Resveratrol Attenuates CSF Markers of Neurodegeneration and Neuroinflammation in Individuals with Alzheimer’s Disease
Abstract
1. Introduction
2. Results
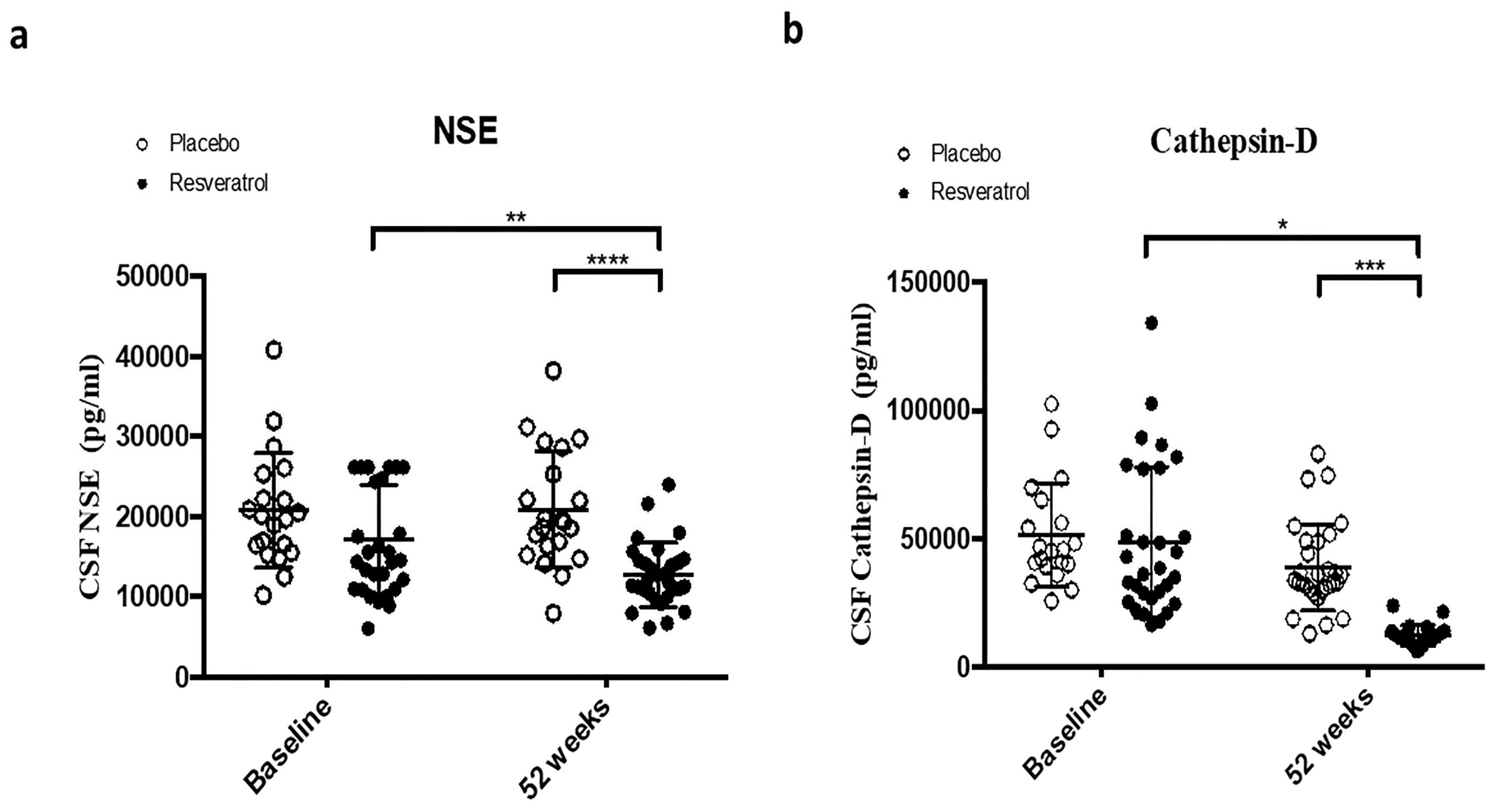
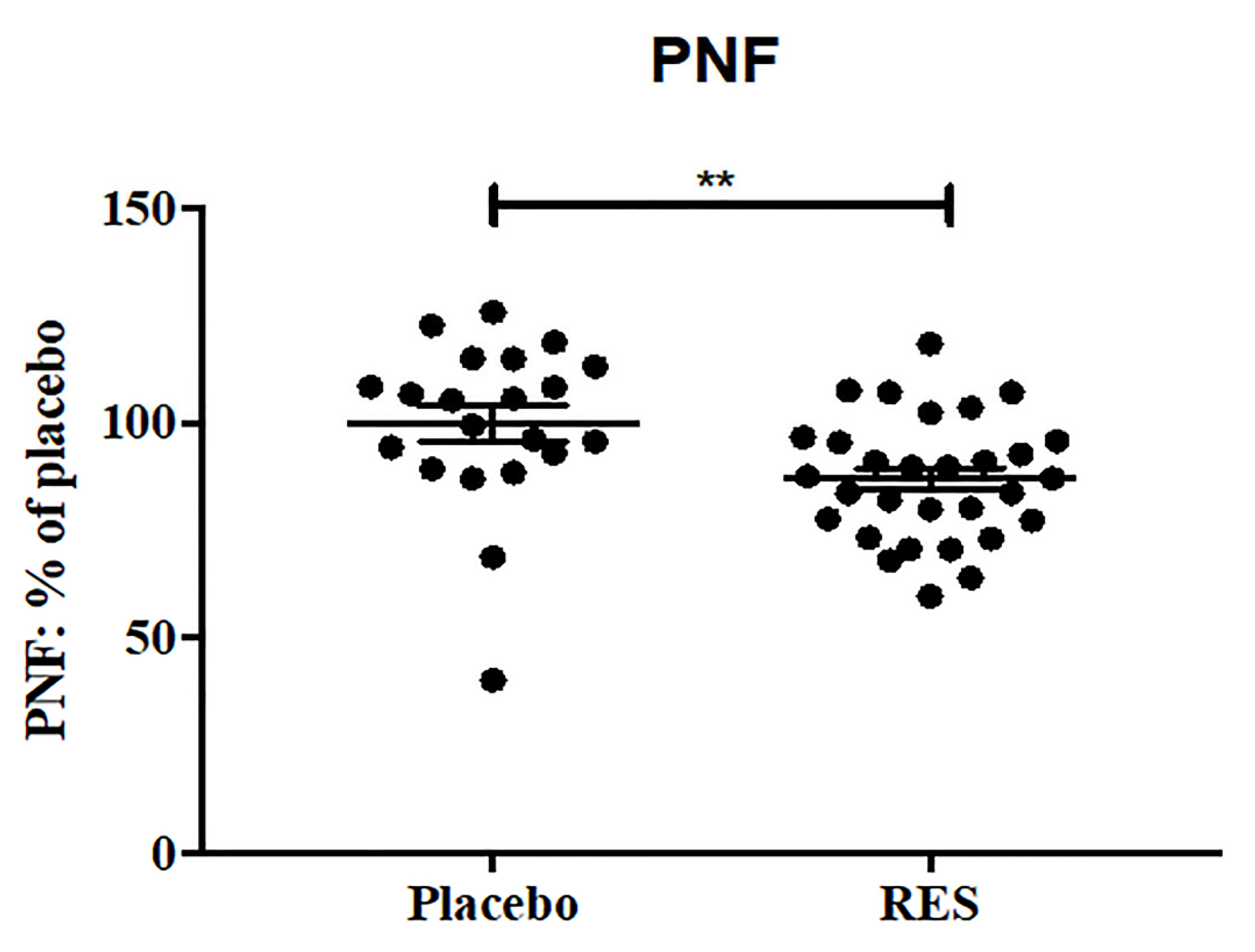
2.1. Resveratrol Reduces CSF Markers of Cell Death
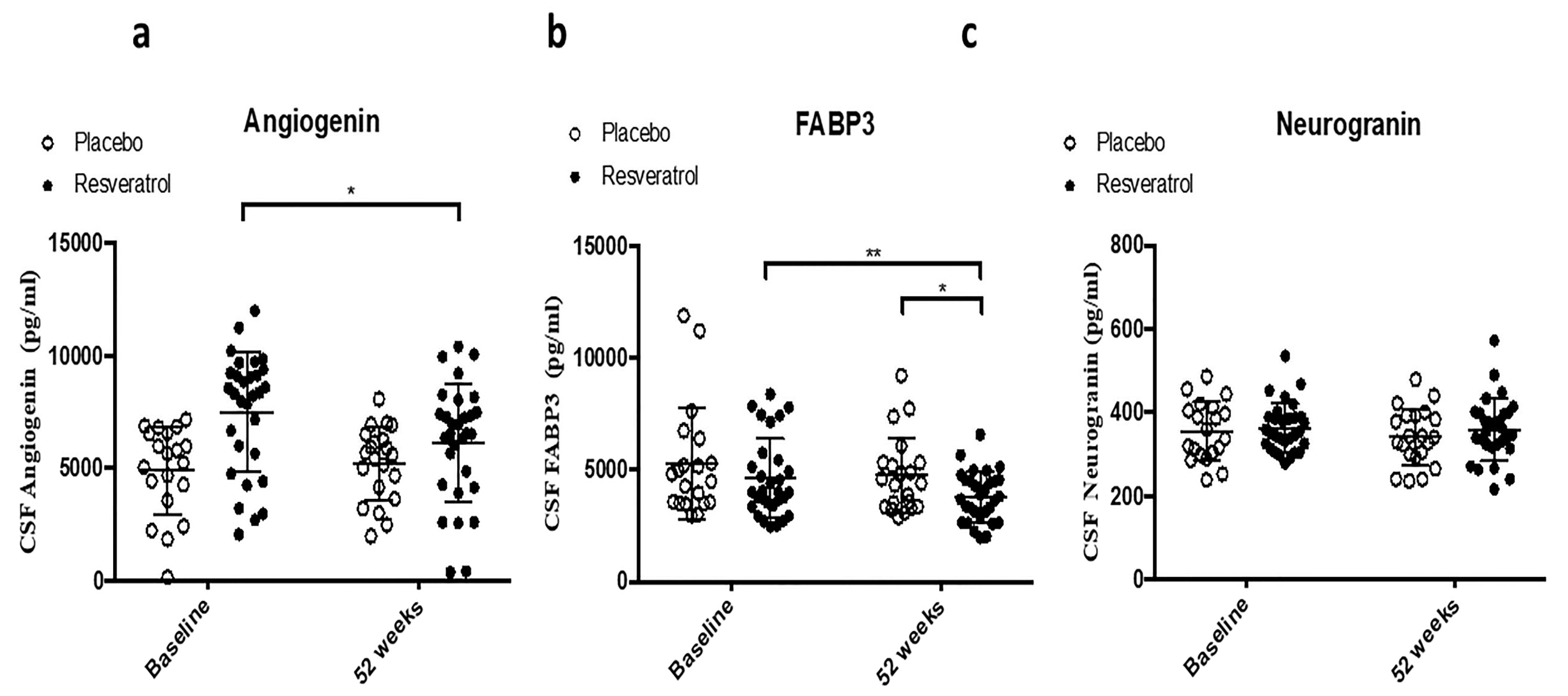
2.2. CSF Biomarker of Angiogenesis and TREM2
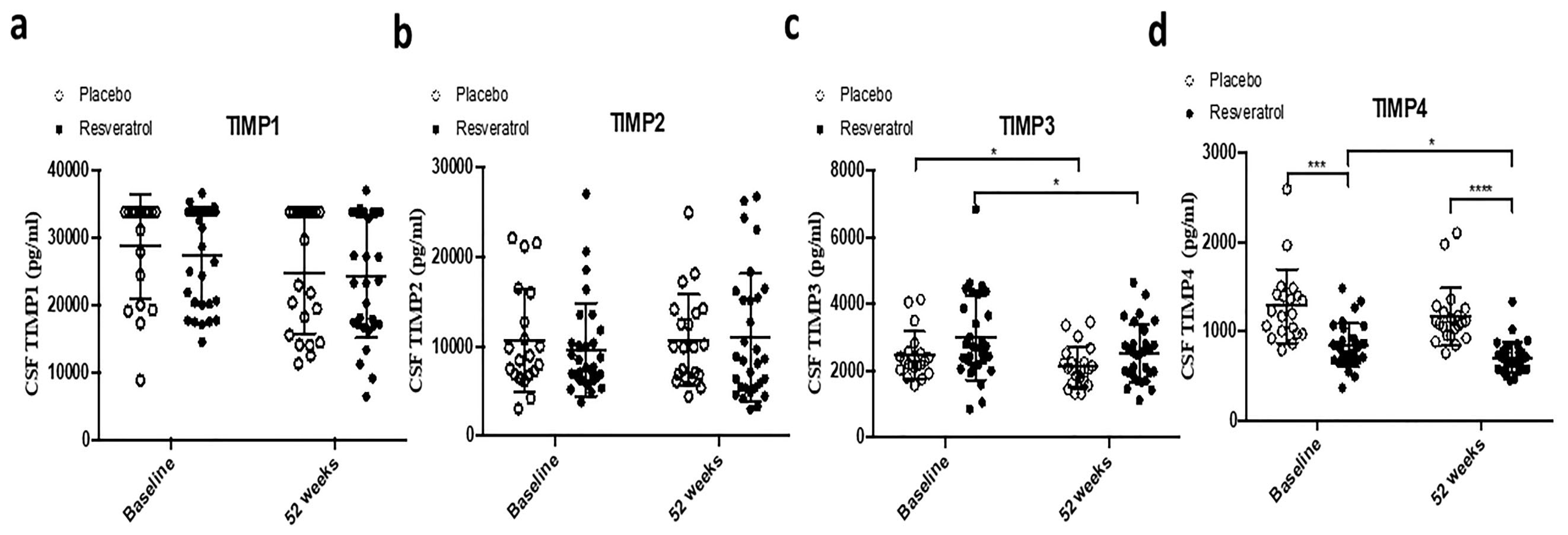
2.3. CSF Biomarkers of Matrix Metalloproteases as Dual Markers of Inflammation and BBB Integrity
3. Discussion
4. Materials and Methods
4.1. Patient Demographics
4.2. Multiplex Xmap ELISA
4.3. Standard Protocol Approvals, Registrations, and Patient Consent
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heneka, M.T.; Carson, M.J.; Khoury, E.J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Wang, Y. TREM2 variants: New keys to decipher Alzheimer disease pathogenesis. Nat. Rev. Neurosci. 2016, 17, 201–207. [Google Scholar] [CrossRef]
- Yang, R.; Dong, H.; Jia, S.; Yang, Z. Resveratrol as a modulatory of apoptosis and autophagy in cancer therapy. Clin. Transl. Oncol. 2022, 24, 1219–1230. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, T.; Wang, Y.; Guo, L. The Role and Mechanism of SIRT1 in Resveratrol-regulated Osteoblast Autophagy in Osteoporosis Rats. Sci. Rep. 2019, 9, 18424. [Google Scholar] [CrossRef]
- D’Arcy, M.S. A review of the chemopreventative and chemotherapeutic properties of the phytochemicals berberine, resveratrol and curcumin, and their influence on cell death via the pathways of apoptosis and autophagy. Cell Biol. Int. 2020, 44, 1781–1791. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Marino, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef]
- Wang, J.; Huang, P.; Pan, X.; Xia, C.; Zhang, H.; Zhao, H.; Yuan, Z.; Liu, J.; Meng, C.; Liu, F. Resveratrol reverses TGF-beta1-mediated invasion and metastasis of breast cancer cells via the SIRT3/AMPK/autophagy signal axis. Phytother. Res. 2023, 37, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, S.; Zhu, X.; Lv, Z. Autophagy-related 7 proteindependent autophagy mediates resveratrol-caused upregulation of mitochondrial biogenesis and steroidogenesis in aged Leydig cell. Mol. Biol. Rep. 2023, 51, 28. [Google Scholar]
