The Development of 3D Primary Co-Culture Models of the Human Airway
Abstract
1. Introduction
2. Results
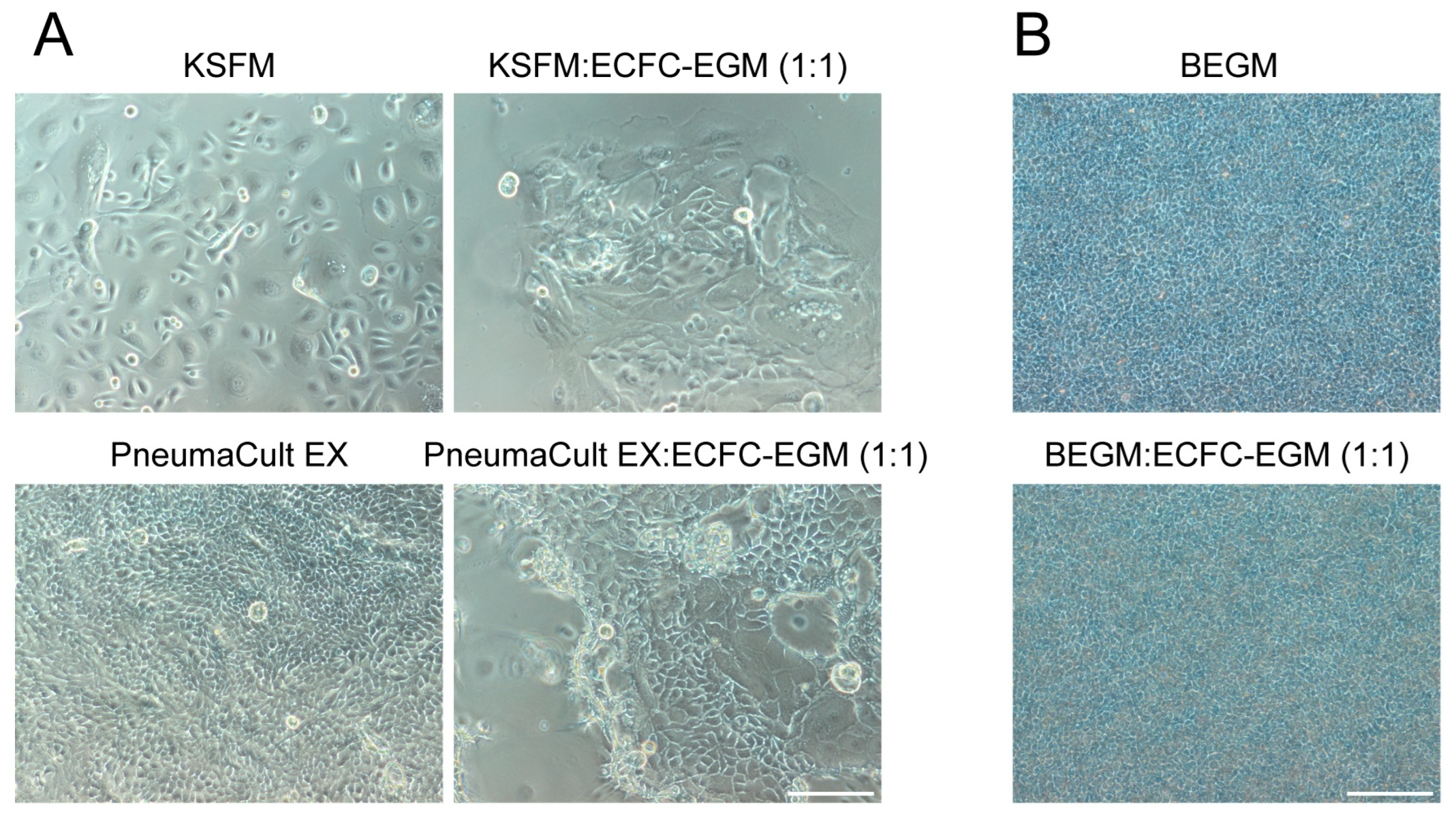
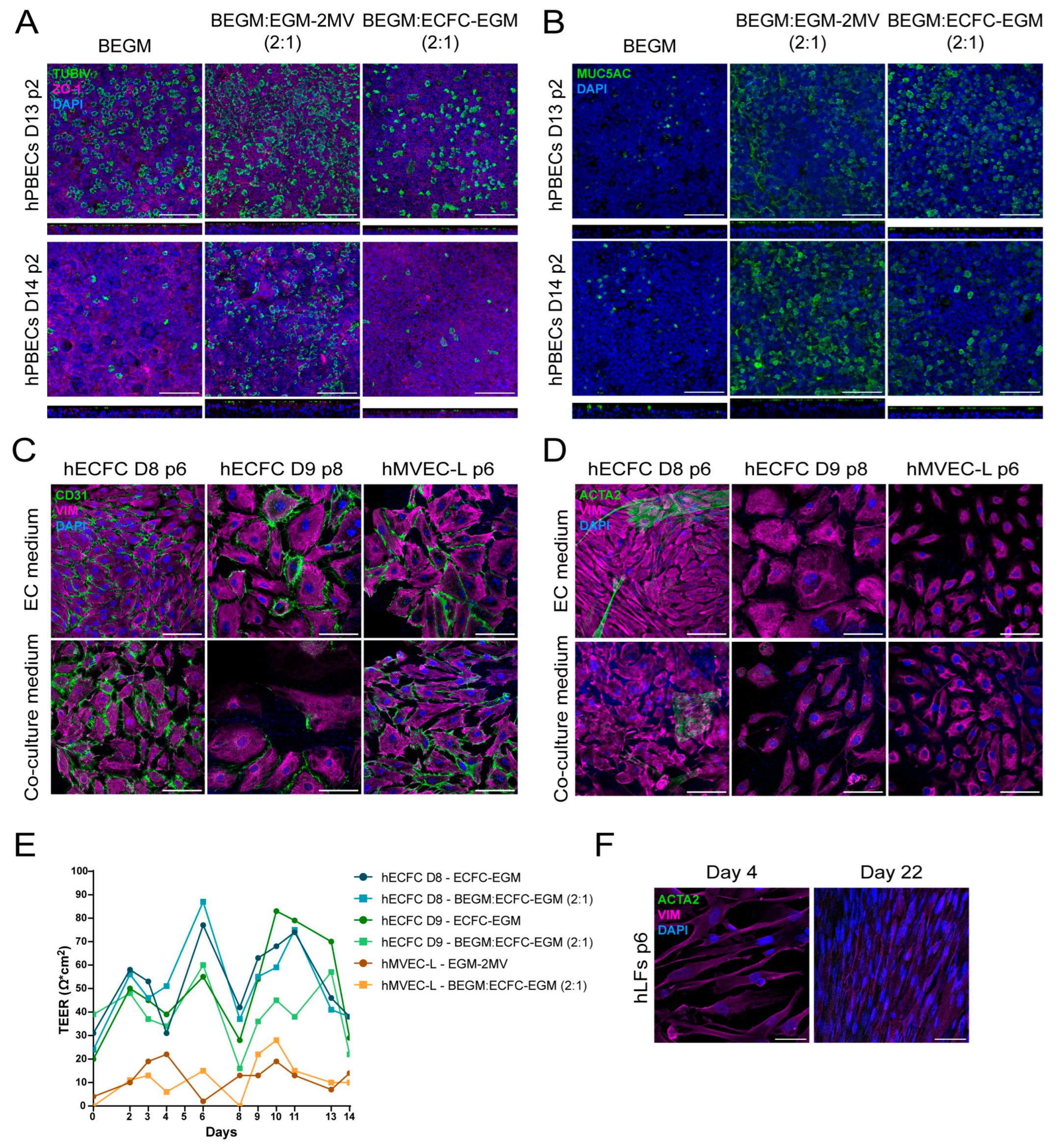
2.1. Selecting Co-Culture Medium for Primary Co-Culture of Lung Cells
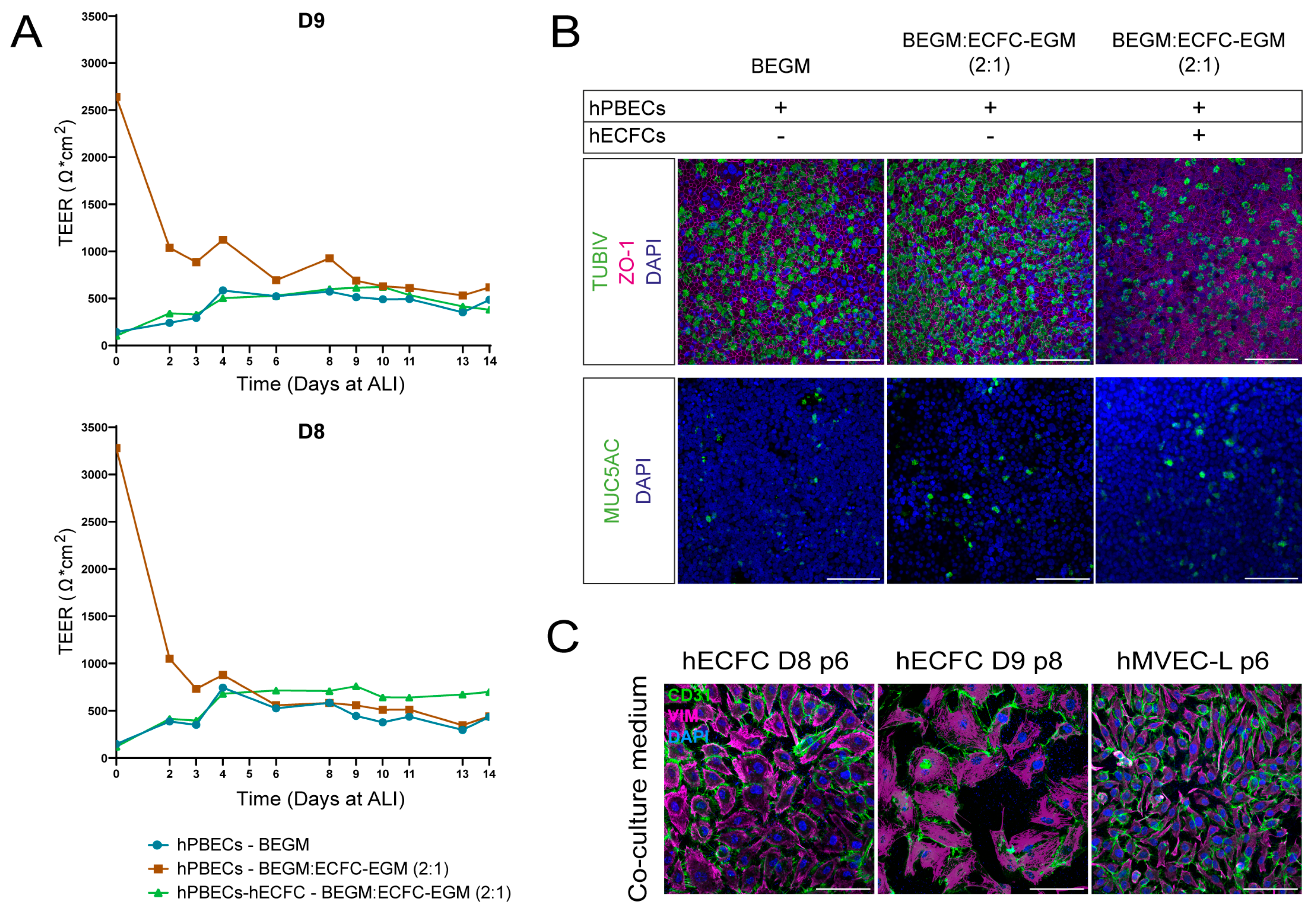
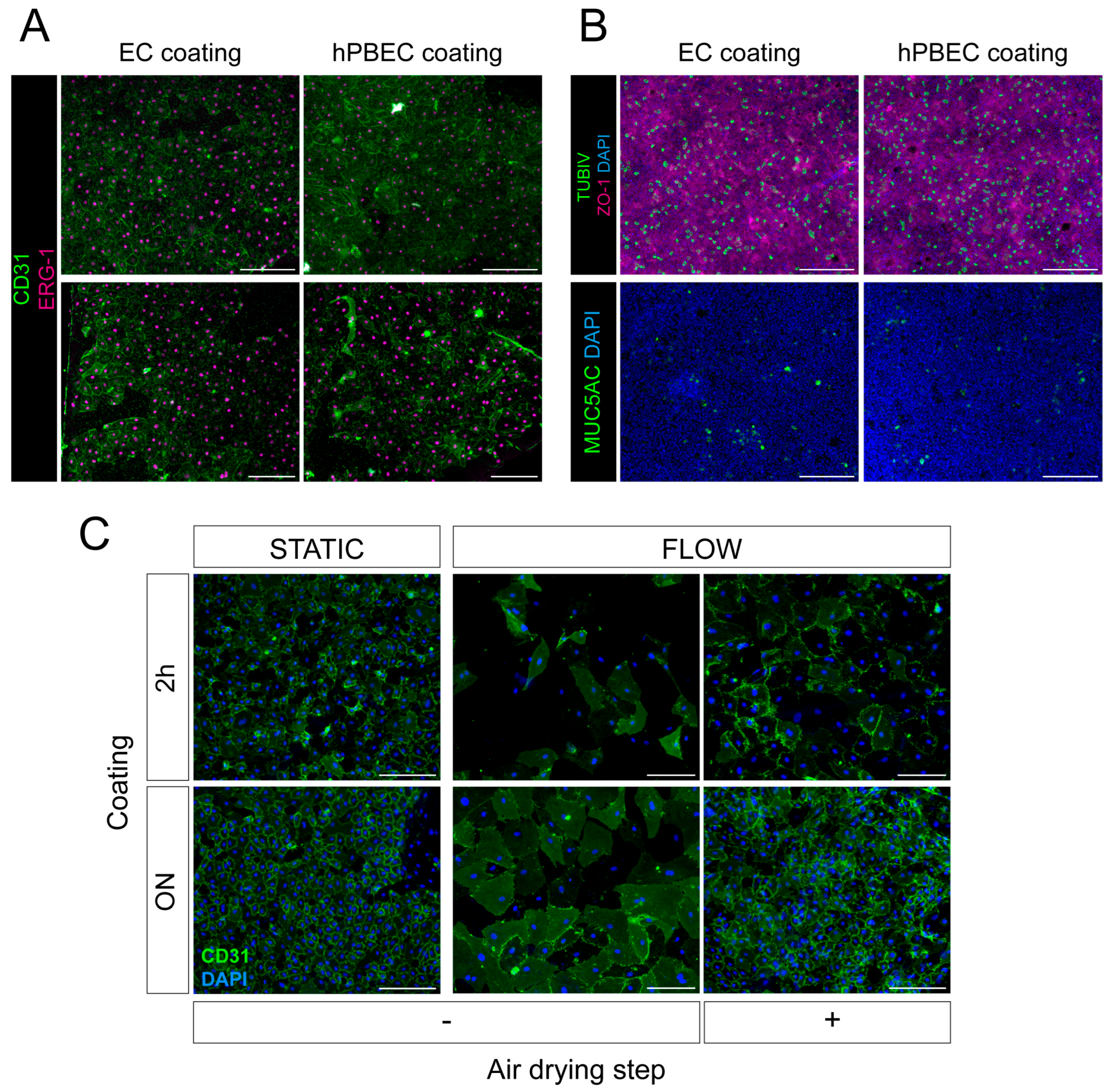
2.2. Selecting Surface Coating for Primary hPBECs-ECs Co-Cultures
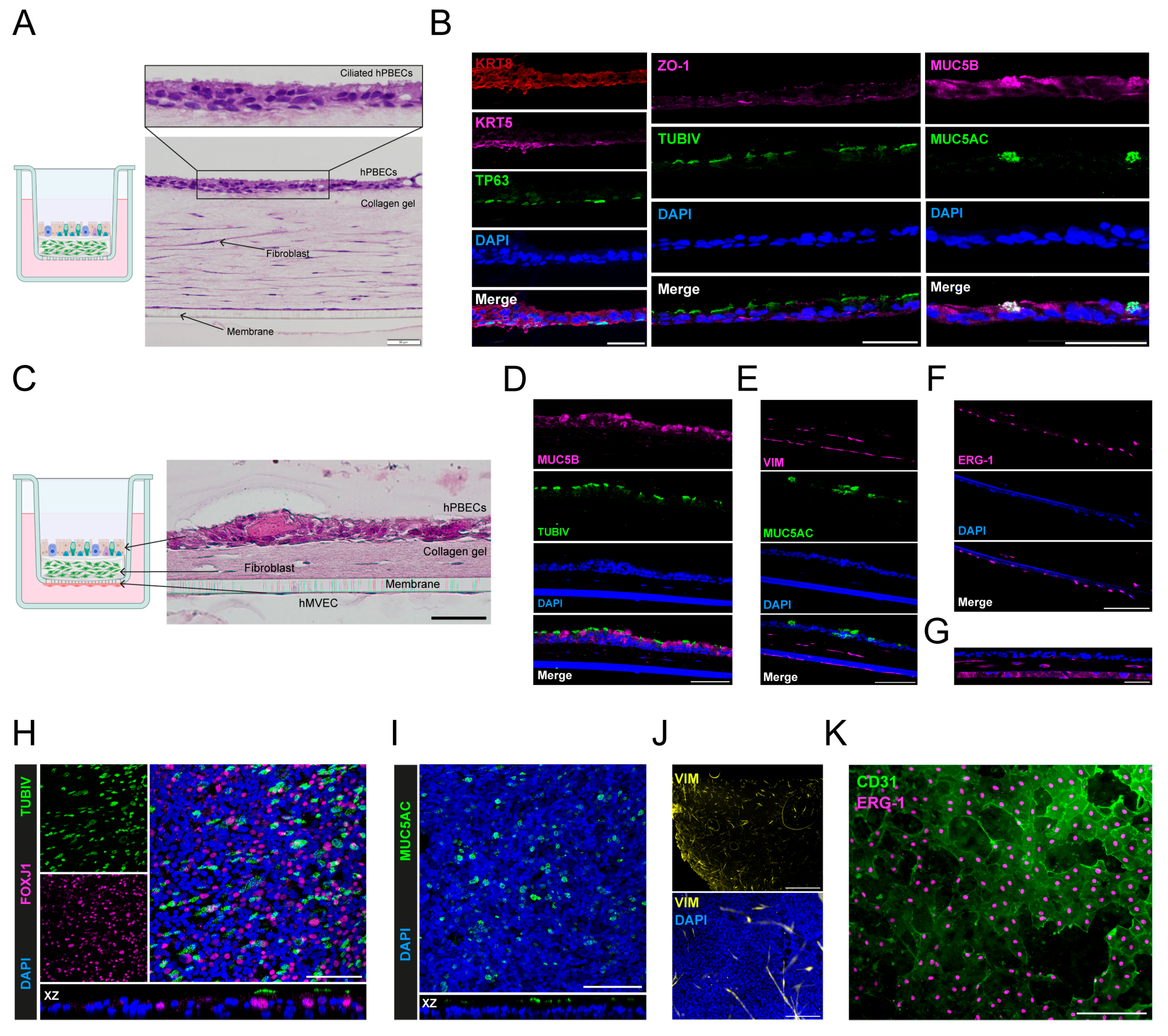
2.3. Selecting a Three-Dimensional Hydrogel for In Vitro Cultures
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zar, H.J.; Ferkol, T.W. The global burden of respiratory disease-Impact on child health. Pediatr. Pulmonol. 2014, 49, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.A.; Kalin, T.V.; Xu, Y.; Kalinichenko, V.V. Building and Regenerating the Lung Cell by Cell. Physiol. Rev. 2019, 99, 513–554. [Google Scholar] [CrossRef] [PubMed]
- Schilders, K.A.A.; Eenjes, E.; van Riet, S.; Poot, A.A.; Stamatialis, D.; Truckenmüller, R.; Hiemstra, P.S.; Rottier, R.J. Regeneration of the lung: Lung stem cells and the development of lung mimicking devices. Respir. Res. 2016, 17, 44. [Google Scholar] [CrossRef]
- Bailey, K.E.; Floren, M.L.; D’Ovidio, T.J.; Lammers, S.R.; Stenmark, K.R.; Magin, C.M. Tissue-informed engineering strategies for modeling human pulmonary diseases. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 316, L303–L320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burgstaller, G.; Oehrle, B.; Gerckens, M.; White, E.S.; Schiller, H.B.; Eickelberg, O. The instructive extracellular matrix of the lung: Basic composition and alterations in chronic lung disease. Eur. Respir. J. 2017, 50, 1601805. [Google Scholar] [CrossRef] [PubMed]
- Loewa, A.; Feng, J.J.; Hedtrich, S. Human disease models in drug development. Nat. Rev. Bioeng. 2023, 1, 545–559. [Google Scholar] [CrossRef]
- Marx, U.; Andersson, T.B.; Bahinski, A.; Beilmann, M.; Beken, S.; Cassee, F.R.; Cirit, M.; Daneshian, M.; Fitzpatrick, S.; Frey, O.; et al. Biology-inspired microphysiological system approaches to solve the prediction dilemma of substance testing. Altex 2016, 33, 272–321. [Google Scholar] [CrossRef]
