG CCA CTC CGG-3′ | |
| IL-4 | F | 5′-ATC CTG CTC TTC TTT CTC GAA TGT-3′ |
| R | 5′-GCC GAT GAT CTC TCT CAA GTG ATT-3′ | |
| MMP9 | F | 5′-TGA ATC AGC TGG CTT TTG TG-3′ |
| R | 5′-ACC TTC CAG TAG GGG CAA CT-3′ | |
| NOS2 | F | 5′-GGC AGC CTG TGA GAC CTT TG-3′ |
| R | 5′-GAA GCG TTT CGG GAT CTG AA-3′ | |
| TNFα | F | 5′-CAT CTT CTC AAA ATT CGA GTG ACA A-3′ |
| R | 5′-TGG GAG TAG ACA AGG TAC AAC CC-3′ | |
| IFN-γ | F | 5′-TCA AGT GGC ATA GAT GTG GAA GAA-3′ |
| R | 5′-TGG CTC TGC AGG ATT TTC ATG-3′ | |
| IL12 | F | 5′-AAG CTC TGC ATC CTG CTT CAC-3′ |
| R | 5′-GAT AGC CCA TCA CCC TGT TGA-3′ | |
| IL15 | F | 5′-GAT TGA AGG GAA GCA ACG GG-3′ |
| R | 5′-GCA CTC TCC AAC CCA CTT GA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Han, I.-H.; Lee, S.; Park, D.; Lee, H.; Kim, J.; Kim, J.; Jeon, J.-W.; Bae, H. The Combination of CD300c Antibody with PD-1 Blockade Suppresses Tumor Growth and Metastasis by Remodeling the Tumor Microenvironment in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2025, 26, 5045. https://doi.org/10.3390/ijms26115045
Kim S, Han I-H, Lee S, Park D, Lee H, Kim J, Kim J, Jeon J-W, Bae H. The Combination of CD300c Antibody with PD-1 Blockade Suppresses Tumor Growth and Metastasis by Remodeling the Tumor Microenvironment in Triple-Negative Breast Cancer. International Journal of Molecular Sciences. 2025; 26(11):5045. https://doi.org/10.3390/ijms26115045
Chicago/Turabian StyleKim, Soyoung, Ik-Hwan Han, Suin Lee, DaeHwan Park, Hyunju Lee, Jongyeob Kim, Joon Kim, Jae-Won Jeon, and Hyunsu Bae. 2025. "The Combination of CD300c Antibody with PD-1 Blockade Suppresses Tumor Growth and Metastasis by Remodeling the Tumor Microenvironment in Triple-Negative Breast Cancer" International Journal of Molecular Sciences 26, no. 11: 5045. https://doi.org/10.3390/ijms26115045
APA StyleKim, S., Han, I.-H., Lee, S., Park, D., Lee, H., Kim, J., Kim, J., Jeon, J.-W., & Bae, H. (2025). The Combination of CD300c Antibody with PD-1 Blockade Suppresses Tumor Growth and Metastasis by Remodeling the Tumor Microenvironment in Triple-Negative Breast Cancer. International Journal of Molecular Sciences, 26(11), 5045. https://doi.org/10.3390/ijms26115045