- Tao, G.; Wang, X.; Wang, J.; Ye, Y.; Zhang, M.; Lang, Y.; Ding, S. Dihydro-resveratrol ameliorates NLRP3 inflammasome-mediated neuroinflammation via Bnip3-dependent mitophagy in Alzheimer’s disease. Br. J. Pharmacol. 2025, 182, 1005–1024. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, H.; Li, F.; Liu, S.; Wang, Y.; Zhang, Z.; Wang, J.; Wu, Q. Resveratrol alleviates acute lung injury in mice by promoting Pink1/Parkin-related mitophagy and inhibiting NLRP3 inflammasome activation. Biochim. Biophys. Acta Gen. Subj. 2024, 1868, 130612. [Google Scholar] [CrossRef] [PubMed]
- Cen, X.; Zhang, M.; Zhou, M.; Ye, L.; Xia, H. Mitophagy Regulates Neurodegenerative Diseases. Cells 2021, 10, 1876. [Google Scholar] [CrossRef]
- Li, A.; Zhang, S.; Li, J.; Liu, K.; Huang, F.; Liu, B. Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol. Cell. Endocrinol. 2016, 434, 36–47. [Google Scholar] [CrossRef]
- Li, M.; Wu, X.; An, P.; Dang, H.; Liu, Y.; Liu, R. Effects of resveratrol on autophagy and the expression of inflammasomes in a placental trophoblast oxidative stress model. Life Sci. 2020, 256, 117890. [Google Scholar] [CrossRef]
- Mo, Y.; Sun, Y.Y.; Liu, K.Y. Autophagy and inflammation in ischemic stroke. Neural Regen. Res. 2020, 15, 1388–1396. [Google Scholar]
- Chang, Y.P.; Ka, S.M.; Hsu, W.H.; Chen, A.; Chao, L.K.; Lin, C.C.; Hsieh, C.C.; Chen, M.C.; Chiu, H.W.; Ho, C.L.; et al. Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J. Cell. Physiol. 2015, 230, 1567–1579. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Li, X.; Chen, J.; Yu, Z.; Li, X.; Sun, C.; Hu, L.; Wu, M.; Liu, L. Polydatin protects against atherosclerosis by activating autophagy and inhibiting pyroptosis mediated by the NLRP3 inflammasome. J. Ethnopharmacol. 2023, 309, 116304. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Isgro, M.A.; Bottoni, P.; Scatena, R. Neuron-Specific Enolase as a Biomarker: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 125–143. [Google Scholar] [PubMed]
- Vos, P.E.; Jacobs, B.; Andriessen, T.M.; Lamers, K.J.; Borm, G.F.; Beems, T.; Edwards, M.; Rosmalen, C.F.; Vissers, J.L. GFAP and S100B are biomarkers of traumatic brain injury: An observational cohort study. Neurology 2010, 75, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Meric, E.; Gunduz, A.; Turedi, S.; Cakir, E.; Yandi, M. The prognostic value of neuron-specific enolase in head trauma patients. J. Emerg. Med. 2010, 38, 297–301. [Google Scholar] [CrossRef]
- Guzel, A.; Er, U.; Tatli, M.; Aluclu, U.; Ozkan, U.; Duzenli, Y.; Satici, O.; Guzel, E.; Kemaloglu, S.; Ceviz, A.; et al. Serum neuron-specific enolase as a predictor of short-term outcome and its correlation with Glasgow Coma Scale in traumatic brain injury. Neurosurg. Rev. 2008, 31, 439–444; discussion 444–445. [Google Scholar] [CrossRef]