- Pezzulo, A.A.; Starner, T.D.; Scheetz, T.E.; Traver, G.L.; Tilley, A.E.; Harvey, B.-G.; Crystal, R.G.; McCray, P.B., Jr.; Zabner, J. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2011, 300, L25–L31. [Google Scholar] [CrossRef]
- Silva, S.; Bicker, J.; Falcão, A.; Fortuna, A. Air-liquid interface (ALI) impact on different respiratory cell cultures. Eur. J. Pharm. Biopharm. 2023, 184, 62–82. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Cozens, D.; Grahame, E.; Sutherland, E.; Taylor, G.; Berry, C.C.; Davies, R.L. Development and optimization of a differentiated airway epithelial cell model of the bovine respiratory tract. Sci. Rep. 2018, 8, 853. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bukowy-Bieryllo, Z. Long-term differentiating primary human airway epithelial cell cultures: How far are we? Cell Commun. Signal. 2021, 19, 63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. SLAS Technol. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Eenjes, E.; van Riet, S.; Kroon, A.A.; Slats, A.M.; Khedoe, P.P.S.J.; Boerema-de Munck, A.; Kempen, M.J.B.-V.; Ninaber, D.K.; Reiss, I.K.M.; Clevers, H.; et al. Disease modeling following organoid-based expansion of airway epithelial cells. Am. J. Physiol.-Lung Cell. Mol. Physiology. 2021, 321, L775–L786. [Google Scholar] [CrossRef]
- Nawroth, J.C.; Roth, D.; van Schadewijk, A.; Ravi, A.; Maulana, T.I.; Senger, C.N.; van Riet, S.; Ninaber, D.K.; de Waal, A.M.; Kraft, D.; et al. Breathing on chip: Dynamic flow and stretch accelerate mucociliary maturation of airway epithelium in vitro. Mater. Today Bio 2023, 21, 100713. [Google Scholar] [CrossRef]
- Iriondo, C.; Koornneef, S.; Skarp, K.P.; Buscop-van Kempen, M.; Boerema-de Munck, A.; Rottier, R.J. Simple-Flow: A 3D-Printed Multiwell Flow Plate to Coculture Primary Human Lung Cells at the Air-Liquid Interface. ACS Biomater. Sci. Eng. 2025, 11, 451–462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gkatzis, K.; Taghizadeh, S.; Huh, D.; Stainier, D.Y.R.; Bellusci, S. Use of three-dimensional organoids and lung-on-a-chip methods to study lung development, regeneration and disease. Eur. Respir. J. 2018, 52, 1800876. [Google Scholar] [CrossRef]