- Brea, D.; Sobrino, T.; Blanco, M.; Cristobo, I.; Rodriguez-Gonzalez, R.; Rodriguez-Yanez, M.; Moldes, O.; Agulla, J.; Leira, R.; Castillo, J. Temporal profile and clinical significance of serum neuron-specific enolase and S100 in ischemic and hemorrhagic stroke. Clin. Chem. Lab. Med. 2009, 47, 1513–1518. [Google Scholar] [CrossRef]
- Cataldo, A.M.; Paskevich, P.A.; Kominami, E.; Nixon, R.A. Lysosomal hydrolases of different classes are abnormally distributed in brains of patients with Alzheimer disease. Proc. Natl. Acad. Sci. USA 1991, 88, 10998–11002. [Google Scholar] [CrossRef]
- Cataldo, A.M.; Barnett, J.L.; Berman, S.A.; Li, J.; Quarless, S.; Bursztajn, S.; Lippa, C.; Nixon, R.A. Gene expression and cellular content of cathepsin D in Alzheimer’s disease brain: Evidence for early up-regulation of the endosomal-lysosomal system. Neuron 1995, 14, 671–680. [Google Scholar] [CrossRef]
- Schwagerl, A.L.; Mohan, P.S.; Cataldo, A.M.; Vonsattel, J.P.; Kowall, N.W.; Nixon, R.A. Elevated levels of the endosomal-lysosomal proteinase cathepsin D in cerebrospinal fluid in Alzheimer disease. J. Neurochem. 1995, 64, 443–446. [Google Scholar] [CrossRef]
- Tome-Carneiro, J.; Larrosa, M.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Sawda, C.; Moussa, C.; Turner, R.S. Resveratrol for Alzheimer’s disease. Ann. N.Y. Acad. Sci. 2017, 1403, 142–149. [Google Scholar] [CrossRef]
- Zhou, D.D.; Luo, M.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Gan, R.Y.; Li, H.B. Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Ntais, C.; Polycarpou, A.; Ioannidis, J.P. Meta-analysis of the association of the cathepsin D Ala224Val gene polymorphism with the risk of Alzheimer’s disease: A HuGE gene-disease association review. Am. J. Epidemiol. 2004, 159, 527–536. [Google Scholar] [CrossRef][Green Version]
- Lowry, J.R.; Klegeris, A. Emerging roles of microglial cathepsins in neurodegenerative disease. Brain Res. Bull. 2018, 139, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Heckler, I.; Venkataraman, I. Phosphorylated neurofilament heavy chain: A potential diagnostic biomarker in amyotrophic lateral sclerosis. J. Neurophysiol. 2022, 127, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Loveland, A.B.; Koh, C.S.; Ganesan, R.; Jacobson, A.; Korostelev, A.A. Structural mechanism of angiogenin activation by the ribosome. Nature 2024, 630, 769–776. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Wang, H.; Yang, K.; Luan, J.; Wang, S. TREM2: Potential therapeutic targeting of microglia for Alzheimer’s disease. Biomed. Pharmacother. 2023, 165, 115218. [Google Scholar] [CrossRef]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.; Younkin, S.; et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef]
- Ulland, T.K.; Colonna, M. TREM2—A key player in microglial biology and Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Yu, J.T.; Zhu, X.C.; Tan, L. TREM2 in Alzheimer’s disease. Mol. Neurobiol. 2016, 53, 3876–3887. [Google Scholar] [CrossRef] [PubMed]
- Jay, T.R.; Miller, C.M.; Cheng, P.J.; Graham, L.C.; Bemiller, S.; Broihier, M.L.; Xu, G.; Margevicius, D.; Karlo, J.C.; Sousa, G.L.; et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J. Exp. Med. 2017, 214, 2873–2889. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011, 12, 233. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; la Rosa, C.C.-D.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Aksnes, M.; Schibstad, M.H.; Chaudhry, F.A.; Neerland, B.E.; Caplan, G.; Saltvedt, I.; Eldholm, R.S.; Myrstad, M.; Edwin, T.H.; Persson, K.; et al. Differences in metalloproteinases and their tissue inhibitors in the cerebrospinal fluid are associated with delirium. Commun. Med. 2024, 4, 124. [Google Scholar] [CrossRef]
- Kettlun, A.M.; Cartier, L.; Garcia, L.; Collados, L.; Vasquez, F.; Ramirez, E.; Valenzuela, M.A. TIMPs and MMPs expression in CSF from patients with TSP/HAM. Life Sci. 2003, 72, 2863–2876. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Neurological diseases in relation to the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1139–1151. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Matrix metalloproteinases in neuroinflammation. Glia 2002, 39, 279–291. [Google Scholar] [CrossRef]
- Malec, J.F.; Brown, A.W.; Leibson, C.L.; Flaada, J.T.; Mandrekar, J.N.; Diehl, N.N.; Perkins, P.K. The mayo classification system for traumatic brain injury severity. J. Neurotrauma 2007, 24, 1417–1424. [Google Scholar] [CrossRef]
- Ghiam, M.K.; Patel, S.D.; Hoffer, A.; Selman, W.R.; Hoffer, B.J.; Hoffer, M.E. Drug Repurposing in the Treatment of Traumatic Brain Injury. Front. Neurosci. 2021, 15, 635483. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.T. Experimental models of repetitive brain injuries. Prog. Brain Res. 2007, 161, 253–261. [Google Scholar] [PubMed]