- Phutane, P.; Telange, D.; Agrawal, S.; Gunde, M.; Kotkar, K.; Pethe, A. Biofunctionalization and Applications of Polymeric Nanofibers in Tissue Engineering and Regenerative Medicine. Polymers 2023, 15, 1202. [Google Scholar] [CrossRef]
- op ‘t Veld, R.C.; van den Boomen, O.I.; Lundvig, D.M.S.; Bronkhorst, E.M.; Kouwer, P.H.J.; Jansen, J.A.; Middelkoop, E.; Hoff, J.W.V.D.; Rowan, A.E.; Wagener, F.A. Thermosensitive biomimetic polyisocyanopeptide hydrogels may facilitate wound repair. Biomaterials 2018, 181, 392–401. [Google Scholar] [CrossRef]
- Gustafsson, L.; Tasiopoulos, C.P.; Jansson, R.; Kvick, M.; Duursma, T.; Gasser, T.C.; van der Wijngaart, W.; Hedhammar, M. Recombinant Spider Silk Forms Tough and Elastic Nanomembranes that are Protein-Permeable and Support Cell Attachment and Growth. Adv. Funct. Mater. 2020, 30, 2002982. [Google Scholar] [CrossRef]
- O’Leary, C.; Gilbert, J.L.; O’Dea, S.; O’Brien, F.J.; Cryan, S.-A. Respiratory Tissue Engineering: Current Status and Opportunities for the Future. Tissue Eng. Part B Rev. 2015, 21, 323–344. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Eckerle, I.; Lenk, M.; Ulrich, R. More Novel Hantaviruses and Diversifying Reservoir Hosts-Time for Development of Reservoir-Derived Cell Culture Models? Viruses 2014, 6, 951–967. [Google Scholar] [CrossRef]
- Richter, M.; Piwocka, O.; Musielak, M.; Piotrowski, I.; Suchorska, W.M.; Trzeciak, T. From Donor to the Lab: A Fascinating Journey of Primary Cell Lines. Front. Cell Dev. Biol. 2021, 9, 711381. [Google Scholar] [CrossRef]
- Inoue, H.; Akimoto, K.; Homma, T.; Tanaka, A.; Sagara, H. Airway Epithelial Dysfunction in Asthma: Relevant to Epidermal Growth Factor Receptors and Airway Epithelial Cells. J. Clin. Med. 2020, 9, 3698. [Google Scholar] [CrossRef]
- Leung, C.; Wadsworth, S.J.; Yang, S.J.; Dorscheid, D.R. Structural and functional variations in human bronchial epithelial cells cultured in air-liquid interface using different growth media. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 318, L1063–L1073. [Google Scholar] [CrossRef]
- Gray, T.E.; Guzman, K.; Davis, C.W.; Abdullah, L.H.; Nettesheim, P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1996, 14, 104–112. [Google Scholar] [CrossRef]
- Kreimendahl, F.; Ossenbrink, S.; Köpf, M.; Westhofen, M.; Schmitz-Rode, T.; Fischer, H.; Jockenhoevel, S.; Thiebes, A.L. Combination of vascularization and cilia formation for three-dimensional airway tissue engineering. J. Biomed. Mater. Res. Part A. 2019, 107, 2053–2062. [Google Scholar] [CrossRef]
- Luengen, A.E.; Cheremkhina, M.; Gonzalez-Rubio, J.; Weckauf, J.; Kniebs, C.; Uebner, H.; Buhl, E.M.; Taube, C.; Cornelissen, C.G.; Schmitz-Rode, T.; et al. Bone Marrow Derived Mesenchymal Stromal Cells Promote Vascularization and Ciliation in Airway Mucosa Tri-Culture Models in Vitro. Front. Bioeng. Biotechnol. 2022, 10, 872275. [Google Scholar] [CrossRef]
- Mejías, J.C.; Nelson, M.R.; Liseth, O.; Roy, K. A 96-well format microvascularized human lung-on-a-chip platform for microphysiological modeling of fibrotic diseases. Lab Chip 2020, 20, 3601–3611. [Google Scholar] [CrossRef]
- Pasman, T.; Baptista, D.; van Riet, S.; Truckenmüller, R.K.; Hiemstra, P.S.; Rottier, R.J.; Hamelmann, N.M.; Paulusse, J.M.J.; Stamatialis, D.; Poot, A.A. Development of an In Vitro Airway Epithelial–Endothelial Cell Culture Model on a Flexible Porous Poly(Trimethylene Carbonate) Membrane Based on Calu-3 Airway Epithelial Cells and Lung Microvascular Endothelial Cells. Membranes 2021, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Sellgren, K.L.; Butala, E.J.; Gilmour, B.P.; Randell, S.H.; Grego, S. A biomimetic multicellular model of the airways using primary human cells. Lab Chip 2014, 14, 3349–3358. [Google Scholar] [CrossRef]
- Leslie Fulcher, M.; Gabriel, S.; Burns, K.A.; Yankaskas, J.R.; Randell, S.H. Well-Differentiated Human Airway Epithelial Cell Cultures. In Human Cell Culture Protocols; Humana Press: Totowa, NJ, USA, 2005; pp. 183–206. [Google Scholar]
- You, Y.; Brody, S.L. Culture and Differentiation of Mouse Tracheal Epithelial Cells. In Epithelial Cell Culture Protocols, 2nd ed.; Randell, S.H., Fulcher, M.L., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 123–143. [Google Scholar]