- Ates, O.; Cayli, S.; Altinoz, E.; Gurses, I.; Yucel, N.; Sener, M.; Kocak, A.; Yologlu, S. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol. Cell. Biochem. 2007, 294, 137–144. [Google Scholar] [CrossRef]
- Shi, Z.; Qiu, W.; Xiao, G.; Cheng, J.; Zhang, N. Resveratrol Attenuates Cognitive Deficits of Traumatic Brain Injury by Activating p38 Signaling in the Brain. Med. Sci. Monit. 2018, 24, 1097–1103. [Google Scholar] [CrossRef]
- Atalay, T.; Gulsen, I.; Colcimen, N.; Alp, H.H.; Sosuncu, E.; Alaca, I.; Ak, H.; Ragbetli, M.C. Resveratrol Treatment Prevents Hippocampal Neurodegeneration in a Rodent Model of Traumatic Brain Injury. Turk. Neurosurg. 2017, 27, 924–930. [Google Scholar]
- Rahman, M.H.; Akter, R.; Bhattacharya, T.; Abdel-Daim, M.M.; Alkahtani, S.; Arafah, M.W.; Al-Johani, N.S.; Alhoshani, N.M.; Alkeraishan, N.; Alhenaky, A.; et al. Resveratrol and Neuroprotection: Impact and Its Therapeutic Potential in Alzheimer’s Disease. Front. Pharmacol. 2020, 11, 619024. [Google Scholar] [CrossRef] [PubMed]
- Vingtdeux, V.; Giliberto, L.; Zhao, H.; Chandakkar, P.; Wu, Q.; Simon, J.E.; Janle, E.M.; Lobo, J.; Ferruzzi, M.G.; Davies, P.; et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 2010, 285, 9100–9113. [Google Scholar] [CrossRef]
- Shakibaei, M.; Csaki, C.; Nebrich, S.; Mobasheri, A. Resveratrol inhibits IL-1β-induced inflammatory signaling and apoptosis in human articular chondrocytes: Potential for use as a therapeutic agent for osteoarthritis. Biochem. Pharmacol. 2009, 77, 676–687. [Google Scholar]
- Zamzam, A.; Syed, M.H.; Rotstein, O.D.; Eikelboom, J.; Klein, D.J.; Singh, K.K.; Abdin, R.; Qadura, M. Validating fatty acid binding protein 3 as a diagnostic and prognostic biomarker for peripheral arterial disease: A three-year prospective follow-up study. eClinicalMedicine 2023, 55, 101766. [Google Scholar] [CrossRef]
- Zhong, F.F.; Wei, B.; Bao, G.X.; Lou, Y.P.; Wei, M.E.; Wang, X.Y.; Xiao, X.; Tian, J.J. FABP3 Induces Mitochondrial Autophagy to Promote Neuronal Cell Apoptosis in Brain Ischemia-Reperfusion Injury. Neurotox. Res. 2024, 42, 35. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef]
- Goedert, M. Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2001, 2, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008, 7, 97–109. [Google Scholar] [CrossRef]
- Vives-Bauza, C.; de Vries, R.L.; Tocilescu, M.A.; Przedborski, S. Is there a pathogenic role for mitochondria in Parkinson’s disease? Park. Relat Disord. 2009, 3, S241–S244. [Google Scholar] [CrossRef]