- Alphonse, R.S.; Vadivel, A.; Zhong, S.; McConaghy, S.; Ohls, R.; Yoder, M.C.; Thebaud, B. The isolation and culture of endothelial colony-forming cells from human and rat lungs. Nat. Protoc. 2015, 10, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.A.; Mead, L.E.; Tanaka, H.; Meade, V.; Fenoglio, A.; Mortell, K.; Ferkowicz, M.J.; Gilley, D.; Yoder, M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004, 104, 2752–2760. [Google Scholar] [CrossRef]
- Comhair, S.A.; Xu, W.; Mavrakis, L.; Aldred, M.A.; Asosingh, K.; Erzurum, S.C. Human primary lung endothelial cells in culture. Am. J. Respir. Cell Mol. Biol. 2012, 46, 723–730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van der Heijden, M.; van Nieuw Amerongen, G.P.; van Bezu, J.; Paul, M.A.; Groeneveld, A.B.J.; van Hinsbergh, V.W.M. Opposing Effects of the Angiopoietins on the Thrombin-Induced Permeability of Human Pulmonary Microvascular Endothelial Cells. PLoS ONE 2011, 6, e23448. [Google Scholar] [CrossRef]
- Gupta, K.; Ramakrishnan, S.; Browne, P.V.; Solovey, A.; Hebbel, R.P. A Novel Technique for Culture of Human Dermal Microvascular Endothelial Cells under either Serum-Free or Serum-Supplemented Conditions: Isolation by Panning and Stimulation with Vascular Endothelial Growth Factor. Exp. Cell Res. 1997, 230, 244–251. [Google Scholar] [CrossRef]
- Labitzke, R.; Friedl, P. A serum-free medium formulation supporting growth of human umbilical cord vein endothelial cells in long-term cultivation. Cytotechnology 2001, 35, 87–92. [Google Scholar] [CrossRef]
- Liu, Z.; Mackay, S.; Gordon, D.M.; Anderson, J.D.; Haithcock, D.W.; Garson, C.J.; Tearney, G.J.; Solomon, G.M.; Pant, K.; Prabhakarpandian, B.; et al. Co-cultured microfluidic model of the airway optimized for microscopy and micro-optical coherence tomography imaging. Biomed. Opt. Express 2019, 10, 5414–5430. [Google Scholar] [CrossRef]
- Dupont-Gillain, C.C.; Pamula, E.; Denis, F.A.; Rouxhet, P.G. Nanostructured layers of adsorbed collagen: Conditions, mechanisms and applications. In Surface and Colloid Science; Springer: Berlin/Heidelberg, Germany, 2004; pp. 98–104. [Google Scholar]
- Dupont-Gillain, C.C. Understanding and controlling type I collagen adsorption and assembly at interfaces, and application to cell engineering. Colloids Surf. B Biointerfaces 2014, 124, 87–96. [Google Scholar] [CrossRef]