- Gautier, C.A.; Corti, O.; Brice, A. Mitochondrial dysfunctions in Parkinson’s disease. Rev. Neurol. 2014, 170, 339–343. [Google Scholar] [CrossRef]
- Cabreira, V.; Massano, J.J. [Parkinson’s Disease: Clinical Review and Update]. Acta Med. Port. 2019, 32, 661–670. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Obeso, J.A.; Stocchi, F. Drug insight: Continuous dopaminergic stimulation in the treatment of Parkinson’s disease, Nature clinical practice. Neurology 2006, 2, 382–392. [Google Scholar]
- Alqahtani, T.; Deore, S.L.; Kide, A.A.; Shende, B.A.; Sharma, R.; Chakole, R.D.; Nemade, L.S.; Kale, N.K.; Borah, S.; Deokar, S.S.; et al. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease, and Parkinson’s disease, Huntington’s disease and Amyotrophic Lateral Sclerosis-An updated review. Mitochondrion 2023, 71, 83–92. [Google Scholar] [CrossRef]
- Mahadevan, H.M.; Hashemiaghdam, A.; Ashrafi, G.; Harbauer, A.B. Mitochondria in Neuronal Health: From Energy Metabolism to Parkinson’s Disease. Adv. Biol. 2021, 5, e2100663. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Wang, Y.D.; Yu, X.M.; Li, D.W.; Li, G.R. Mitochondria-mediated damage to dopaminergic neurons in Parkinson’s disease. Int. J. Mol. Med. 2018, 41, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, E.; Schiemann, J.; Liss, B. Dopamine midbrain neurons in health and Parkinson’s disease: Emerging roles of voltage-gated calcium channels and ATP-sensitive potassium channels. Neuroscience 2015, 284, 798–814. [Google Scholar] [CrossRef] [PubMed]
- Trinh, D.; Israwi, A.R.; Arathoon, L.R.; Gleave, J.A.; Nash, J.E. The multi-faceted role of mitochondria in the pathology of Parkinson’s disease. J. Neurochem. 2021, 156, 715–752. [Google Scholar] [CrossRef]
- Mischley, L.K.; Shankland, E.; Liu, S.Z.; Bhayana, S.; Fox, D.J.; Marcinek, D.J. ATP and NAD+ Deficiency in Parkinson’s Disease. Nutrients 2023, 15, 943. [Google Scholar] [CrossRef]
- Siddique, A.H.H.; Kale, P.P. Importance of glucose and its metabolism in neurodegenerative disorder, as well as the combination of multiple therapeutic strategies targeting alpha-synuclein and neuroprotection in the treatment of Parkinson’s disease. Rev. Neurol. 2024, 180, 736–753. [Google Scholar] [CrossRef]
- Ribeiro, G.; Rodrigues, F.R.; Pasqualotto, E.; Dantas, J.M.; Di Luca, D.G. Exposure to Glycolysis-Enhancing Drugs and Risk of Parkinson’s Disease: A Meta-Analysis. J. Park. Dis. 2024, 14, 1237–1242. [Google Scholar] [CrossRef]
- Schultz, J.L.; Brinker, A.N.; Xu, J.; Ernst, S.E.; Tayyari, F.; Rauckhorst, A.J.; Liu, L.; Uc, E.Y.; Taylor, E.B.; Simmering, J.E.; et al. A pilot to assess target engagement of terazosin in Parkinson’s disease. Park. Relat. Disord. 2022, 94, 79–83. [Google Scholar] [CrossRef]
- Li, L.; Voullaire, L.; Sandi, C.; Pook, M.A.; Ioannou, P.A.; Delatycki, M.B.; Sarsero, J.P. Pharmacological screening using an FXN-EGFP cellular genomic reporter assay for the therapy of Friedreich ataxia. PLoS ONE 2013, 8, e55940. [Google Scholar] [CrossRef]
- Yiu, E.M.; Tai, G.; Peverill, R.E.; Lee, K.J.; Croft, K.D.; Mori, T.A.; Scheiber-Mojdehkar, B.; Sturm, B.; Praschberger, M.; Vogel, A.P.; et al. An open-label trial in Friedreich ataxia suggests clinical benefit with high-dose resveratrol, without effect on frataxin levels. J. Neurol. 2015, 262, 1344–1353. [Google Scholar] [CrossRef]
- Campos, D.; Monaga, M. Mucopolysaccharidosis type I: Current knowledge on its pathophysiological mechanisms. Metab. Brain Dis. 2012, 27, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Muenzer, J.; Wraith, J.E.; Clarke, L.A.; International Consensus Panel on Management and Treatment of Mucopolysaccharidosis I. Management and treatment guidelines. Pediatrics 2009, 123, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Giugliani, R.; Federhen, A.; Rojas, M.V.; Vieira, T.; Artigalas, O.; Pinto, L.L.; Azevedo, A.C.; Acosta, A.; Bonfim, C.; Lourenco, C.M.; et al. Mucopolysaccharidosis I, and VI: Brief review and guidelines for treatment. Genet. Mol. Biol. 2010, 33, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Ohno, K.; Maita, N.; Sakuraba, H. Structural and clinical implications of amino acid substitutions in alpha-L-iduronidase: Insight into the basis of mucopolysaccharidosis type I. Mol. Genet. Metab. 2014, 111, 107–112. [Google Scholar] [CrossRef]
- Rintz, E.; Ziemian, M.; Kobus, B.; Gaffke, L.; Pierzynowska, K.; Wegrzyn, G. Synergistic effects of resveratrol and enzyme replacement therapy in the Mucopolysaccharidosis type I. Biochem. Pharmacol. 2024, 229, 116467. [Google Scholar] [CrossRef]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef]

| Analytes | Baseline 52 Weeks | Baseline | 52 Weeks | |||||
|---|---|---|---|---|---|---|---|---|
| Paired t-Test | Wilcoxon Signed-Rank Test | Baseline: Active vs. Placebo | 52 Weeks: Active vs. Placebo | |||||