- Jacquemart, I.; Pamuła, E.; De Cupere, V.M.; Rouxhet, P.G.; Dupont-Gillain, C.C. Nanostructured collagen layers obtained by adsorption and drying. J. Colloid Interface Sci. 2004, 278, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Keresztes, Z.; Rouxhet, P.G.; Remacle, C.; Dupont-Gillain, C. Supramolecular assemblies of adsorbed collagen affect the adhesion of endothelial cells. J. Biomed. Mater. Res. Part A 2006, 76A, 223–233. [Google Scholar] [CrossRef]
- Nadzir, M.M.; Kino-oka, M.; Sugawara, K.; Taya, M. Effect of preservation conditions of collagen substrate on its fibril formation and rabbit chondrocyte morphology. J. Biosci. Bioeng. 2012, 114, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raub, C.B.; Putnam, A.J.; Tromberg, B.J.; George, S.C. Predicting bulk mechanical properties of cellularized collagen gels using multiphoton microscopy. Acta Biomater. 2010, 6, 4657–4665. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pageau, S.C.; Sazonova, O.V.; Wong, J.Y.; Soto, A.M.; Sonnenschein, C. The effect of stromal components on the modulation of the phenotype of human bronchial epithelial cells in 3D culture. Biomaterials 2011, 32, 7169–7180. [Google Scholar] [CrossRef]
- Dallon, J.C.; Ehrlich, H.P. A review of fibroblast-populated collagen lattices. Wound Repair Regen. 2008, 16, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.G.; Ko, J.A.; Iwata, W.; Okumichi, H.; Kiuchi, Y. An in vitro study of scarring formation mediated by human Tenon fibroblasts: Effect of Y-27632, a Rho kinase inhibitor. Cell Biochem. Funct. 2019, 37, 113–124. [Google Scholar] [CrossRef]
- Zhang, T.; Day, J.H.; Su, X.; Guadarrama, A.G.; Sandbo, N.K.; Esnault, S.; Denlinger, L.C.; Berthier, E.; Theberge, A.B. Investigating Fibroblast-Induced Collagen Gel Contraction Using a Dynamic Microscale Platform. Front. Bioeng. Biotechnol. 2019, 7, 196. [Google Scholar] [CrossRef]
- Cross, V.L.; Zheng, Y.; Won Choi, N.; Verbridge, S.S.; Sutermaster, B.A.; Bonassar, L.J.; Fischbach, C.; Stroock, A.D. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 2010, 31, 8596–8607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishihara, S.; Haga, H. Matrix Stiffness Contributes to Cancer Progression by Regulating Transcription Factors. Cancers 2022, 14, 1049. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, M.E.; Papakonstantinou, E.; Stolz, D. Matrix Metalloproteinases in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2023, 24, 3786. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martin-Martin, B.; Tovell, V.; Dahlmann-Noor, A.H.; Khaw, P.T.; Bailly, M. The effect of MMP inhibitor GM6001 on early fibroblast-mediated collagen matrix contraction is correlated to a decrease in cell protrusive activity. Eur. J. Cell biol. 2011, 90, 26–36. [Google Scholar] [CrossRef]
- Ishikawa, S.; Ishimori, K.; Ito, S. A 3D epithelial–mesenchymal co-culture model of human bronchial tissue recapitulates multiple features of airway tissue remodeling by TGF-β1 treatment. Respir. Res. 2017, 18, 195. [Google Scholar] [CrossRef]
- Choe, M.M.; Sporn, P.H.; Swartz, M.A. An in vitro airway wall model of remodeling. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2003, 285, L427–L433. [Google Scholar] [CrossRef] [PubMed]
- Choe, M.M.; Tomei, A.A.; Swartz, M.A. Physiological 3D tissue model of the airway wall and mucosa. Nat. Protoc. 2006, 1, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Leach, T.; Gandhi, U.; Reeves, K.D.; Stumpf, K.; Okuda, K.; Marini, F.C.; Walker, S.J.; Boucher, R.; Chan, J.; Cox, L.A.; et al. Development of a novel air–liquid interface airway tissue equivalent model for in vitro respiratory modeling studies. Sci. Rep. 2023, 13, 10137. [Google Scholar] [CrossRef]
- Doryab, A.; Schmid, O. Towards a gold standard functional readout to characterize In Vitro lung barriers. Eur. J. Pharm. Sci. 2022, 179, 106305. [Google Scholar] [CrossRef] [PubMed]
- Eenjes, E.; Mertens, T.C.J.; Buscop-van Kempen, M.J.; van Wijck, Y.; Taube, C.; Rottier, R.J.; Hiemstra, P.S. A novel method for expansion and differentiation of mouse tracheal epithelial cells in culture. Sci. Rep. 2018, 8, 7349. [Google Scholar] [CrossRef]