| Placebo | Resveratrol | Placebo | Active | Unpaired t-Test (Unequal) | Unpaired t-Test (Equal) | Unpaired t-Test (Unequal) | Unpaired t-Test (Equal) | |
| NSE | 0.8594 | 0.0051 ** | 0.3371 | 0.0047 ** | 0.0387 * | 0.0773 | <0.0001 **** | <0.0001 **** |
| Cathepsin D | 0.2053 | 0.0971 | 0.1153 | 0.0701 | 0.6863 | 0.7071 | 0.0002 *** | 0.0001 *** |
| TREM2 | 0.3622 | 0.2754 | 0.1153 | 0.1746 | 0.2755 | 0.243 | 0.0023 ** | 0.0048 ** |
| Angiogenin | 0.5284 | 0.0437 * | 0.4492 | 0.0249 * | 0.0002 *** | 0.0005 *** | 0.0636 | 0.1581 |
| FABP3 | 0.2216 | 0.0066 ** | 0.252 | 0.0027 ** | 0.3111 | 0.2762 | 0.164 * | 0.0205 * |
| Neurogranin | 0.3955 | 0.777 | 0.2152 | 0.2274 | 0.6684 | 0.656 | 0.2013 | 0.41 |
| p-NF | NA | NA | NA | NA | NA | NA | 0.0131 * | 0.0081 ** |
| MMP-2 | 0.1692 | 0.6759 | 0.229 | 0.8067 | NA | NA | NA | NA |
| MMP-9 | 0.3621 | 0.0003 *** | 0.3778 | 0.0004 *** | >0.9999 | >0.9999 | 0.0092 ** | 0.0058 ** |
| MMP-10 | 0.064 | 0.2527 | 0.0843 | 0.1502 | NA | NA | NA | NA |
| TIMP-1 | 0.1177 | 0.1368 | 0.0946 | 0.0698 | 0.5233 | 0.5187 | 0.8294 | 0.8293 |
| TIMP-2 | 0.9452 | 0.3568 | 0.8983 | 0.6702 | 0.4965 | 0.4867 | 0.8517 | 0.8600 |
| TIMP-3 | 0.0275 * | 0.0371 * | 0.0153 * | 0.0323 * | 0.3613 | 0.3692 | 0.0504 | 0.0688 |
| TIMP-4 | 0.0968 | 0.0126 * | 0.0583 | 0.0086 ** | 0.0002 *** | <0.0001 **** | <0.0001 **** | <0.0001 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Baxley, S.; Hebron, M.; Turner, R.S.; Moussa, C. Resveratrol Attenuates CSF Markers of Neurodegeneration and Neuroinflammation in Individuals with Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 5044. https://doi.org/10.3390/ijms26115044
Liu X, Baxley S, Hebron M, Turner RS, Moussa C. Resveratrol Attenuates CSF Markers of Neurodegeneration and Neuroinflammation in Individuals with Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(11):5044. https://doi.org/10.3390/ijms26115044
Chicago/Turabian StyleLiu, Xiaoguang, Sean Baxley, Michaeline Hebron, Raymond Scott Turner, and Charbel Moussa. 2025. "Resveratrol Attenuates CSF Markers of Neurodegeneration and Neuroinflammation in Individuals with Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 11: 5044. https://doi.org/10.3390/ijms26115044
APA StyleLiu, X., Baxley, S., Hebron, M., Turner, R. S., & Moussa, C. (2025). Resveratrol Attenuates CSF Markers of Neurodegeneration and Neuroinflammation in Individuals with Alzheimer’s Disease. International Journal of Molecular Sciences, 26(11), 5044. https://doi.org/10.3390/ijms26115044