- Dekkers, J.F.; Alieva, M.; Wellens, L.M.; Ariese, H.C.R.; Jamieson, P.R.; Vonk, A.M.; Amatngalim, G.D.; Hu, H.; Oost, K.C.; Snippert, H.J.G.; et al. High-resolution 3D imaging of fixed and cleared organoids. Nat. Protoc. 2019, 14, 1756–1771. [Google Scholar] [CrossRef] [PubMed]
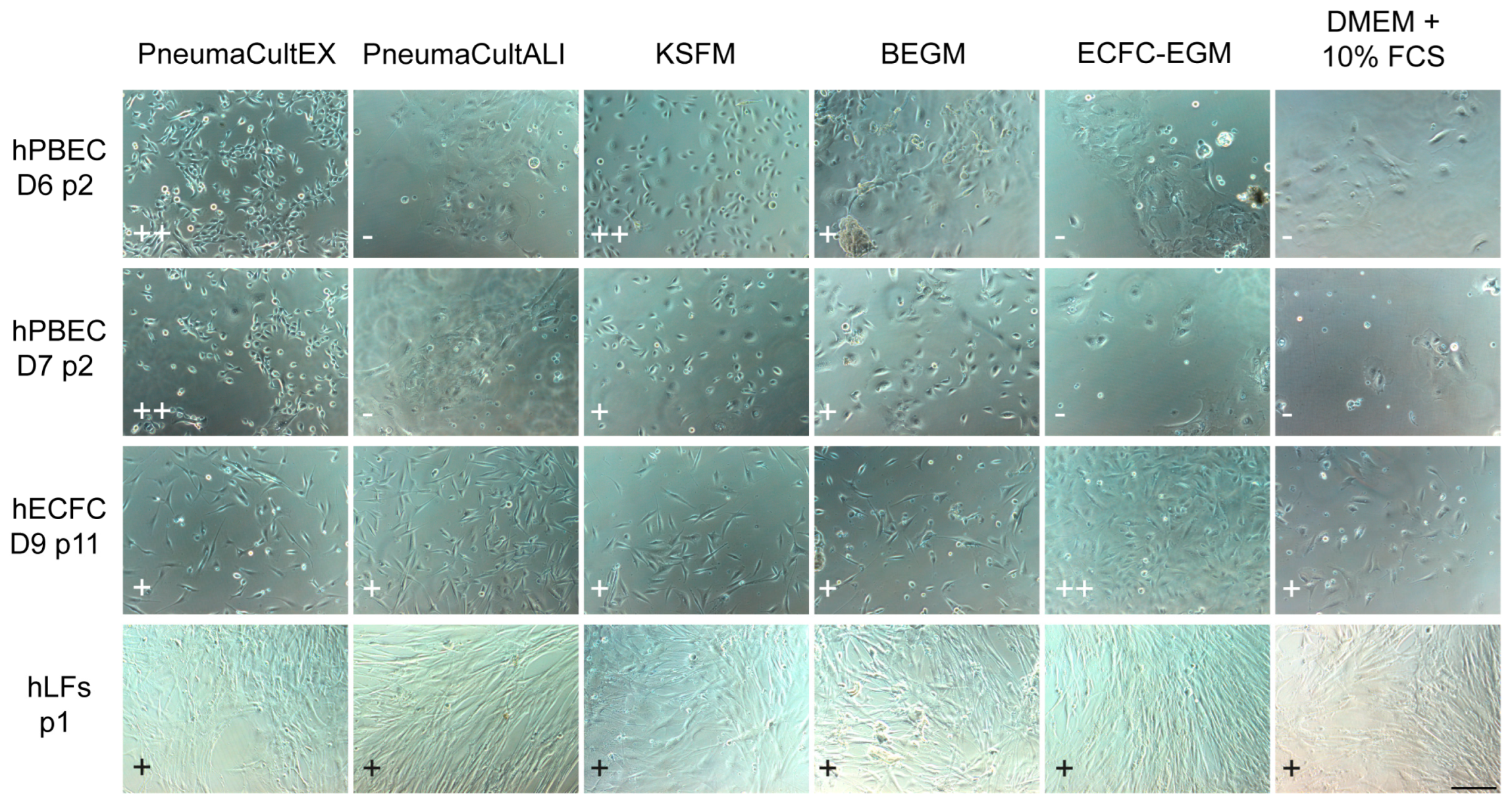
| Cells/Medium | Complete KSFM | Complete BEGM | ECs Medium | DMEM + 10% FBS | Co-Culture Medium |
|---|---|---|---|---|---|
| hPBECs proliferation | ++ | + | − | − | − |
| hPBECs differentiation | + | ++ | − | − | + |
| ECs | − | − | ++ | + | + |
| hLFs | − | − | ++ | ++ | + |
| Tri-culture | − | − | − | − | + |
| Primary Cell Type | Source Cells | Medium | Coating |
|---|---|---|---|
| hPBECs | Erasmus MC | Complete KSFM (growth medium) | hPBECs coating |
| Complete BEGM (differentiation medium) | |||
| hMVEC | Erasmus MC | EGM-2MV | EC coating |
| hMVEC-L | Lonza | EGM-2MV | No coating or EC coating |
| HUVEC | Lonza | HUVEC-EGM | No coating or EC coating |
| hECFCs | Erasmus MC | ECFC-EGM | EC coating |
| hLFs | Erasmus MC | DMEM + 10% FBS | No coating |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iriondo, C.; Koornneef, S.; Skarp, K.-P.; Buscop-van Kempen, M.; Boerema-de Munck, A.; Rottier, R.J. The Development of 3D Primary Co-Culture Models of the Human Airway. Int. J. Mol. Sci. 2025, 26, 5027. https://doi.org/10.3390/ijms26115027
Iriondo C, Koornneef S, Skarp K-P, Buscop-van Kempen M, Boerema-de Munck A, Rottier RJ. The Development of 3D Primary Co-Culture Models of the Human Airway. International Journal of Molecular Sciences. 2025; 26(11):5027. https://doi.org/10.3390/ijms26115027
Chicago/Turabian StyleIriondo, Cinta, Sem Koornneef, Kari-Pekka Skarp, Marjon Buscop-van Kempen, Anne Boerema-de Munck, and Robbert J. Rottier. 2025. "The Development of 3D Primary Co-Culture Models of the Human Airway" International Journal of Molecular Sciences 26, no. 11: 5027. https://doi.org/10.3390/ijms26115027
APA StyleIriondo, C., Koornneef, S., Skarp, K.-P., Buscop-van Kempen, M., Boerema-de Munck, A., & Rottier, R. J. (2025). The Development of 3D Primary Co-Culture Models of the Human Airway. International Journal of Molecular Sciences, 26(11), 5027. https://doi.org/10.3390/ijms26115027






